Abstract
Here, we report that diesel exhaust particles (DEPs), a major constituent of urban air pollution, affect blood-brain barrier function at the tissue, cellular, and molecular levels. Isolated rat brain capillaries exposed to DEPs showed increased expression and transport activity of the key drug efflux transporter, P-glycoprotein (6 h EC50 was ∼5 μg/ml). Up-regulation of P-glycoprotein was abolished by blocking transcription or protein synthesis. Inhibition of NADPH oxidase or pretreatment of capillaries with radical scavengers ameliorated DEP-induced P-glycoprotein up-regulation, indicating a role for reactive oxygen species in signaling. DEP exposure also increased brain capillary tumor necrosis factor-α (TNF-α) levels. DEP-induced P-glycoprotein up-regulation was abolished when TNF-receptor 1 (TNF-R1) was blocked and was not evident in experiments with capillaries from TNF-R1 knockout mice. Inhibition of JNK, but not NF-κB, blocked DEP-induced P-glycoprotein up-regulation, indicating a role for AP-1 in the signaling pathway. Consistent with this, DEPs increased phosphorylation of c-jun. Together, our results show for the first time that a component of air pollution, DEPs, alters blood-brain barrier function through oxidative stress and proinflammatory cytokine production. These experiments disclose a novel blood-brain barrier signaling pathway, with clear implications for environmental toxicology, CNS pathology, and the pharmacotherapy of CNS disorders.—Hartz, A. M. S., Bauer, B., Block, M. L., Hong, J.-S., Miller, D.-S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier.
Keywords: NADPH oxidase; reactive oxygen species; TNF-α, TNF-receptor 1; JNK
Particulate matter is the particle component of air pollution that is associated with the deaths of more than 500,000 people per year (1, 2). A major constituent of urban air pollution is diesel exhaust, a complex mixture of gases, chemicals and particles (3,4,5). Recent evidence suggests that exposure to air pollution can increase the risk of fatal stroke, cause cerebrovascular damage, and induce neuroinflammation and oxidative stress that may trigger neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases (6,7,8,9,10). Ultrafine particles such as diesel exhaust particles (DEPs) are considered the most toxic component of particulate matter. DEPs consist of a carbon core and heavy hydrocarbons derived from fuel and lubricant oils and hydrated sulfuric acid derived from the fuel sulfur. In addition, DEPs have adsorbed to them polycyclic aromatic hydrocarbons (PAHs). About 40 PAHs have been identified to date, but it is estimated that more than 300 PAHs are adsorbed to DEPs; partial lists of adsorbed chemicals have been reported (3,4,5). Thus, DEPs are a complex, yet very real environmental toxin that billions of people are exposed to on a daily basis. Once inhaled, DEPs can enter the circulation and translocate to tissues throughout the body, including the brain (11). At present, however, we lack understanding of how DEPs exert their deleterious effects in the central nervous system (CNS).
One pathway through which DEPs are predicted to enter the brain is by crossing the blood-brain barrier (11), a unique, tightly regulated, and dynamic capillary endothelium separating the peripheral blood circulation from the CNS. This barrier is composed of highly specialized brain capillary endothelial cells that are in close contact with pericytes, astrocytes, and neurons. Major physiological functions of this so-called neurovascular unit are maintenance of brain homeostasis and protection from potentially harmful neurotoxicants (12). A key component of the barrier is P-glycoprotein, an ATP-driven efflux transporter that limits brain penetration of xenobiotics, including a large number of CNS-acting drugs (13, 14). Because of its location, high expression in the luminal membrane of the brain capillary endothelial cells, and ability to actively transport a wide spectrum of structurally diverse compounds, P-glycoprotein is considered the primary obstacle to pharmacotherapy of CNS disorders (15,16,17). For example, P-glycoprotein prevents successful chemotherapy of brain tumors, has been implicated in drug-resistant epilepsy, and has been postulated to contribute to patient-to-patient variability in response to CNS drugs (18, 19).
Impairment of the blood-brain barrier in response to chemical toxins, stroke, or neurodegenerative diseases is often marked by changes in P-glycoprotein expression and activity (20, 21). Certainly, understanding mechanisms and signals that modulate P-glycoprotein expression and activity at the blood-brain barrier could result in new therapeutic targets for improved treatment of CNS disorders. In this regard, previous studies from our laboratory show that blocking P-glycoprotein function selectively opens the blood-brain barrier to drugs that are P-glycoprotein substrates (22), whereas increasing P-glycoprotein expression and functional transport activity drastically reduces efficacy of CNS drugs (23). We have also shown that proinflammatory mediators such as lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α) and endothelin-1 (ET-1) regulate expression and transport activity of P-glycoprotein (24,25,26).
Because DEPs can enter the body and reach the brain, where they cause oxidative stress and inflammation, we hypothesized that they target the brain capillary endothelium. Here we test this hypothesis by exposing freshly isolated, functionally intact brain capillaries from rats and mice to DEPs [National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 2975; refs. 27, 28] and measuring changes in P-glycoprotein expression and transport activity.
