Abstract
Airway hyperresponsiveness (AHR), a hallmark of asthma and several other diseases, can be modulated by γδ T cells. In mice sensitized and challenged with ovalbumin, AHR depends on allergen-specific αβ T cells, but Vγδ1+ γδ T cells spontaneously enhance AHR, whereas Vγ4+ γδ T cells after being induced by airway challenge suppress AHR. The activity of these γδ T cell modulators is allergen-nonspecific, and how they develop is unclear. We now show that CD8 is essential for the development of both the AHR-suppressor and enhancer γδ T cells although neither type needs to express CD8 itself. Both cell types encounter CD8-expressing non-T cells in the spleen, and their functional development in an otherwise CD8-negative environment can be restored with transferred spleen cell preparations containing CD8+ DC, but not CD8+ T cells or CD8− DC. Our findings suggest that CD8+ DC in the lymphoid tissues enable an early step in the development of γδ T cells, through direct cell-contact. DC-expressed CD8 might take part in this interaction.
Keywords: Rodent, dendritic cells, T cells, cell differentiation, spleen and lymph nodes
Introduction
Airway hyperresponsiveness (AHR) is a symptom in several potentially life-threatening diseases, including asthma and chronic obstructive pulmonary disease. The pathways leading to AHR are not yet completely mapped. In allergic asthma, antigen-specific T cells are initiators of a cascade of immune-dependent mechanisms that result in the production of the critical cytokine interleukin 13, eosinophilic airway inflammation, allergen-specific IgE antibodies, mast cell activation, goblet cell differentiation and mucus production, and AHR (1, 2). The development and role of allergen-specific αβ T cells have been studied extensively in animal models of allergic airway inflammation and AHR. In the process, it became apparent that innate antigen-nonspecific T cells were involved as well, which are capable of suppressing and enhancing AHR in allergic airway disease and other pathological conditions of the lung (3-7). Both αβ T cells and γδ T cells are included among these innate AHR-modulating T cells and are potentially attractive targets for immune intervention (8). However, little is known about these cells and their development. We have studied AHR-modulating γδ T cells in vivo. In mice sensitized and challenged with ovalbumin (OVA), which display hypersensitivity when given the cholinergic agonist methacholine (MCh) (9), we found that γδ T cells can dramatically alter AHR whereas their effect on airway inflammation is comparatively small (5). The γδ T cells capable of modulating AHR belong to two subsets distinguished by their T cell receptors (TCR): A Vγ1+ subset which contains γδ T cells spontaneously capable of enhancing AHR (10, 11) and a Vγ4+ subset which contains γδ T cells that can be induced to suppress AHR (12-15). Neither cell-type requires specific Ag-priming (11, 15) and it is unclear how these AHR-modulators develop. However, both are found in spleen and lung (16). Moreover, following airway challenge, selectively depleting either subset in the lung (using aerosolized inhaled anti TCR-antibodies) alters AHR in the predicted fashion, i.e. depletion of Vγ1+ cells decreases and depletion of Vγ4+ cell increases AHR (8, 12), suggesting that the γδ T cells within the challenged lung are functionally competent.
In the development of antigen-specific T cells, dendritic cells (DC) are critical (17). The role of DC in the development of innate T cells is less well defined. Evidence that γδ T cells and DC interact has come mainly from studies in vitro. These revealed maturational effects on both the DC and the γδ T cells (18-24), raising the question of whether such interactions might also be critical for the functional development of γδ T cells in vivo. However, DC are heterogeneous and their functions diverse (25-31). The present study began with the finding that AHR-suppression by γδ T cells could not be demonstrated in mice genetically deficient in CD8α, and our attempts to determine which cells express the required CD8. Using adoptive cell transfer, we find that the γδ T cells themselves need not express CD8, but that CD8 is required in the donor mice in which the γδ T cells develop. Further, the development of γδ T cells capable of AHR-suppression can be restored in CD8-deficient mice by reconstituting them with cell preparations containing CD8+DC, but not with CD8-expressing T cells or CD8− DC. Mice deficient in CD8α also fail to give rise to γδ T cells capable of AHR-enhancement and again, their development can be restored by transfer of CD8+ DC, suggesting that interactions with CD8+ DC in lymphoid tissues represent a critical step in the development of γδ T cells towards functional competence.
Materials and Methods
Animals
C57BL/6, B6.CD8α−/−, B6.TCR-β−/−, B6.TCR-δ−/−, and B6.TCR-β−/−/δ−/− mice were purchased from The Jackson Laboratory (Bar Harbor, Maine); TCR-Vγ4/6−/− mice deficient in Vγ4+ and Vγ6+ γδ T cells (32) were a kind gift from K. Ikuta (Kyoto, Japan). They were backcrossed to the C57BL/6 genetic background and established after 11 backcrossed generations (designated B6.TCR-Vγ4/6−/−). B6.TCRVγ4/6−/−CD8α−/− mice were generated starting from the F2 generation of a cross between B6.CD8α−/− and B6.TCR-Vγ4/6−/− mice. All mice were maintained on an OVA-free diet, and were cared for at National Jewish Medical and Research Center (Denver, Colorado), following guidelines for immune deficient animals when necessary. All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
Sensitization and airway challenge
Mice received the following treatments: no OVA treatment (NT) or sensitization to OVA by intraperitoneal injection of 20μg of OVA (Grade V; Sigma) emulsified in 2.25mg alum (AlumImject®; Pierce, Rockford, Illinois) in a total volume of 100μl on days 0 and 14 (2ip), or sensitization followed by airway exposure to nebulized OVA (1% in saline), using ultrasonic nebulization (particle size 3−5μm) for 20 mins on days 28, 29 and 30 (2ip3N). Donors of DC-preparations either received no treatment or only the sensitizing treatment (2ip). Airway responsiveness was assessed 48h after the last nebulized OVA exposure for 2ip3N treated mice.
Depletion with monoclonal antibody against TCR
Cell-depletion was achieved by injection into the tail vein of 200μg hamster mAb against Vγ4 (UC3) (33), 3 days before the first OVA challenge, and depletion was assessed as described (10). Sham depletion was carried out using hamster Ig (Jackson Laboratories, Bar Harbor, Maine). Throughout this article, we use the nomenclature for murine TCR-Vγ genes introduced by Heilig and Tonegawa (34).
Determination of airway responsiveness
Airway responsiveness was assessed as a change in respiratory system resistance after provocation with aerosolized methacholine (MCh) using a method previously described in detail (5). MCh aerosol was administered for 10 s (60 breaths/min, 0.5 ml of tidal volume) in increasing concentrations as indicated in the figures. Maximum values of lung resistance (RL) and minimum values of dynamic compliance (Cdyn) were recorded and expressed as percentage change from baseline after saline aerosol.
Histological analysis of the spleen
For the tissue preparation, sectioning and staining, essentially the same methods were used as described previously for the analysis of γδ T cells in the lung (16). Briefly, sections of 5−10 mm thickness cut from frozen blocks were mounted on microscopic slides, air dried, dehydrated in acetone, hydrated, blocked with a mixture of normal mouse serum and Avidin, washed, stained with primary and secondary antibodies, and finally mounted with coverslips. Slides were viewed using a Leica (Knowhill, UK) DMRXMA upright fluorescent microscope, and digital images were generated on an Apple MacIntosh computer connected with the microscope, using the SlideBook imaging program (3I Inc., Atlanta, GA). The tissue autofluorescence (green) was used to distinguish overall tissue organization, especially the localization of central arterioles, and the density of the cellular distribution. The higher cell-density of the white pulp around the arterioles was used to determine the extent of the PALS. Cells and cell-contacts were enumerated and compared either across the entire tissue (whole spleen) or limited to the PALS. Antibodies used for histology: anti TCR-δ (mAb GL3), anti Vγ4 (mAb UC3), anti Vγ1 (mAb 2.11), anti-CD8α (mAb 53−6.7, eBioscience), anti-CD11c (mAb HL3, Pharmingen), anti CD103 (mAb 2E7, eBisocience), anti DEC-205 (mAb ATCC# HB 290), plus secondary reagents as described(16).
