Abstract
Previous work suggested that altered Ca2+ homeostasis might contribute to dysfunction of nebulin-free muscle, as gene expression analysis revealed that the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-inhibitor sarcolipin (SLN) is up-regulated >70-fold in nebulin knockout mice, and here we tested this proposal. We investigated SLN protein expression in nebulin-free and wild-type skeletal muscle, as well as expression of other Ca2+-handling proteins. Ca2+ uptake capacity was determined in isolated sarcoplasmic reticulum vesicles and in intact myofibers by measuring Ca2+ transients. Muscle contractile performance was determined in skinned muscle activated with exogenous Ca2+, as well as in electrically stimulated intact muscle. We found profound up-regulation of SLN protein in nebulin-free skeletal muscle, whereas expression of other Ca2+-handling proteins was not (calsequestrin and phospholamban) or was minimally (SERCA) affected. Speed of Ca2+ uptake was >3-fold decreased in sarcoplasmic reticulum vesicles isolated from nebulin-free muscle as well as in nebulin-free intact myofibers. Ca2+-activated stress in skinned muscle and stress produced by intact nebulin-free muscle were reduced to a similar extent compared with wild type. Half-relaxation time was significantly longer in nebulin-free compared with wild-type muscle. Thus, the present study demonstrates for the first time that nebulin might also be involved in physiological Ca2+ handling of the SR-myofibrillar system.—Ottenheijm, C. A. C., Fong, C., Vangheluwe, P., Wuytack, F., Babu, G. J., Periasamy, M., Witt, C. C., Labeit, S., Granzier, H. Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle.
Keywords: sarcolipin, SERCA function, muscle contractility, nebulin KO model
Nebulin is a giant protein(molecular mass ∼800 kDa) found in skeletal muscle, where it spans the entire length of the thin filament (1). Recently, nebulin knockout (KO) mouse models have been generated, making it possible for the first time to investigate the in vivo functions of nebulin (2, 3). These studies established that nebulin is a regulator of thin filament length. In its absence, the thin filaments vary in length and are on average shorter than in wild-type (WT) muscle (2, 3). Phenotypically, the nebulin KO model recapitulates human nemaline myopathy, a group of nondystrophic congenital myopathies frequently caused by nebulin gene mutations (4). The nebulin KO model and the nemaline myopathy patients have in common the formation of nemaline rods combined with severe depressed contractility (muscle weakness).
The mechanisms that underlie the depressed contractility in nebulin KO mice are incompletely understood. Possible explanations for muscle dysfunction are reduced thin filament length and sarcomere disruption, both observed in the nebulin KO model (2,3). Previously, we postulated that altered Ca2+ homeostasis might also be involved, as gene expression analysis revealed that sarcolipin (SLN) is up-regulated >70-fold in nebulin KO mice (2), and here we tested this proposal.
SLN is a sarcoplasmic reticulum (SR) membrane protein. The release of Ca2+ from the SR on muscle stimulation triggers muscle contraction. Subsequent muscle relaxation occurs through reuptake of Ca2+ in to the SR by ATP-dependent Ca2+ pumps [sarco(endo)plasmic reticulum Ca2+-ATPase (SERCAs)]. SLN is a potent inhibitor of SERCA function (5,6,7,8) and is considered a phospholamban (PLB) homologue. In vitro, both SLN and PLB inhibit SERCA1 (expressed in fast-twitch skeletal muscle) and SERCA2a (expressed in cardiac and slow-twitch skeletal muscle) to the same extent (9). Traditionally, SLN has been viewed as the physiological regulator of SERCA1 and PLB as the corresponding SERCA2 regulator, but recent work (10) has shown that PLB and SLN are coexpressed in vivo and can regulate either SERCA isoform.
Overexpressing SLN in rat soleus muscle inhibits SERCA and decreases the SR Ca2+ store, significantly impairing contractility (reduced force generation and speed of relaxation; ref. 5). Similarly, contractility was depressed after overexpression of SLN in rat cardiac myocytes (7, 8). Importantly, a novel SLN KO mouse model shows enhanced SR Ca2+ reuptake and atrial contractility (11). Thus, SLN plays an important role in regulating the SERCA pump activity and contractile function.
Considering its important role in Ca2+ homeostasis and its up-regulation at the transcript level in nebulin KO mice, we hypothesized that increased SLN protein expression in the skeletal muscles of nebulin KO mice impairs Ca2+ handling and muscle contraction. To test this hypothesis, we investigated SLN protein expression in nebulin KO mice, as well as Ca2+ uptake of isolated SR vesicles, Ca2+ transients of intact myofibers, and muscle contractile performance.