MATERIALS AND METHODS
Chemicals
DEPs were obtained from NIST (Gaithersburg, MD, USA) (27, 28). Carbon black (CB) mock particles were a kind gift from Degussa Corporation (Akron, OH, USA). 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) was from Molecular Probes (Eugene, OR, USA). Apocynin, catalase, nuclear factor-κB (NF-κB) SN50, NF-κB SN50M, SULT1A1–2 antibody, TNF-α antibody, and IgG control antibody were purchased from Calbiochem-Novabiochem (La Jolla, CA, USA). H398 and antibodies to multidrug resistance-associated proteins 1, 2, and 4 (Mrp1, Mrp2, and Mrp4) and breast cancer resistance protein (BCRP) were from Alexis-Axxora (San Diego, CA, USA); SP600125 was from A.G. Scientific (San Diego, CA, USA). Antibody to gp91PHOX was from BD Transduction Laboratories (San Jose, CA, USA). DPI and cyclooxygenase 2 (COX-2) antibody were purchased from Cayman (Ann Arbor, MI, USA), and C219 antibody was from Signet (Dedham, MA, USA). Antibodies to glucose transporter 1 (GLUT-1), β-actin, c-jun, c-jun-P, and gluthathione sulfotransferase π (GSTπ) were purchased from Abcam (Cambridge, MA, USA). Na+/K+-ATPase antibody was from Upstate (Chicago, IL, USA), TLR4 antibody was from Santa Cruz (Santa Cruz, CA, USA), and TNF-receptor 1 (TNF-R1) antibody was from U.S. Biological (Swampscott, MA, USA). Iba-1 antibody was from Wako Pure Chemical Industries (Osaka, Japan). Antibodies to ZO-1, occludin, and claudin-1 and -5 were purchased from Zymed (Carlsbad, CA, USA). [N-ε (4-nitrobenzofurazan-7-yl)-d-Lys (8)]-cyclosporine A (NBD-CSA) was custom-synthesized by R. Wenger (Basel, Switzerland) (29). PSC833 was a kind gift from Novartis (Basel, Switzerland). All other chemicals were obtained from Sigma (St. Louis, MO, USA).
Animals
Male TNF-R1-deficient mice (C57BL/6-Tnfrsf1atm1Imx) and wild-type mice (C57BL/6 background) were a generous gift from Dr. Perry J. Blackshear (National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA) (30, 31). Male Sprague-Dawley rats (retired breeders) were purchased from Taconic Farms (Germantown, NY, USA). Animal housing protocols were approved by the Institutional Animal Care and Use Committees of the National Institute of Environmental Health Sciences (NIEHS)/National Institutes of Health (NIH) and were in accordance with NIEHS/NIH guidelines.
Isolation of brain capillaries
Brain capillaries from rats and mice were isolated as described previously (23,24,25,26, 32). For each preparation, 10 rats or 15 mice were euthanized by CO2 inhalation and decapitated. Brains were dissected and homogenized in PBS buffer (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, and 8.1 mM Na2HPO4 supplemented with 5 mM d-glucose and 1 mM sodium pyruvate, pH 7.4). After the addition of Ficoll (final concentration 15%), the homogenate was centrifuged at 5800 g for 20 min at 4°C. The pellet was resuspended in PBS containing 1% BSA and passed over a glass bead column. Capillaries adhering to the glass beads were collected by gentle agitation in PBS (1% BSA), washed with PBS, and then used for experiments.
Preparation of DEP working suspension
DEPs (SRM 2975) used were collected by the manufacturer from a filtering system designed specifically for diesel-powered forklifts (27, 28). In our laboratory, DEP working suspensions were prepared according to Block et al. (6). Briefly, 2 mg of DEPs was suspended in 10 ml PBS buffer, vortexed for 1 min, and sonicated for 45 min using an ultrasonic processor (Ultrasonic LC 20 H, Elma Hans Schmidbauer GmbH & Co KG, Singen, Germany). The suspension was filtered through a 0.22-μm filter (MillexGS; Millipore, Billerica, MA, USA) prior to experiments. Freshly isolated capillaries were exposed to DEPs at the concentrations indicated for 6 h at room temperature without or with modulators. CB mock particles were prepared accordingly.
P-glycoprotein-mediated transport
Details of the transport assay in functionally intact rat and mouse brain capillaries were reported previously (23,24,25,26, 32). After 6 h exposure to DEPs, isolated brain capillaries were incubated for 1 h at room temperature with 2 μM NBD-CSA, a fluorescent P-glycoprotein substrate. For each treatment, images of 10 capillaries were acquired by confocal microscopy (Zeiss 410 Meta laser scanning confocal microscope, ×40 oil-immersion objective, numerical aperture 1.2, 488-nm line of argon laser; Carl Zeiss Inc., Thornwood, NY, USA). Images were analyzed by measuring luminal NBD-CSA fluorescence intensity using Scion Image software (Scion Corp., Frederick, MD, USA) as described previously (25, 26, 33). P-glycoprotein-specific, luminal NBD-CSA fluorescence was taken as the difference between total luminal fluorescence and fluorescence in the presence of the P-glycoprotein inhibitor, PSC833 (24).
Western blot analysis
Protein expression levels were analyzed by Western blot analysis as described before (23, 24, 26). In brief, isolated brain capillaries were homogenized and samples were centrifuged at 10,000 g for 15 min; denucleated supernatants were then centrifuged at 100,000 g for 90 min. Pellets (crude plasma membranes) were resuspended, and protein concentrations were determined. Western blotting was performed using the Invitrogen NuPage Bis-Tris electrophoresis and blotting system (Invitrogen, Carlsbad, CA, USA). After blocking, blotting membranes were incubated with primary antibody. Membranes were washed and incubated with horseradish peroxidase-conjugated ImmunoPure secondary IgG (1:15,000; Pierce, Rockford, IL, USA) for 1 h. Proteins were detected using SuperSignal West Pico Chemoluminescent Substrate (Pierce). Bands were visualized and recorded using a Bio-Rad Gel Doc 2000 gel documentation system (Bio-Rad, Hercules, CA, USA).
Reactive oxygen species (ROS) measurement
Generation of ROS in isolated rat brain capillaries was measured using CM-H2DCFDA (Molecular Probes). The nonfluorescent ester CM-H2DCFDA penetrates into cells and undergoes deacetylation to nonfluorescent 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein (CM-H2DCF) by cellular esterases. ROS rapidly oxidizes CM-H2DCF to highly fluorescent 5-(and-6)-chloromethyl-2′,7′-DCF (CM-DCF). Thus, CM-DCF fluorescence is a measure of ROS levels.
