Abstract
The recent release of draft genome sequences of two of the major human schistosomes has underscored the pressing need to develop functional genomics approaches for these significant pathogens. The sequence information also makes feasible genome-scale investigation of transgene integration into schistosome chromosomes. Retrovirus-mediated transduction offers a means to establish transgenic lines of schistosomes, to elucidate schistosome gene function and expression, and to advance functional genomics approaches for these parasites. We investigated the utility of the Moloney murine leukemia retrovirus (MLV) pseudotyped with vesicular stomatitis virus glycoprotein (VSVG) for the transduction of Schistosoma mansoni and delivery of reporter transgenes into schistosome chromosomes. Schistosomula were exposed to virions of VSVG-pseudotyped MLV, after which genomic DNA was extracted from the transduced schistosomes. Southern hybridization analysis indicated the presence of proviral MLV retrovirus in the transduced schistosomes. Fragments of the MLV transgene and flanking schistosome sequences recovered using an anchored PCR-based approach demonstrated definitively that somatic transgenesis of schistosome chromosomes had taken place and, moreover, revealed widespread retrovirus integration into schistosome chromosomes. More specifically, MLV transgenes had inserted in the vicinity of genes encoding immunophilin, zinc finger protein Sma-Zic, and others, as well as near the endogenous schistosome retrotransposons, the fugitive and SR1. Proviral integration of the MLV transgene appeared to exhibit primary sequence site specificity, targeting a gGATcc-like motif. Reporter luciferase transgene activity driven by the schistosome actin gene promoter was expressed in the tissues of transduced schistosomula and adult schistosomes. Luciferase activity appeared to be developmentally expressed in schistosomula with increased activity observed after 1 to 2 wk in culture. These findings indicate the utility of VSVG-pseudotyped MLV for transgenesis of S. mansoni, herald a tractable pathway forward toward germline transgenesis and functional genomics of parasitic helminths, and provide the basis for comparative molecular pathogenesis studies of chromosomal lesions arising from retroviral integration into human compared with schistosome chromosomes.—Kines, K. J., Morales, M. E., Mann, V. H., Gobert, G. N., Brindley, P. J. Integration of reporter transgenes into Schistosoma mansoni chromosomes mediated by pseudotyped murine leukemia virus.
Keywords: schistosome, transgenesis, VSVG, luciferase, provirus, functional genomics
Retroviruses have been used for the transgenesis of eukaryotes with complex genomes, for example, the genome of the zebrafish (1, 2). Indeed, transgenesis techniques have been developed for a number of model and medically and economically important species, including the malaria parasite, mosquito vectors of infectious diseases, and human cell lines (3,4,5). By contrast, transgenic technologies and tools for schistosomes and other parasitic helminths are not as well advanced (6,7,8,9,10,11,12,13). Among the potential tools for gene manipulation, transgenesis mediated by retroviruses offers a potentially tractable method to introduce foreign genes, a means to determine the importance of schistosome genes, including those that could be targeted in novel interventions and the potential to undertake large-scale genetic analysis by insertional mutagenesis.
Schistosomiasis is considered the most important of the human helminthiases in terms of both morbidity and mortality (14, 15). Advances in molecular genetics and immunology hold the promise to control the spread of schistosomiasis and to guide development of new tools to combat this neglected tropical disease. At present, control of schistosomiasis largely relies on chemotherapy with praziquantel but wide-scale use of this medication has led to concerns about development of drug resistance (16). No vaccine is yet available for control of schistosomiasis, although new antigens are being developed (17). It is anticipated that the genome sequences of two of the main species of schistosomes that parasitize humans will be published soon, Schistosoma japonicum and Schistosoma mansoni, following on reasonably comprehensive descriptions of their transcriptomes and proteomes (18,19,20,21).
We modified a murine leukemia virus (MLV) vector to incorporate the reporter gene firefly luciferase under control of endogenous schistosome gene promoters, including the actin promoter, which was then incubated with developmental stages of S. mansoni, including schistosomula. The vesicular stomatitis virus glycoprotein (VSVG) -pseudotyped replication incompetent retroviruses transduced the cultured schistosomes, leading to integration of proviral forms of the retrovirus into schistosome chromosomes. A PCR technique utilizing endogenous mobile elements as anchors was employed to recover proviral integration junctions, which revealed that MLV showed primary sequence specificity for a gGATcc-like target motif and that the proviral transgenes were located within or near protein encoding genes and other sites. Furthermore, the actin promoter drove transcription of the luciferase transgene in schistosomula and adult mixed-sex schistosomes. These findings definitively demonstrated chromosomal integration of transgenes and somatic transgenesis of schistosomes mediated by pseudotyped retrovirus, and provide a foundation for functional genomics investigations of schistosomes.