Cell preparation from spleen and T cell-sorting using the MO FLO
A suspension of splenocytes was prepared by mechanical dispersion. Suspended cells were treated with Gey's red cell lysis solution. Nylon wool non-adherent (NAD) cells were prepared from spleens of OVA-sensitized and challenged B6.TCR-β−/− mice 2 days after the last challenge. These preparations contained >75% T cells. Total cell counts were determined using a Coulter counter. NAD cells in PBS/5% FBS were incubated with FITC conjugated anti-Vγ4 mAb (UC3) and PE-conjugated anti-CD8α (53−6.7, Pharmingen) or PE-conjugated anti-CD8β (53−5.8, Pharmingen) (20mins, 4°C), and then washed. Cells were next sorted based on their expression of Vγ4 and CD8 (α or β) using a MO FLO cell sorter. Purified Vγ4+/CD8+ or Vγ4+/CD8− cells were washed in PBS and resuspended to 5 × 104 cells/ml PBS, and 1 × 104 cells/mouse injected in 200μl of PBS via the tail vein into OVA sensitized B6.TCR-Vγ4/6−/− mice within 1 hour prior to the first airway challenge.
Adoptive transfer of γδ T cells
Nylon wool nonadherent (NAD) cells were prepared from spleens of OVA-sensitized and challenged B6.TCR-β−/− or B6.CD8α−/− mice 2 days after the last challenge. These cells contained >75% T cells. Total cell counts were determined using a Coulter counter. NAD cells in PBS/5% FBS were incubated with biotinylated anti-Vγ4 mAb (UC3) or anti-Vγ1 mAb (2.11) (35) (20 mins, 4°C) then washed and incubated with streptavidin-conjugated magnetic beads (streptavidin microbeads; Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 mins at 4°C and passed twice through magnetic columns to purify Vγ4+ or Vγ1+ cells. This produced a cell population containing >80% Vγ4+ or Vγ1+ viable cells as determined by two-color staining with anti-TCR-δ and anti-Vγ4 or anti-Vγ1 mAbs. These splenic Vγ4+ or Vγ1+ cells were washed in PBS and resuspended to 5 × 104 cells/ml PBS and 1 × 104 cells/mouse were injected in 200μl of PBS via the tail vein into OVA sensitized B6.TCR-Vγ4/6−/− or B6.TCR-δ−/− mice, within 1 hour prior to the first airway challenge.
Phenotype of splenocytes and enriched DC from B6.TCR-β/δ−/− mice
Red cell depleted splenocytes from naïve or 2ip treated B6.TCR-β/δ−/− mice (or magnetically enriched DC) were incubated with APC conjugated anti-CD8α mAb (53−6.7, eBioscience), FITC conjugated anti-CD11c mAb (HL3, Pharmingen), biotinylated anti CD45R (B220, eBioscience, clone RA3−6B2), biotinylated anti CD3ε mAb (KT3) and PE conjugated anti-MHC II (M5/114.15.2, Pharmingen) in PBS/5% FBS (20min, 4°C), then washed and analyzed on a FACSCAN.
Adoptive transfer of accessory non-T cells
Cells were isolated from the spleen of untreated or 2ip treated B6.TCR-β−/−/δ−/− and were treated with Gey's red cell lysis solution. To reconstitute with whole splenocytes, one “spleen equivalent” (∼ 5×107 cells) was then transferred per 2ip treated B6.CD8α−/− mouse. A small sample of these cells was analysed for CD8 expression by flow cytometry (mAb 53−6.7-PE, Pharmingen). Approx. 9% were found to be CD8+. Reconstituted B6.CD8α−/− mice were then nebulized as described earlier, and used as donors of Vγ4+ or Vγ1+ cells. To reconstitute with CD8-enriched or CD8-depleted splenocytes, the red cell depleted splenocytes from 2ip treated B6.TCR-β−/−/δ−/− mice, or B6.TCR-δ−/− mice (for CD8+ T cell adoptive transfer), were incubated with biotinylated anti-CD8 mAb 53−6.7 in PBS/5% FBS (20 min, 4°C), then washed and incubated with streptavidin-conjugated magnetic beads (streptavidin microbeads; Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 mins at 4°C and passed twice over MS magnetic columns to purify CD8+ cells. This produced a CD8-enriched fraction containing approx. 45% CD8+ cells as determined by flow cytometry, and a CD8-depleted fraction containing < 1% CD8+ cells. Approx. 1 ×106 viable enriched cells were transferred to 2ip-treated B6.CD8α−/− recipients. The B6.CD8α−/− mouse was then nebulized as described earlier and these mice were used as donors of Vγ4+ cells. Finally, to reconstitute with more highly enriched CD8+ DC, a CD8+ purification kit (Miltenyi Biotec, Bergisch Gladbach, Germany) was used as per the manufacturer's recommendations. Briefly, freshly isolated red cell depleted splenocytes derived from the B6.TCR-β−/−/δ−/− mice were incubated with biotinylated antibody cocktail (anti-CD90, anti-CD45R and anti-CD49b) in PBS/5% FBS (10min, on ice), then incubated with anti-biotin microbeads (15min, on ice), then washed and passed over a LD magnetic column to enrich DCs. The enriched DCs were incubated with anti-CD8α (Ly-2) microbeads (30mins, on ice) then washed and passed twice over a MS magnetic column retaining the CD8+ DCs. In the worst case (with 2ip-treated mice), this still produced a preparation containing 60−70% viable CD8α+ DC, based on the dual criterion of MHC class II and CD11c expression. The concentration of CD8+ DC in this preparation might actually be higher due to variable MHC class II expression. 1.3 ×105 of these enriched cells containing approx. 105 CD8+DC were transferred to 2ip-treated B6.CD8α−/− recipients, which were then nebulized as described above and used as donors of Vγ4+ cells. Donors of Vγ1+ cells (non-sensitized B6.CD8α−/− mice that received enriched CD8+ DC from non-sensitized B6.TCR-β−/−/δ−/− mice - these could be obtained at a higher purity than those from 2ip-treated mice) remained non-challenged.
Statistical analysis
Data are presented as means +/− SEM. The unpaired T test was used for two group comparisons and ANOVA for analysis of differences in three or more groups. Pairwise comparisons were performed using the Tukey-Kramer honest significant difference test. Statistically significant levels are indicated as follows: 1 symbol (*, #, +) = p < 0.05; 2 symbols = p < 0.01; 3 symbols = p < 0.001.