Isolated brain capillaries were exposed to DEPs for 30 min. CM-H2DCFDA was added at a final concentration of 10 μM and incubated for 30–45 min at room temperature. Capillaries were washed with PBS, and after a short recovery time (5 min), CM-DCF fluorescence was read at 485 nm in a microplate reader (Tecan GENios Pro; Tecan, Research Triangle Park, NC, USA).
TNF-α ELISA
TNF-α levels in isolated rat brain capillaries were measured using an ELISA kit from R&D Systems (Minneapolis, MN, USA). Isolated brain capillaries were homogenized in lysis buffer (Sigma) containing Complete® protease inhibitor (Roche, Mannheim, Germany). Capillary homogenate was centrifuged at 10,000 g for 15 min and the protein concentration of the denucleated supernatant was determined. ELISA assays were conducted according to the manufacturer’s protocol.
Statistical analysis
Data are presented as mean ± se. One- or two-tailed unpaired Student’s t test was used to evaluate differences between control and treated groups; values of P < 0.05 were considered statistically significant.
RESULTS
DEP exposure up-regulates P-glycoprotein in brain capillaries
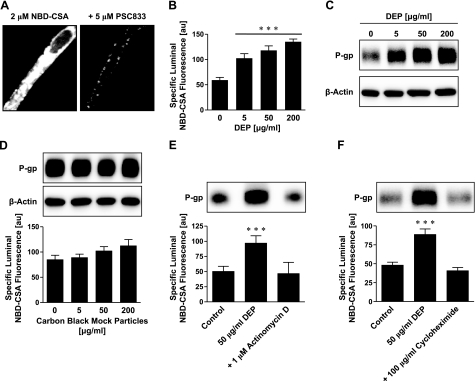
As before, we assessed P-glycoprotein activity in isolated brain capillaries by measuring accumulation of the fluorescent P-glycoprotein-specific substrate NBD-CSA in capillary lumens, using confocal microscopy and quantitative image analysis (25, 26). For this assay, capillaries were incubated to steady state in medium containing 2 μM NBD-CSA. We previously demonstrated that NBD-CSA accumulation in brain capillary lumens is concentrative, specific, and sensitive to inhibitors of cellular metabolism and P-glycoprotein, whereas inhibitors of other efflux transporters, e.g., Mrps and BCRP, are without effect (25, 32). Thus, this assay provides a specific measure of P-glycoprotein transport activity in isolated brain capillaries. Figure 1A shows a control capillary with high luminal NBD-CSA fluorescence (left panel) compared to the incubation medium, indicating concentrative transport into the capillary lumen. Exposing capillaries to the P-glycoprotein-specific inhibitor, PSC833, substantially decreased luminal NBD-CSA fluorescence (Fig. 1A, right panel). Simple diffusion and unspecific binding of NBD-CSA to tissue account for luminal fluorescence remaining after inhibition with PSC833 (25, 26, 33).
Figure 1.
DEPs increase P-glycoprotein expression and transport activity in isolated rat brain capillaries. A) Left panel: representative image of an isolated brain capillary after 1 h of exposure to 2 μM NBD-CSA, showing steady-state NBD-CSA fluorescence. Note that NBD-CSA transport is concentrative from bath (no visible fluorescence) to endothelium (low fluorescence) to capillary lumen (high fluorescence). Right panel: the P-glycoprotein-specific inhibitor PSC833 blocks concentrative NBD-CSA transport from capillary endothelium to capillary lumen. B) DEPs increase specific luminal NBD-CSA fluorescence after 6 h in a concentration-dependent manner. C) Western blot analysis showing concentration-dependent P-glycoprotein up-regulation through DEPs in brain capillary plasma membranes. β-Actin was used as loading control. D) CB mock particles had no effect on P-glycoprotein expression or transport activity after 6 h. P-glycoprotein transport activity is shown as specific luminal NBD-CSA fluorescence. E) Inhibition of transcription with actinomycin D abolishes DEP-induced increase in P-glycoprotein expression (Western blot) and transport activity (specific luminal NBD-CSA fluorescence). F) Inhibition of protein synthesis with cycloheximide also abolishes DEP up-regulation of P-glycoprotein. For Specific luminal NBD-CSA fluorescence values are means ± se for 10 capillaries from a single preparation (pooled tissue from 10 rats); arbitrary units (au; scale 0–255). ***P < 0.001 vs. control.
Six-hour exposure of freshly isolated rat brain capillaries to DEPs (5–200 μg/ml) increased specific P-glycoprotein transport activity in a concentration-dependent manner; the EC50 was estimated to be ∼5 μg/ml (Fig. 1B). Consistent with increased activity, we found a concentration-dependent increase of P-glycoprotein expression in plasma membranes isolated from brain capillaries that were treated with DEPs (Fig. 1C). Note that β-actin protein levels were the same in membranes from control and DEP-treated capillaries (loading control). In separate experiments, we exposed brain capillaries to CB mock particles and examined P-glycoprotein expression and transport function but found no effect (Fig. 1D). Thus, up-regulation of P-glycoprotein seems to be associated with DEPs rather than particulate matter in general.
To determine the chain of events that connect DEP exposure to up-regulation of P-glycoprotein, we exposed brain capillaries to DEPs for 6 h in the absence and presence of specific inhibitors of cell signaling and measured both specific P-glycoprotein transport activity (PSC833-sensitive, luminal NBD-CSA accumulation) and protein expression of P-glycoprotein (Western blots). As before, preliminary experiments showed that by themselves the pharmacological tools we used to dissect signaling pathways had no effect on transport activity or transporter expression (data not shown). Figure 1E, F shows that inhibiting transcription with actinomycin D or inhibiting translation with cycloheximide blocked the effect of DEPs on P-glycoprotein activity and expression. Thus, both P-glycoprotein transcription and protein synthesis were stimulated by DEPs.
