Abstract
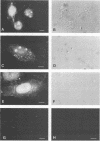
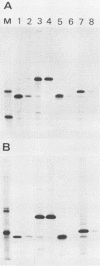
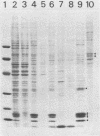
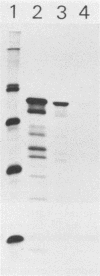
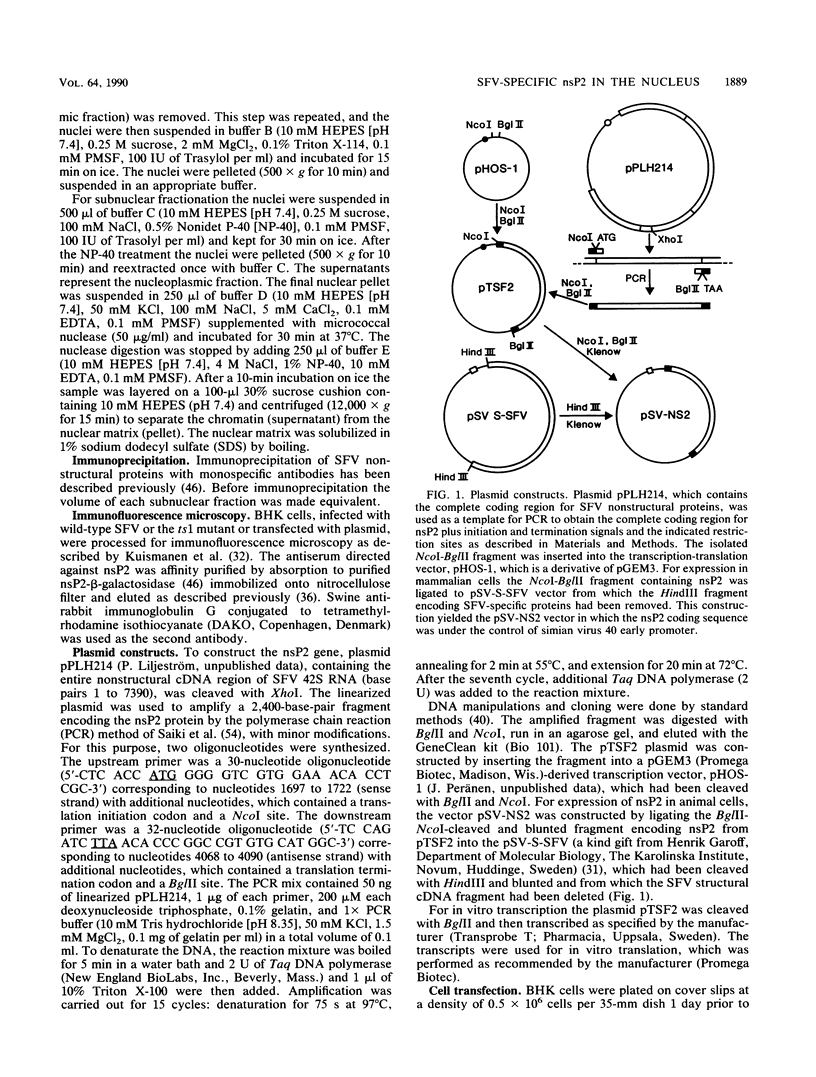
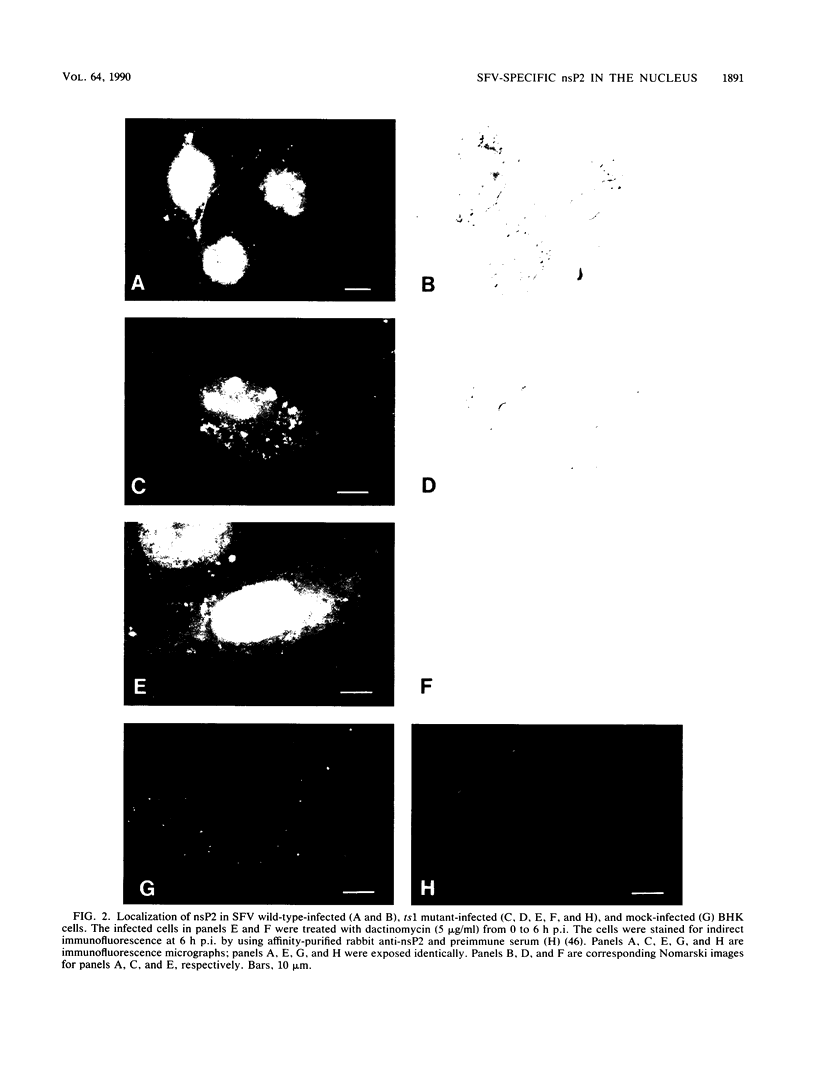
About 50% of Semliki Forest virus-specific nonstructural protein nsP2 is associated with the nuclear fraction in virus-infected BHK cells. Transport into the nucleus must be specific, since only trace amounts of nsP3 and nsP4 and about 13% of nsP1, all derived from the same polyprotein, were found in the nucleus. Subfractionation of [35S]methionine-labeled Semliki Forest virus-infected cells showed that 80 to 90% of the nuclear nsP2 was associated with the nuclear matrix. Indirect immunofluorescence, with anti-nsP2 antiserum, showed the most intensive staining of structures which by Nomarski optics appeared to be nucleoli. In the presence of 1 to 5 micrograms of dactinomycin per ml the nuclei were stained evenly and no nucleoli could be found. Transport of nsP2 into the nucleus occurred early in infection and was fairly rapid. A cDNA encoding the complete nsP2 was isolated by the polymerase chain reaction technique and ligated into a simian virus 40 expression vector derivative. When BHK cells were transfected with this pSV-NS2 vector by the lipofection procedure, nsP2 was expressed in about 1 to 5% of the cells, as shown by indirect immunofluorescence. In positively transfected cells the immunofluorescence stain was most intensive in the nucleoli. Thus, Semliki Forest virus-specific nsP2 must have information which directs it into the nuclear matrix and, more specifically, into the nucleoli.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton D. J., Sawicki S. G., Sawicki D. L. Demonstration in vitro of temperature-sensitive elongation of RNA in Sindbis virus mutant ts6. J Virol. 1988 Oct;62(10):3597–3602. doi: 10.1128/jvi.62.10.3597-3602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Abulafia R., Bratosin S. Herpes simplex virus and protein transport are associated with the cytoskeletal framework and the nuclear matrix in infected BSC-1 cells. Virology. 1983 Sep;129(2):501–507. doi: 10.1016/0042-6822(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Benavente R., Rose K. M., Reimer G., Hügle-Dörr B., Scheer U. Inhibition of nucleolar reformation after microinjection of antibodies to RNA polymerase I into mitotic cells. J Cell Biol. 1987 Oct;105(4):1483–1491. doi: 10.1083/jcb.105.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Lampert M. A., Li A. C., Baluda M. A. Nuclear compartmentalization of the v-myb oncogene product. Mol Cell Biol. 1985 Nov;5(11):3017–3023. doi: 10.1128/mcb.5.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Gomatos P. J. Concomitant methylation and synthesis in vitro of Semliki Forest virus (SFV) ss RNAs by a fraction from infected cells. Virology. 1981 Oct 30;114(2):542–554. doi: 10.1016/0042-6822(81)90234-8. [DOI] [PubMed] [Google Scholar]