MATERIALS AND METHODS
Developmental stages of S. mansoni
Mice and Biomphalaria glabrata snails infected with the NMRI (Puerto Rican) strain of S. mansoni were supplied by Dr. Fred Lewis (Biomedical Research Institute, Rockville, MD, USA). Schistosomula were mechanically transformed from cercariae released from infected B. glabrata snails. Briefly, cercariae were concentrated by centrifugation (2000 rpm for 10 min) and washed once with schistosomulum wash medium (RPMI 1640 supplemented with 200 U/ml penicillin G sulfate, 200 μg/ml streptomycin sulfate, 500 ng/ml amphotericin B, and 10 mM HEPES). Cercarial tails were removed by 20 passes through 22-gauge emulsifying needles, after which schistosomulum bodies were isolated free from tails by Percoll gradient centrifugation (22). Schistosomula were washed 3× in wash medium and cultured at 37°C under 5% CO2 in air in modified Basch’s medium (23) supplemented with washed human erythrocytes, 2 μl erythrocytes/ml culture medium. Adult schistosomes perfused from mice were cultured at 37°C under 5% CO2 in air in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U of penicillin and streptomycin, and washed human erythrocytes (as above). Schistosomula and adult schistosomes were maintained in culture for up to 3 wk.
Transduction of schistosomes with pseudotyped retrovirus
Plasmid constructs were assembled from the pLNHX-based plasmid pLNHX-SmSL-luciferase (24) by insertion of the cassette encoding the S. mansoni actin gene promoter and the firefly luciferase gene (25) to derive pLNHX-SmACT-Luc. The actin gene promoter was amplified using Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA) designed with restriction sites for XhoI and BglII in the primer clamp region to directionally clone the promoters into pLNHX. The S. mansoni SL RNA gene promoter was removed using XhoI and BglII and replaced with the actin gene promoter to derive pLNHX-SmACT-Luc. Production of VSVG-pseudotyped virions in packaging cells was undertaken as described previously (24). In brief, GP2–293 packaging cells (Clontech, Mountain View, CA, USA) (modified HEK-293 cells) were transfected with plasmids pLNHX-SmACT-Luc or pLNHX-SmSL-Luc along with a plasmid encoding VSVG, delivered in liposomes (Lipofectamine 2000, Invitrogen). Subsequently, pantropic virus in the culture supernatants was concentrated by high-speed centrifugation (Sorvall SS-34 rotor, Thermo Scientific, Waltham, MA, USA; 50,000 g, 90 min, 4°C). The pellet of concentrated virions (from 50 ml of packaging cell culture supernatants) was resuspended in TNE (50 mM Tris, 130 mM NaCl, 1 mM EDTA, pH 7.8) at 4°C overnight, after which the virions were stored at −80°C. Viral titers were determined using target NIH-3T3 fibroblast cells cultured in the presence of the antibiotic geneticin. Schistosomula (103–104) or mixed-sex adult S. mansoni (10–100) were cultured in 35-mm tissue culture wells in 2 to 3 ml of medium containing ∼200 μl of virion stock with an infectivity of 1 × 104 to 1 × 107 colony forming units (CFU)/ml, i.e., in the range of one to several thousand virions per worm.
Detection of provirus in the schistosome genome
Total genomic DNAs (gDNAs) were isolated from transduced or control schistosomes using the AquaPure system (Bio-Rad, Hercules, CA, USA). Primers specific for genes encoding neomycin phosphotransferase II, 5′-TGTGCTCGACGTTGTCACTGAA and 5′-ATGAATCCAGAAAAGCGGCCA, and firefly luciferase, 5′-GTGCCAGAGTCCTTCGATAG and 5′-ACAACTTTACCGACCGCGCC, were employed to amplify proviral reporter transgenes. Primers for the S. mansoni cytochrome oxidase 1 (cox I) gene [GenBank AF101196; National Center for Biotechnology Information (NCBI), Bethesda, MD, USA], 5′-TGAGTGTCATTTTAGGGTGGTG and 5′-ACAAACCAATGAAAATATCCAAGA were used to confirm the integrity of schistosome gDNAs. At the time of harvest of the cultured schistosomes, an aliquot of culture media was also collected for analysis to investigate the presence of residual, contaminating pLNHX plasmids carried over in virion inocula. PCR products were separated by electrophoresis through 1% agarose, stained with ethidium bromide, visualized under UV illumination and digital images captured (Versa-Doc, Bio-Rad). PCRs were carried out using Master Mix (Promega, Madison, WI, USA) reagents, as well as 35 thermal cycles of 94°C for 1 min, 52°C for 1 min, and 72°C for 2 min. PCR products were Southern blotted onto Zeta-Probe (Bio-Rad) nylon membranes. A ∼4.5 kb, KpnI fragment of pLNHX-SmSL-Luc (24) was labeled with 32P-dCTP by random oligomer priming (RadPrime DNA Labeling System, Invitrogen); this probe includes transgenes encoding neomycin phosphotransferase and luciferase. Southern blots were hybridized to the labeled probe for 18 h and washed at high stringency (26), and hybridization signals were detected by autoradiography and X-ray film (Biomax; Kodak, Rochester, NY, USA).