DEPs signal through NADPH oxidase and ROS
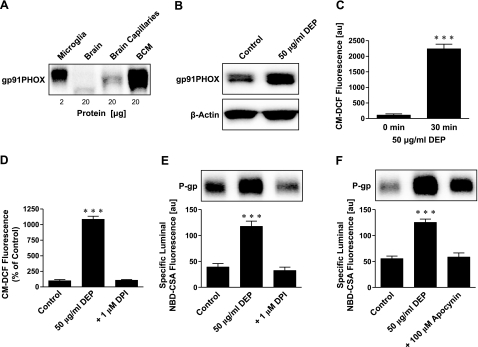
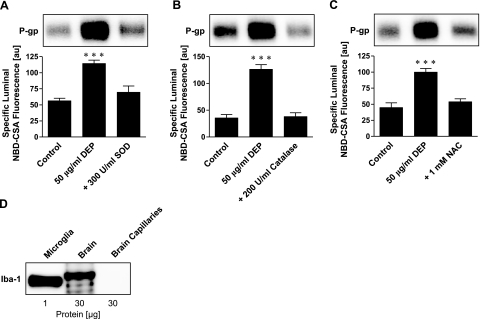
Oxidative stress is an early and potentially important event in tissues exposed to diesel exhaust (34). Block et al. (6) previously demonstrated that DEPs activate NADPH oxidase to generate ROS in microglia. We assayed expression of gp91PHOX, the major subunit of NADPH oxidase, in isolated brain capillaries and capillary membranes and found increased expression after exposure to DEPs (Fig. 2A, B). ROS levels, measured as CM-DCF fluorescence, increased in brain capillaries exposed to DEPs for 30 min (Fig. 2C). This increase was abolished by the NADPH oxidase inhibitor DPI, indicating that increased ROS levels were generated by activation of NADPH oxidase (Fig. 2D). Moreover, blocking NADPH oxidase with DPI or with apocynin prevented the DEP-induced increase in P-glycoprotein expression and activity (Fig. 2E, F). We used superoxide dismutase (SOD) and catalase to scavenge superoxide radicals. When added to the medium, both enzymes abolished DEP effects on P-glycoprotein activity and expression (Fig. 3A, B). Consistent with this, the cell-permeant antioxidant, N-acetyl-L-cysteine (NAC), was equally effective (Fig. 3C). These results indicate that NADPH oxidase activation and ROS production are essential for the DEP-induced increase in P-glycoprotein in isolated brain capillaries.
Figure 2.
NADPH oxidase and ROS are involved in DEP up-regulation of P-glycoprotein. A) Western blot showing expression of NADPH oxidase subunit gp91PHOX in microglia and brain capillary membranes. B) Exposing brain capillaries to DEPs for 6 h increased gp91PHOX expression in membranes. C) DEP exposure of brain capillaries for 30 min increased CM-DCF fluorescence, indicating increased ROS levels. ROS was measured using CM-H2DCFDA; values are means ± se (au; n=3) for capillaries from a single preparation (pooled tissue from 10 rats). D) NADPH oxidase inhibition with DPI abolished the DEP-induced increase of ROS (measured as CM-DCF fluorescence) in isolated brain capillaries. Data are percentages of control levels. E) Inhibition of NADPH oxidase with DPI blocked DEP up-regulation of P-glycoprotein expression (Western blot) and transport activity (specific luminal NBD-CSA fluorescence). F) Inhibition of NADPH oxidase with apocynin also blocked the DEP effect on P-glycoprotein. Specific luminal NBD-CSA fluorescence values are means ± se for 10 capillaries from a single preparation (pooled tissue from 10 rats); scale 0–255 au. ***P < 0.001.
Figure 3.
ROS scavengers prevent P-glycoprotein up-regulation. A) The ROS scavenger SOD abolishes DEP up-regulation of P-glycoprotein expression (Western blot) and transport activity (specific luminal NBD-CSA fluorescence). B) Catalase, another ROS scavenger, also abolishes the effect of DEPs on P-glycoprotein. C) The antioxidant N-acetyl-l-cysteine (NAC) blocks DEP up-regulation of P-glycoprotein. D) Western blot showing expression of the microglia marker Iba-1. Iba-1 is expressed in microglia (positive control) and total brain, but not in brain capillaries. Specific luminal NBD-CSA fluorescence values are means ± se for 10 capillaries from a single preparation (pooled tissue from 10 rats); scale 0–255 au. ***P < 0.001.
Because the main source of ROS within the brain is microglia, we assayed the expression of Iba-1, a specific microglial protein marker, in isolated microglia (positive control), whole rat brain lysate, and brain capillary lysate. We detected Iba-1 protein in microglia and total brain, but not in brain capillaries (Fig. 3D). Thus, our isolated brain capillary preparation was free of detectable microglial contamination. This confirms that the primary source of ROS generated in response to DEPs was the brain capillaries themselves.