MATERIALS AND METHODS
Animal model
The nebulin KO mouse model has been described previously (2). In brief, destruction of the TATA motif and murine nebulin exon 1, including the start ATG, was achieved by homologous recombination. Three mouse lines were raised from independent embryonic stem-cell clones. KO mice typically die within ∼10 days after birth due to severe muscle weakness resulting in respiratory failure, and mice used for the present study were 5–10 days old. All animal experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
Western blotting
Western blotting procedures were as described previously (10). Briefly, quadriceps muscles from nebulin KO and littermate control WT mice were solubilized in a buffer (1 mg/50 μl buffer) containing 10 mM imidazole (pH 7.0), 300 mM sucrose, complete protease inhibitor cocktail (Roche, Basel, Switzerland), lithium dodecyl sulfate, and β-mercaptoethanol. Solubilized samples (20 μl) were separated on 3–12% Bis-Tris gradient gels (200 V for 35 min at 20°C, NuPAGE electrophoresis system; Invitrogen, Carlsbad, CA, USA) and blotted onto polyvinylidene difluoride membranes (pore size=0.22 μm for SLN and PLB and 0.45 μm for calsequestrin (CSQ) and SERCA; Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. Blots were probed with the homemade polyclonal SLN antibody SLNAP78 directed against the N terminus of SLN (1:150), a monoclonal anti-PLB antibody (mA1; 1:1000, Upstate Biotechnology, Charlottesville, VA, USA), a homemade pan-specific polyclonal anti-SERCA antibody TRY1 (1:20,000), and a homemade skeletal muscle CSQ antibody (1:5000; refs. 10, 12). Detection was performed with alkaline phosphatase-coupled secondary antibodies, using the Vistra enhanced chemifluorescence system (Amersham Biosciences, Piscataway, NJ, USA). In a second Western blotting assay, quadricep, extensor digitorum longus (EDL), soleus, and atrium homogenates were electrophoretically separated on a 16% tricine gel and transferred to nitrocellulose membrane. Membranes were immunoprobed with the novel primary antibody anti-rabbit SLN directed to the C terminus of SLN (1:3000; ref. 13) followed by horseradish-conjugated secondary antibody. Signals were detected by Super Signal WestDura substrate (Pierce, Rockford, IL, USA) and quantified by densitometry.
SR Ca2+ transport assay
We used the relatively large quadriceps muscle for Ca2+ uptake experiments, as these assays require large amounts of tissue. Ca2+ transport activity of microsomal quadriceps homogenates at 1 μg protein/20 μl was assayed at 20°C in 200 μl of a reaction mixture containing the following (in mM): 20 MOPS, 100 KCl, 5 MgCl2, 5 ATP, 5 potassium oxalate, 0.5 EGTA, 10 μg of protein, and varying concentrations of CaCl2 (with 106 cpm/μmol of [45Ca2+]), as described previously (5). Free [Ca2+] was determined according to Fabiato and Fabiato (14). Data were normalized for total protein concentration (protein concentration determined using the bicinchoninic acid protein assay; Pierce) and analyzed by nonlinear regression (Hill equation) using Graphpad Prism software (Graphpad, San Diego, CA, USA). Values for maximal transport activity (Vmax) and pCa of half-maximal transport activity (pCa50) were obtained.
Determination of Ca2+ transients in single muscle fibers
For these experiments, we selected the flexor digitorum brevis muscle because of its very short fibers that reduce movement artifacts, making it a popular muscle for Ca2+-handling studies (15,16,17,18,19). The procedure for isolating intact fibers was as described previously (15), with minor modifications. Briefly, muscles were dissected and incubated for ∼30 min at 36°C in mammalian Ringer solution (in mM: 145 NaCl, 2.5 KCl, 1.0 MgSO4, 1.0 CaCl2, 10 glucose, and 10 HEPES, pH 7.4, at 20°C) containing 2 mg/ml collagenase type II (Worthington CLS2). After incubation, the muscles were washed four times with mammalian Ringer solution and gently separated from tendons by sucking the muscle through a small-bore pipette. Subsequently, the dissociated single muscle fibers were loaded with the ratiometric Ca2+ indicator fura 2FF/AM (the ester form of fura 2FF; Teflabs, Austin, TX, USA) for 45 min at 20°C at a concentration of 4 μM. Fibers were transferred to the experimental chamber and allowed to adhere to its glass bottom. The experimental chamber was mounted on the stage of an inverted Olympus IX70 microscope equipped for fluorescence(Olympus, Tokyo, Japan). A 360/380 nm excitation wavelength pair was used with a 510 nm emission filter set, which permitted continuous excitation at 380 nm, while fluorescence excited at 360 nm was interpolated (at this wavelength fura 2FF fluorescence yield is independent of Ca2+ concentration). Fluorescence signals were collected from an area of ∼20 × 20 μm, using a photomultiplier system (IonOptix, Milton, MA, USA). Intact fibers were activated with electrical field stimulation (at 1 Hz for 10 s), and the change in the ratio of the fluorescence measured at 380 and 360 nm excitation was calculated to determine Ca2+ transients. This procedure allowed Ca2+ transient recordings from numerous fibers located within the chamber. The myosin inhibitor N-benzyl-p-toluene sulfonamide (10 μM) was used to depress contractions and limit movement artifacts. Temperature was maintained at 20°C during the whole experiment. The recordings of 10 consecutive transients per fiber were averaged and analyzed using Ionwizard software (IonOptix).