In addition, schistosome gDNAs and plasmid pLNHX-SmACT-Luc were cleaved with NcoI and separated by electrophoresis through 1% agarose, and the fragments were transferred by capillary action to Zeta-probe nylon membranes (Bio-Rad). A gene probe was prepared by isolation of a ∼5.3 kb KpnI fragment pLNHX-SmACT-Luc, which includes the luciferase coding sequence. The ∼5.3 kb KpnI fragment was isolated from the digestion products by agarose gel electrophoresis, eluted from the gel, and labeled with 32P-dCTP. Southern blots were hybridized to this probe for 18 h and washed at high stringency at 65°C, and hybridization signals were detected as above.
Reverse transcription PCR
Pellets of schistosomula were homogenized using a motorized pestle, after which total RNA was isolated from the homogenates using the Versagene RNA Purification kit (Gentra, Minneapolis, MN, USA). The RNA preparations were incubated with RNase-free DNase I (Gentra) to remove contaminating gDNA. cDNAs were synthesized from the DNase-treated RNA using iSCRIPT reverse transcriptase (Bio-Rad). Endpoint PCR was performed on dilutions of cDNA and control DNase I-treated RNA using the Promega Mastermix system, and 35 thermal cycles of 94°C, 1 min, 52°C, 1 min, and 72°C, 2 min. The control S. mansoni housekeeping gene cox I (GenBank AF101196), neomycin phosphotransferase II gene (neo), and firefly luciferase gene were amplified using the primers described above. Negative controls included total (virus exposed schistosome) RNA that had not been reverse transcribed as a template, and reactions in which water replaced the template nucleic acids. Amplification products were sized by electrophoresis through 1% agarose, visualized after staining with ethidium bromide and UV illumination, and the images were recorded (Versa-Doc, Bio-Rad). PCR products were transferred to nylon membranes by Southern blot analysis and probed with the 32P-labeled pLNHX-SmSL-Luc/KpnI probe (above).
Retrotransposon-anchored PCR (RAP)
We developed an anchored PCR-based approach, RAP, to investigate retrovirus integrations into the schistosome genome. In brief, RAP employs a primer specific for the luciferase (luc) transgene from the donor pLNHX construct in tandem with second primer specific for endogenous retrotransposons present at high copy number and interspersed throughout the genome of natural populations of S. mansoni (27). Specifically, the primers included sequences specific for the retrotransposons SR1 and fugitive (28,29,30,31,32,33); SR1 reverse 5′-CGTCGTGGTACCAACCTTTGCACTCATC, fugitive reverse 5′-CAACTGCGTTACTGTCTCCTCGAAACTG, and luciferase left 5′-CTGCGAAATGCCCATACTGTTGAGC. Primers targeting other endogenous retrotransposons were also tested, as described previously (11). The RAP primers were employed with ∼100 ng template gDNA from populations of MLV-transduced schistosomula and Platinum Taq DNA Polymerase High Fidelity (Invitrogen). The RAP cycling conditions were 94°C for 2 min followed by 38 cycles of 94°C for 30 s, 57.5°C for 30 s and 68°C for 10 min, with a final extension at 68°C for 10 min. RAP products were analyzed by ethidium-stained agarose gel (1%) electrophoresis, after which the products were transferred to nylon (Zeta-probe) and hybridized under stringent conditions to a luciferase gene probe. This probe was amplified from pGL3 Basic (Promega) with primers Luc-F 5′-ATGGAAGACGCCAAAAACAT and Luc-R 5′-TACACGGCGATCTTTCCGCC, and cycling conditions of 1 min at 94°C followed by 38 cycles of 30 s at 94°C, 30 s at 50°C, 2 min at 68°C, and a final extension at 72°C for 7 min. The ∼1.6 kb product was isolated by agarose gel electrophoresis, eluted from the gel and labeled with 32P-dCTP using the random oligomer priming method. From the RAP products that appeared to be positive in the Southern hybridizations, fragments ranging from ∼8 to 2 kb in size were isolated from a crystal violet gel and ligated into plasmid pCR-XL TOPO (Invitrogen) to establish libraries of integration junction fragments. About 1000 randomly selected colonies were screened by colony hybridization. Nylon membranes were probed with the labeled luciferase gene fragment; from the ∼1000 colonies, ∼10% were positive for luciferase-encoding sequences. Minipreps of plasmids from the ∼100 positive colonies were isolated (GenElute Plasmid Miniprep Kit, Sigma-Aldrich, St. Louis, MO, USA), digested with EcoR I to release the inserts, and sized by agarose gel electrophoresis; the fragments were then transferred to nylon, and the membranes were probed with the labeled luciferase probe, as above. Nucleotide sequences of the positive clones were determined using vector-specific and insert-specific primers.
