Abstract
Traveling, living and working in space is now a reality. The number of people and length of time in space is increasing. With new horizons for exploration it becomes more important to fully understand and provide countermeasures to the effects of the space environment on the human body. In addition, space provides a unique laboratory to study how life and physiologic functions adapt from the cellular level to that of the entire organism. Caenorhabditis elegans is a genetic model organism used to study physiology on Earth. Here we provide a description of the rationale, design, methods, and space culture validation of the ICE-FIRST payload, which engaged C. elegans researchers from four nations. Here we also show C. elegans growth and development proceeds essentially normally in a chemically defined liquid medium on board the International Space Station (10.9 day round trip). By setting flight constraints first and bringing together established C. elegans researchers second, we were able to use minimal stowage space to successfully return a total of 53 independent samples, each containing more than a hundred individual animals, to investigators within one year of experiment concept. We believe that in the future, bringing together individuals with knowledge of flight experiment operations, flight hardware, space biology, and genetic model organisms should yield similarly successful payloads.
Keywords: Caenorhabditis elegans, Spaceflight, Development, Axenic culture, Astrobiology
1. Introduction
On Earth, there is a long history of using model systems to understand physiologic processes. This remains the case, as evidenced by recent Nobel prizes in Medicine or Physiology awarded for experiments utilizing eukaryotic genetic model organisms: zebrafish and Drosophila melanogaster in 1995 (Ringertz, 1997), yeast in 2001 (Frangsmyr, 2002), Caenorhabditis elegans in 2002 and 2006 (Frangsmyr, 2003, 2007), and mice in 2007 (Manis, 2007). As we strive to understand physiologic alterations in the space environment, studies on non-human models will be essential. While rats are a historically important model for physiology, they are still being developed into a genetic model organism (Twigger et al., 2005). At least until this developments is complete, the use of already well developed genetic models affords several advantages.
In the case of C. elegans, experimentation is relatively easy and inexpensive with animals having a short gestation period and producing large numbers of progeny on Earth (Bahls et al., 2003). The complete anatomical description of the cell contacts and innervations of each muscle cell is available (White et al., 1986). The completion of the genome sequence (Consortium, 1998) provides a powerful reservoir of information that has led to genome-wide RNAi libraries (Kamath and Ahringer, 2003), genome-wide mapping of protein–protein interactions (Li et al., 2004), and identification of C. elegans homologues of positionally cloned genes mutated in human diseases (Culetto and Sattelle, 2000). Excellent bioinformatics tools such as Worm-base (www.wormbase.org) allow practical use of this information (Stein et al., 2001). Mutant strains are easily isolated and characterized with mutations in many genes of biomedical interest available. For example, mutations affecting neuromuscular transmission and signal transduction are readily available (Chervitz et al., 1998; Bargmann and Kaplan, 1998). A number of excellent texts reviewing C. elegans biology and experimental methods have been produced, including a free on-line text produced by the C. elegans research community (www.wormbook.org). Combined, these resources make C. elegans a leading organism in which to conduct systems biology experiments (Lehner, 2007).
The promise of C. elegans as a model system for space biology studies has previously been reviewed (Johnson and Nelson, 1991; Zhao et al., 2005), and a number of laboratories, on the ground, are already studying processes relevant to astronaut health. These include radiation biology, muscle physiology, immune response, and stress response. We sought to harness the C. elegans research community to understand how spaceflight affects physiology by bringing together C. elegans investigators from four nations. In the past, C. elegans experiments in space were principally conducted by one investigator interested in one specific question. Prior to ICE-FIRST, there were four spaceflights involving live C. elegans. On STS-42, males were found to mate successfully and C. elegans was observed to successfully complete two continuous full life cycles in space without gross developmental abnormalities (Nelson et al., 1994a,b). On STS-42 and STS-76, an increased rate of mutation was observed and demonstrated to be the direct effect of cosmic radiation and not micro-gravity (Hartman et al., 2001; Nelson et al., 1994b). On STS-95, the flown, and ground control, animals died, presumably from anoxia or some other bio-incompatibility of the flight hardware (E. Moss, personal communication). On STS-107, in addition to surviving the breakup (Szewczyk and McLamb, 2005), the lack of developmental abnormalities on STS-42 was confirmed and the validation of a chemically defined medium for C. elegans growth in flight was begun (Szewczyk et al., 2005). For ICE-FIRST, we theorized that if we focused on setting a general experiment design first, using standardized culturing conditions, and bringing on experienced C. elegans researchers second, we could conduct more science than on a single PI driven flight.
