Abstract
We identify calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors on human neural progenitor cells (NPCs) and present a physiological role in neurogenesis. RNA editing of the GluR2 subunit at the Q/R site is responsible for making most AMPA receptors impermeable to calcium. Because a single-point mutation could eliminate the need for editing at the Q/R site and Q/R-unedited GluR2 exists during embryogenesis, the Q/R-unedited GluR2 subunit presumably has some important actions early in development. Using calcium imaging, we found that NPCs contain calcium-permeable AMPA receptors, whereas NPCs differentiated to neurons and astrocytes express calcium-impermeable AMPA receptors. We utilized reverse-transcription polymerase chain reaction and BbvI digestion to demonstrate that NPCs contain Q/R-unedited GluR2, and differentiated cells contain Q/R-edited GluR2 subunits. This is consistent with the observation that the nuclear enzyme responsible for Q/R-editing, adenosine deaminase (ADAR2), is increased during differentiation. Activation of calcium-permeable AMPA receptors induces NPCs to differentiate to the neuronal lineage and increases dendritic arbor formation in NPCs differentiated to neurons. AMPA-induced differentiation of NPCs to neurons is abrogated by overexpression of ADAR2 in NPCs. This elucidates the role of AMPA receptors as inductors of neurogenesis and provides a possible explanation for why the Q/R editing process exists.—Whitney, N. P., Peng, H., Erdmann, N. B., Tian, C., Monaghan, D. T., Zheng, J. C. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons.
Keywords: glutamate, Q/R editing, neurogenesis, dendritic arborization, ADAR2
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionicacid (AMPA) receptors are ionotropic glutamate receptors that mediate the majority of fast excitatory neurotransmission in the mammalian central nervous system (CNS) (1, 2). AMPA receptors are composed of GluR1, 2, 3, and 4 subunits in a tetraheteromeric complex, with the vast majority of AMPA receptors containing GluR2 subunits (3,4,5). Incorporation of GluR2 subunits typically renders AMPA receptors impermeable to calcium (6,7,8,9). Calcium impermeability is a consequence of editing at the Q/R site of GluR2 pre-mRNA in which a gene-encoded glutamine (Q) codon in the channel-forming intramembrane segment is changed to an arginine (R) codon (7, 8, 10, 11). This Q/R editing is mediated by the enzyme adenosine deaminase (ADAR2), which edits nearly 100% of GluR2 pre-mRNA in the adult CNS (10, 12, 13). Q/R editing is necessary for survival, as demonstrated by Higuchi et al. (12), who showed that a mouse ADAR2 knockout was fatal. Transgenic replacement of edited GluR2, substituting an arginine in the Q/R editing site, restored viability. Although unedited GluR2 has been identified (14,15,16,17), a physiological role for this type of receptor has not yet been identified.
Neurogenesis is dependent on the proper proliferation, migration, differentiation, and survival of neural progenitor cells (NPCs) (18, 19). NPCs are self-renewing multipotent cells capable of differentiating into neurons, astrocytes, and oligodendrocytes (18, 20). l-Glutamate (Glu) is a key factor influencing neurogenesis (21, 22), an effect mediated in part by AMPA receptors. Potentiation of AMPA receptors has been shown to increase cell proliferation in the adult rat hippocampus (23), enhance performance of rats in cognitive assays (24, 25), and increase brain-derived neurotrophic factor (BDNF) expression in the dentate gyrus of the rat hippocampus (26). We sought to characterize the nature of the Glu-mediated neurogenesis and to determine the effects of Glu on NPC function. Here, we describe Glu stimulation in cultured NPCs directing differentiation to the neuronal lineage.
We observed that Glu stimulation induced calcium influx in NPCs that lacked functional N-methyl-d-aspartate (NMDA) receptor expression but expressed AMPA receptors. NPCs expressed significant amounts of Q/R-unedited GluR2 subunits, identifying a population of cells expressing calcium-permeable AMPA receptors. Activation and potentiation of calcium-permeable AMPA receptors induced differentiation of NPCs to neurons and stimulated dendritic arbor formation. NPCs differentiated to astrocytes and neurons expressed AMPA receptors with Q/R-edited GluR2 subunits, and Q/R editing during differentiation correlated with an increase in the expression of ADAR2. Overexpression of ADAR2 in NPCs resulted in the increase of Q/R-edited GluR2 and prevented AMPA-mediated differentiation to neurons. In identifying a physiological role for these calcium-permeable AMPA receptors, we present a feasible explanation for the existence of Q/R-unedited GluR2 subunits and the Q/R-editing process.
MATERIALS AND METHODS
Human fetal NPC, differentiated neuron, and differentiated astrocyte culture
Human fetal NPCs were isolated from human fetal brain tissue (gestational age 13–16 wk) from elective aborted specimens as described previously (27), in full compliance with University of Nebraska Medical Center (UNMC) and U.S. National Institutes of Health (NIH) ethical guidelines. Briefly, NPCs were cultured in substrate-free tissue culture flasks and grown in suspension in neurosphere initiation medium (NPIM), which consisted of X-Vivo 15 (BioWhittaker, Walkersville, ME, USA) with N2 supplement (Gibco Invitrogen Corporation, Carlsbad, CA, USA), basic fibroblast growth factor (bFGF; 20 ng/ml; Sigma-Aldrich, St. Louis, MO, USA), epidermal growth factor (EGF; 20 ng/ml; Sigma-Aldrich), leukemia inhibitory factor (LIF; 10 ng/ml; Chemicon, Temecula, CA, USA), neural cell survival factor-1 (NSF-1; Cambrex BioScience, Walkersville, MD, USA), and 60 ng/ml N-acetylcysteine (Sigma-Aldrich) (28). NPCs were passaged at 2-wk intervals as described previously (27) and utilized between passages 4 and 14.
After a 15-min trypsin incubation and mechanical disassociation of NPCs, 2 × 106 NPCs for astrocyte differentiation and 5 × 106 NPCs for neuron differentiation were plated in poly-d-lysine-coated T75 flasks. NPCs are differentiated to neurons in neuron differentiation medium, which consists of neurobasal medium with B27 supplement (Gibco Invitrogen), 100 IU/ml penicillin/streptomycin, and 500 μM l-glutamine. NPCs are differentiated to astrocytes in astrocyte differentiation medium, which consists of Dulbecco’s modified eagle medium with F12 (Gibco Invitrogen), 10% fetal bovine serum, 100 IU/ml penicillin/streptomycin, and fungizone.
RNA extraction and TaqMan real-time reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated with TRIzol Reagent (Invitrogen) and RNeasy Mini Kit according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). Assay-on-Demand primers for GluR1 (Hs00181348_m1), GluR2 (Hs00181331_m1), GluR3 (Hs00241485_m1), GluR4 (Hs00168163_m1), ADAR2 (Hs00210562_m1), and GAPDH (4310884E) were purchased from Applied Biosystems (Foster City, CA, USA). Real-time RT-PCR was carried out using the one-step quantitative TaqMan Real-time RT-PCR system (Applied Biosystems). Relative GluR1–4 and ADAR2 levels were determined and standardized with GAPDH as an internal control.