- Cross R. K. Identification of a unique guanine-7-methyltransferase in Semliki Forest virus (SFV) infected cell extracts. Virology. 1983 Oct 30;130(2):452–463. doi: 10.1016/0042-6822(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Ding M. X., Schlesinger M. J. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. doi: 10.1016/0042-6822(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Hancock D. C. Studies on the interaction of the human c-myc protein with cell nuclei: p62c-myc as a member of a discrete subset of nuclear proteins. Cell. 1985 Nov;43(1):253–261. doi: 10.1016/0092-8674(85)90030-3. [DOI] [PubMed] [Google Scholar]
- Faaberg K. S., Peeples M. E. Strain variation and nuclear association of Newcastle disease virus matrix protein. J Virol. 1988 Feb;62(2):586–593. doi: 10.1128/jvi.62.2.586-593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J Gen Virol. 1975 Feb;26(2):183–196. doi: 10.1099/0022-1317-26-2-183. [DOI] [PubMed] [Google Scholar]
- Froshauer S., Kartenbeck J., Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988 Dec;107(6 Pt 1):2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Käriäinen L., Keränen S., Ranki M., Sawicki D. L. Semliki Forest virus replication complex capable of synthesizing 42S and 26S nascent RNA chains. J Gen Virol. 1980 Jul;49(1):61–69. doi: 10.1099/0022-1317-49-1-61. [DOI] [PubMed] [Google Scholar]
- Hahn Y. S., Grakoui A., Rice C. M., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol. 1989 Mar;63(3):1194–1202. doi: 10.1128/jvi.63.3.1194-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügle B., Scheer U., Franke W. W. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985 Jun;41(2):615–627. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- Ishida I., Simizu B., Koizumi S., Oya A., Yamada M. Nucleoside triphosphate phosphohydrolase produced in BHK cells infected with western equine encephalitis virus is probably associated with the 82K dalton nonstructural polypeptide. Virology. 1981 Jan 15;108(1):13–20. doi: 10.1016/0042-6822(81)90523-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Okret S., Wikström A. C., Gustafsson J. A., Shaper J. H. Binding of the glucocorticoid receptor to the rat liver nuclear matrix. The role of disulfide bond formation. J Biol Chem. 1986 Sep 15;261(26):11962–11967. [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Isolation and basic characterization of temperature-sensitive mutants from Semliki Forest virus;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):810–820. doi: 10.1111/j.1699-0463.1974.