On Earth, C. elegans growth is more or less standardized by using Nematode Growth Medium (NGM, Brenner, 1974). NGM has been an immensely successful tool in producing the wealth of information about C. elegans biology. However, this medium has some important disadvantages for use in prolonged spaceflight. Cultures on NGM require weekly manual transfer to new plates, requiring astronaut time that is often limited (Board, 1998). Use of the chemically defined axenic medium, C. elegans Maintenance Medium (CeMM, Lu and Goetsch, 1993), allows both liquid and solid cultivation. Three features of CeMM culturing minimize the need for astronaut intervention. First, animals on CeMM will grow and reproduce considerably longer than on NGM without subculturing (Szewczyk et al., 2003). Second, culture automation is possible using liquid CeMM. Third, automated analysis of liquid-grown animals is possible using either video recordings or fluorescence sensors. The second two features are major potential advantages for future experiments in space where down mass may be limited or non-existent. Therefore, we standardized experiments carried out on ICE-FIRST to use CeMM rather than NGM.
While CeMM affords numerous advantages for conducting experiments upon C. elegans in space, the majority of data on C. elegans come from NGM grown animals. Past studies show that there are clear differences in animals grown on NGM vs. in or on CeMM (Szewczyk et al., 2003, 2006). Specifically, in CeMM vs. NGM grown animals: development is slower, fecundity is reduced, lifespan is increased, lipid and protein stores are decreased, and gene expression is altered (Szewczyk et al., 2006). Additionally, CeMM grown animals appear to enter the developmentally arrested dauer state at a temperature slightly lower than NGM grown animals do, suggesting CeMM grown animals may be slightly more sensitive to stress (Szewczyk et al., 2005). Because the bulk of observed changes are consistent both with altered metabolism and with DAF-16 (FOXO) controlled phenotypes it has been proposed that the bulk of changes are due to altered insulin-like signaling in CeMM grown vs. NGM grown animals (Szewczyk et al., 2006). The differences in CeMM vs. NGM grown animals along with the, at present, limited data on CeMM grown animals make validation of culture methods and experimental procedures important prior to carrying out experiments in space.
Here we report that growth and development are essentially normal in liquid CeMM on board the International Space Station. Having thus validated the use of liquid CeMM to grow C. elegans in space, we were able to return 53 samples to investigators from four space agencies while using a limited amount of stowage space. Results of experiments by the individual investigators are reported elsewhere (Higashitani et al., 2005; Higashibata et al., 2006; Zhao et al., 2006; Leandro et al., 2007; Adachi et al., in press; Selch et al., in press) and will be reviewed in a forthcoming issue. The robust scientific return obtained with only one year elapsing between in-flight experiment conception and completion suggests that the use of genetic model organisms with standardized culturing conditions can speed research advances in space life science.
2. Methods
2.1. Nematode handling
For the validation studies presented here, wild-type C. elegans strain CC1, derived from the wild-type strain N2 (Szewczyk et al., 2003), was maintained in CeMM (Lu and Goetsch, 1993) obtained on contract from Fisher (Santa Clara, CA). For studies involving solidified CeMM, plates were composed of 1.7% agar and CeMM. For studies starting from eggs, eggs were obtained by bleaching (Szewczyk et al., 2003). Population densities were obtained by counting as previously described (Lu and Goetsch, 1993). Lengths of cuticular sheaths were obtained by measuring as previously described (Jantunen, 1964). Videos of animals were recorded at the landing site within 2 h of return utilizing a Proscope (Bodelin Technologies, www.theproscope.com).