DEPs signal through TNF-α
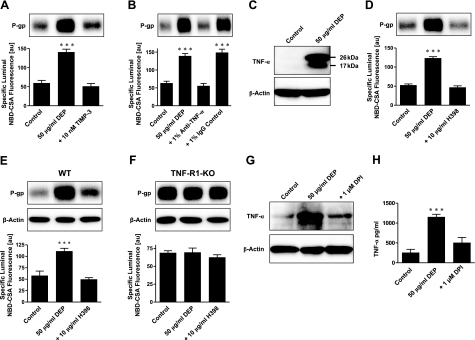
DEPs trigger release of proinflammatory cytokines like TNF-α from a number of cells (35, 36). We previously showed that TNF-α acting through TNF-R1 regulates P-glycoprotein activity and expression in brain capillaries (24). TNF-α is produced in a proform (TNF-α precursor), which is released from cells and cleaved by TNF-α converting enzyme (TACE) to the active cytokine. Figure 4A shows that inhibiting TACE with tissue inhibitor of metalloproteinase 3 (TIMP3) abolished DEP-induced increases of P-glycoprotein expression and activity. Exposing capillaries to an antibody against TNF-α also blocked P-glycoprotein up-regulation, while a control IgG had no effect (Fig. 4B). These results indicate DEP-induced release of TNF-α from the capillaries. Consistent with this, Fig. 4C shows a Western blot analysis for TNF-α in capillaries exposed to DEPs. Two bands were found in DEP-treated capillaries, one for the TNF-α precursor (26 kDa) and one for mature TNF-α (17 kDa); neither protein was detected in control capillaries.
Figure 4.
DEP activation of NADPH oxidase is followed by TNF-α release and TNF-α signaling through TNF-R1 to up-regulate P-glycoprotein. A) TIMP-3, an inhibitor of TNF-α converting enzyme, blocks the DEP-mediated increase in P-glycoprotein expression (Western blot) and transport function (specific luminal NBD-CSA fluorescence). B) An antibody against TNF-α blocks the effect of DEPs on P-glycoprotein; an IgG control antibody has no effect. C) DEPs induce TNF-α in brain capillaries (26 kDa: TNF-α precursor protein, 17 kDa: TNF-α mature protein). D) Blocking TNF-R1 with H398 abolishes DEP up-regulation of P-glycoprotein. E) In isolated capillaries of wild-type mice, DEPs increase P-glycoprotein expression (Western blot) and transport activity (specific luminal NBD-CSA fluorescence). The specific TNF-R1 blocker, H398, blocks the DEP effect. F) In capillaries isolated from TNF-R1-deficient mice, DEPs had no effect on P-glycoprotein expression levels or transport activity. Note that different band intensities between controls in the Western blots of wild-type and TNF-R1 knockout mice result from different exposure times of the blotting membrane. G) Western blot showing that the NADPH oxidase inhibitor DPI blocks DEP up-regulation of P-glycoprotein. H) ELISA showing that blocking NADPH oxidase with DPI brings TNF-α levels back to control levels. Specific luminal NBD-CSA fluorescence values are means ± se for 10 capillaries from a single preparation (pooled tissue from 10 rats or 15 mice); scale 0–255 au. ***P < 0.001.
TNF-R1 with H398 abolished the DEP effect on P-glycoprotein expression and transport function (Fig. 4D), indicating that released TNF-α signaled through TNF-R1. To confirm this, we compared the response to DEPs of brain capillaries isolated from mice lacking TNF-R1 with those from wild-type mice. In brain capillaries from wild-type mice, DEPs increased P-glycoprotein expression and function; this effect was abolished by blocking TNF-R1 with H398 (Fig. 4E). In contrast, DEPs did not affect P-glycoprotein expression or transport function in capillaries isolated from TNF-R1 knockout mice (Fig. 4F; note that different band intensities between controls in the Western blots of wild-type and TNF-R1 knockout mice result from different exposure times of the blotting membrane). Together, these results indicate that DEPs triggered release of TNF-α, which signaled through TNF-R1 to up-regulate P-glycoprotein.
To determine which DEP-initiated signal, TNF-α release or NADPH oxidase activation (ROS production), occurred first, we blocked NADPH oxidase using DPI and measured TNF-α expression in DEP-treated capillaries. Both Western blots and ELISA for TNF-α showed increased TNF-α levels in DEP-treated capillaries (Fig. 4G, H). This increase was abolished by DPI, indicating that DEPs activated NADPH oxidase, which then caused TNF-α release from brain capillaries.
DEP signaling involves nitric oxide synthase (NOS) and c-jun
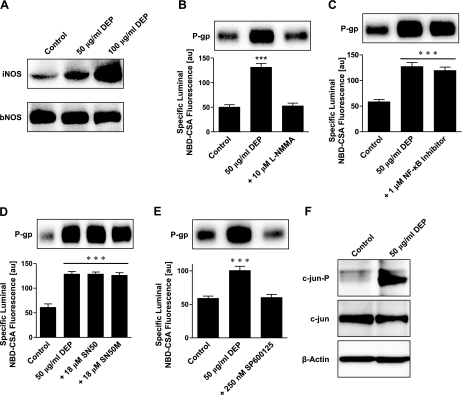
Our previous studies showed that capillaries release ET-1 on exposure to TNF-α, leading to activation of NOS, protein kinase C (PKC), and the transcription factor NF-κB, and thus upregulates P-glycoprotein expression (24). In the present study, we found no evidence of ET-1 or PKC involvement. Blocking ETA and ETB receptors using RES-701–1 and JKC-301, respectively, or inhibiting of PKC with BIM, did not alter DEP-mediated up-regulation of P-glycoprotein (data not shown). However, our data do indicate that NOS played a role in DEP signaling. Exposure to DEPs increased protein levels of inducible NOS (iNOS) in capillaries, but not brain NOS (bNOS) (Fig. 5A). Inhibition of NOS with L-NMMA abolished the DEP effect, indicating that at least one NOS isoform played a role in the induction of P-glycoprotein (Fig. 5B).
Figure 5.