Results
AHR-suppressive γδ T cells depend on the CD8-molecule but need not themselves express CD8
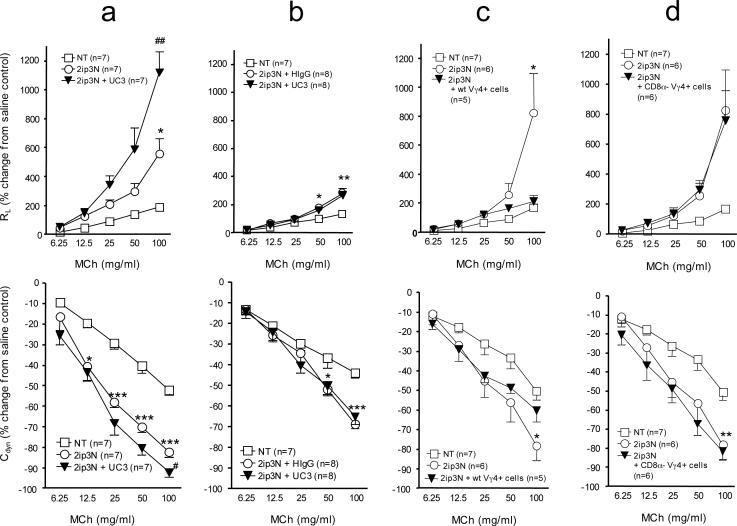
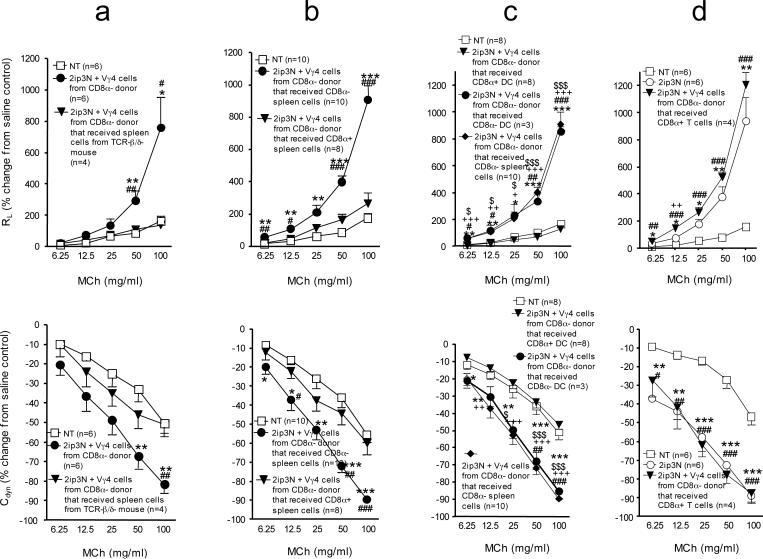
We have previously found that Vγ4+ γδ T cells suppress AHR in several different mouse strains including C57BL/6 (B6) (12-15). Here, we have used B6 mice to take advantage of available mutations on this background. As predicted by earlier studies (36), by comparison with wild-type mice (Fig.1a), mice deficient in CD8 (B6.CD8α−/−) (Fig.1b) exhibited much-reduced AHR after being sensitized and challenged with OVA. Unexpectedly, however, i.v. injection of a cell-depleting anti TCR-Vγ4 antibody (mAb UC3), which increases AHR in wild-type B6 mice (Fig.1a), failed to increase AHR in the CD8-deficient mice (Fig. 1b). This difference suggested a role for CD8 in the suppression of AHR by γδ T cells but it remained unclear when the molecule might be needed and which cells must express it. To address these questions, we chose a cell-transfer approach. We compared mice adoptively transferred with purified Vγ4+ γδ T cells derived from the spleens of OVA-sensitized and challenged wild-type B6 (Fig. 1c) or B6.CD8α−/− donors (Fig. 1d), using recipients deficient in Vγ4+ cells but expressing normal levels of CD8 (B6.TCR-Vγ4/6−/−). These B6.TCR-Vγ4/6−/− mice develop strong AHR following OVA-sensitization and challenge (13). Following cell transfer, the B6.TCR-Vγ4/6−/− mice that received Vγ4+ cells from wild-type-mice exhibited reduced AHR (Fig. 1c), but those that received Vγ4+ cells from B6.CD8α−/− mice did not (Fig. 1d). In a similar comparative experiment transferring γδ T cells derived from the lung, again the wild-type-derived cells suppressed AHR whereas the cells from CD8-deficient mice failed to do so (not shown). These results indicated that CD8-expression in the cell-transfer recipients is not sufficient to support the AHR-modulating function of the γδ T cells. CD8-expression by the donor cells is required, either by the γδ T cells themselves or by other cells in the donors that might determine the development of the γδ T cells. Whether in addition CD8 might play a role during γδ T cell-mediated AHR-suppression in the cell transfer-recipients was not pursued because the CD8-deficient recipient-mice bred for this purpose (B6.TCR-Vγ4/6−/−CD8α−/−) failed to develop AHR (not shown).
Figure 1.
AHR in mice after systemic sensitization and airway challenge with OVA (2ip3N protocol). (a-d) Lung resistance (RL) and dynamic compliance (Cdyn) as percent changes from saline controls in relation to increasing doses of aerosolized MCh. Treatments: none (NT), intra-peritoneal sensitization and airway challenge (2ip3N), 2ip3N plus hamster IgG i.v., 2ip3N plus anti Vγ4 mAb UC3 i.v., 2ip3N plus purified Vγ4+ cells from 2ip3N-treated donors (wt or CD8α−/−) i.v. (a) Normal C57BL/6 mice after 2ip3N or 2ip3N plus depletion of Vγ4+ cells with mAb UC3 (* non-treated compared with 2ip3N, # 2ip3N compared with 2ip3N plus UC3); (b) B6.CD8α−/− mice after 2ip3N plus depletion of Vγ4+ cells with mAb UC3 (* non-treated compared with 2ip3N); (c) B6.TCR-Vγ4/6−/− mice after 2ip3N or 2ip3N plus Vγ4+ cells from B6.TCR-β−/−(CD8αwt) donors (* non-treated compared with 2ip3N; there was no significant difference between non-treated and 2ip3N plus WT Vγ4 cells) ; (d) B6.TCR-Vγ4/6−/− mice after 2ip3N or 2ip3N plus Vγ4+ cells from B6.CD8α−/− donors (there was no significant difference between 2ip3N and 2ip3N plus CD8−/− Vγ4 cells at any dose of MCh).