2.2. Animals flown on the Delta Mission
Animals flown on the European Space Agency's Delta Mission were prepared in Toulouse, France 5−7 days prior to launch, depending on each individual experiment. Animals were loaded into prearranged flight hardware by the Principal Investigators. Photographs of all of the hardware used for this mission can be found in the Centre National d'Estudes Spatiales public outreach booklet “ICE-FIRST of worms and men” which is available as a portable document file on the web (http://www.gsbms.ups-tlse.fr/actu.html or http://weboflife.nasa.gov/celegans/questionsice.htm) or on request. Most of the hardware used is shown in Fig. 1. For samples fixed on orbit, animals were loaded into Culture Chamber Andromede like (CCA) inserts (Fig. 1d, Horn and Sebastian, 1999), which were housed in Biorack Type I Experimental Containers (EC1) (Nelson et al., 1995). For samples returned alive, animals were loaded in cell culture bags previously validated for use during spaceflight (Fig. 1c, Vassy et al., 2001), housed in a Vented EC1 (Horn and Sebastian, 1999). Once loaded, samples were placed in a Thermocase (Comat, Toulouse, France) and held at 12 °C until 14 h prior to launch. Samples were transported, in the Thermocase, to Moscow by Air France and to Baikonur by an Energia contracted air carrier. The day before launch, flight samples in culture bags were examined for overall appearance and potential contaminates. In some cases flight samples were replaced by transport controls (details in Section 2.3). Approximately 14 h prior to launch, samples were transferred to Kubik Topaz (Comat, Toulouse, France) and held at 12 °C. Upon docking with the International Space Station, 2 days after launch, samples were transferred to Aquarius (Comat, Toulouse, France) and held at 20 °C. Three days after launch, samples were transferred to Kubik Amber (Comat, Toulouse, France) and held at 20 °C for the duration of the mission. Autonomous temperature recorders housed with the samples indicate that there was a brief (<1 min) temperature increase to 25 °C during the transfer to the International Space Station, that the internal temperature of the Aquarius was actually 26 °C for the day the samples were in Aquarius, and that there was a brief (<1 min) temperature increase to 31 °C during reentry of the Soyuz. Some samples were fixed by manual activation at launch plus 6 days and 4 h (designated samples ICE-01 and ICE-03) and launch plus 8 days and 4 h (designated samples ICE-02 and ICE-04). All samples were returned to Earth in a passive thermal case. Samples were recovered within 2 h of landing. The majority of samples were flash frozen (freezing time < 1 min) by contact with a metal bar that had been housed in liquid/gaseous Nitrogen (−196 °C/−135 °C) overnight. Following freezing samples were stored in the gaseous Nitrogen transport container until return to the Principal Investigators (the container was refreshed with liquid Nitrogen upon return to Moscow and prior to return to Toulouse the next day). A small minority of samples were returned to the Principal Investigators alive with incubation from the landing site to Toulouse at 12 °C in a Thermocase. Air carriers were the same as above. Two flight samples were video recorded on site and returned alive as detailed above.
Fig. 1.
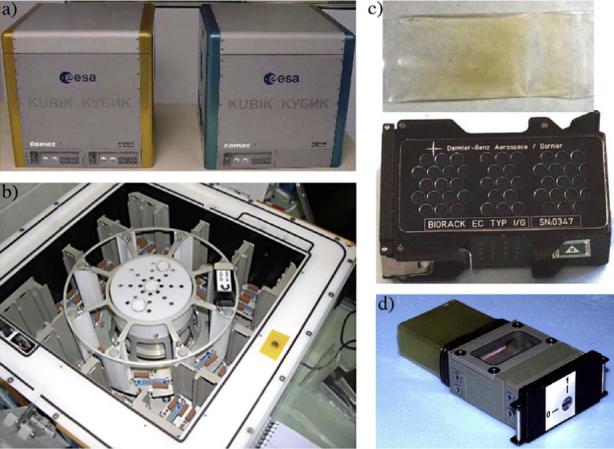
Sample images of flight hardware. (a) The Kubik incubators Amber (left) and Topaz (right). The outer dimensions of the Kubiks are 37 × 37 × 37 cm. (b) Internal view of Kubik Amber. Amber is equipped with a centrifuge that can hold up to eight EC1s, with an additional 16 maintained around the edges. (c) Live culture hardware. Worms we grown in culture bags (above), housed inside of vented EC1s (below). The culture bag shown contains 5 ml of cultured worms; 2.5, 5, and 10 ml culture sizes were tested and utilized. The internal dimensions of the EC1 are approximately 81 × 40 × 20 mm. Each EC1 can hold approximately 40 ml total volume of cultured worms within 4−16 culture bags. (d) Hardware for fixation on orbit. The CCA was housed within a non-vented EC1 (not shown). The CCA contains an air reservoir (top left), a culture chamber (middle, beneath glass), and a fixative (bottom right). Fixation is achieved by turning the indicator to the on position, releasing fixative into the culture chamber.
2.3. Transport controls
A second set of flight samples was identically prepared. To allow for healthy flight samples and diagnosis of preflight problems, this set of samples was transported to Baikonur with the flight samples. The day before launch, flight samples in culture bags were examined for overall appearance and potential contaminates. In 7 of 53 cases flight samples were replaced by transport controls. After this health check, culture bags were photographed and reinte-grated into the flight cassettes. Post flight examination revealed only one sample had developed contamination, and it was one that had replaced a contaminated sister culture. A small set of transport controls were fixed 3 h post launch to allow for later examination by the Principal Investigators. The transport controls were subsequently used as backup ground controls with temperature maintained using the transport Thermocase and operations on a 12 h delay.
2.4. Ground controls
A third set of flight samples was identically prepared. These samples remained in Toulouse for the duration of the flight. On orbit operations were completed on a 12 h delay. Temperature was maintained utilizing a Thermocase. None of the temperature abnormalities recorded during the mission were simulated in the ground controls as they were only observed following the mission and did not appear to impact the validation studies presented here.