DEP up-regulation of P-glycoprotein involves NO synthase, JNK, and c-jun. A) Western blot showing that DEPs increase expression of iNOS, but not of bNOS. B) Inhibition of NOS with L-NMMA abolishes the DEP-mediated induction of P-glycoprotein expression and transport activity. C) An NF-κB activation inhibitor has no effect on DEP up-regulation of P-glycoprotein. D) SN50, an inhibitor of NF-κB nuclear translocation, and SN50M, the corresponding inactive control peptide, also have no effect on DEP up-regulation of P-glycoprotein. E) Inhibition of JNK with SP600125 blocks the DEP-mediated increase of P-glycoprotein expression (Western blot) and transport activity (specific luminal NBD-CSA fluorescence). F) Western blot showing that DEPs increase phosphorylated c-jun (c-jun-P) in capillaries exposed to DEPs for 6 h; c-jun expression was slightly decreased. Specific luminal NBD-CSA fluorescence values are means ± se for 10 capillaries from a single preparation (pooled tissue from 10 rats); scale 0–255 au. ***P < 0.001.
TNF-α acting at TNF-R1 can activate multiple signal transduction pathways (37, 38). The major pathway leads to activation of the transcription factor NF-κB, which translocates to the nucleus on activation and regulates transcription of target genes such as P-glycoprotein (39, 40). We previously demonstrated that TNF-α-induced activation of NF-κB increased expression and transport function of P-glycoprotein in isolated rat brain capillaries (24). As described above, this pathway also involved signaling through ET-1, NOS, and PKC, components that were not involved in DEP signaling. Nevertheless, we looked for activation of NF-κB in DEP-treated capillaries and found none. That is, both, blocking NF-κB activation or nuclear translocation were without effect (Fig. 5C, D).
Another TNF-R1 signaling pathway involves activation of the stress-activated protein kinase, c-Jun N-terminal kinase (JNK) (41). In response to stimuli, JNK phosphorylates and thus activates c-jun, a key component of the transcription factor AP-1 (activator protein-1). We found that inhibition of JNK with SP600125 blocked the increase of P-glycoprotein expression and function in capillaries exposed to DEPs (Fig. 5E). Furthermore, in capillaries exposed to DEPs for 6 h, we detected a slight decrease of c-jun protein levels and a large increase of phosphorylated c-jun (Fig. 5F). These data suggest that in DEP-exposed brain capillaries JNK phosphorylates c-jun to activate the downstream transcription factor, AP-1, and to up-regulate P-glycoprotein.
DEPs alter expression of other blood-brain barrier proteins
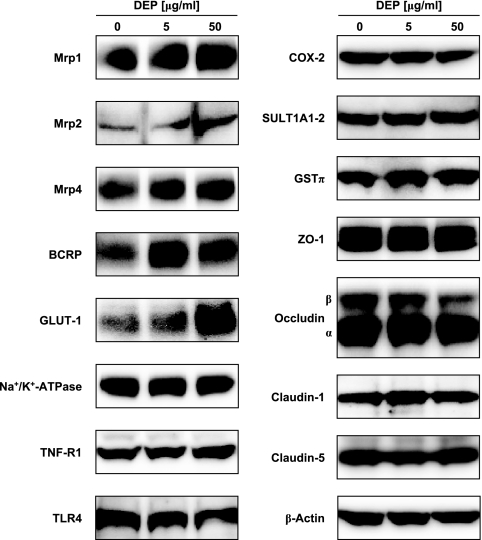
In addition to P-glycoprotein, DEPs altered levels of several important proteins expressed in brain capillaries (Fig. 6). Using Western blot analysis, we found increased expression of Mrp1, Mrp2, Mrp4, BCRP, and GLUT-1. Expression of the metabolizing enzyme GSTπ was also increased, whereas expression of the proinflammatory enzyme COX-2 was decreased. DEPs did not alter expression of TNF-R1, TLR4, Na+/K+-ATPase and SULT1A1/2. Levels of the tight junction proteins, ZO-1, occludin, claudin-1, and claudin-5, as well as β-actin, were unchanged. Thus, in the brain capillary endothelium, DEPs affected multiple proteins, including several ATP-driven transporters, and therefore it could have a profound impact on blood-brain barrier function.
Figure 6.
Effect of DEPs on the expression of selected proteins in isolated rat brain capillaries.
DISCUSSION
Diesel exhaust, the main particulate component of polluted air in the urban environment, is a worldwide health concern, affecting a large number of people and dramatically increasing susceptibility to cardiovascular, respiratory, and CNS disease (1, 42). Autopsy samples from individuals living in air-polluted environments show deposition of DEPs within the brain, indicating that these particles can cross barrier tissues, including the blood-brain barrier (10, 43). Recent studies show that DEPs are capable of inducing an oxidative and inflammatory response in microglia, the primary immune responsive cells of the brain (6). Given the important role inflammation plays in a number of CNS diseases, exposure to DEPs has the potential to cause substantial CNS pathology. The results of the present study demonstrate that exposing brain capillaries from rats and mice to DEPs increased, in parallel, protein expression and functional activity of the drug efflux transporter P-glycoprotein, a key element of the blood-brain barrier. Transporter up-regulation could be blocked by inhibitors of transcription and translation and was signaled through a novel pathway that involved elements of the endothelial cell responses to both oxidative stress (NADPH oxidase activation and superoxide production, Fig. 2) and inflammation (release of the proinflammatory cytokine TNF-α, Fig. 4). Importantly, our findings indicate that all of these events occurred on or within the endothelial cells themselves. This is supported by the fact that we could not find any evidence for contaminating microglia in our brain capillary preparations. Finally, DEP exposure also increased expression of several other luminal plasma membrane efflux transporters, including Mrp1, Mrp2, Mrp4, and BCRP. It is not yet clear whether a common signaling pathway is responsible for the up-regulation of all these ABC transporters.