Intact muscle contractility protocol
Contractility experiments were performed as described previously (20), with minor modifications. Briefly, soleus muscles were dissected and, with the use of silk suture, mounted horizontally in a tissue bath between a force transducer and a fixed hook. The tissue bath was perfused continuously with oxygenated (95% O2-5% CO2) mammalian Ringer solution, pH 7.40, at 30°C. Temperature of the solution was maintained at 30°C during the experiment. The muscle was stimulated directly by using platinum plate electrodes placed in close apposition to the muscle. Muscle preload force was adjusted until optimal fiber length for maximal twitch force was achieved (pulse duration of 1.0 ms). A 1 s tetanus was then induced using a stimulation frequency of 80 Hz (which was sufficient in soleus muscle to induce maximal tetanic force; data not shown). After the contractile measurements were completed, length and weight of the soleus muscles were determined. Cross-sectional area was calculated by dividing soleus muscle weight (g) by muscle length (cm) multiplied by specific density (1.056). Force was normalized to muscle cross-sectional area (in mN/mm2).
Skinned muscle contractility protocol
The procedures for skinned muscle contractility were as described previously (2, 20), with minor modifications. In short, soleus muscle from nebulin KO and WT mice were skinned for 6 h at ∼4°C in relaxing solution (in mM: 20 BES, 10 EGTA, 6.56 MgCl2, 5.88 NaATP, 1 DTT, 46.35 K-propionate, and 15 creatine phosphate, pH 7.0, at 20°C) containing 1% (w/v) Triton X-100. The skinning procedure renders the membranous structures in the muscle fibers permeable, which enables activation of the myofilaments with exogenous Ca2+. Preparations were washed thoroughly with relaxing solution and stored in 50% glycerol/relaxing solution at −20°C. Small muscle bundles (diameter of ∼0.01 mm2) were dissected from the skinned muscles. Muscle bundles were attached to a strain gauge and a high-speed motor using aluminum foil clips. Experiments were performed at 20°C. Sarcomere length was measured with laser diffraction using a He-Ne laser beam and adjusted to 2.15 and 2.5 μm for KO and WT muscle, respectively (unpublished data revealed that at these respective sarcomere lengths, KO and WT soleus muscle produce maximum Ca2+-activated force). The xy (width) and xz (depth, using a prism) bundle diameters were measured with an ×40 objective. The muscle bundle cross-sectional area was calculated from the average of three width, and depth measurements made along the length of the muscle bundle while it was mounted in relaxing solution at a sarcomere length of 2.15 μm. The preparation was activated at pCa 4.5 to obtain maximal Ca2+-activated force. Maximal stress was determined by dividing the force generated at pCa 4.5 by cross-sectional area.
Statistical analysis
Data are presented as mean ± se. Statistical analyses were performed by t test between nebulin KO and WT mice; P < 0.05 was considered statistically significant.
RESULTS
Increased expression of SLN in nebulin-free skeletal muscle
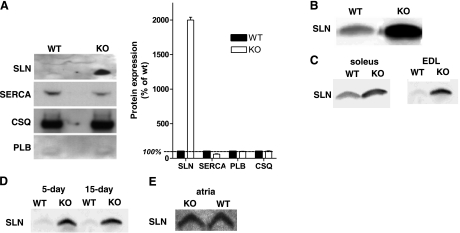
SLN gene transcription has previously been shown to be highly up-regulated in skeletal muscle of nebulin KO mice (2), but no protein studies were conducted at the time due to the unavailability of SLN antibodies. After the successful production of SLN antibodies by two independent laboratories (10,13), we studied SLN expression at the protein level. We also included antibodies directed against additional Ca2+-regulating components of the SR in our analysis. For loading normalization, we used both total protein (determined using the bicinchoninic acid protein assay) and the relative myosin heavy chain content determined by gel electrophoresis, and identical results were obtained with both methods. Figure 1A shows results with quadriceps muscle. SLN is just detectable in WT mice and highly up-regulated in nebulin KO mice. Protein expression levels of other Ca2+-handling proteins were affected either minimally or not at all. Expression of CSQ, a Ca2+-binding protein in the SR that facilitates muscle relaxation, was unchanged in KO mice (104±4% of WT values; n=5; P<0.05). SERCA expression levels were slightly down-regulated in quadriceps muscle of KO mice (60±5% of WT values; n=5; P<0.001), and PLB expression was not significantly different in KO mice (101±1% of WT values; n=5; P>0.05). The profound up-regulation of SLN expression in nebulin KO quadriceps muscle was confirmed by using a second SLN antibody directed against the opposite end of the SLN molecule (13; Fig. 1B). In addition, this SLN antibody was used to investigate SLN expression across slow- and fast-twitch muscle and to investigate age dependence of SLN expression. Figure 1C illustrates that SLN was found to be highly up-regulated in both soleus (slow-twitch) and EDL (fast-twitch) muscle, and Fig. 1D shows that SLN up-regulation in EDL of nebulin KO mice was independent of age. Finally, SLN expression in atria was clearly detectable in WT mice, consistent with previous work (10, 11, 13), and was unaltered in nebulin KO mice (Fig. 1E). Thus, a consistent finding of our protein expression analysis is the high degree of SLN up-regulation in skeletal muscle of nebulin KO mice. Overall these findings are consistent with previous transcript data that indicate that SLN gene expression is highly up-regulated in skeletal muscle of nebulin KO mice and that other Ca2+-handling proteins are not significantly different (2).