Immunocytochemistry
Cells were fixed using 1:1 acetone/methanol for 20 min at −20°C, after which the acetone/methanol was removed, and the cells were allowed to air dry and then were rehydrated with PBS. Cells were blocked for 1 h with 0.1% Triton X-100 and 2% BSA in PBS and incubated overnight at 4°C with primary antibodies in PBS with 0.1% Triton X-100 and 2% BSA. Secondary antibodies, Alexa Fluor 488 (green) and 594 (red) (Molecular Probes Invitrogen, Carlsbad, CA, USA) were applied for 1 h at room temperature, and then stained with Hoechst 33342 for 20 min. The primary antibodies used were anti-GluR1 (polyclonal, 2.0 μg/ml; Chemicon), anti-GluR2 (monoclonal, 2.0 μg/ml; Chemicon), anti-GluR3 (monoclonal, 2.0 μg/ml; Chemicon), anti-GluR4 (polyclonal, 2.0 μg/ml; Chemicon), nestin (polyclonal, 1:100, Chemicon; monoclonal, 5.0 μg/ml, R&D Systems, Minneapolis, MN, USA), anti-β-tubulin isotype III (monoclonal, 1:400; Sigma-Aldrich), and anti-glial fibrillary acidic protein (GFAP) (polyclonal, 1:1000; DAKO, Carpinteria, CA, USA).
Protein extraction and Western blot analysis
Nuclear proteins were extracted using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL, USA), and total protein was isolated using M-PER mammalian protein extraction reagent (Pierce) according to the manufacturer’s protocol. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrophoretically transferred to an Immobilon-P membrane (polyvinyldifluoridene membranes) (Bio-Rad, Hercules, CA, USA). Membranes were incubated with primary antibodies for ADAR2 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and loading control β-actin (1:10,000; Sigma-Aldrich) or Tata box binding protein (TBP) (1:2,000; Abcam, Cambridge, MA, USA) overnight at 4°C, followed by a horseradish peroxidase-linked secondary anti-mouse antibody (1:10,000; Cell Signaling Technologies, Danvers, MA, USA) or anti-goat antibody (1:10,000; Santa Cruz Biotechnology) for 1 h at room temperature. Antigen-antibody complexes were visualized by enhanced chemiluminescence Western blot analysis and captured with Hyperfilm ECL (Pierce), and immunoblot images were quantified using NIH Image 1.63 (NIH, Bethesda, MD, USA).
Calcium imaging
NPCs (2×105)were plated into 6-well plates with poly-d-lysine-coated 25-mm glass coverslips in NPIM, neuron differentiation medium, or astrocyte differentiation medium. Calcium imaging was performed on NPCs between 1 and 3 days after plating; differentiated neurons and astrocytes were used between 4 and 6 wk after plating. Medium was removed, and cells were loaded with 7 μM Fura-II AM (Molecular Probes, Eugene, OR, USA) in calcium buffer (145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose) with 0.018% pluronic acid (Molecular Probes) and 0.09% dimethyl sulfoxide (DMSO) for 30–60 min at 37°C. Cells were rinsed 4 times with calcium buffer, mounted into a perfusion chamber, and perfused with the calcium buffer or calcium-free buffer (CaCl2 was omitted and 3 mM EGTA was added). A field including 15–20 cells was selected, and the change in intracellular calcium level was measured by monitoring the excitation intensity at 340 and 380 nm wavelengths using a photometer (Photon Technology International, London, ON, Canada) coupled to an inverted Nikon TMD Diaphot epifluorescent microscope (Nikon, Melville, NY, USA) as described previously (29). The intracellular calcium concentrations were calculated as follows: [Ca2+] = Kd [(R − Rmin)/(Rmax − R)] (380min/380max), where Rmin and Rmax are the fluorescence ratios in the absence (with 3 mM EGTA) or presence of saturating calcium (3 mM), respectively, and Kd = 224 nM.
Q/R editing assay
Nested PCR products containing the Q/R editing site were digested with BbvI as described previously (13). cDNA (50 ng) was subjected to PCR in a 50-μl reaction containing 5 μl 10× PCR buffer with MgCl2 (Roche Applied Science, Indianapolis, IN, USA), 0.8 mM dNTP mix (Applied Biosystems), 0.25 μl TaqDNA polymerase (Roche Applied Science), and 200 μM primer. The PCR amplification protocol was 95°C for 2 min, followed by 36 cycles of 95°C for 30 s, 64°C for 30 s, and 72°C for 1 min, and finished with a final extension of 72°C for 7 min. The primers used for the first-round reaction were forward (5′-TTCCTGGTCAGCATTTAGCC-3′) and reverse (5′-TTCCCTTTGGACTTCCGCAC-3′), and for the second-round reaction, the primers used were the same forward and a second reverse (5′-TGGGAGACACCATCCTCTCTACA G-3′) (13). A nested PCR was then performed on 2 μl of the first-round product in a 100-μl reaction containing 10 μl of 10× PCR buffer with MgCl2 (Roche Applied Science), 0.8 mM of dNTP mix (Applied Biosystems), 0.5 μl of TaqDNA polymerase (Roche Applied Science), and 200 μM of primer. PCR products were run on a 1% agarose gel; the 277-bp band (the expected size of the second-round PCR product) was excised and gel purified using a QIAquick Gel Extraction Kit (Qiagen), according to the manufacturer’s protocol, yielding 48–50 μl of product. The gel-purified product was digested with BbvI overnight at 37°C, and digested products were run on a 2.5% agarose gel. The products were visualized and photographed with a UVP Transilluminator (UVP Inc., Upland, CA, USA), the bands were quantified with NIH Image 1.63, and the percentage of unedited GluR2 was calculated by comparing the relative intensities of the 228 and the sum of 147- and 81-bp bands.
Differentiation assay
NPCs were plated into 24-well poly-d-lysine-coated plates at a concentration of 5 × 104 cells/well (Fig. 1) or 1 × 105 (Fig. 6) in neuron differentiation medium. Cells were treated after plating with control neuron differentiation medium alone or with progressive addition of 10 μM l-glutamate, 10 μM cyclothiazide, and 30 μM MK-801 or 10 μM CNQX (Fig. 1); or control neuron differentiation medium alone or with progressive addition of 3 μM AMPA, 10 μM cyclothiazide, and 10 μM 1-napthyl acetyl spermine (Naspm) in the presence of 0.1% DMSO (Fig. 6). After 1 wk, cells were stained for GFAP, β-tubulin-III and Hoechst 333342. Ten (Fig. 1) or 15 pictures per condition (Fig. 6) were taken at ×200, and the numbers of neurons and astrocytes for each condition were manually counted: control, 10 μM l-glutamate, 10 μM l-glutamate with 10 μM cyclothiazide, 10 μM l-glutamate with 10 μM cyclothiazide and 30 μM MK-801, and 10 μM l-glutamate with 10 μM cyclothiazide and 10 μM CNQX (Fig. 1); or control, 3 μM AMPA, 3 μM AMPA with 10 μM cyclothiazide, and 3 μM AMPA with 10 μM cyclothiazide and 10 μM Naspm (Fig. 6). The data are expressed as the percentage of neurons or astrocytes; representative pictures are shown for each condition.
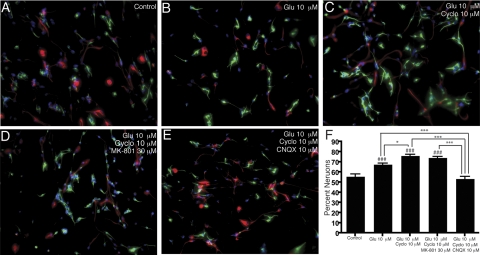
Figure 1.