Four aspects of the present study require further discussion. First, DEPs themselves are a complex component of air pollution. They contain a core of elemental carbon with adsorbed heavy hydrocarbons derived from fuel and lubricant oils and hydrated sulfuric acid derived from the fuel sulfur. DEPs also contain a large number of PAHs. The Certificate of Analysis for the DEPs we have used (NIST SRM 2975; refs. 27, 28) lists 39 selected PAHs adsorbed to the particles. However, it is anticipated that DEPs have more than 300 compounds adsorbed to them (3,4,5). Our data in Fig. 1D show that CB mock particles did not up-regulate P-glycoprotein, suggesting that chemicals adsorbed to the DEPs rather than the particles themselves are responsible for this effect. Given that more than 300 compounds are reported to be adsorbed to DEPs, it is impossible at this time to identify those responsible for up-regulation of P-glycoprotein in our brain capillaries.
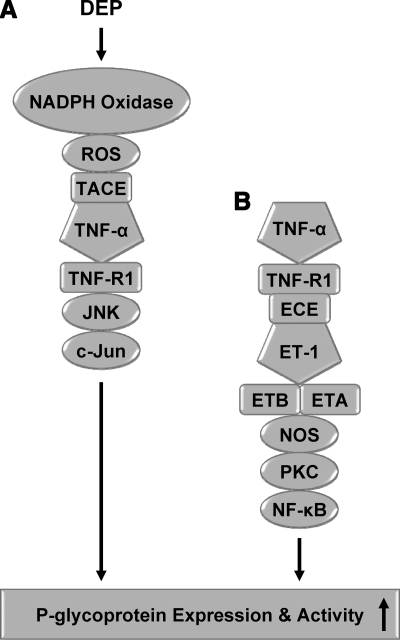
Second, Fig. 7 shows the postulated chain of events in brain capillaries that connects DEP exposure to increased P-glycoprotein expression as well as a parallel signaling pathway initiated by the proinflammatory cytokine TNF-α, which was mapped in a previous study (24). Note that signaling through TNF-R1 is common to both. When signaling is initiated by TNF-α in the absence of ROS, downstream events include ET-1 release and binding to the ETA/B receptors, activation of NOS and PKC, and translocation of NF-κB to the nucleus where it increases P-glycoprotein expression and activity (Fig. 7B) (24). However, it is clear from the present results that when signaling through TNF-α/TNF-R1 was initiated by DEPs, neither ET-1, PKC, or NF-κB was involved in downstream events that led to P-glycoprotein up-regulation (Fig. 7A). Rather, when DEPs activated NADPH oxidase to produce ROS, stimulate TNF-α release, and activate TNF-R1, the increase in P-glycoprotein expression was signaled through JNK kinase, c-jun and likely AP-1. NOS was activated by DEP exposure, but from the present results, it is not clear where in the pathway NO acts. Together, these studies have disclosed an interesting aspect of TNF-R1 signaling to P-glycoprotein in brain capillary endothelial cells: context-dependent determination of downstream signaling events. Apparently, in the context of NADPH oxidase activation and ROS generation, TNF-R1 activates one transcription factor (AP-1) and in the absence of ROS, it activates another (NF-κB). Such complex, context-dependent, switchlike behavior has been seen for other receptors (37, 44).
Figure 7.
Comparison of DEP signaling (present study) and TNF-α signaling (24, 26).
Third, multiple ATP-driven efflux transporters are expressed at the luminal plasma membrane of brain capillary endothelial cells, where they can both prevent xenobiotics from entering the CNS and remove potentially toxic metabolites from the brain (15, 45). The present study shows up-regulation of protein expression of all efflux transporters assayed in DEP-exposed brain capillaries. These increases were not accompanied by changes in expression of tight junction proteins. For P-glycoprotein, a critical determinant of blood-brain barrier function, we found a parallel increase in transport activity. Given the important role that P-glycoprotein plays in blood-brain barrier function, an increase in transport activity would be expected to tighten the barrier to the large number of therapeutic drugs that are P-glycoprotein substrates and thus reduce efficacy of many CNS-acting drugs in the clinic. Indeed, our recent study with the P-glycoprotein substrate, methadone, shows in an animal model that doubling P-glycoprotein expression at the blood-brain barrier reduces methadone’s analgesic effects by ∼70% (23). Certainly, the magnitude of this effect would vary with substrate and the extent of transporter up-regulation. The importance of other efflux transporters to blood-brain barrier function is not as well defined. However, it is likely that the substantial increases in their expression levels in DEP-exposed brain capillaries would tighten the barrier further.
Finally, the present study shows for the first time that blood-brain barrier function can be altered by DEPs, an environmental pollutant that acts directly on the capillary endothelium. This is of importance, as diesel exhaust is a ubiquitous component of polluted air worldwide, and DEPs have been shown to enter the brain (10, 43). Thus, DEP-stimulated brain capillaries could serve as an additional source of oxidative stress and inflammatory mediators for the brain parenchyma, contributing to CNS pathology and possibly disease. Altered brain capillary function could contribute to DEP-exacerbated cerebrovascular disease, in particular, and to our ability to treat CNS disease with drugs in general.
Acknowledgments
We thank Dr. Deborah Stumpo and Dr. Perry Blackshear, Laboratory of Signal Transduction, National Institute of Environmental Health Sciences (NIEHS)/National Institutes of Health (NIH), Research Triangle Park, NC, for providing TNF-R1-deficient mice and wild-type mice. This research was supported by the Intramural Research Program of the NIH, NIEHS. M.L.B. was supported by the NIH Pathway to Independence award (R00ES015409).