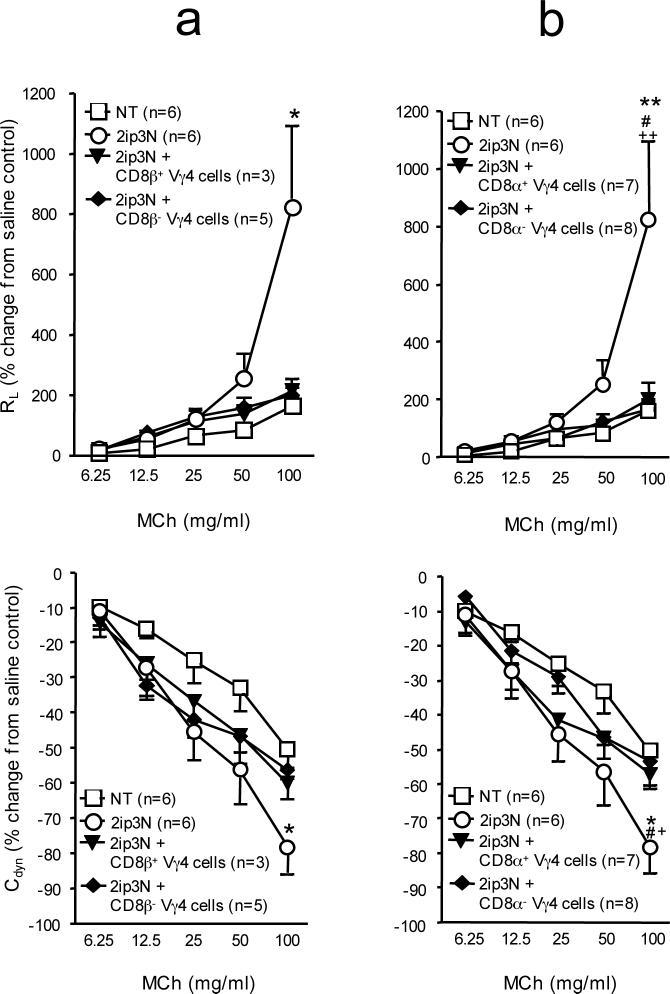
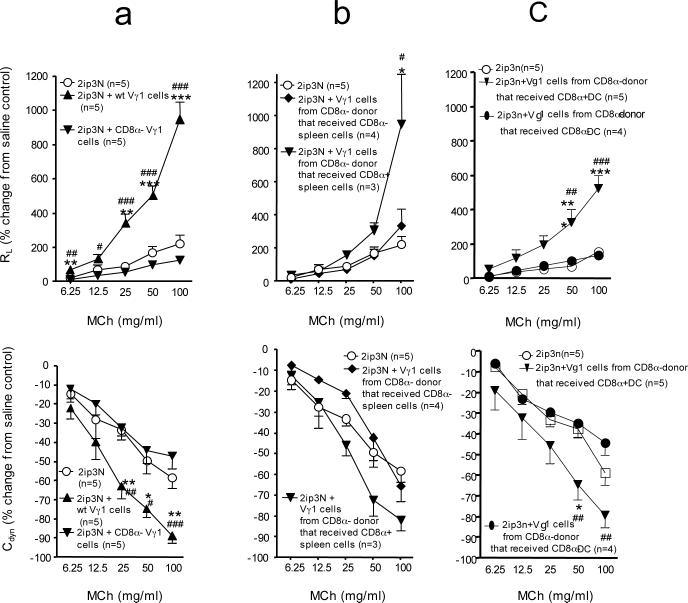
We previously found that some of the Vγ4+ γδ T cells in spleen and lung express CD8, including CD8αβ and CD8αα (12). To test whether the AHR-suppressive γδ T cells must themselves express CD8αβ or CD8αα, we separated purified Vγ4+ cells from the spleen of sensitized and challenged donors into CD8β-positive or –negative, or into CD8α-positive or –negative fractions, and tested each fraction separately in the cell-transfer model (Fig. 2a, b). All fractions suppressed AHR, indicating that CD8 expression by the γδ T cells is not required for the suppressive function. This was confirmed by our subsequent experiments with γδ T cells derived from B6.CD8α−/− mice (see below). The finding that γδ T cells need not express CD8 implied that the required CD8 must be expressed by another cell-type. Furthermore, since CD8 is required in the donor, the “other” CD8-expressing cell-type might be involved in the development of the AHR-suppressive γδ T cells.
Figure 2.
AHR in γδ T cell-deficient recipients reconstituted with CD8-selected Vγ4+ cells, after systemic sensitization and airway challenge with OVA (2ip3N protocol). (a,b) Lung resistance (RL) and dynamic compliance (Cdyn) as percent changes from saline controls in relation to increasing doses of aerosolized MCh. Recipients: B6.TCR-Vγ4/6−/− mice; donors: 2ip3N-treated B6.TCR-β−/− or C57BL/6 mice. Treatments: none (NT), systemic sensitization and airway challenge (2ip3N), 2ip3N plus selected purified Vγ4+ /CD8β+ or − or Vγ4+ /CD8α+ or − cells from the donors. (a) Recipients untreated or after 2ip3N or 2ip3N plus selected Vγ4+ /CD8β+ or − cells from B6.TCR-β−/− donors (* non-treated compared with 2ip3N); (b) Recipients untreated or after 2ip3N or 2ip3N plus selected Vγ4+/CD8α+ or − cells from C57BL/6 donors (* non-treated compared with 2ip3N, # 2ip3N compared with 2ip3N plus CD8α+ Vγ4 cells, + 2ip3N compared with 2ip3N plus CD8α− Vγ4 cells).
Encounters between γδ T cells and CD8+ non-T cells in the spleen
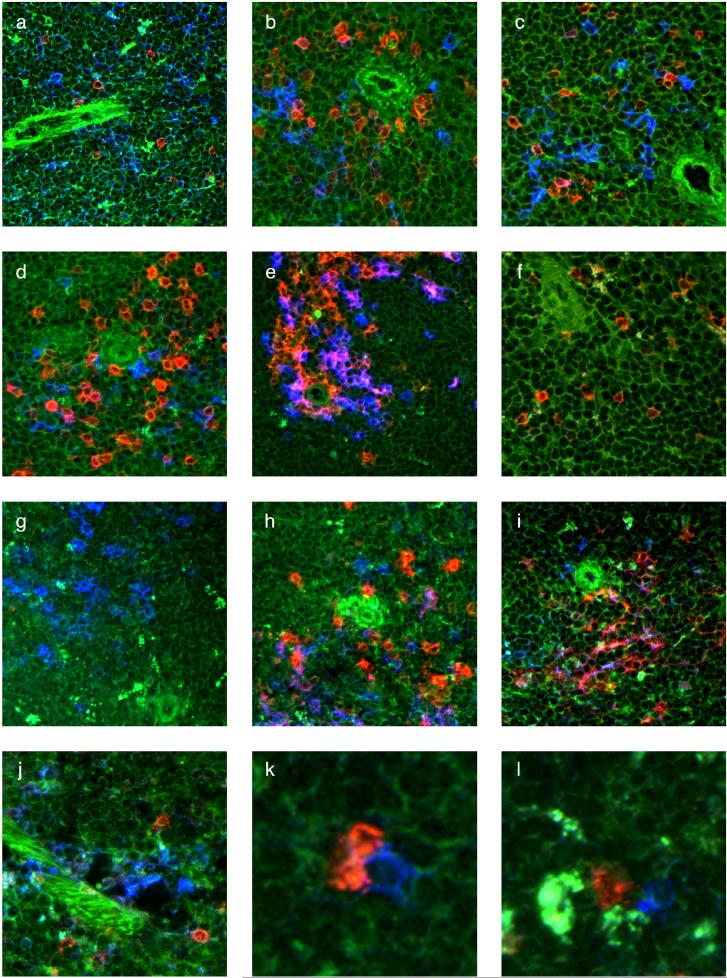
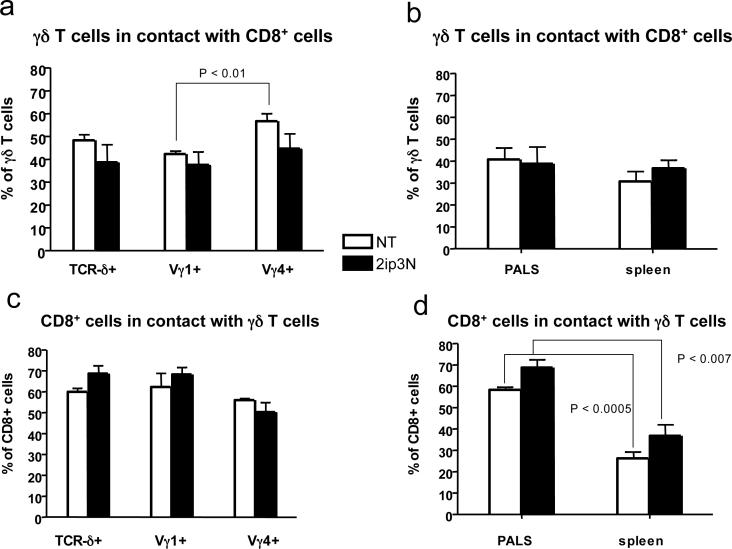
Because γδ T cells prepared from the spleen are capable of modulating AHR, we examined their distribution and cell-contacts here. In C57BL/6 mice (Fig. 3a), and in B6.TCR-β−/− mice (Fig. 3b), both of which give rise to functionally normal AHR-regulatory γδ T cells (11, 13), γδ T cells were mainly present in the peri-arteriolar lymphoid sheath (PALS), although some were also detected outside the PALS. The γδ T cells were often in close proximity/contact with DEC-205+ or CD8+ cells (Fig. 3a and 3b). The CD8+ cells are not αβ T cells because they are present in the spleen of B6.TCR-β−/− mice. Both Vγ4+ and Vγ1+ cells were found in such interactions (Fig.3c, d), with little change following sensitization and challenge (Fig. 4). The frequency of CD8+ non-T cells in contact with γδ T cells was higher inside the PALS (50−60%) than in the spleen in general (30−40%) (Fig. 4). However, the high frequency of such contacts might be a peculiarity of B6.TCR-β−/− mice. In accordance with the phenotype of “conventional” CD8+ DC (31), the CD8+ non-T cells in the PALS of the B6.TCR-β−/− mice co-expressed CD11c (Fig.3e), and many co-expressed CD103 and DEC-205 (Fig.3h, i). Collectively, these findings suggest that γδ T cells, including those types implicated in the modulation of AHR, encounter CD8+ DC in the spleen.