Glutamate directs the differentiation of NPCs to neurons. Human NPCs were cultured in neuron differentiation medium alone (A); with 10 μM Glu (B); 10 μM Glu and 10 μM cyclo (C); 10 μM Glu, 10 μM cyclo, and 30 μM MK-801 (D); and 10 μM Glu, 10 μM cyclo, and 10 μM CNQX (E) for 1 wk and then stained for β-tubulin-III (green), GFAP (red), and Hoechst (blue). The numbers of neurons and astrocytes were counted in 10 pictures for each condition (n=10) (A–E) and expressed as the percentage of neurons (β-tubulin+) (F). *P < 0.05; ***P < 0.001. ###P < 0.001 vs. control. Data are representative of 3 separate experiments with NPCs from 3 separate donors.
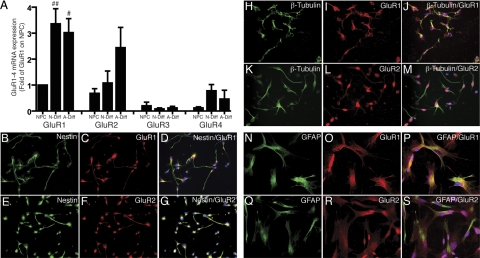
Figure 2.
GluR1–4 AMPA subunit mRNA and GluR1–2 protein are expressed on NPCs, differentiated neurons, and differentiated astrocytes. Real-time RT-PCR was used to determine GluR1–4 mRNA expression; values are expressed as fold of GluR1 from NPCs and standardized to GAPDH (n=5) (A). NPCs (B–G), N-diff cells (H–M), and A-diff cells (N–S) were stained for nestin (B, E), β-tubulin-III (H, K), GFAP (N, Q), GluR1 (C, I, O) and GluR2 (F, L, R). #P < 0.05, ##P < 0.01 vs. GluR1 on NPCs. Data are representative of 3 separate experiments with NPCs from 3 separate donors.
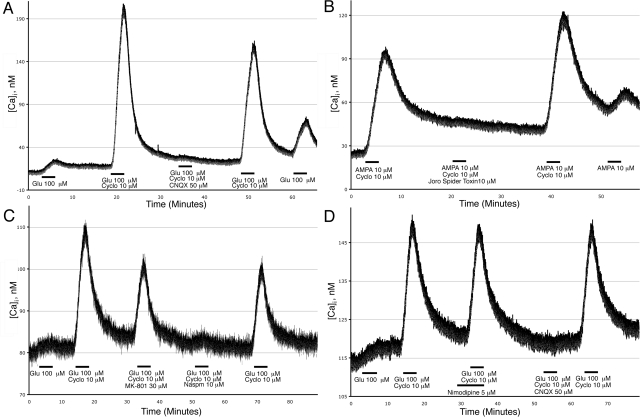
Figure 3.
NPCs contain calcium-permeable AMPA receptors. Calcium imaging was performed on a group of 20 NPCs; intracellular calcium concentrations are expressed as nanomoles of calcium ions. A) Glu (100 μM) caused calcium influx into NPCs, which was increased with 10 μM cyclo and blocked with 50 μM CNQX. B) Joro spider toxin (10 μM) inhibited AMPA-induced calcium influx. C) Glu-induced calcium influx was blocked by 10 μM Naspm, but not by 30 μM MK-801. D) The voltage-gated ion channel blocker nimodipine (5 μM) did not prevent glutamate-induced calcium influx. Data are representative of multiple experiments with NPCs from 3 separate donors.
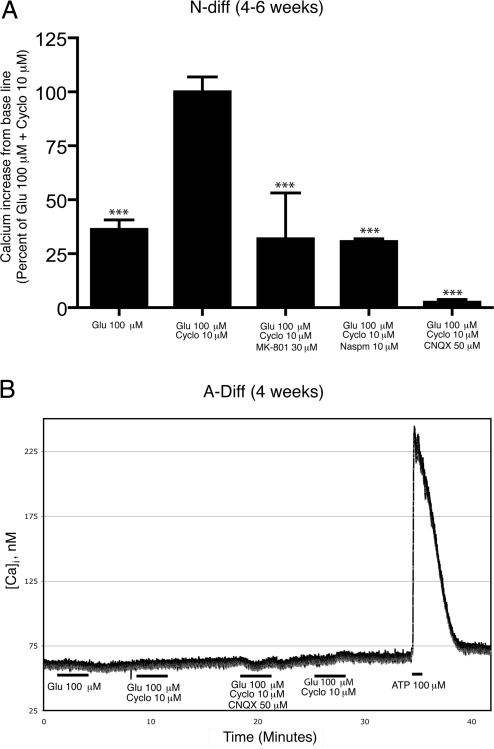
Figure 4.
Differentiated astrocytes lack calcium-permeable AMPA receptors, and differentiated neurons have reduced expression of calcium-permeable AMPA receptors. Calcium imaging was performed on N-diff (A) and A-diff cells (B). Intracellular calcium levels are expressed as the increase in calcium level from the baseline before stimulation and expressed as a percentage calcium influx as compared to the calcium influx induced by 100 μM Glu and 10 μM cyclo (A) or as nanomoles of calcium ion concentration (B). A) Glu (100 μM; n=4) caused calcium influx into differentiated neurons, which was increased with 10 μM cyclo (n=10). The majority of calcium influx was blocked with 30 μM Mk-801 (n=3) and completely blocked with 50 μM CNQX (n=4). B) Glu (100 μM) alone and with 10 μM cyclo did not induce calcium influx, but 100 μM ATP did stimulate an increase in calcium. ***P < 0.001 vs. 100 μM Glu and 10 μM cyclo. Data are representative of multiple experiments with NPCs from 3 separate donors.
Figure 5.
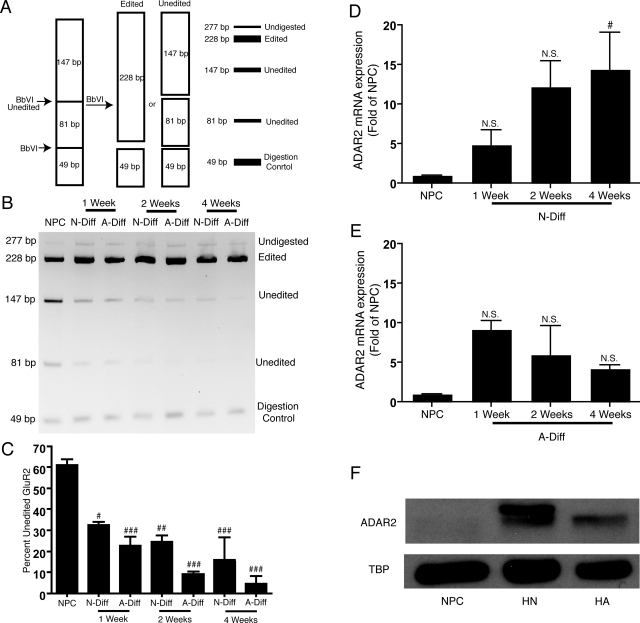
NPCs express AMPA receptors with Q/R-unedited GluR2 subunits, whereas differentiated cells have Q/R-edited GluR2 subunits, which correlates to an increased expression of ADAR2 as NPCs differentiate. A) Schematic diagram of BbVI restriction enzyme digestion to distinguish between Q/R-edited and unedited GluR2 mRNA. B) BbVI digestion product run on a 2% agarose gel and visualized with EtBr; image is inverted to assist the presentation. Undigested (277 bp), Q/R-edited (228 bp), Q/R-unedited (147 and 81 bp), and digestion control (49 bp) are shown for one representative donor. C) NIH Image 1.63 analysis of bands from panel B (n=3); data are expressed as percentage of unedited GluR2, [(147+81 bp)/228 bp]100. D) Real-time RT-PCR analysis to determine ADAR2 mRNA expression; values are fold of NPCs and standardized to GAPDH. ADAR2 levels were measured in NPCs and N-diff cells for 1, 2, and 4 wk. E) ADAR2 mRNA levels were measured in NPCs and A-diff cells for 1, 2, and 4 wk. F) Western blot analysis of nuclear extraction protein levels for ADAR2 and TBP for NPCs and primary neurons and astrocytes. N.S., not significant; P > 0.05. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. NPCs. Data are representative of 3 separate experiments with NPCs from 3 separate donors.