3. Results
3.1. Flight hardware validation
Type I cassettes have previously been utilized for growth of C. elegans in flight (Nelson et al., 1994a,b). However, the prior experiments were conducted with animals grown on NGM and not in CeMM and results from STS-107 suggest that CeMM grown animals may be more sensitive to biocompatibility issues and/or environmental stressors than NGM grown animals (Szewczyk et al., 2005). Additionally, growth in CeMM is associated with altered metabolism (Szewczyk et al., 2006) and the use of hermetically sealed cassettes presents the problem of limited oxygen for the animals. To circumvent the problem of limited oxygen, two approaches were taken. First, samples to be returned alive were cultured in gas permeable culture bags within modified type I cassettes equipped with a Gortex membrane for gas exchange (Fig. 1c, Horn and Sebastian, 1999). Second, for samples to be fixed on orbit, samples were cultured in CCA inserts which contain an air reservoir (Fig. 1d, Horn and Sebastian, 1999). To assure that animals were obtaining sufficient oxygen and to rule out other potential biocompatibility issues, growth in the proposed flight hardware was examined.
Population growth for a starting population of 500 eggs was calculated from previously obtained data (Szewczyk et al., 2006) as a reference point (Table 1). Growth of 500 eggs was then measured in culture bags either placed in a petri dish, a vented type I cassette, or an unvented type I cassette (Table 1). At 18 °C growth in culture bags in a vented type I cassette was within ten percent of that calculated for growth in standard laboratory hardware and similar to that for growth in other hardware proposed for culturing C. elegans in space (Fahlen et al., 2005). This growth was significantly better than in unvented cassettes or culture bags alone. At 25 °C growth in culture bags in a vented type I cassette was well below that calculated for growth in standard laboratory hardware.
Table 1.
Preflight growth in CeMM in type I cassettes
| 18 °C (n = 2 trials) (mean ± sd) | 25 °C (n = 2 trials) (mean ± sd) | |
|---|---|---|
| Normal laboratory growth (Reference value) | 67,500 | 42,000 |
| Culture bag only | 37,500 ± 1000 | 17,500 ± 1000 |
| Vented type I cassette | 62,500 ± 1000 | 19,375 ± 1000 |
| Unvented type I cassette | 6250 ± 500 | 1000 ± 100 |
Experiments designed to be performed in the CCA hardware called for worms to be at specific life stages at specified times during the flight. To accomplish this, rate of development from egg was compared between animals grown in the CCAs and in standard laboratory hardware. No differences in rate of development were observed under any of the various temperature profiles that were considered with animals reaching early adulthood at the time of the first fixation.
3.2. CeMM cultured C. elegans grow normally on the International Space Station
To validate in flight use of CeMM, growth on orbit was compared to growth on the ground. Animals grown on orbit display no statistically significant defects in population growth (Table 2). Growth from various larval stages on solid CeMM was normal as was growth from eggs in liquid.
Table 2.
Growth in flight
| Liquid CeMM (n = 2 trials) (mean ± sd) | Solid CeMM (n = 2 trials) (mean ± sd) | |
|---|---|---|
| Starting population | 500, eggs | 2000, mixed stages |
| Final flight population | 14,800 ± 500 | 41,000 ± 1000 |
| Final ground population | 15,000 ± 500 | 40,000 ± 1000 |
3.3. Development is essentially the same on board the International Space Station
We determined the number of larval stages animals undergo in flight by measuring and plotting the shed cuticular sheath lengths (Table 3). Consistent with past flights (Nelson et al., 1994b; Szewczyk et al., 2005), development in flight appears grossly normal. Animals undergo the usual four larval stages and displayed no evidence of a dauer diapause, associated with starvation or stress (Riddle et al., 1987). Animal length at molts on the ground were the same as previously observed for growth in CeMM (Szewczyk et al., 2006). The length of animals in space at the time of the L1 molt was significantly smaller than expected and smaller than previously reported for C. elegans under any conditions we are aware of.
Table 3.
Molting in flight
| Flight (n = 2 trials, 100 animals each) (mean ± sd) | Ground (n = 2 trials, 100 animals each) (mean ± sd) | |
|---|---|---|
| Length of first cuticular sheath (mm) | 0.20 ± .01 | 0.24 ± .01 |
| Length of second cuticular sheath (mm) | 0.45 ± .01 | 0.46 ± .02 |
| Length of third cuticular sheath (mm) | 0.64 ± .03 | 0.64 ± .02 |
| Length of fourth cuticular sheath (mm) | 0.76 ± .02 | 0.76 ± .02 |
4. Discussion
4.1. Hardware testing
There did not appear to be any biocompatibility issues with the flight hardware selected. Growth in culture bags in vented type I cassettes was not significantly different than that predicted for growth in standard laboratory hardware at 18 °C and development in the CCA proceeded normally.