Figure 1.
SLN protein expression. A) Using the polyclonal SLN antibody SLNAP78 (20), quadriceps SLN expression is just detectable in WT mice but highly up-regulated in nebulin KO mice. In the same muscles, SERCA expression is slightly down-regulated, whereas CSQ and PLB expression do not differ between KO and WT mice. B) A second SLN antibody (2) confirms the profound up-regulation of SLN expression in nebulin KO quadriceps muscle compared with WT. C) SLN expression is highly up-regulated in both soleus (slow-twitch) and EDL (fast-twitch) muscle. D) SLN expression is similar in 5-day-old compared with 15-day-old EDL muscle, both in nebulin KO and WT mice. E) SLN expression is comparable in atria of WT and KO mice. (Note that identical protein loadings were used, see text.)
Speed of SR Ca2+ uptake is depressed both in microsomes and in intact fibers of nebulin-free skeletal muscle
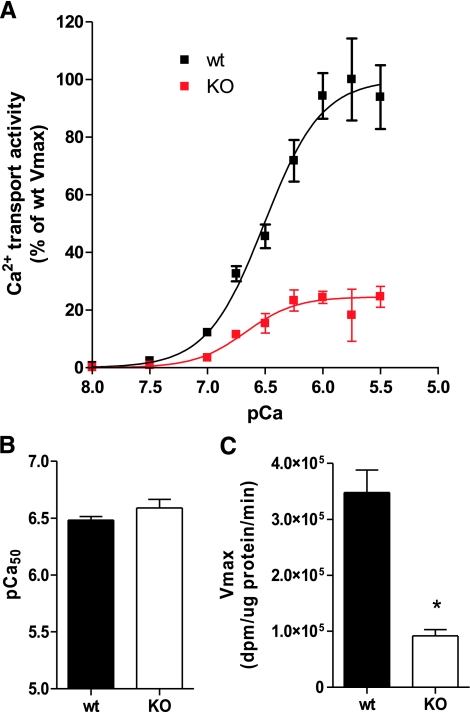
We next measured SR Ca2+ uptake in the quadriceps of nebulin KO and WT mice using microsomal homogenates. As illustrated by Fig. 2, Ca2+ uptake of isolated SR vesicles from nebulin KO mice was reduced across a range of free Ca2+ levels from pCa 7 to pCa 5.5 (Fig. 2A). There was no significant change in Ca2+ affinity, as pCa50 was not different between groups (Fig. 2B). On the other hand, Vmax was reduced by >3-fold (n=6; P<0.001; Fig. 2C). Thus, Ca2+ uptake speed by SR vesicles from KO mice is greatly reduced compared with WT, without a change in Ca2+ affinity.
Figure 2.
SR Ca2+ transport assay. Ca2+ dependence of Ca2+ uptake was determined in microsomal quadriceps homogenates from nebulin KO and WT mice. Ca2+ uptake in nebulin KO mice is reduced across a range of free Ca2+ levels from pCa 7 to pCa 5.5 (A); Vmax is reduced by >3-fold (C). pCa50 does not differ between groups (B), indicating no significant change in Ca2+ affinity between KO and WT. *Significantly different from WT, P < 0.05.