Figure 6.
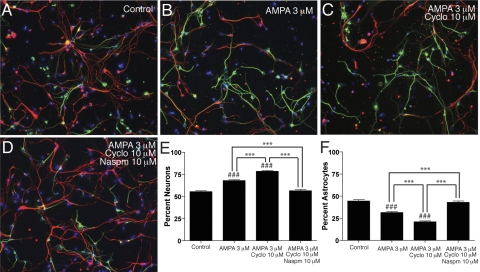
Stimulation and potentiation of AMPA receptors directs NPC differentiation to neurons. Human NPCs were cultured in neuron differentiation medium alone (A), with 3 μM AMPA (B), 3 μM AMPA and 10 μM cyclo (C), or 3 μM AMPA, 10 μM cyclo, and 10 μM Naspm (D) for 1 wk, and then stained for β-tubulin-III (green), GFAP (red), and Hoechst (blue). The numbers of neurons and astrocytes were counted in 15 pictures for each condition (n=15) (A–D) and expressed as percentages of neurons (E) and astrocytes (F). ***P < 0.001. ###P < 0.001 vs. control. Data are representative of 2 separate experiments with NPCs from 2 separate donors.
Dendritic arbor quantification
Dendritic arborization was quantified as described previously (30). Briefly, NPCs were plated into 24-well plates with 12-mm poly-d-lysine-coated coverslips at a concentration of 5 × 104 cells/well in neuron differentiation medium. Cells were treated after plating with control neuron differentiation medium alone or with progressive addition of 3 μM AMPA, 10 μM cyclothiazide, and 10 μM Naspm. After 1 wk in culture, the cells were stained for GFAP, β-tubulin-III, and Hoechst. Five pictures per condition were taken at ×200, and numbers of neurites per cell, arbors per cell, branch nodes per cell, and average arbor length were calculated for neurons not physically associated with astrocytes for each condition: control, 3 μM AMPA, 3 μM AMPA with 10 μM cyclothiazide; and 3 μM AMPA with 10 μM cyclothiazide and 10 μM Naspm.
Electroporation
NPCs were transfected with recombinant pECFP-ADAR2 plasmid (CFP-ADAR2) (generously provided by Dr. Andrew MacMillan, University of Alberta, Edmonton, AB, Canada) and pECFP-N1 alone using a basic nucleofector kit for primary mammalian neurons (Amaxa, Gaithersburg, MD, USA). NPCs (5×106) were suspended in 100 μM nucleofector solution with 8 μg recombinant plasma or empty vector. Electroporation was preformed using the Amaxa nucleofector device (Amaxa, Gaithersburg, MD, USA) and nucleofector program A-033.
Statistical analysis
Data are expressed as means ± se. The data were evaluated statistically by analysis of variance (ANOVA), followed by the Tukey test for paired observations. Significance was considered to be P < 0.05.
RESULTS
Glutamate induces differentiation of NPCs to neurons
Using our human NPC culture, which is >90% nestin positive (27), we determined the effect of Glu on NPC differentiation. NPCs were cultured in neuronal differentiation medium for 7 days, and then they were stained for β-tubulin-III, GFAP, and Hoechst. NPCs were treated with neuronal differentiation medium alone (Fig. 1A); Glu (Fig. 1B); Glu and cyclothiazide (cyclo, an AMPA receptor-specific potentiator; Fig. 1C); Glu, cyclo, and MK-801 (an NMDA receptor antagonist; Fig. 1D); and Glu, cyclo, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, an AMPA and kainate receptor antagonist; Fig. 1E). Cells were counted and scored as neurons (GFAP±/β-tubulin-III+ cells) or astrocytes(GFAP+/β-tubulin-III− cells), with data expressed as a percentage of neurons (Fig. 1F). Treatment with only cyclo, MK-801, or CNQX had no significant effect (data not shown). Chronic stimulation with Glu caused a significant increase (P<0.001) in the number of NPCs that differentiated to neurons. Neuronal differentiation was increased by the AMPA receptor-specific potentiator, cyclo (P<0.001), blocked by the AMPA/kainate receptor antagonist, CNQX (P<0.001), but not prevented by the NMDA receptor antagonist, MK-801 (Fig. 1F). These findings suggest that Glu-induced differentiation of NPCs to neurons is dependent on the activation of non-NMDA receptors.
AMPA receptor expression on NPCs, differentiated neurons, and differentiated astrocytes
NPCs were differentiated in culture to a predominant neuronal culture (N-diff) or astrocyte culture (A-diff). A majority of β-tubulin-III-positive (β-tubulin+) cells was induced by serum-free neurobasal medium supplemented with B27. A majority of GFAP-positive (GFAP+) cells was induced by DMEM/F12 with 10% fetal bovine serum. Real-time RT-PCR was used to determine the expression pattern of AMPA receptor subunits in NPCs, N-diff cells, and A-diff cells. The results were standardized with GAPDH as an internal control. AMPA receptor subunits GluR1, 2, 3, and 4 mRNA (n=5 donors) were present in NPCs, N-diff cells, and A-diff cells (Fig. 2A). GluR1 and GluR2 subunits are preferred binding partners in the heteromeric receptor assembly (31,32,33). To confirm the expression of AMPA receptor subunit protein, GluR1 and GluR2 were demonstrated with immunocytochemistry on NPCs (Fig. 2B–G), N-diff cells (Fig. 2H–M), and A-diff cells (Fig. 2N–S). NPCs, N-diff cells, and A-diff cells were all shown to express GluR1 and GluR2 subunit protein.
NPCs contain calcium-permeable AMPA receptors
Using calcium imaging on groups of 20 NPCs, we found that 100 μM Glu stimulation caused calcium influx. The observed calcium influx was greatly increased using the AMPA receptor-specific potentiator, cyclo, but was almost completely blocked using an AMPA/kainate receptor antagonist, CNQX (Fig. 3A). To more specifically test whether calcium-permeable AMPA receptors were responsible for the Glu-induced increase in intracellular calcium, we used an AMPA receptor-specific antagonist, Joro Spider toxin (Fig. 3B), and a synthetic Joro Spider toxin analog, Naspm (Fig. 3C). Both agents specifically block calcium-permeable AMPA receptors while minimally blocking calcium-impermeable AMPA receptors (34,35,36). Joro Spider toxin and Naspm eliminated the increases in intracellular calcium of Glu-stimulated NPCs. The use of calcium-free buffer eliminated the Glu response in NPCs, indicating the calcium response is dependent on extracellular calcium (data not shown). Furthermore,neither the NMDA receptor antagonist MK-801 (Fig. 3C) nor the voltage-gated ion channel blocker nimodipine (Fig. 3D) prevented glutamate-induced calcium influx. Thus, Glu-mediated calcium elevations in NPCs are primarily through calcium-permeable AMPA receptors and not through NMDA receptors, kainate receptors, voltage-gated ion channels, or intracellular stores.