Figure 3.
Localization of γδ T cells, CD8α+ DCs and CD8α− OVA/alum-containing phagocytes in the spleen (a-l) Frozen sections of spleen stained with mAbs, tissue autofluorescence (green), antibody staining (red or blue), coincidence of antibody staining (pink/purple), (a) C57BL/6 spleen, stained with anti TCR-δ mAb (red) and anti DEC-205 mAb (blue); (b) B6.TCR-β−/− spleen, stained with anti TCR-δ mAb (red) plus anti CD8α mAb (blue); (c) B6.TCR-β−/− spleen, stained with anti TCR-Vγ4 mAb (red) plus anti CD8α mAb (blue), or with (d) anti TCR-Vγ1 mAb (red) plus anti CD8α mAb (blue); (e) B6.TCR-β−/− spleen, stained with anti CD11c mAb (red) plus anti CD8α mAb (blue), cells co-expressing these markers appear violet; (f) B6.CD8α−/− spleen, stained with anti TCR-δ mAb (red); (g) B6.TCR-β−/−δ−/− spleen, stained with anti CD8α mAb; (h) B6.TCR-β−/− spleen, stained with an antibody against CD103 (red) plus anti CD8α mAb (blue), cells co-expressing these markers appear violet; (i) B6.TCR-β−/− spleen, stained with an antibody against DEC-205 (red) plus anti CD8α mAb 53−6/7 (blue), cells co-expressing these markers appear violet; (j) B6.CD8α−/− spleen, stained with anti TCR-δ mAb (red) plus anti DEC-205 mAb (blue); (k) 2ip-treated B6.CD8α−/− spleen after transfer of CD8+ cells from the spleen of a 2ip-treated B6.TCR-β−/−δ−/− mouse, stained with anti TCR-δ mAb (red) plus anti CD8α mAb (blue), enlarged detail showing two endogenous γδ T cells in contact with transferred CD8+ DC; (l) same tissue and staining as in (k), additional detail: OVA/alum appears as bright white/yellow fluorescence in CD8α−, TCR-δ− phagocytes, which are found in close proximity to both a γδ T cell and a CD8+ DC.
Figure 4.
Distribution of contacts between γδ T cells and CD8+ DCs in the spleen (a-d) Frozen sections of spleen stained with mAbs were evaluated for relative frequencies of γδ T cell-DC contacts (a) B6.TCR-β−/− spleen non-treated and 2ip3N, fractions of TCR-δ+, Vγ1+ and Vγ4+ cells in contact with CD8+ non-T cells; (b) B6.TCR-β−/− spleen non-treated and 2ip3N, comparison of PALS and “outside the PALS”, fractions of TCR-δ+ cells in contact with CD8+ non-T cells. (c) B6.TCR-β−/− spleen non-treated and 2ip3N, fractions of CD8+ non-T cells in contact with TCR-δ+, Vγ1+ and Vγ4+ cells; (d) B6.TCR-β−/−spleen non-treated and 2ip3N, comparison of PALS and “outside the PALS”, fractions of CD8+ non-T cells in contact with TCR-δ+ cells.
Enriched CD8+ DC enable the development of AHR-suppressive γδ T cells in mice lacking CD8
Because CD8-negative γδ T cells were capable of suppressing AHR (Fig. 2), and because of our previous finding that the AHR-suppressors can develop in mice lacking all αβ T cells (5, 12, 13), we concluded that CD8+ non-T cells must satisfy the CD8-requirement. Furthermore, the histological data of this study suggested CD8+ DC as candidate CD8+ non-T cells. Therefore, we tested whether B6.CD8α−/− mice might be able to generate Vγ4+ AHR-suppressors if reconstituted with CD8+ DC. To avoid all CD8+ T cells, we chose B6.TCR-β−/−/δ−/− mice as a source of the CD8+ accessory cells. Furthermore, because the AHR-suppressive Vγ4+ γδ T cells from the spleen require prior sensitization and challenge (15), we OVA-sensitized (2ip) both the donors of the CD8+ accessory cells and the CD8α− recipients, prior to i.v. transfer of the accessory cells. The reconstituted B6.CD8α−/− mice were then immediately challenged with OVA (3N) so that all cells in these mice were exposed to the full protocol of sensitization and challenge (2ip3N). Vγ4+ γδ T cells of these mice were subsequently tested for their ability to suppress AHR in B6.TCR-Vγ4/6−/− recipients, using the same method as described in Fig.1. Indeed, these Vγ4+ cells suppressed AHR, indicating that genetically CD8-deficient Vγ4+ γδ T cells still retain the potential of developing into AHR-suppressors, and that B6.TCR-β−/−/δ−/− mice (CD8wt) contain non-T cells capable of restoring their development (Fig. 5a). To determine if this capability was contained within the CD8+ non-T cells themselves, we compared CD8α+ and CD8α− splenocytes from B6.TCR-β−/−/δ−/− mice for their ability to restore γδ T cell-development. Only the CD8α+ fraction enabled the development of the AHR-suppressors, suggesting that the reconstituting cells themselves must express CD8 (Fig. 5b). This experiment also ruled out the possibility that the mere process of cell-transfer induced the AHR-suppressors. Next, to better enrich splenic CD8α+ DC from the B6.TCR-β−/−/δ−/− mice, we used a purification kit for CD8α+ DC, which depletes CD90 (Thy-1.2), CD45R (B220) and CD49b-positive cells, followed by positive magnetic bead-selection of CD8α+ cells. This cell-fraction contained at a minimum 60% CD8+ DC (MHC class II+, CD11c+, CD8α+). These enriched cells, when injected i.v. (105 CD8α+ DC/inoculum), were sufficient to support the development of AHR-suppressive Vγ4+ γδ T cells in B6.CD8α−/− mice (Fig. 5c). In contrast, neither CD8α− splenocytes nor enriched CD8α− DC (Fig. 5c) nor splenic CD8+ αβ T cells (from B6.TCR-δ−/− donors) had any reconstituting activity (Fig. 5d), albeit provided in larger numbers. These data suggest that CD8+ DC function as accessory cells in the development of the Vγ4+ AHR-suppressor γδ T cells whereas other DC-types or T cells do not.
Figure 5.