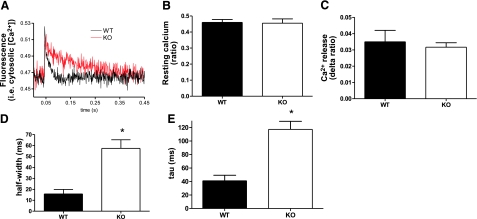
Our studies had to be carried out on neonatal mice (average age ∼7 days; nebulin KO mice typically die before an age of 10 days), which constituted a considerable challenge, especially for the intact fiber experiments. (Note that earlier work by others on neonatal mice was restricted to mice older than 7 days; ref. 15.) We decided to isolate fibers enzymatically from the flexor digitorum brevis muscle, as this muscle is widely used for these experiments due to its very short fibers that reduce movement artifacts (15,16,17,18,19). The fibers that we obtained were well suited to measure Ca2+ transients of twitch contractions, and many twitches could be induced with identical Ca2+ transients. However, the neonatal single fibers tended to visually deteriorate after tetanic stimulation, and results were not well reproducible. Thus, the Ca2+ transient experiments were restricted to twitch activation protocols. Figure 3A depicts Ca2+ transient recordings from twitch-activated single fibers. On average, there was no significant difference in baseline Ca2+ levels (Fig. 3B) or in the amount of Ca2+ released on twitch stimulation (Fig. 3C) between nebulin KO and WT fibers (n=6; ∼5 fibers/mouse analyzed). To investigate the effect of twitch activation history on these parameters we compared the average of the first 5 transients of a 10 twitch pulse train (twitch frequency 1 Hz) to the average of the last 5 transients. No difference was found within WT or KO fibers (data not shown). A significant finding of our work was that the rate of Ca2+ reuptake into the SR was significantly reduced in nebulin KO fibers compared with WT fibers, as indicated by increased values for half-width (i.e., the duration at half-maximal amplitude, Fig. 3D) and tau (i.e., the time course of transient decay, derived from a mono-exponential fit, Fig. 3E).
Figure 3.
Ca2+ transients from intact single muscle fibers. A) Typical Ca2+ transient recording from flexor digitorum brevis muscle fibers from nebulin KO and WT mice, using the ratiometric Ca2+ dye fura 2FF/AM. Note the slower Ca2+ reuptake in KO fibers. B–D) Baseline Ca2+ levels do not differ between KO and WT fibers (B), nor does the amount of Ca2+ release on stimulation (C). The rate of Ca2+ reuptake by the SR is significantly reduced in nebulin KO mice, as indicated by the increased duration at half-maximal amplitude (i.e., half-width; D) and increased time constant of transient decay (i.e., tau; E). *Significantly different from WT, P < 0.05.
Slowed relaxation in nebulin-free skeletal muscle
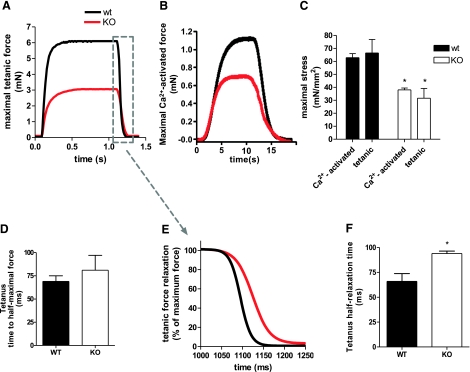
For force measurements, we used the soleus muscle because it has tendons that are already well defined in young WT and KO mice, making it possible to perform intact muscle experiments. Figure 4A shows a typical contractile response in soleus muscle to tetanic stimulation. Maximal tetanic stress was reduced by ∼52% in nebulin KO mice (31.7±7.5 vs. 66.5±10.4 mN/mm2, KO vs. WT, respectively). We also studied skinned muscle and measured maximal Ca2+-activated stress (pCa 4.0). This stress was reduced by ∼40% in nebulin KO compared with WT mice (38.0±1.5 vs. 62.9±3.1 mN/mm2, KO vs. WT, respectively). As depicted in Fig. 4C, results from intact and skinned muscle were comparable in both WT and KO mice, indicating that the altered Ca2+ handling in KO muscle does not significantly affect force generating capacity.
Figure 4.
Intact and skinned soleus contractility. A, B)Typical force tracings in response to tetanic stimulation (intact soleus; A) and exogenous Ca2+ (skinned soleus; B) of nebulin KO and WT soleus with similar cross sectional areas. C) Maximal intact soleus stress generation is significantly reduced in nebulin KO compared with WT mice and to a similar extent as maximal skinned soleus stress production. D) Time to half-maximal force during tetanic stimulation does not differ between KO and WT soleus. E) Magnification of the relaxation phase of tracings shown in A, but normalized to maximal force. F) Tetanic half-relaxation time is significantly increased in nebulin KO vs. WT soleus. *Significantly different from WT, P < 0.05.