Reduced expression of calcium-permeable AMPA receptors on differentiated neurons and astrocytes
After identifying calcium-permeable AMPA receptors on NPCs, we sought to determine whether calcium-permeable AMPA receptors were present on N-diff and A-diff cells. N-diff cells responded to Glu, and the calcium influx was amplified with cyclo (P<0.001; Fig. 4A), suggesting that there may be calcium-permeable AMPA receptors on N-diff cells. However, Naspm failed to completely block the Glu and cyclo-induced calcium influx (P<0.001; Fig. 4A), demonstrating that calcium-permeable AMPA receptors are present on N-diff cells, but the calcium influx involves other receptors or channels. MK-801 prevented nearly 70% of the Glu- and cyclo-induced calcium influx (P<0.001; Fig. 4A), suggesting that functional NMDA receptors are present. CNQX blocked 97% of the Glu and cyclo-induced calcium influx (P<0.001; Fig. 4A). These findings indicate that the Glu-induced calcium influx in N-diff cells is likely through NMDA, kainate, and AMPA receptors. Most mature glutamatergic neurons express calcium-impermeable AMPA receptors; thus, it is likely that the presence of calcium-permeable AMPA receptors is due to immature neurons in the culture. Neurons not fully differentiated may still possess some features of NPCs, such as calcium-permeable AMPA receptors.
A-diff cultures were stimulated with 100 μM Glu and 10 μM cyclo. A-diff cultures exhibited no calcium influx when stimulated by Glu and cyclo but did respond to ATP-positive control, demonstrating the viability of the cells (Fig. 4B). These results indicate that A-diff cells no longer express calcium-permeable AMPA receptors.
NPCs express AMPA receptors with Q/R-unedited GluR2 subunits, and expression of Q/R-edited GluR2 and ADAR2 increases as NPCs differentiate
AMPA receptors are generally impermeable to calcium, although small populations of GluR2-lacking AMPA receptors are calcium permeable. We observed substantial GluR2 expression on NPCs, leading to the hypothesis that the calcium permeability of AMPA receptors in NPCs is due to the presence of Q/R-unedited GluR2 subunits. We determined the amount of Q/R editing of GluR2 mRNA using nested RT-PCR and BbVI restriction enzyme digestion. As diagrammed in Fig. 5A, BbVI cuts Q/R-edited GluR2 cDNA once, creating two fragments (228 and 49 bp) and cuts Q/R-unedited GluR2 subunits into three fragments (147, 81, and 49 bp). The digestion fragments from NPCs and NPCs differentiated to neurons and astrocytes for 1, 2, and 4 wk were separated on an agarose gel and visualized with ethidium bromide (Fig. 5B). The majority (61±2.5%) of GluR2 subunit mRNA was Q/R-unedited in NPCs, and the percentage of Q/R-unedited GluR2 subunits decreased as NPCs differentiated (Fig. 5C).
Expression of ADAR2 increases as NPCs differentiate to neurons and astrocytes
Q/R editing of GluR2 is dependent on the enzyme ADAR2. ADAR2 mRNA levels were analyzed from NPCs and NPCs differentiated to neurons and astrocytes for 1, 2, and 4 wk (n=3) by real-time RT-PCR. The results were standardized with GAPDH and expressed as fold increase of mRNA relative to ADAR2 in NPCs. ADAR2 levels were elevated in differentiated cells as compared to NPCs. The expression of ADAR2 mRNA was upregulated in neurons (14-fold by 4 wk; Fig. 5D) and upregulated in astrocytes (4-fold at 4 wk; Fig. 5E). Nuclear protein from NPCs and isolated primary neurons and astrocytes were analyzed by Western blotting. ADAR2 protein was not detected in NPCs but was present in both neurons and astrocytes (Fig. 5F). ADAR2 expression increases as NPCs differentiate, which correlates to the change in Q/R editing of the GluR2 subunit.
Stimulation and potentiation of AMPA receptors directs NPC differentiation to neurons
Intracellular calcium ions play a key role in cell differentiation (37); thus, we hypothesized that a possible role of calcium-permeable AMPA receptors on NPCs might be neurogenesis. NPCs were treated with neuron differentiation medium alone (Fig. 6A), AMPA (Fig. 6B), AMPA and cyclo (Fig. 6C), or AMPA, cyclo, and Naspm (Fig. 6D) for 7 days, and then stained for β-tubulin-III, GFAP, and Hoechst. The cells were counted and scored as neurons (GFAP+/−/β-tubulin+ cells) or astrocytes (GFAP+/β-tubulin− cells), and the data are expressed as percentage neurons (Fig. 6E) and percentage astrocytes (Fig. 6F). Treatment with cyclo or Naspm alone had no significant effect (data not shown). AMPA stimulation of NPCs caused a significant increase in differentiation of NPCs to neurons (P<0.001). This effect was enhanced by potentiating AMPA receptors with cyclo (P<0.001) and was completely blocked with the calcium-permeable AMPA receptor antagonist Naspm (P<0.001; Fig. 6E). Therefore, activation of calcium-permeable AMPA receptors directed the differentiation of NPCs to the neuronal lineage.
Stimulation and potentiation of AMPA receptors stimulate dendrite arbor formation in NPCs differentiated to neurons
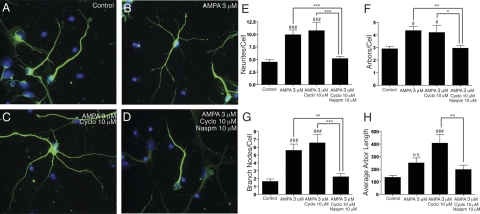
Dendritic arborization is an important component of neurogenesis and correlates to successful integration of new neurons into the neuronal circuitry (38). NPCs were treated with control neuron differentiation medium alone (Fig. 7A), AMPA (Fig. 7B), AMPA and cyclo (Fig. 7C), or AMPA, cyclo, and Naspm (Fig. 7D) for 7 days, and then stained for β-tubulin-III, GFAP, and Hoechst. The numbers of neurites, arbors, and branch nodes per cell and the average arbor length were calculated for neurons not associated with astrocytes in 5 pictures per condition. Administration of cyclo or Naspm alone showed no significant effects on dendritic arborization (data not shown). Activation of AMPA receptors significantly increased the numbers of neurites per cell (Fig. 7E), arbors per cell (Fig. 7F) and branch nodes per cell (Fig. 7G) and the average arbor length (Fig. 7H) for N-diff cells. These effects were prevented by the AMPA antagonist Naspm, indicating calcium-permeable AMPA receptor stimulation-induced dendritic arborization.
Figure 7.