AHR in γδ T cell-deficient recipients reconstituted with genetically CD8-deficient Vγ4+ cells from “CD8-restored donors”, after intra-peritoneal sensitization and airway challenge with OVA (2ip3N protocol). (a-d) Lung resistance (RL) and dynamic compliance (Cdyn) as percent changes from saline controls in relation to increasing doses of aerosolized MCh. Recipients: B6.TCR-Vγ4/6−/− mice; donors: B6.CD8α−/− mice. Treatments: none (NT), systemic sensitization and airway challenge (2ip3N), 2ip3N plus Vγ4+ cells from 2ip3N-treated donors. Donors: The donors of the Vγ4+ cells either remained non-restored or were restored with splenocytes from 2ip-treated B6.TCR-β−/−/δ−/− mice, including the following fractions: (a) whole spleen, (b) magnetic bead-selected CD8α+ or CD8α− splenocytes, or (c) DC-enriched, magnetic bead-selected CD8α+ or CD8α− DCs. (d) Donors of Vγ4+ cells were restored with nylon wool non-adherent, magnetic bead-selected CD8α+ T cells from 2ip-treated B6.TCR-δ−/− mice.
Recipients: (a) untreated or after 2ip3N plus Vγ4+ cells from non-restored donors or donors that were restored with whole spleen (* 2ip3N plus Vγ4 cells compared with 2ip3N plus Vγ4 cells from donor restored with whole spleen, # non-treated compared with 2ip3N plus Vγ4 cells from non-restored donor); (b) untreated or after 2ip3N plus Vγ4+ cells from 2ip3N-treated donors restored with CD8-enriched or –depleted splenocytes (* non-treated compared with 2ip3N plus Vγ4 cells from donors reconstituted with CD8− splenocytes, # 2ip3N plus Vγ4 cells from donors reconstituted with CD8− splenocytes compared with 2ip3N plus Vγ4 cells from donor reconstituted with CD8+ splenocytes); (c) untreated or after 2ip3N plus Vγ4+ cells from donors restored with CD8+ or CD8− splenic DCs or with non-DCs (mostly CD8−) (* non-treated compared with 2ip3N plus Vγ4 cells from donor restored with non-DCs or # with CD8− DCs, $ 2ip3N plus Vγ4 cells from donor restored with CD8+ DCs compared with 2ip3N plus Vγ4 cells from donor restored with non-DCs or # with CD8− DCs; (d) untreated or after 2ip3N or 2ip3N plus Vγ4+ cells from donors restored with CD8α+ T cells (* non-treated compared with 2ip3N, # non-treated compared with 2ip3N plus Vγ4 cells from donors reconstituted with CD8α+ T cells, + there was no significant difference in RL between 2ip3N and 2ip3N plus Vγ4 cells from donors reconstituted with CD8α+ T cells, with exception of the response to 12.5 mg MCh).
AHR-enhancing γδ T cells also depend on CD8, and enriched CD8+ DC enable their development
Vγ1+ γδ T cells enhance AHR, as shown by cell-depletion and transfer experiments (10). In fact, development of AHR in young mice depends of these cells (10, 11) whereas AHR in older mice can develop in their absence while remaining subject to the influence of AHR-suppressive γδ T cells (5, 14). In contrast to the AHR-suppressors, the AHR-enhancing γδ T cells develop spontaneously, without need for sensitization or challenge of the cell-donors (11). However, we found that B6.CD8α−/− mice were also unable to produce these AHR-enhancers (Fig. 6a). Therefore, we tested whether reconstituting these mice with CD8+ non-T cells would also restore development of the AHR-enhancing γδ T cells, in the absence of any sensitization or challenge. Indeed, we found that reconstituting B6.CD8α−/− mice with the CD8α+ splenocyte fraction of B6.TCR-β−/−/δ−/− mice (Fig. 6b), or with small numbers of enriched CD8α+ DC (Fig. 6c), restored their ability to produce the AHR-enhancers, whereas the CD8α− splenocyte fraction had no effect (Fig. 6b). Because the restoration of the AHR-enhancers occurred without sensitizing the source of the DC, and without sensitizing or challenging the donors of the γδ T cells, this result suggested that interactions between CD8+ DC and γδ T cells are an integral part of normal and spontaneous γδ T cell-development.
Figure 6.
AHR in γδ T cell-deficient recipients reconstituted with Vγ1+ cells from normal or CD8-deficient mice, or with CD8-deficient Vγ1+ cells from “CD8-restored donors”, after systemic sensitization and airway challenge with OVA (2ip3N protocol). (a-c) Lung resistance (RL) and dynamic compliance (Cdyn) as percent changes from saline controls in relation to increasing doses of aerosolized MCh. Recipients: B6.TCR-δ−/− mice; donors: C57BL/6 or B6.CD8α−/− mice. Treatment of recipients: none (NT), intra-peritoneal sensitization and airway challenge (2ip3N), 2ip3N plus selected purified Vγ1+ cells from non-treated donors, 2ip3N plus Vγ1+ cells from non-treated B6.CD8α−/− mice restored with with magnetic bead-selected CD8α+ or CD8α− splenocytes from 2ip-treated B6.TCR-β−/−/δ−/− mice, or 2ip3N plus Vγ1+ cells from non-treated B6.CD8α−/− mice restored with DC-enriched, magnetic bead-selected CD8α+ or CD8α− DCs from 2ip-treated B6.TCR-β−/−/δ−/− mice.
Recipients: (a) after 2ip3N or 2ip3N plus Vγ1+ cells from C57BL/6 or from B6.CD8α−/− donors (* 2ip3N compared with 2ip3N plus Vγ1+ cells from C57BL/6 donors, # 2ip3N plus Vγ1+ cells from C57BL/6 donors compared with 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors); (b) after 2ip3N or 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors restored with CD8+ or − spleen cells from non-treated B6.TCR-β−/−/δ−/−mice (* 2ip3N compared with 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors restored with CD8+ spleen cells, # 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors restored with CD8+ spleen cells compared with 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors restored with CD8− spleen cells); (c) after 2ip3N or 2ip3N plus Vγ1+ cells from non-treated B6.CD8α−/− donors restored with CD8+ or CD8− splenic DCs from non-treated B6.TCR-β−/−/δ−/− mice (*2ip3N compared with 2ip3N plus Vγ1+ cells from B6.CD8α−/−donors restored with CD8+ DCs, # 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors restored with CD8+ DCs compared with 2ip3N plus Vγ1+ cells from B6.CD8α−/− donors restored with CD8− DCs).
Are the encounters between γδ T cells and CD8+ DC in the spleen functionally relevant?
In B6.CD8α−/− mice, γδ T cells are still present in the PALS (Fig. 3f), while in T cell-deficient mice (B6.TCR-β−/−/δ−/−), CD8+ non-T cells still populate the general vicinity of the central arterioles (Fig. 3g). Although there likely are quantitative differences in cell distributions by comparison with wt mice, this indicates that neither cell type absolutely requires the other to accumulate at this site.
To test whether the transferred CD8+ DCs that restore the development of AHR-regulatory γδ T cells in B6.CD8α−/− mice indeed appear in the spleen and engage γδ T cells, we transferred purified CD8+ non-T cells from B6.TCR-β−/−/δ−/− spleen in larger numbers (1×106) to facilitate their detection in situ. Five days later, when the regulatory γδ T cells are normally harvested, the CD8+ non-T cells were indeed detectable in the PALS of the B6.CD8α−/− mice, proximal to and frequently in direct contact with the endogenous γδ T cells (Fig. 3k). Thus, the transferred CD8+ non-T cells (in the B6.CD8α−/− mice) accumulated and encountered γδ T cells in the same splenic location as the endogenous CD8+ non-T cells in the B6.TCR-β−/− mice. Because these transferred cells also enable splenic γδ T cell function (Figs. 5, 6), their interactions with γδ T cells in the spleen are likely to be critical for the functional development of the AHR-regulators.