References
- Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- U.N. Environment Programme, and W. H. O. Report Air pollution in the world’s megacities. Environment. 1994;36:2–13, 25–37. [Google Scholar]
- Singh P, DeMarini D M, Dick C A, Tabor D G, Ryan J V, Linak W P, Kobayashi T, Gilmour M I. Sample characterization of automobile and forklift diesel exhaust particles and comparative pulmonary toxicity in mice. Environ Health Perspect. 2004;112:820–825. doi: 10.1289/ehp.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper W. Quantitation of nitro and dinitropolycyclic aromatic hydrocarbons in diesel exhaust particulate matter. Chemosphere. 1986;15:437–447. [Google Scholar]
- Schuetzle D. Sampling of vehicle emissions for chemical analysis and biological testing. Environ Health Perspect. 1983;47:65–80. doi: 10.1289/ehp.834765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M L, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong J S, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot R R, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt A C, Altenburg M, Torres-Jardon R, Swenberg J A. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Maronpot R R, Torres-Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, Villarreal-Calderon A, Nakamura J, Fernando R, Reed W, Azzarelli B, Swenberg J A. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31:524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- Wellenius G A, Schwartz J, Mittleman M A. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Franco-Lira M, Torres-Jardon R, Henriquez-Roldan C, Barragan-Mejia G, Valencia-Salazar G, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Reed W. Pediatric respiratory and systemic effects of chronic air pollution exposure: nose, lung, heart, and brain pathology. Toxicol Pathol. 2007;35:154–162. doi: 10.1080/01926230601059985. [DOI] [PubMed] [Google Scholar]
- Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen L C, Geiser M, Reed W, Rothen-Rutishauser B, Schurch S, Schulz H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo F. The blood-brain barrier. New aspects to the function of the cerebral endothelium. Nature. 1986;321:197–198. doi: 10.1038/321197a0. [DOI] [PubMed] [Google Scholar]
- Kim R B. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34:47–54. doi: 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- Taylor E M. The impact of efflux transporters in the brain on the development of drugs for CNS disorders. Clin Pharmacokinet. 2002;41:81–92. doi: 10.2165/00003088-200241020-00001. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz A M, Fricker G, Miller D S. Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med (Maywood) 2005;230:118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- Jette L, Beliveau R. P-glycoprotein is strongly expressed in brain capillaries. Adv Exp Med Biol. 1993;331:121–125. doi: 10.1007/978-1-4615-2920-0_20. [DOI] [PubMed] [Google Scholar]
- Hartz A M S, Bauer B, Baehr C H, Miller D S, Fricker G. Drug delivery across the blood-brain barrier. Curr Nanosci. 2005;1:203–209. [Google Scholar]
- Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- Lin J H, Yamazaki M. Clinical relevance of P-glycoprotein in drug therapy. Drug Metab Rev. 2003;35:417–454. doi: 10.1081/dmr-120026871. [DOI] [PubMed] [Google Scholar]
- Desai B S, Monahan A J, Carvey P M, Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16:285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- Dazert P, Suofu Y, Grube M, Popa-Wagner A, Kroemer H K, Jedlitschky G, Kessler C. Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience. 2006;142:1071–1079. doi: 10.1016/j.neuroscience.2006.07.056. [DOI] [PubMed] [Google Scholar]
- Fellner S, Bauer B, Miller D S, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz A M, Olson E R, Zhao R, Kalvass J C, Pollack G M, Miller D S. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz A M, Miller D S. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- Hartz A M, Bauer B, Fricker G, Miller D S. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- Hartz A M, Bauer B, Fricker G, Miller D S. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- National Institute of Standards and Technology Certificate of analysis for standard reference material 2975, diesel particulate matter (industrial forklift) 2000 https://srmors.nist.gov/view_cert.cfm?srm=2975. [Google Scholar]
- National Institute of Standards and Technology Material safety data sheet for SRM 2975. 2006 https://srmors.nist.gov/view_msds.cfm?srm=2975. [Google Scholar]
- Schramm U, Fricker G, Wenger R, Miller D S. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol Renal Physiol. 1995;268:F46–F52. doi: 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Carballo E, Blackshear P J. Roles of tumor necrosis factor-alpha receptor subtypes in the pathogenesis of the tristetraprolin-deficiency syndrome. Blood. 2001;98:2389–2395. doi: 10.1182/blood.v98.8.2389. [DOI] [PubMed] [Google Scholar]
- Carballo E, Lai W S, Blackshear P J. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood. 2000;95:1891–1899. [PubMed] [Google Scholar]
- Bauer B, Hartz A M, Fricker G, Miller D S. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Miller D S, Nobmann S N, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58:1357–1367. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- Xiao G G, Wang M, Li N, Loo J A, Nel A E. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- Rao K M, Ma J Y, Meighan T, Barger M W, Pack D, Vallyathan V. Time course of gene expression of inflammatory mediators in rat lung after diesel exhaust particle exposure. Environ Health Perspect. 2005;113:612–617. doi: 10.1289/ehp.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Xiao G G, Li N, Xie Y, Loo J A, Nel A E. Use of a fluorescent phosphoprotein dye to characterize oxidative stress-induced signaling pathway components in macrophage and epithelial cultures exposed to diesel exhaust particle chemicals. Electrophoresis. 2005;26:2092–2108. doi: 10.1002/elps.200410428. [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel D V. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc Natl Acad Sci U S A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D A, O'Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- U.S. EPA Washington, DC: National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency; Health assessment document for diesel engine exhaust. 2002 [Google Scholar]
- Sugamata M I T, Takano H, Oshio S, Takeda K. Maternal diesel exhaust exposure damages newborn murine brains. J Health Sci. 2006;52:82–84. [Google Scholar]
- Kyriakis J M. Life-or-death decisions. Nature. 2001;414:265–266. doi: 10.1038/35104735. [DOI] [PubMed] [Google Scholar]
- Demeule M, Regina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Beliveau R. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol. 2002;38:339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]