Stimulation and potentiation of AMPA receptors stimulates dendrite arbor formation in NPCs differentiated to neurons. Human NPCs were cultured in control neuron differentiation medium alone (A), with 3 μM AMPA (B), 3 μM AMPA and 10 μM cyclo (C), or 3 μM AMPA, 10 μM cyclo, and 10 μM Naspm (D) for 1 wk, and then stained for β-tubulin-III (green) and Hoechst (blue). The numbers of neurites/cell (E), arbors/cell (F), and branch nodes/cell (G) and the average arbor length (H) of neurons not physically associated with astrocytes were measured for each condition in panels A–D (n=5). N.S., not significant; P > 0.05. *P < 0.05; **P < 0.01; ***P < 0.001. #P < 0.05, ###P < 0.001 vs. control. Data are representative of 2 separate experiments with NPCs from 2 separate donors.
Overexpression of ADAR2 prevents AMPA-induced differentiation of NPCs to neurons
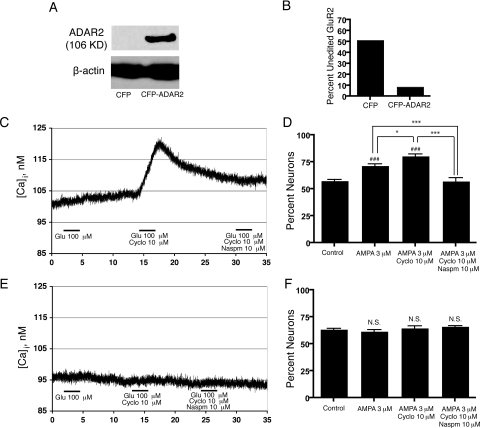
Expression of Q/R-edited GluR2 subunits is essential to confirm that AMPA-induced neural differentiation of NPCs is dependent on activation of calcium-permeable AMPA receptors. Thus, we transfected NPC cultures with a vector containing ADAR2 cDNA with a cyan fluorescent protein tag (CFP-ADAR2) to induce premature ADAR2 expression and Q/R editing of GluR2 subunits. Expression of ADAR2 protein in NPCs was demonstrated by Western blot analysis (Fig. 8A). ADAR2 expression in NPCs reduced the amount of Q/R-unedited GluR2 subunits (Fig. 8B) to levels comparable to A-diff cells (Fig. 5C), resulting in a lack of calcium-permeable AMPA receptors (Fig. 8E). Delivery of ADAR2 to NPCs prevented AMPA-induced neural differentiation of NPCs (Fig. 8F). NPCs transfected with the CFP plasmid contained calcium-permeable AMPA receptors (Fig. 8C) and underwent typical AMPA-induced neural differentiation, as previously demonstrated in Fig. 6 (Fig. 8D). AMPA or glutamate stimulation induces differentiation of NPCs to neurons by activation of calcium-permeable AMPA receptors. The overexpression of ADAR2, and resulting Q/R-edited GluR2, abrogates the effect of AMPA on NPC differentiation.
Figure 8.
Overexpression of ADAR2 prevents AMPA-induced differentiation of NPCs to neurons. A) Western blot analysis demonstrates ADAR2 overexpression. B) Percentage of unedited GluR2 in NPCs overexpressing CFP and CFP-ADAR2. C–F) Human NPCs were electroporated with plasmids containing CFP (C, D) or CFP-ADAR2 (E, F) Calcium imaging was performed on NPCs overexpressing CFP (C) and CFP-ADAR2 (E) using 100 μM Glu, 10 μM cyclo, and 10 μM Naspm. Forty-eight hours after eletroporation, NPCs were cultured in control neuron differentiation medium alone, 3 μM AMPA, 3 μM AMPA and 10 μM cyclo, or 3 μM AMPA, 10 μM cyclo, and 10 μM Naspm for 1 wk, and then stained for β-tubulin-III, GFAP, and Hoechst. The numbers of neurons and astrocytes counted in 5 pictures for each condition are expressed as the percentage of neurons (D, F). N.S., not significant; P > 0.05. *P < 0.01; ***P < 0.001. ###P < 0.001 vs. control. Data are representative of 2 separate experiments with NPCs from 2 separate donors.
DISCUSSION
In this study, we identified human NPCs expressing calcium-permeable AMPA receptors that contain Q/R-unedited GluR2 subunits. As NPCs matured and differentiated to neural or glial lineages, cells began to express calcium-impermeable AMPA receptors comprising Q/R-edited GluR2 subunits. The transition from unedited to edited GluR2 subunits correlated with increased ADAR2 expression. Stimulation of calcium-permeable AMPA receptors was found to steer the differentiation of NPCs to neuronal cells in favor of glial cells, and promoted dendritic arborization. Overexpression of ADAR2 in NPCs resulted in Q/R editing and the expression of calcium-impermeable AMPA receptors, which prevented AMPA-mediated differentiation to neurons. These findings suggest a physiological role for the Q/R editing process and identify calcium-permeable AMPA receptors containing Q/R-unedited GluR2 subunits as important to neurogenesis.
We initially observed that glutamate stimulation of cultured human NPCs led to a notable intracellular calcium spike and sought to identify the source of calcium. Removal of calcium from the medium of NPCs prevented the calcium spike, indicating an influx of extracellular calcium (data not shown). NMDA receptors are typically responsible for glutamate-induced calcium influx; however, the expression of NMDA receptors on neural precursors is still unclear (39). Microarray analysis failed to identify any NMDA subunit mRNA, and Western blotting demonstrated the lack of NR1 subunit protein expression in our NPC culture (data not shown). This observation was validated by treatment with the NMDA receptor antagonist MK-801, which did not block calcium influx following stimulation with 100 μM Glu (data not shown) or 100 μM Glu and 10 μM cyclo (Fig. 3C), and 100 μM NMDA failed to induce calcium response (data not shown). Having ruled out NMDA-dependent calcium influx, we investigated non-NMDA glutamate receptors on NPCs. The AMPA and kainate receptor antagonist CNQX completely blocked the glutamate-induced calcium influx (Fig. 3A, D). To decipher whether this was a consequence of AMPA or kainate receptor activation, we used cyclo, an AMPA receptor-specific potentiator. Cyclo significantly enhanced glutamate-induced calcium influx (Fig. 3A, C, D), supporting AMPA receptors as the primary source of the observed calcium spikes. Furthermore, the AMPA receptor-specific antagonist Joro spider toxin and its synthetic analog, Naspm, both blocked the glutamate- or AMPA-induced calcium influx (Fig. 3B, C). The use of the AMPA-specific potentiator and antagonists demonstrates that the glutamate-induced calcium influx in NPCs is dependent on AMPA receptors.
Although AMPA receptor activation generally does not facilitate calcium influx, a variety of mechanisms could explain this phenomenon. Membrane depolarization due to sodium influx through AMPA receptors could remove the magnesium blockade from NMDA receptors or open voltage-gated calcium ion channels. However, the NMDA antagonist MK-801 did not prevent the glutamate-mediated calcium influx; further, the calcium ion channel blocker nimodipine had no effect (Fig. 3C, D). AMPA assembly without the GluR2 subunit results in a higher-conductance channel that permits calcium entrance (8). Low expression of GluR2 subunits leads to tetraheteromeric complexes lacking GluR2 and the presence of calcium-permeable AMPA receptors (40). However, our cultures were found to express significant amounts of GluR2 subunits at levels comparable to GluR1 (Fig. 2A, F, G). Cells expressing GluR2 may contain the occasional AMPA receptor lacking GluR2 incorporation (6, 41), but this is unlikely to explain the high amounts of calcium influx observed. Thus, we hypothesized that the observed calcium influx in NPCs was due to the presence of AMPA receptors containing Q/R-unedited GluR2 subunits.