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Senechek D., Rice S. A., Smith J. L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987 Feb;61(2):276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Simizu B., Hashimoto K., Oya A., Yamada M. Inhibition of DNA synthesis in BHK cells infected with western equine encephalitis virus. 1. Induction of an inhibitory factor of cellular DNA polymerase activity. Virology. 1979 Apr 30;94(2):314–322. doi: 10.1016/0042-6822(79)90464-1. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Simizu B., Ishida I., Oya A., Yamada M. Inhibition of DNA synthesis in BHK cells infected with western equine encephalitis virus. 2. Properties of the inhibitory factor of DNA polymerase induced in infected cells. Virology. 1979 Oct 30;98(2):439–447. doi: 10.1016/0042-6822(79)90566-x. [DOI] [PubMed] [Google Scholar]
- Kondor-Koch C., Burke B., Garoff H. Expression of Semliki Forest virus proteins from cloned complementary DNA. I. The fusion activity of the spike glycoprotein. J Cell Biol. 1983 Sep;97(3):644–651. doi: 10.1083/jcb.97.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Hedman K., Saraste J., Pettersson R. F. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982 Nov;2(11):1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Takkinen K., Keränen S., Söderlund H. Replication of the genome of alphaviruses. J Cell Sci Suppl. 1987;7:231–250. [PubMed] [Google Scholar]
- Lachmi B. E., Käriäinen L. Sequential translation of nonstructural proteins in cells infected with a Semliki Forest virus mutant. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1936–1940. doi: 10.1073/pnas.73.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmi B., Käriäinen L. Control of protein synthesis in Semliki forest virus-infected cells. J Virol. 1977 Apr;22(1):142–149. doi: 10.1128/jvi.22.1.142-149.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Last R. L., Woolford J. L., Jr Identification and nuclear localization of yeast pre-messenger RNA processing components: RNA2 and RNA3 proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2103–2112. doi: 10.1083/jcb.103.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. P., Rice C. M. Mutagenesis of the in-frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J Virol. 1989 Mar;63(3):1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Puddington L., McCreedy B. J., Jr Vesicular stomatitis virus M protein in the nuclei of infected cells. J Virol. 1988 Nov;62(11):4387–4392. doi: 10.1128/jvi.62.11.4387-4392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y., Tadano M., Anzai T., Ma S. P., Yasuda S., Fukunaga T. Detection of dengue 4 virus core protein in the nucleus. II. Antibody against dengue 4 core protein produced by a recombinant baculovirus reacts with the antigen in the nucleus. J Gen Virol. 1989 Jun;70(Pt 6):1417–1425. doi: 10.1099/0022-1317-70-6-1417. [DOI] [PubMed] [Google Scholar]