The data demonstrating poor growth in the flight hardware (Table 1) appear to reflect the fact that worms require oxygen to live. Growth at 25 °C is likely close to the metabolic limits of life for animals in CeMM (Szewczyk et al., 2005, 2006). It was expected that any growth problems would be more acute with growth at 25 °C than 18 °C and that oxygen would be limited in unvented cassettes. This appears to have been the case. Growth was decreased at 25 °C and most drastically reduced at 25 °C in unvented cassettes. Of note, at 18 °C, growth was better in culture bags in vented type I cassettes than in a petri dish. This likely represents the fact that animals had access to oxygen on both sides of the bag in the vented cassettes but not in the petri dish. Growth was not similarly improved in vented cassettes at 25 °C. This possibly indicates that animals grown in the culture bags are limited for oxygen, but that given other favorable growth conditions they are able to compensate. In support of this hypothesis, gene expression levels indicate that a sample (23) adjacent to the membrane of a vented type I cassette did not experience a stress response while two samples (4 and 22) that were not adjacent to the membrane did experience a stress response, including induction of hypoxia induced genes (Selch et al., in press).
Taken together, these data suggest that biocompatibility testing should not just be performed for planned optimal growth conditions but also for suboptimal conditions where biocompatibility issues may be more noticeable. This is especially important given that spaceflight experiments are often not conducted under the optimal conditions planned, for example our animals spent a day at 26 °C rather than the planned 20 °C. Testing under suboptimal conditions cannot only reveal underlying biocompatibility issues that are masked under optimal conditions but can also reveal what the margins for error in maintaining experiment viability in non-nominal flight situations actually are.
4.2. Caenorhabditis elegans is mostly normal in liquid CeMM on board the International Space Station
4.2.1. Media validation
Use of liquid culturing enables automated experimentation and analysis. Automated experimentation is valuable when astronaut time is limited and on autonomous satellites. Automated analysis is valuable under the same circumstances and allows data to be stored and transmitted in situations where sample return is not feasible. To enable liquid culturing of C. elegans in space, we chose to first validate the use of liquid medium in flight. These experiments were deemed important because of the life history alteration that animals grown in CeMM undergo (Szewczyk et al., 2006), a consequence of which is increased sensitivity to environmental stressors (Szewczyk et al., 2005).
Animals grew as well in flight as on the ground demonstrating that there are no gross problems with using CeMM to grow C. elegans in flight. The lack of a detectable difference in population growth in flight is consistent with results from previous flights (Nelson et al., 1994a; Szewczyk et al., 2005) and likely indicates that there are no gross problems with C. elegans development or reproduction. Detailed future studies may detect problems with development or reproduction, however our findings and prior studies (Nelson et al., 1994a; Szewczyk et al., 2005) suggest that any problems are not severe enough to prevent a population from successfully propagating itself. These results open the possibility of using CeMM to conduct automated culturing and/or experiments onboard the ISS, autonomous satellites, and/or interplanetary missions.
4.2.2. Culturing C. elegans on solid vs. liquid medium
Caenorhabditis elegans is a soil nematode. Neither standard laboratory cultivation on NGM nor the cultivation in liquid CeMM used here represent growth in soil. However, both are likely to represent variants of natural conditions encountered by C. elegans (Viglierchio, 1992). On NGM, animals are on a solid surface with abundant food, perhaps similar to growth on rotting vegetation. In CeMM animals are not on a solid surface and food is present but not necessarily abundant. This may parallel growth in stagnant water. Little research has been preformed comparing C. elegans biology on solid vs. liquid medium. The ability to effectively use CeMM in both a solid and liquid form presents a unique tool for such studies and it is clear that gene expression changes in response to the form of the medium (Selch et al., in press).
In flight, surface tension forces on the order of 10,000×G are predicted to hold C. elegans to the surface of solid media (Nelson et al., 1994a; Szewczyk et al., 2005), potentially masking the effects of microgravity. This is in contrast to other systems where microgravity is believed to unmask the effects of surface tension (Oser and Battrick, 1989). To differentiate failure of animals to grow in liquid CeMM from failure to grow on solid CeMM both forms of the medium were employed. Our finding that animals grow normally in liquid CeMM suggests that previous studies finding normal growth and development in flight (Nelson et al., 1994a; Szewczyk et al., 2005) do not represent a masking of defects by surface tension forces.