Finally, because development of the AHR-suppressive γδ T cells in the spleen depends on donor sensitization with OVA, we also examined the histological distribution of i.p. injected OVA/alum (Fig. 3l). We found OVA/alum in the spleen, in aggregates or contained within CD8− phagocytes, but not within CD8+ non-T cells or γδ T cells. However, both the CD8+ non-T cells and the γδ T cells were seen in proximity/contact with the OVA/alum in the spleen (Fig. 3l).
Discussion
Because innate T cells can become powerful modulators of AHR, it is important to understand their development. We have examined the development of two γδ T cell-types in mice, capable of either suppressing or enhancing allergen-induced AHR. These cells express Vγ4 and Vγ1, respectively, and they are present in the lung and in secondary lymphoid tissues (10, 16). The AHR-suppressive Vγ4+ cells are induced by allergen-sensitization and challenge (15) whereas the AHR-enhancing Vγ1+ cells develop spontaneously (11). Here, we report three observations related to the development of these two cell-types, namely that the process requires CD8, that the γδ T cells encounter CD8+ non-T cells which appear to be DC in the splenic PALS, and that enriched CD8+ DC from the spleen can restore the development of these two γδ T cell types when transferred into genetically CD8-deficient mice. Crosstalk between γδ T cells and DC occurs in vitro (20-22, 24), but little is known about their cellular interactions in the spleen and other lymphoid tissues. In mice lacking αβ T cells (B6.TCR-β−/−), we found many of the γδ T cells within the splenic PALS in proximity or contact with what appear to be CD8+DC based on morphology, phenotype and tissue location. In wt mice (C57BL/6), there are fewer γδ T cells. These still accumulate in the PALS (Fig. 3a), but due to their smaller numbers and perhaps also because of competition with the αβ T cells (37, 38), one might expect to find overall fewer contacts with DC. However, in mice lacking CD8 which contain αβ T cells, the γδ T cells were found in proximity of what appeared to be endogenous DEC-205+DC in the PALS, and they were not prevented from contacts with transferred splenic CD8+ non-T cells which probably are CD8+DC (Fig.3k). Contacts with endogenous splenic CD8+ non-T cells seem to occur continuously as there was little change in frequency following OVA-sensitization/challenge, and both Vγ1+ cells and Vγ4+ cells take part. Likewise, enriched CD8+ DC can restore the development of both Vγ1+ AHR-enhancers and Vγ4+ AHR-suppressors. Our preliminary data (not shown) suggest that the transfer of CD8+ DC drives the γδ T cells into cell cycle (C.L.R. et al., unpublished). However, the CD8+ DC appear to exert their effect at an early stage in the development of the AHR-modulating γδ T cells, and these interactions alone seem insufficient to explain the functional differentiation of the AHR-enhancers and suppressors. Notably, development of the suppressors requires airway challenge, and thus may depend on a lung-derived signal (11, 15).
Our data suggest a strict requirement for CD8 in the development of the AHR-regulatory γδ T cells although these cells themselves need not express CD8. A role for CD8 “in trans” in γδ T cell development has not been reported (39). Since transferred enriched CD8+ DC can restore development of the AHR-modulators in the absence of other CD8+ cells in the recipients, it seems likely that the DC-expressed CD8 satisfies the requirement for CD8 although other more complex scenarios cannot be excluded. A direct mechanism would be interesting because, although CD8+ DC in the lymphoid tissues have been characterized as a phenotypically and functionally distinct population (40), no functional significance has been assigned to the CD8 they express. In fact, in one study comparing the largely overlapping populations of CD8+ and DEC-205+ DC for their ability to support αβ T cell-responses, DC-expressed CD8 appeared to be of no consequence as CD8+ DC could be replaced with DEC-205+ DC without change (41). Our findings contrast with this study, inasmuch as endogenous DEC-205+ cells, which are plentiful in the spleen of CD8− mice and co-localize here with γδ T cells (Fig. 3j), failed to support the development of the AHR-modulating γδ T cells. Thus, DC-expressed CD8α might have a function after all, in connection with the development of γδ T cells, and perhaps as an activating receptor (42, 43).
CD8+ DC, which reside in the lymphoid tissues(28, 29), have been found to receive Ag and activating signals from phagocytes that shuttle from peripheral tissues such as skin and lung to the secondary lymphoid organs (44, 45), to excel at Ag-cross-presentation, and to prime naïve αβ T cells to become Ag-specific CTLs and type 1 helper (Th1) cells (46-48). To our knowledge, they have not previously been connected with γδ T cells. However, a dependence on CD8+ DC aligns the γδ T cells with the above-mentioned types of αβ T cells (CD8+ and Th1), and sets them apart from others (Th2), and from NK cells, neither of which depend on CD8+ DC although they require DC-priming (25, 49). We have not yet determined which factors might be involved in the DC-γδ T interaction. Candidates include TNF-α and IL-15, both of which are produced by CD8+ DC, and TNF-α, because the function and development of AHR-regulatory γδ T cells depends on it (50, 51), and IL-15, because it supports homeostatic expansion of γδ T cells (37). Incidentally, IL-15 is “trans”-presented by DC, requires DC-lymphocyte contact, and also is essential in the “priming” of NK cells (49).
Connecting the observations reported in this study, we propose that developing γδ T cells must interact with CD8+DC in the lymphoid tissues as an early step on their way to functional competence (e.g. towards becoming AHR-modulators). Because this interaction appears to involve direct cell-contact, because CD8 is absolutely required for γδ T cell-development, and because development can take place when the only available CD8 appears to be expressed by the DC, we envisage that the DC-expressed CD8-molecules might engage ligands on the γδ T cells they contact. Known ligands for CD8 are MHC class I or class I-like molecules (12, 52). We have not yet examined AHR-enhancement, but we found that AHR-suppression by Vγ4+ γδ T cells indeed depends on MHC class I expression (Cook et al., unpublished). Thus, our data are consistent with the scenario outlined, but fall short of proving it. However, in another study with naturally occurring Treg whose functional activation requires both CD8 and MHC I, a similar cell-cell interaction was proposed (53).
The interaction between γδ T cells and CD8+DC documented here likely represents only one of several steps in the development of the γδ T cells. As both AHR-suppressive and AHR-enhancing types depend on CD8+DC, their functional differences might be determined elsewhere, earlier or later in their development (54). Additional accessory cells such as the CD8− phagocytes shown in Fig. 3l, which engulf OVA/alum particles and appear following sensitization in the splenic PALS near the CD8+DC and the γδ T cells, are perhaps involved as well (44, 45). These cells or cells from the challenged lung might mediate the functional differentiation of the splenic AHR-suppressors, which require induction by OVA-sensitization and challenge (15).
Acknowledgements
We thank Drs. Katsuyuki Takeda, M. Kemal Aydintug and Jena French for advice and support, Drs. Philippa Marrack and Michael Lahn for discussion, and Joshua Loomis and Bill Townend for their assistance with cell sorts and fluorescence microscopy.