- Mi S., Durbin R., Huang H. V., Rice C. M., Stollar V. Association of the Sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989 Jun;170(2):385–391. doi: 10.1016/0042-6822(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Pienta K. J., Barrack E. R., Coffey D. S. The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- Newport J. W., Forbes D. J. The nucleus: structure, function, and dynamics. Annu Rev Biochem. 1987;56:535–565. doi: 10.1146/annurev.bi.56.070187.002535. [DOI] [PubMed] [Google Scholar]
- Nickerson J. A., Krochmalnic G., Wan K. M., Penman S. Chromatin architecture and nuclear RNA. Proc Natl Acad Sci U S A. 1989 Jan;86(1):177–181. doi: 10.1073/pnas.86.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Nuclear function and organization: the potential of immunochemical approaches. Int Rev Cytol. 1988;110:27–92. doi: 10.1016/s0074-7696(08)61847-1. [DOI] [PubMed] [Google Scholar]
- Peränen J., Takkinen K., Kalkkinen N., Käriäinen L. Semliki Forest virus-specific non-structural protein nsP3 is a phosphoprotein. J Gen Virol. 1988 Sep;69(Pt 9):2165–2178. doi: 10.1099/0022-1317-69-9-2165. [DOI] [PubMed] [Google Scholar]
- Pinard M. F., Simard R., Bibor-Hardy V. DNA-binding proteins of herpes simplex virus type 1-infected BHK cell nuclear matrices. J Gen Virol. 1987 Mar;68(Pt 3):727–735. doi: 10.1099/0022-1317-68-3-727. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Nuclear localization of herpesvirus proteins: potential role for the cellular framework. Mol Cell Biol. 1983 Mar;3(3):315–324. doi: 10.1128/mcb.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M., Käriäinen L. Solubilized RNA replication complex from Semliki Forest virus-infected cells. Virology. 1979 Oct 30;98(2):298–307. doi: 10.1016/0042-6822(79)90553-1. [DOI] [PubMed] [Google Scholar]
- Ranki M., Ulmanen I., Käriäinen L. Semliki Forest virus-specific nonstructural protein is associated with ribosomes. FEBS Lett. 1979 Dec 1;108(1):299–302. doi: 10.1016/0014-5793(79)81232-6. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Rihs H. P., Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989 May;8(5):1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saraste J., Käiäinen L., Söderlund H., Keränen S. RNA synthesis directed by a temperature-sensitive mutant of Semliki Forest virus. J Gen Virol. 1977 Nov;37(2):399–406. doi: 10.1099/0022-1317-37-2-399. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Functional analysis of the A complementation group mutants of Sindbis HR virus. Virology. 1985 Jul 15;144(1):20–34. doi: 10.1016/0042-6822(85)90301-0. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of loss of regulation of minus-strand RNA synthesis on Sindbis virus replication. Virology. 1986 Jun;151(2):339–349. doi: 10.1016/0042-6822(86)90054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of overproduction of nonstructural proteins on alphavirus plus-strand and minus-strand RNA synthesis. Virology. 