4.2.3. Growth and development in flight
Our results confirm and extend those of two previous flights (Nelson et al., 1994a; Szewczyk et al., 2005). C. elegans appear to have no detectable difficulties with growth and reproduction during spaceflight. Animals appear to grow well both under conditions similar to those commonly employed in the laboratory and in or on CeMM. The fact that animals grow well in flight should not be misconstrued as an argument against further study of C. elegans. Rather, it should be viewed as a validation of culture techniques and a demonstration that C. elegans can be employed to study biology in flight. The data we present here document some of the concerns related to how culture conditions are different than on the ground (for example limited oxygen and temperature control) and explain how preflight testing can limit these differences impacting experiments in flight.
Previous flights have already established that even though C. elegans populations grow well in flight, close examination reveals an increased rate of mutation (Hartman et al., 2001). Closer study of C. elegans physiology as it relates to known space flight induced physiologic changes in other systems will likely be productive. Behavioral, biochemical, histologic, and microarray data suggest that C. elegans muscle undergoes altered development in flight with molecular changes that parallel those seen in mammalian muscle (Higashibata et al., 2006; Adachi et al., in press; Selch et al., in press). These findings suggest that spaceflight induced muscle alterations may be effectively studied in the future using C. elegans. Additionally, while development in flight appears grossly normal with animals undergoing the usual four larval stages and displaying no evidence of a dauer diapause, associated with starvation or stress (Riddle et al., 1987), flown animals do appear to have undergone the first larval molt at a smaller size than the ground control animals. This is of interest because the L1 transition state appears to be regulated by insulin-like signaling (Baugh and Sternberg, 2006) and insulin-like signaling appears to be altered in spaceflown C. elegans (Selch et al., in press). Because altered metabolism likely underlies many phenotypes observed in response to spaceflight (Oser and Battrick, 1989), study of spaceflight induced metabolic alterations in C. elegans may also be an area of future research interest.
4.3. Use of genetic model organisms allows international collaboration within limited stowage space and more rapidly than single hypothesis driven biology experiments
The total stowage space required for the eight type I cassettes was minimal. Despite this limited size and mass we were able to house experiments for investigators from four space agencies and return a total of 53 independent samples each of which contained more than 100 individual animals. The concept of accommodating a number of experiments within a limited available volume and upmass appears to have merit.
By setting flight constraints first and bringing together established C. elegans researchers second, it was possible to design and successfully execute the flight portion of these experiments within one year. In the past, the time from NASA flight grant solicitation to completion of a flight experiment has been longer than 3 years. The shortened administrative time coupled with the number of publications from this experiment to date (Higashitani et al., 2005; Higashibata et al., 2006; Zhao et al., 2006; Leandro et al., 2007; Adachi et al., in press; Selch et al., in press) suggest that use of genetic model organisms and standardized culturing conditions can increase productivity in space life sciences. It is our belief that future payloads which bring together individuals with knowledge of flight experiment operations, flight hardware, space biology, and genetic model organisms should be able to be similarly successful.
Acknowledgements
The Dutch Soyuz mission, DELTA, was facilitated by the Dutch Government. Funding for this experiment was provided by CNES, NASA, JAXA, and CSA. Thanks to Professor Eberhard Horn for use of the modified EC1s. Thanks to Dr. Andre Kuipers, Cdr. Gannady Padalka, Flt. Eng. Michael Fincke, Cdr. Michael Foale, and Flt. Eng. Alexander Kaleri for in-flight payload operations and support. Thanks to Comat, ESA, Roscosmos and Energia for payload support.