AMPA receptors are typically composed of GluR2 subunits that express an arginine at residue 607 (2, 3, 7). This residue is actually genetically coded as a glutamine, but the pre-mRNA is edited, resulting in incorporation of an arginine. We found significant levels of Q/R-unedited GluR2 subunits in NPCs using an established restriction enzyme digestion protocol (13) to assay the Q/R editing status of GluR2. The expression ratio of Q/R-unedited GluR2 mRNA to edited mRNA was found to decrease as NPCs differentiated. The assay indicates roughly 60% of GluR2 mRNA is unedited in NPC cultures; whereas Q/R-unedited mRNA is significantly reduced in both neuronal and glial cultures 4 wk later (Fig. 5C). Given that our NPC culture is contaminated with ∼5% differentiated cells (27), the actual percentage of unedited GluR2 in NPCs may be even higher than indicated in Fig. 5C.
The enzyme responsible for editing GluR2 pre-mRNA at the Q/R site is ADAR2 (10, 12, 13). ADAR2 edits nearly 100% of GluR2 mRNA to the arginine encoding form. In support of our finding in NPCs, ADAR2 (42, 43) and Q/R editing (44) were previously shown to be upregulated during embryonic development, and, similarly, ADAR2 increases during differentiation (42, 43). To confirm the role of calcium-permeable AMPA receptors with Q/R-unedited GluR2 in neuronal differentiation, we induced GluR2 Q/R editing in cultured NPCs. In lieu of overexpressing edited GluR2 subunits, we transfected NPCs with the ADAR2 enzyme to prematurely induce GluR2 editing. ADAR2 overexpression increased observed Q/R editing from ∼50% to more than 90% (Fig. 8B). Although CFP vector transfection had no effect on AMPA-induced differentiation to neurons (Fig. 8D), ADAR2 overexpression in NPCs prevented AMPA-induced neuronal differentiation (Fig. 8F). Cumulatively, these findings demonstrate that calcium-permeable AMPA receptors with Q/R-unedited GluR2 are responsible for glutamate-induced differentiation of NPCs to neurons.
Neurogenesis is the process of generating functionally integrated neurons from progenitor cells (45). There are two paths of differentiation for NPCs: neurogenesis (neurons) and gliogenesis (astrocytes and oligodendrocytes). We used immunocytochemistry to evaluate NPC differentiation to β-tubulin-III+ neurons and to quantify dendritic arborization. Activation and/or potentiation of calcium-permeable AMPA receptors were found to enhance neuronal differentiation. Successful neurogenesis requires the generation of new neurons, as well as formation of functional interactions with other neurons. The potential of neurons to form connections can be quantified through measurement of arbors, neurites, and branch nodes, as we have described previously (30). We found that stimulation of AMPA receptors on NPCs differentiated to neurons increased the number of arbors, neurites, and branch nodes per cell, indicating that AMPA receptor stimulation is important to forming synaptic connections and integration in the CNS. This was consistent with the prior finding that AMPA receptor potentiation increases neurite length (46). Previous in vivo studies demonstrated that a gene-encoded arginine in the Q/R-editing site led to normal physiological functions and no gross anatomical defects (12, 47). However, studies specifically investigating neurogenesis in these animals have not been reported.
Q/R editing of the GluR2 subunit is essential for survival, as demonstrated by the premature death of ADAR2 knockout mice (12) Furthermore, disruption of Q/R-editing propagates neurodegeneration in amyotrophic lateral sclerosis (ALS) (17) and ischemia (14). The use of mRNA modification in favor of a gene-encoded arginine at the Q/R site is a unique biological circumstance. If this arrangement had no biological significance, mutation of a single nucleotide changing the CAG (Q) codon to a CGG (R) codon would have likely replaced this inefficient system. However, the presence of a gene-encoded glutamine and the Q/R-editing process is highly conserved (48). Tracing back to the appearance of cartilaginous fish, the Q/R-editing process is conserved with very few exceptions (48, 49), implying a strong selective pressure for calcium-permeable AMPA receptors with Q/R-unedited GluR2 subunits. Our results suggest a physiological role for calcium-permeable AMPA receptors with Q/R-unedited GluR2 as a mechanism in neurogenesis.
Whether NPC differentiation is dependent on calcium influx in general or through AMPA-specific signaling is not clear. Calcium from AMPA receptor activation can increase BDNF transcription through calcium response elements on the BDNF promoter (24). AMPA receptors physically associate with the tyrosine kinase Lyn. Lyn activation through AMPA receptors can stimulate mitogen-activated protein kinases (MAPK), leading to increased BDNF expression (24). BDNF promotes neurogenesis in the CNS by promoting survival and differentiation of NPCs to a neuronal lineage (52, 53). Further, AMPA receptors with Q/R-unedited GluR2 subunits are also permeable to zinc (50), and this may be involved in differentiation (51). Other mechanisms of AMPA-induced differentiation may include regulation of immediate early genes, such as, c-fos, c-jun, and NGFI-A (54) and basic helix-loop-helix (bHLH) genes such as Mash1, Math, and Ngn that play critical roles in the regulation of neural stem cell differentiation (55, 56). The intracellular signaling pathways responsible for the induction of NPC differentiation to neurons require further investigation.
Our findings further support the growing evidence that AMPA receptor potentiators may be a useful therapeutic tool to enhance neurogenesis and treat neurodegenerative disorders (23,24,25,26). AMPA receptor potentiators have been shown to enhance NPC proliferation in rodents (23) and to provide neuroprotective stimuli for neurons in vivo (24, 60). Drugs such as 1-(quinoxalin-6-yl-carbonyl)piperidine (CX516) and LY451395 have been found to be well tolerated by adult humans, with minor adverse effects (24, 55,56,57). This therapeutic avenue may provide a safe intervention to protect neurons and to promote neurogenesis in the setting of neurodegenerative disorders.
Acknowledgments
This work was supported, in part, by research grants by the National Institutes of Health: R01 NS 41858, P20 RR15635, and P01 NS043985 to J.Z. We kindly acknowledge Dr. Yumei Wu, Dr. Anuja Ghorpade, Li Wu, and Danielle Williams, who provided technical support for this work. Drs. Tsuney Ikezu, Myron Toews, Terry Hexum, Yong Zhao, and Xu Luo provided valuable comments and suggestions about the project and manuscript. Julie Ditter, Johnna Belling, Robin Taylor, Myhanh Che, Na Ly, and Emilie Scoggins provided outstanding administrative support.