Finally, we measured the speed of tetanic activation of intact muscle and the speed of relaxation (Fig. 4D–F). The speed of activation (time to half-maximal force) was not different in KO vs. WT muscle (81±17 vs. 69±6 ms, respectively; Fig. 4D). In contrast, the tetanus half-relaxation time was significantly increased in nebulin KO mice (94.0±2.5 vs. 66.0±7.8 ms, KO vs. WT, respectively; Fig. 4E, F).
DISCUSSION
Nebulin is a major component of skeletal muscle, comprising ∼3% of total myofibrillar protein (1), that interacts in vitro with multiple other myofibrillar proteins (21,22). This, together with the capability of nebulin to modulate acto-myosin driven in vitro motility, has led to the proposal that nebulin constitutes a contractile regulatory system (21,22). Also consistent with a role of nebulin in contractility is the observation that, phenotypically, the nebulin KO model recapitulates a major symptom of human nemaline myopathy, i.e., severe muscle weakness (4). Here, we used the nebulin KO mouse model to investigate the contribution of nebulin to contractile regulation in skeletal muscle in an in vivo context. We discovered changes in Ca2+ handling and muscle relaxation in nebulin-free skeletal muscle. Thus, the present study demonstrates for the first time that nebulin might also be involved in physiological Ca2+ handling of the SR-myofibrillar system.
Up-regulation of SLN protein in nebulin-free skeletal muscle
SLN regulates Ca2+ uptake by inhibiting the SERCA pump activity (11, 23,24,25). Furthermore, CSQ is a Ca2+-binding protein located within the SR that facilitates Ca2+ reuptake and thus muscle relaxation (26). Recent studies (2) revealed that in nebulin-free skeletal muscle SLN transcript is up-regulated >70-fold, prompting us to examine SLN at the protein level in skeletal muscle of WT and nebulin KO mice. We found a low level of SLN protein in skeletal muscle of WT mice, which is in line with previous work (13). Importantly, in KO mice SLN protein was significantly up-regulated (Fig. 1), which is consistent with the high degree of up-regulation of SLN transcript. Studies of SLN protein expression have been hampered by the lack of a specific antibody. In the present study, we used two different SLN antibodies that were recently generated, both directed against two different epitopes (10, 13). Because both antibodies produced comparable results, the high degree of SLN up-regulation in nebulin-free muscle can be considered well established. In line with recent data from a SLN KO mouse model (11) in which protein levels of other Ca2+-handling proteins were unaffected, the profound up-regulation of SLN in nebulin-free muscle did not affect the expression of CSQ and PLB and only to a limited extent affected the expression of SERCA. Up-regulation of SLN was found across different skeletal muscle types (i.e., soleus, gastrocnemius, and EDL muscle), and within the complete age-range that we studied (Fig. 1C, D). However, we did not detect SLN up-regulation in atrial tissue, indicating that the up-regulation of SLN is specific for skeletal muscles of nebulin KO mice. The finding that SLN expression is similar in atria from WT and nebulin KO mice is consistent with the absence of nebulin in the heart (2) and is in line with a role for nebulin in regulating SLN expression.
Slowed Ca2+ reuptake in nebulin-free skeletal muscle
Overexpression of SLN in rat soleus muscle (5) and murine cardiac muscle (7, 8) has been shown to inhibit SERCA activity, thereby reducing SR Ca2+ uptake and impairing contractile function. In line with these studies, we found >3-fold reduced in vitro Ca2+ uptake rate by SR vesicles isolated from quadriceps muscle of nebulin KO mice (Fig. 2), without a change in Ca2+ affinity (i.e., pCa50). This finding was supported by studies on intact fibers from flexor digitorum brevis muscle that revealed an ∼3-fold increased Ca2+ transient decay time in KO mice. Given that the expression of the other Ca2+-regulating proteins PLB and CSQ is unaltered, it is likely that the greatly increased SLN/SERCA ratio is responsible for the impaired SR Ca2+ reuptake. This increased ratio is largely explained by the up-regulation of SLN expression, estimated at ∼20-fold, with a minor contribution made by the 40% reduction in the SERCA expression level.
Theoretically, a lower rate of Ca2+ removal from the cytosol might reduce the amount of Ca2+ stored in the SR and thus reduce the Ca2+ transient amplitude during stimulation. However, unlike previous SLN overexpression studies in cardiac muscle (7, 8), we did not observe a decreased Ca2+ transient amplitude in nebulin KO fibers, suggesting no effect on the amount of Ca2+ released during stimulation. Apparently, the size of the Ca2+ store in the SR of nebulin KO fibers is sufficient for the release of normal Ca2+ levels. It should be noted, however, that in contrast to the present study, the cardiomyocytes used in the previously mentioned studies (7, 8) were continuously paced. Thus, one can hypothesize that the different findings are explained by differences in protocols (short-term pacing in our study vs. continuous pacing in other studies). When comparing the first 5 Ca2+ transients of a 10-pulse stimulation train at 1 Hz with the last 5 transients, we did not find a difference in nebulin KO fibers or in WT fibers, inconsistent with the aforementioned hypothesis. A more rigorous test consisting of continuous pacing of neonatal KO and WT fibers was not possible due to the high vulnerability of neonatal fibers. Taken together, our findings using isolated SR vesicles as well as intact muscle fibers show that the profound up-regulation of SLN protein results in significantly decreased speed of SR Ca2+ uptake in skeletal muscle of nebulin KO compared with WT mice.