References
- Adachi R, Takaya T, Kuriyama K, Higashibata A, Ishioka N, Kagawa H. Spaceflight results in increase of thick filament but not thin filament proteins in the paramyosin mutant of Caenorhabditis elegans. Adv. Space Res. 2008;41:816–823. [Google Scholar]
- Bahls C, Weitzman J, Gallagher R. Biology's models. The Scientist. 2003;17:5. [Google Scholar]
- Bargmann CI, Kaplan JM. Signal transduction in the Caenorhabditis elegans nervous system. Annu. Rev. Neurosci. 1998;21:279–308. doi: 10.1146/annurev.neuro.21.1.279. [DOI] [PubMed] [Google Scholar]
- Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 2006;16:780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Board SS. A Strategy for Research in Space Biology and Medicine in the New Century. National Research Council; Washington, DC: 1998. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervitz SA, Aravind L, Sherlock G, Ball CA, Koonin EV, Dwight SS, Harris MA, Dolinski K, Mohr S, Smith T, Weng S, Cherry JM, Botstein D. Comparison of the complete protein sets of worm and yeast: orthology and divergence. Science. 1998;282(5396):2022–2028. doi: 10.1126/science.282.5396.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium C.e.S. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Culetto E, Sattelle DB. A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum. Mol. Genet. 2000;9:869–877. doi: 10.1093/hmg/9.6.869. [DOI] [PubMed] [Google Scholar]
- Fahlen T, Sunga J, Rask J, Herrera A, Lam K, Sing L, Sato K, Ramos RA, Kirven-Brooks M, Reiss-Bubenheim D. Methods for the culture of C. elegans and S. cerevisiae in microgravity. Gravit. Space Biol. Bull. 2005;18:91–92. [PubMed] [Google Scholar]
- Frangsmyr T, editor. The Nobel Prizes 2001. Almqvist & Wiksell International; Stockholm: 2002. Les Prix Nobel. [Google Scholar]
- Frangsmyr T, editor. The Nobel Prizes 2002. Almqvist & Wiksell International; Stockholm: 2003. Les Prix Nobel. [Google Scholar]
- Frangsmyr T, editor. The Nobel Prizes 2006. Almqvist & Wiksell International; Stockholm: 2007. Les Prix Nobel. [Google Scholar]
- Hartman PS, Hlavacek A, Wilde H, Lewicki D, Schubert W, Kern RG, Kazarians GA, Benton EV, Benton ER, Nelson GA. A comparison of mutations induced by accelerated iron particles versus those induced by low earth orbit space radiation in the FEM-3 gene of Caenorhabditis elegans. Mutat. Res. 2001;474:47–55. doi: 10.1016/s0027-5107(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Higashibata A, Szewczyk NJ, Conley CA, Imamizo-Sato M, Higashitani A, Ishioka N. Decreased expression of myogenic transcription factors and myosin heavy chains in Caenorhabditis elegans muscles developed during spaceflight. J. Exp. Biol. 2006;209:3209–3218. doi: 10.1242/jeb.02365. [DOI] [PubMed] [Google Scholar]
- Higashitani A, Higashibata A, Sasagawa Y, Sugimoto T, Miyazawa Y, Szewcyk NJ, Viso M, Gasset G, Eche B, Fukui K, Shimazu T, Fujimoto N, Kuriyama K, Ishioka N. Checkpoint and physiological apoptosis in germ cells proceeds normally in spaceflown Caenorhabditis elegans. Apoptosis. 2005;10:949–954. doi: 10.1007/s10495-005-1323-3. [DOI] [PubMed] [Google Scholar]
- Horn E, Sebastian CE. Biorack on Spacehab. ESA SP-1222ESA Publications Division; Noordwijk: 1999. A comparison of normal vestibulo-ocular reflex development under gravity and in the absences of gravity. [Google Scholar]
- Jantunen R. Moulting of Caenorhabditis briggsae (Rhabditidae). Nematologica. 1964;10:419–424. [Google Scholar]
- Johnson TE, Nelson GA. Caenorhabditis elegans: a model system for space biology studies. Exp. Gerontol. 1991;26:299–309. doi: 10.1016/0531-5565(91)90024-g. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Leandro LJ, Szewczyk NJ, Benguría A, Herranz R, Laván D, Medina FJ, Gasset G, van Loon J, Conley CA, Marco R. Comparative analysis of Drosophila melanogaster and Caenorhabditis elegans gene expression experiments in the European Soyuz flights to the International Space Station. Adv. Space Res. 2007;40:506–512. doi: 10.1016/j.asr.2007.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner D. Modelling genotype–phenotype relationships and human disease with genetic interaction networks. J. Exp. Biol. 2007;210:1559–1566. doi: 10.1242/jeb.002311. [DOI] [PubMed] [Google Scholar]