References
- Monaghan D, Bridges R, Cotman C. The excitatory amino acid receptors. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis S F. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Wenthold R J, Petralia R S, Blahos J, II, Niedzielski A S. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens C F. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Konig N, Poluch S, Estabel J, Durand M, Drian M J, Exbrayat J M. Synaptic and non-synaptic AMPA receptors permeable to calcium. Jpn J Pharmacol. 2001;86:1–17. doi: 10.1254/jjp.86.1. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg P H, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Geiger J R, Melcher T, Koh D S, Sakmann B, Seeburg P H, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg P H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Kohr G, Melcher T, Seeburg P H. Candidate editases for GluR channels in single neurons of rat hippocampus and cerebellum. Neuropharmacology. 1998;37:1411–1417. doi: 10.1016/s0028-3908(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single F N, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg P H. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Kanazawa I, Kwak S. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur J Neurosci. 2003;18:23–33. doi: 10.1046/j.1460-9568.2003.02718.x. [DOI] [PubMed] [Google Scholar]
- Peng P L, Zhong X, Tu W, Soundarapandian M M, Molner P, Zhu D, Lau L, Liu S, Liu F, Lu Y. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron. 2006;49:719–733. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Maas S, Patt S, Schrey M, Rich A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci U S A. 2001;98:14687–14692. doi: 10.1073/pnas.251531398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- Kwak S, Kawahara Y. Deficient RNA editing of GluR2 and neuronal death in amyotropic lateral sclerosis. J Mol Med. 2005;83:110–120. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- Gage F H. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Emsley J G, Mitchell B D, Kempermann G, Macklis J D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Palmer T D, Takahashi J, Gage F H. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nelson A D, Eickstaedt J B, Wallace K, Wright L S, Svendsen C N. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24:645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- Luk K C, Kennedy T E, Sadikot A F. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23:2239–2250. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Bergeron M, Nelson D L. Chronic AMPA receptor potentiator ( LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44:1013–1021. doi: 10.1016/s0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- O'Neill M J, Bleakman D, Zimmerman D M, Nisenbaum E S. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Quirk J C, Nisenbaum E S. LY404187: a novel positive allosteric modulator of AMPA receptors. CNS Drug Rev. 2002;8:255–282. doi: 10.1111/j.1527-3458.2002.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak M, O'Neill M J, Hicks C A, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43:1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1 mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck D W, He D, Reitsma M J, Masek M, Phan T V, Tsukamoto A S, Gage F H, Weissman I L. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Thylin M, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y, Gelbard H, Shepard R, Swartz J, Gendelman H. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhuang W, Yan N, Kou G, Peng H, McNally C, Erichsen D, Cheloha A, Herek S, Shi C, Shi Y. Classification of HIV-1 mediated neuronal dendritic and synaptic damage using multiple criteria linear programming. Neuroinformatics. 2004;2:303–326. doi: 10.1385/ni:2:3:303. [DOI] [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring R B, Clements J D, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Sans N, Vissel B, Petralia R S, Wang Y X, Chang K, Royle G A, Wang C Y, O'Gorman S, Heinemann S F, Wenthold R J. Aberrant formation of glutamate receptor complexes in hippocampal neurons of mice lacking the GluR2 AMPA receptor subunit. J Neurosci. 2003;23:9367–9373. doi: 10.1523/JNEUROSCI.23-28-09367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C L, Cotton L, Henley J M. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn M S, Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther. 1996;278:669–678. [PubMed] [Google Scholar]
- Iino M, Koike M, Isa T, Ozawa S. Voltage-dependent blockage of Ca(2+)-permeable AMPA receptors by joro spider toxin in cultured rat hippocampal neurones. J Physiol. 1996;496:431–437. doi: 10.1113/jphysiol.1996.sp021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke M, Keller B U, Rivosecchi R, Hollmann M, Heinemann S, Konnerth A. A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc Natl Acad Sci U S A. 1993;90:6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S E, Moreau M, Leclerc C, Miller A L. Calcium transients and neural induction in vertebrates. Cell Calcium. 2005;37:375–385. doi: 10.1016/j.ceca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Miller F D, Kaplan D R. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol. 2003;13:391–398. doi: 10.1016/s0959-4388(03)00072-2. [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwen B S. The role of N-methyl-d-asparate receptors in neurogenesis. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D E, Gorter J A, Bennett M V, Zukin R S. The GluR2 (GluR-B) hypothesis: Ca(2+)-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- Brorson J R, Zhang Z, Vandenberghe W. Ca(2+) permeation of AMPA receptors in cerebellar neurons expressing glu receptor 2. J Neurosci. 1999;19:9149–9159. doi: 10.1523/JNEUROSCI.19-21-09149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupard M C, O'Connell M A, Gerber A P, Zukin R S. Patterns of developmental expression of the RNA editing enzyme rADAR2. Neuroscience. 2000;95:869–879. doi: 10.1016/s0306-4522(99)00431-5. [DOI] [PubMed] [Google Scholar]
- Barbon A, Vallini I, La Via L, Marchina E, Barlati S. Glutamate receptor RNA editing: a molecular analysis of GluR2, GluR5, and GluR6 in human brain tissues and in NT2 cells following in vitro neural differentiation. Brain Res Mol Brain Res. 2003;117:168–178. doi: 10.1016/s0169-328x(03)00317-6. [DOI] [PubMed] [Google Scholar]
- Lee J C, Greig A, Ravindranathan A, Parks T N, Rao M S. Molecular analysis of AMPA-specific receptors: subunit composition, editing, and calcium influx determination in small amounts of tissue. Brain Res Brain Res Protoc. 1998;3:142–154. doi: 10.1016/s1385-299x(98)00035-x. [DOI] [PubMed] [Google Scholar]
- Ming G L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Voss O P, Milne S, Sharkey J, O'Neill M J, McCulloch J. Molecular mechanisms of neurite growth with AMPA receptor potentiation. Neuropharmacology. 2007;52:590–597. doi: 10.1016/j.neuropharm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Kask K, Zamanillo D, Rozov A, Burnashev N, Sprengel R, Seeburg P H. The AMPA receptor subunit GluR-B in its Q/R site-unedited form is not essential for brain development and function. Proc Natl Acad Sci U S A. 1998;95:13777–13782. doi: 10.1073/pnas.95.23.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov D, Gardiner K. Phylogenetic comparison of the pre-mRNA adenosine deaminase ADAR2 genes and transcripts: conservation and diversity in editing site sequence and alternative splicing patterns. Gene. 2002;299:83–94. doi: 10.1016/s0378-1119(02)01016-8. [DOI] [PubMed] [Google Scholar]
- Kung S S, Chen Y C, Lin W H, Chen C C, Chow W Y. Q/R RNA editing of the AMPA receptor subunit 2 (GRIA2) transcript evolves no later than the appearance of cartilaginous fishes. FEBS Lett. 2001;509:277–281. doi: 10.1016/s0014-5793(01)03183-0. [DOI] [PubMed] [Google Scholar]
- Jia Y, Jeng J M, Sensi S L, Weiss J H. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol. 2002;543:35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mattson M P, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Pende M, Holtzclaw L A, Curtis J L, Russell J T, Gallo V. Glutamate regulates intracellular calcium and gene expression in oligodendrocyte progenitors through the activation of DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1994;91:3215–3219. doi: 10.1073/pnas.91.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro D S, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Ross S E, Greenberg M E, Stiles C D. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Chappell A S, Gonzales C, Williams J, Witte M M, Mohs R C, Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology. 2007;68:1008–1012. doi: 10.1212/01.wnl.0000260240.46070.7c. [DOI] [PubMed] [Google Scholar]
- Lynch G, Granger R, Ambros-Ingerson J, Davis C M, Kessler M, Schehr R. Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol. 1997;145:89–92. doi: 10.1006/exnr.1997.6447. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers G A, Schehr R S, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Murray T K, Whalley K, Robinson C S, Ward M A, Hicks C A, Lodge D, Vandergriff J L, Baumbarger P, Siuda E, Gates M, Ogden A M, Skolnick P, Zimmerman D M, Nisenbaum E S, Bleakman D, O'Neill M J. LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2003;306:752–762. doi: 10.1124/jpet.103.049445. [DOI] [PubMed] [Google Scholar]