SLN up-regulation in nebulin-free skeletal muscle slows relaxation
Consistent with our intact fiber studies and SR Ca2+ transport assay, tetanic half-relaxation time was significantly increased in soleus muscle of nebulin KO mice compared with WT (Fig. 4). Overexpressing SLN in rat soleus muscle has been shown to decrease the rate of force relaxation (i.e., −dF/dt; ref. 5). However, maximal force was also depressed in SLN-overexpressing soleus. Since the rate of relaxation, unlike half-relaxation time, also depends on the absolute force produced, the decreased rate of relaxation in SLN-overexpressing soleus was not merely caused by slower Ca2+ reuptake but was also a reflection of reduced twitch force generation.
The present study allowed a quantitative comparison of the force deficits found in nebulin KO intact muscle (during which Ca2+ homeostasis plays an important role) with that of skinned muscle (when maximally activated with exogenous Ca2+). Maximal tetanic stress production was ∼50% reduced in nebulin KO soleus compared with WT, which is in line with Bang et al. (3), who reported a ∼50% reduction of maximal tetanic stress in nebulin KO tibialis anterior muscle. Considering that maximal Ca2+-activated stress of skinned muscle is reduced to a similar extent (Fig. 4C), these data indicate that the SLN-induced changes in Ca2+ handling in nebulin KO muscle do not have a profound effect on maximal stress production. Apparently, the depressed stress-generating capacity in nebulin KO mice can be largely attributed to the shorter thin filament lengths in KO vs. WT muscle, which reduces the amount of overlap between the thick and thin filaments. Based on previous work (2), at a sarcomere length of 2.5 μm, filament overlap is maximal in WT but ∼50% of maximal in KO muscle. Maximal Ca2+-activated stress is predicted to be reduced accordingly, which is in line with the present data. In addition, up-regulation of SLN slows relaxation.
Nebulin-SR interaction
Up-regulation of SLN expression and the reduced rate of Ca2+ uptake after tetanic stimulation might be a compensatory response, or it might indicate that nebulin functions as a regulator of SLN expression. We found SLN up-regulation in all tested muscle types [EDL (fast-twitch), soleus (slow-twitch), gastrocnemius muscle (intermediate); Fig. 1C] and independent of age (Fig. 1D). This supports the hypothesis that the up-regulated SLN expression in nebulin KO mice is not just a compensatory mechanism, as the need for compensation would be expected to be different in different muscle types or muscle of different ages. To search for a potential physical interaction between nebulin and SLN, we synthesized the cytosolic domain of SLN, but an overlay assay revealed no binding of the biotinylated SLN peptide to nebulin (data not shown). In addition, we performed yeast-two-hybrid screens with the bait M163-M170, a region in the nebulin molecule that interacts with desmin and therefore presumably locates in the Z-disk peripheral region (results not shown). SLN was not identified by this screen. However, in addition to desmin and α-actinin (established nebulin binding partners), the screen identified a prey clone coding for SERCA2. Thus, further research is warranted to explore the potential interaction between nebulin and components of the SR and the role that these interactions play in dysregulated SLN expression. Unraveling the role of nebulin SR function is important for understanding how nebulin mutations in nemaline myopathy (that commonly disrupt nebulin C-terminal region; ref. 4) cause muscle weakness.
Acknowledgments
We thank G. Wright and T. Pecor for outstanding technical assistance. This work was supported by a Rubicon grant from the Dutch Organization for Scientific Research to C.O., by the Deutsche Forschungsgemeinschaft (WI 3278/1–1 to C.W. and La668/10–1 to SL), and by U.S. National Institutes of Health grants AR-053897 and HL-062881 to H.G.