- Li S, Armstrong CM, Bertin N, Ge H, Milstein S, Boxem M, Vidalain PO, Han JD, Chesneau A, Hao T, Goldberg DS, Li N, Martinez M, Rual JF, Lamesch P, Xu L, Tewari M, Wong SL, Zhang LV, Berriz GF, Jacotot L, Vaglio P, Reboul J, Hirozane-Kishikawa T, Li Q, Gabel HW, Elewa A, Baumgartner B, Rose DJ, Yu H, Bosak S, Sequerra R, Fraser A, Mango SE, Saxton WM, Strome S, Van Den Heuvel S, Piano F, Vandenhaute J, Sardet C, Gerstein M, Doucette-Stamm L, Gunsalus KC, Harper JW, Cusick ME, Roth FP, Hill DE, Vidal M. A map of the interactome network of the metazoan C. elegans. Science. 2004;303:540–543. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NC, Goetsch KM. Carbohydrate requirement of Caenorhabditis elegans and the final development of a chemically defined medium. Nematologica. 1993;39:303–331. [Google Scholar]
- Manis JP. Knock out, knock in, knock down – genetically manipulated mice and the Nobel Prize. N. Engl. J. Med. 2007;357:2426–2429. doi: 10.1056/NEJMp0707712. [DOI] [PubMed] [Google Scholar]
- Nelson GA, Schubert WW, Kazarians GA, Richards GF. Development and chromosome mechanics in nematodes: results from IML-1. Adv. Space Res. 1994a;14:209–214. doi: 10.1016/0273-1177(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Nelson GA, Schubert WW, Kazarians GA, Richards GF, Benton ER, Henke RP. Biorack on Spacelab IML-1. ESA SP-1162ESA Publications Division; Noordwijk: 1995. Genetic and molecular dosimetry of HZE radiation. [Google Scholar]
- Nelson GA, Schubert WW, Kazarians GA, Richards GF, Benton EV, Benton ER, Henke R. Radiation effects in nematodes: results from IML-1 experiments. Adv. Space Res. 1994b;14:87–91. doi: 10.1016/0273-1177(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Oser H, Battrick B. Life Sciences Research in Space. European Space Agency Publications Division; Noordwijk: 1989. [Google Scholar]
- Riddle DL, Golden JW, Albert PS. Role of dauer larvae in survival of C. elegans. In: Veech JA, Dickson DW, editors. Vistas on Nematology. Society of Nematologists, Inc.; 1987. pp. 174–179. [Google Scholar]
- Ringertz N, editor. Nobel Lectures Physiology or Medicine 1991–1995. World Scientific Publishing Company; Stockholm: 1997. [Google Scholar]
- Selch F, Higashibata A, Imamizo-Sato M, Higashitani A, Ishioka N, Szewczyk NJ, Conley CA. Genomic response of the nematode Caenorhabditis elegans to spaceflight. Adv. Space Res. 2008;41:807–815. doi: 10.1016/j.asr.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L, Sternberg P, Durbin R, Thierry-Mieg J, Spieth J. Worm-Base: network access to the genome and biology of Caenorhabditis elegans. Nucleic Acids Res. 2001;29:82–86. doi: 10.1093/nar/29.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk NJ, Kozak E, Conley CA. Chemically defined medium and Caenorhabditis elegans. BMC Biotechnol. 2003;3:19. doi: 10.1186/1472-6750-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk NJ, Mancinelli RL, McLamb W, Reed D, Blumberg BS, Conley CA. C. elegans survives atmospheric breakup of STS-107, Space Shuttle Columbia. Astrobiology. 2005;5:690–705. doi: 10.1089/ast.2005.5.690. [DOI] [PubMed] [Google Scholar]
- Szewczyk NJ, McLamb W. Surviving atmospheric spacecraft breakup. Wilderness Environ. Med. 2005;16:27–32. doi: 10.1580/pr21-03.1. [DOI] [PubMed] [Google Scholar]
- Szewczyk NJ, Udranszky IA, Kozak E, Sunga J, Kim SK, Jacobson LA, Conley CA. Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J. Exp. Biol. 2006;209:4129–4139. doi: 10.1242/jeb.02492. [DOI] [PubMed] [Google Scholar]
- Twigger SN, Pasko D, Nie J, Shimoyama M, Bromberg S, Campbell D, Chen J, Dela Cruz N, Fan C, Foote C, Harris G, Hickmann B, Ji Y, Jin W, Li D, Mathis J, Nenasheva N, Nigam R, Petri V, Reilly D, Ruotti V, Schauberger E, Seiler K, Slyper R, Smith J, Wang W, Wu W, Zhao L, Zuniga-Meyer A, Tonellato PJ, Kwitek AE, Jacob HJ. Tools and strategies for physiological genomics – the rat genome database. Physiol. Genomics. 2005;23:246–256. doi: 10.1152/physiolgenomics.00040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassy J, Portet S, Beil M, Millot G, Fauvel-Lafeve F, Karniguian A, Gasset G, Irinopoulou T, Calvo F, Rigaut JP, Schoevaert D. The effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line MCF-7. FASEB J. 2001;15:1104–1106. doi: 10.1096/fj.00-0527fje. [DOI] [PubMed] [Google Scholar]
- Viglierchio DR. The World of Nematodes. AgAccess; Davis, CA: 1992. [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Johnsen R, Baillie D, Rose A. Worms in space? A model biological dosimeter. Gravit. Space Biol. Bull. 2005;18:11–16. [PubMed] [Google Scholar]
- Zhao Y, Lai K, Cheung I, Youds J, Tarailo M, Tarailo S, Rose A. A mutational analysis of Caenorhabditis elegans in space. Mutat. Res. 2006;601:19–29. doi: 10.1016/j.mrfmmm.2006.05.001. [DOI] [PubMed] [Google Scholar]