1986 Jul 30;152(2):507–512. doi: 10.1016/0042-6822(86)90157-1. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. doi: 10.1128/jvi.4.2.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickedanz J., Scheidtmann K. H., Walter G. Kinetics of nuclear transport and oligomerization of simian virus 40 large T antigen. Virology. 1986 Jan 15;148(1):47–57. doi: 10.1016/0042-6822(86)90402-2. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Nuclear subcompartmentalization of simian virus 40 large T antigen: evidence for in vivo regulation of biochemical activities. J Virol. 1989 May;63(5):2308–2316. doi: 10.1128/jvi.63.5.2308-2316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Specific interaction of simian virus 40 large T antigen with cellular chromatin and nuclear matrix during the course of infection. J Virol. 1987 Nov;61(11):3561–3569. doi: 10.1128/jvi.61.11.3561-3569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Boyle W. J., Keith D. E., Press M. F., Golde D. W., Souza L. M. Subnuclear localization of the trans-activating protein of human T-cell leukemia virus type I. J Virol. 1988 Mar;62(3):680–686. doi: 10.1128/jvi.62.3.680-686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos N. M., Chakrabarti S., Moss B., Hare J. D. Expression of polyomavirus virion proteins by a vaccinia virus vector: association of VP1 and VP2 with the nuclear framework. J Virol. 1987 Feb;61(2):516–525. doi: 10.1128/jvi.61.2.516-525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers J. K., Swanepoel R., Shepherd S. P. Protein synthesis in Rift Valley fever virus-infected cells. Virology. 1984 Apr 15;134(1):118–124. doi: 10.1016/0042-6822(84)90277-0. [DOI] [PubMed] [Google Scholar]
- Tadano M., Makino Y., Fukunaga T., Okuno Y., Fukai K. Detection of dengue 4 virus core protein in the nucleus. I. A monoclonal antibody to dengue 4 virus reacts with the antigen in the nucleus and cytoplasm. J Gen Virol. 1989 Jun;70(Pt 6):1409–1415. doi: 10.1099/0022-1317-70-6-1409. [DOI] [PubMed] [Google Scholar]
- Takkinen K. Complete nucleotide sequence of the nonstructural protein genes of Semliki Forest virus. Nucleic Acids Res. 1986 Jul 25;14(14):5667–5682. doi: 10.1093/nar/14.14.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H. J., Schlingemann R. O., Boehmer A. L., van Driel R., Brakenhoff G. J., Ramaekers F. C. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. I. Intracellular localization during interphase. J Cell Sci. 1989 Jan;92(Pt 1):123–130. doi: 10.1242/jcs.92.1.123. [DOI] [PubMed] [Google Scholar]
- van der Velden H. M., Wanka F. The nuclear matrix--its role in the spatial organization and replication of eukaryotic DNA. Mol Biol Rep. 1987;12(2):69–77. doi: 10.1007/BF00368873. [DOI] [PubMed] [Google Scholar]