Footnotes
This work was supported by NIH grants AI40611 and HL65410 to W.K.B., AI44920 and AI063400 to R.L.O. and HL36577 to E.W.G.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 4.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 5.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Koehler G, O'Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nature Medicine. 1999;5:1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 6.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Medicine. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 7.Hadeiba H, Locksley RM. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J.Immunol. 2003;170:5502–5510. doi: 10.4049/jimmunol.170.11.5502. [DOI] [PubMed] [Google Scholar]
- 8.Lahn M, Kanehiro A, Hahn Y-S, Wands JM, Aydintug MK, O'Brien RL, Gelfand EW, Born WK. Aerosolized anti-T-cell-receptor antibodies are effective against airway inflammation and hyperreactivity. Int. Arch. Allergy and Immunology. 2004;134:49–55. doi: 10.1159/000077533. [DOI] [PubMed] [Google Scholar]
- 9.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, Larsen GL, Gelfand EW. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a murine model of airway sensitization. J Exp Medicine. 1996;183:1719–1729. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Kemal Aydintug M, Lahn M, Huber SA, O'Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, TH2 cytokines, and airway inflammation. J.Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 11.Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, J.L. M, Gapin L, O'Brien RL, Gelfand EW, Born WK. Airway hyperresponsiveness through synergy of gd T cells and NKT cells. J.Immunol. 2007;179:2961–2968. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn Y-S, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc. Natl. Acad. Sci (USA) 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn Y-S, Taube C, Jin N, Takeda K, Park J-W, Wands JM, Aydintug MK, Roark CL, Lahn M, O'Brien RL, Gelfand EW, Born WK. Vγ4+ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J.Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 14.Cui Z-H, Joetham A, Aydintug MK, Born WK, Gelfand EW. Reversal of established allergic airway hyperreactivity by long-term allergen challenge depends on γ/δ T cells. Am.J.Respir. Crit. Care Med. 2003;168:1324–1332. doi: 10.1164/rccm.200305-634OC. [DOI] [PubMed] [Google Scholar]
- 15.Jin N, Taube C, Sharp L, Hahn Y-S, Yin X, Wands JM, Roark CL, O'Brien RL, Gelfand EW, Born WK. Mismatched antigen prepares gd T cells for suppression of airway hyperresponsiveness. J. Immunol. 2005;174:2671–2679. doi: 10.4049/jimmunol.174.5.2671. [DOI] [PubMed] [Google Scholar]
- 16.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn Y-S, Cook L, Yin X, Dalporto J, Lahn M, Hyde DM, Gelfand EW, Mason DY, O'Brien RL, Born WK. Distribution and leukocyte contacts of gd T cells in the lung. J. Leukocyte Biology. 2005;78:1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 18.Ismaili J, Olislagers V, Poupot M, Fournie JJ, Goldman M. Human gamma delta T cells induce dendritic cell maturation. Clinical Immunology. 2002;103:296–302. doi: 10.1006/clim.2002.5218. [DOI] [PubMed] [Google Scholar]
- 19.Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated γ/δ T cell maturation of dendritic cells. J.Exp.Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins C, Wolfe J, Roessner K, Shi C, Sigal LH, Budd RC. Lyme arthritis synovial gd T cells instruct dendritic cells via Fas ligand. J.Immunol. 2005;175:5656–5665. doi: 10.4049/jimmunol.175.9.5656. [DOI] [PubMed] [Google Scholar]
- 21.Dieli F, Caccamo N, Meraviglia S, Ivanyi J, Sireci G, Bonanno CT, Ferlazzo V, La Mendola C, Salerno A. Reciprocal stimulation of γδ T cells and dendritic cells during the anti-mycobacterial immune response. Eur. J. Immunol. 2004;34:3227–3235. doi: 10.1002/eji.200425368. [DOI] [PubMed] [Google Scholar]
- 22.Devilder M-C, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen-stimulated Vg9Vd2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J.Immunol. 2006;176:1386–1393. doi: 10.4049/jimmunol.176.3.1386. [DOI] [PubMed] [Google Scholar]
- 23.Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinicpolycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology. 2004;112:364–368. doi: 10.1111/j.1365-2567.2004.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, Poccia F, Gessani S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J.Immunol. 2005;174:252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Medicine. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8a+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 27.Wilson NS, Behrens GMN, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nature Immunology. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 28.Dakic A, Shao Q.-x., D'Amico A, O'Keefe M, Chen W.-f., Shortman K, Wu L. Development of the dendritic cell system during mouse ontogeny. J.Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 29.Heath WR, Belz GT, Behrens GMN, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunological Reviews. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 30.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J. Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 31.Shortman K, Naik SH. Steady-state and inflammatory dendritic cell development. Nature Reviews Immunology. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 32.Sunaga S, Maki K, Komagata Y, Miyazaki J-I, Ikuta K. Developmentally ordered V-J recombination in mouse T cell receptor γ locus is not perturbed by targeted deletion of the Vγ4 gene. J. Immunol. 1997;158:4223–4228. [PubMed] [Google Scholar]
- 33.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive γδ T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 34.Heilig JS, Tonegawa S. T-cell γ gene is allelically but not isotypically excluded and is not required in known functional T-cell subsets. Proc Natl Acad Sci USA. 1987;84:8070–8074. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J. Exp. Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nature Medicine. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 37.French JD, Roark CL, Born WK, O'Brien RL. Gamma delta T cell homeostasis is established in competition with alpha beta T cells and NK cells. Proc. Natl. Acad. Sci (USA) 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willis RA, Kappler JW, Marrack PC. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc. Natl. Acad. Sci (USA) 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rammensee H-G, Bevan MJ, Fink PJ. Antigen specific suppression of T-cell responses – the veto concept. Immunol Today. 1985;6:41–43. doi: 10.1016/0167-5699(85)90044-1. [DOI] [PubMed] [Google Scholar]
- 40.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 41.Kronin V, Vremec D, Winkel K, Classon BJ, Miller RG, Mak TW, Shortman K, Suss G. Are CD8+ dendritic cells (DC) veto cells? The role of CD8 on DC in DC development and in the regulation of CD4 and CD8 T cell responses. Int. Immunol. 1997;9:1061–1064. doi: 10.1093/intimm/9.7.1061. [DOI] [PubMed] [Google Scholar]
- 42.Attinger A, Devine L, Wang-Zhu Y, Martin D, Wang J.-h., Reinherz EL, Kronenberg M, Cheroutre H, Kavathas P. Molecular basis for the high affinity interaction between the thymic leukemia antigen and the CD8αα molecule. J.Immunol. 2005;174:3501–3507. doi: 10.4049/jimmunol.174.6.3501. [DOI] [PubMed] [Google Scholar]
- 43.Kern P, Hussey RE, Spoerl R, Reinherz EL, Chang H-C. Expression, purification and functional analysis of murine ectodomain fragments of CD8αα and CD8αβ dimers. J.Biol.Chemistry. 1999;274:27237–27243. doi: 10.1074/jbc.274.38.27237. [DOI] [PubMed] [Google Scholar]
- 44.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci (USA) 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 46.den Haan JMM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1695. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J.Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: Conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J.Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 49.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanehiro A, Lahn M, Makela MJ, Dakhama A, Fujita M, Joetham A, Mason RJ, Born W, Gelfand EW. Tumor necrosis factor-α negatively regulates airway hyperresponsiveness through γδ T cells. Am. J. Respir. Crit. Care Med. 2001;164:2229–2238. doi: 10.1164/ajrccm.164.12.2012059. [DOI] [PubMed] [Google Scholar]
- 51.Kanehiro A, Lahn M, Makela MJ, Dakhama A, Joetham A, Rha Y-H, Born W, Gelfand EW. Requirement for the p75 TNF-α receptor 2 in the regulation of airway hyperresponsiveness by γδ T cells. J.Immunol. 2002;169:4190–4197. doi: 10.4049/jimmunol.169.8.4190. [DOI] [PubMed] [Google Scholar]
- 52.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang H-C, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8αα and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 53.Joetham A, Takeda K, Miyahara N, Matsubara S, Ohnishi H, Koya T, Dakhama A, Gelfand EW. Activation of naturally occurring lung CD4+CD25+ regulatory T cells requires CD8 and MHC I interaction. Proc. Natl. Acad. Sci (USA) 2007;104:15057–15062. doi: 10.1073/pnas.0706765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. gd T cell receptors: functional correlations. Immunol. Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]