References
- Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988;107:2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt C C, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 2006;25:3843–3855. doi: 10.1038/sj.emboj.7601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M L, Li X, Littlefield R, Bremner S, Thor A, Knowlton K U, Lieber R L, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol. 2006;173:905–916. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelin K, Hilpela P, Donner K, Sewry C, Akkari P A, Wilton S D, Wattanasirichaigoon D, Bang M L, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea J A, Muntoni F, Dubowitz V, Beggs A H, Laing N G, Labeit S, de la Chapelle C A, Wallgren-Pettersson C. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci U S A. 1999;96:2305–2310. doi: 10.1073/pnas.96.5.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupling A R, Asahi M, MacLennan D H. Sarcolipin overexpression in rat slow twitch muscle inhibits sarcoplasmic reticulum Ca2+ uptake and impairs contractile function. J Biol Chem. 2002;277:44740–44746. doi: 10.1074/jbc.M206171200. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Becker S, Khanna V K, Kurzydlowski K, Leisner E, Pette D, MacLennan D H. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1998;273:12360–12369. doi: 10.1074/jbc.273.20.12360. [DOI] [PubMed] [Google Scholar]
- Asahi M, Otsu K, Nakayama H, Hikoso S, Takeda T, Gramolini A O, Trivieri M G, Oudit G Y, Morita T, Kusakari Y, Hirano S, Hongo K, Hirotani S, Yamaguchi O, Peterson A, Backx P H, Kurihara S, Hori M, MacLennan D H. Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci U S A. 2004;101:9199–9204. doi: 10.1073/pnas.0402596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu G J, Bhupathy P, Petrashevskaya N N, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen P M, Ziolo M T, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem. 2006;281:3972–3979. doi: 10.1074/jbc.M508998200. [DOI] [PubMed] [Google Scholar]
- MacLennan D H, Asahi M, Tupling A R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu G J, Bhupathy P, Timofeyev V, Petrashevskaya N N, Reiser P J, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A. 2007;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangheluwe P, Tjwa M, Van Den B A, Louch W E, Beullens M, Dode L, Carmeliet P, Kranias E, Herijgers P, Sipido K R, Raeymaekers L, Wuytack F. A SERCA2 pump with an increased Ca2+ affinity can lead to severe cardiac hypertrophy, stress intolerance and reduced life span. J Mol Cell Cardiol. 2006;41:308–317. doi: 10.1016/j.yjmcc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Babu G J, Bhupathy P, Carnes C A, Billman G E, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol Paris. 1979;75:463–505. [PubMed] [Google Scholar]
- Capote J, Bolanos P, Schuhmeier R P, Melzer W, Caputo C. Calcium transients in developing mouse skeletal muscle fibres. J Physiol. 2005;564:451–464. doi: 10.1113/jphysiol.2004.081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinniger G J, Bruton J D, Westerblad H, Ranatunga K W. Effects of a myosin-II inhibitor (N-benzyl-p-toluene sulphonamide, BTS) on contractile characteristics of intact fast-twitch mammalian muscle fibres. J Muscle Res Cell Motil. 2005;26:135–141. doi: 10.1007/s10974-005-2679-2. [DOI] [PubMed] [Google Scholar]
- Bruton J D, Dahlstedt A J, Abbate F, Westerblad H. Mitochondrial function in intact skeletal muscle fibres of creatine kinase deficient mice. J Physiol. 2003;552:393–402. doi: 10.1113/jphysiol.2003.050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z M, Messi M L, Delbono O. Sustained overexpression of IGF-1 prevents age-dependent decrease in charge movement and intracellular Ca(2+) in mouse skeletal muscle. Biophys J. 2002;82:1338–1344. doi: 10.1016/S0006-3495(02)75489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Grounds M D, Bakker A J. Measurement of sub-membrane [Ca2+] in adult myofibers and cytosolic [Ca2+] in myotubes from normal and mdx mice using the Ca2+ indicator FFP-18. Cell Calcium. 2006;40:299–307. doi: 10.1016/j.ceca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Ottenheijm C A C, Heunks L M, Geraedts M C, Dekhuijzen P N. Hypoxia-induced skeletal muscle fiber dysfunction: role for reactive nitrogen species. Am J Physiol Lung Cell Mol Physiol. 2006;290:L127–L135. doi: 10.1152/ajplung.00073.2005. [DOI] [PubMed] [Google Scholar]
- McElhinny A S, Kazmierski S T, Labeit S, Gregorio C C. Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc Med. 2003;13:195–201. doi: 10.1016/s1050-1738(03)00076-8. [DOI] [PubMed] [Google Scholar]
- Wang K, Knipfer M, Huang Q Q, van Heerden A, Hsu L C, Gutierrez G, Quian X L, Stedman H. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture. Sequence motifs and affinity profiles of tandem repeats and terminal SH3. J Biol Chem. 1996;271:4304–4314. doi: 10.1074/jbc.271.8.4304. [DOI] [PubMed] [Google Scholar]
- MacLennan D H. Ca2+ signalling and muscle disease. Eur J Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Sipido K R, Raeymaekers L, Wuytack F. New perspectives on the role of SERCA2’s Ca2+ affinity in cardiac function. Biochim Biophys Acta. 2006;1763:1216–1228. doi: 10.1016/j.bbamcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Berchtold M W, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]