Luciferase activity assay
Schistosomula and adult schistosomes were transduced in vitro with retrovirus by exposure to MLV-VSVG virions. One day later, media were replaced, and the worms were cultured for 2 to 7 days. At this point, transduced schistosomes were removed from culture, washed 3×, and stored at −80°C until needed. To prepare soluble lysates for investigation of reporter firefly luciferase activity, pellets of schistosomula were subjected to sonication (5×5 s bursts, output cycle 4, Heat Systems–Ultrasonics, Plainview, NY, USA) in 250 μl CCLR lysis buffer (Promega). Protein concentration of soluble sonicates were determined using the bicinchoninic acid assay (Pierce, Rockford, IL, USA). Aliquots of 100 μl of sonicate were injected into 100 μl luciferin substrate (Promega) at 23°C and mixed; the relative light units (RLUs) were determined in the luminometer 10 s later. Duplicate samples were measured; results are presented as the average of the duplicate readings per microgram of soluble protein. Recombinant luciferase (Promega) was included as a positive control (11).
Immunolocalization of luciferase
Schistosomula were harvested from tissue culture into 1.5 ml microcentrifuge tubes by centrifugation for 2 min at 10,000 g, washed in PBS (3× each, 1–2 min) by centrifugation to remove culture media, and fixed in 10% formalin in PBS for 20 min at 23°C or overnight at 4°C. Schistosomulum tissues were permeabilized by incubation in 0.2% Triton X-100 in PBS for 30 min, washed 2× in PBS to remove the detergent, and incubated in 1% nonfat milk in PBS for 30 min at 37°C to block unbound antibody sites. After two further PBS washes, schistosomula were incubated in primary antibody (goat anti-luciferase, diluted 1:200; Promega) for 18 h at 4°C or for 45 min at 37°C. Following removal of the primary antibody (PBS, 3× washes), schistosomula were incubated in secondary antibody [Alexa Fluor 594 donkey anti-goat (red), Molecular Probes, Eugene, OR, USA; 1:250] for 90 min at 37°C and washed 3× in PBS. Samples were mounted in 50% glycerol/PBS and viewed with an inverted microscope (Eclipse TS100; Nikon, Tokyo, Japan) fitted with a red filter (Texas Red) at 540–580 nm excitation, 595 nm emission. Images were captured with a CoolPix 5700 camera (Nikon). In addition, to determine the percentage of the populations of schistosomula positive for luciferase, 120 to 160 individual schistosomula were examined for fluorescence at each time point.
Bioinformatics
Sequences obtained from RAP clones were analyzed with assistance of the Accelrys Gene version 2.0 software (Accelrys, San Diego, CA, USA) and/or or other online bioinformatics tools, as appropriate. Searches of putative integration junction sequences were performed using BLAST search algorithms at the NCBI (http://www.ncbi.nlm.nih.gov/BLAST/) and Sanger Schistosoma mansoni Genome Project (http://www.sanger.ac.uk/Projects/S_mansoni/) databases.
RESULTS
Provirus retroviral transgenes detected in the transduced schistosomes
Direct PCR analysis of genomic DNA (gDNA) from transduced schistosomula indicated the presence of proviral retrovirus in the schistosome chromosomes. In particular, the reporter transgenes encoding luciferase and neomycin phosphotransferase II (schematic of predicted transgene provirus in Fig. 1A) were amplified from the genome of schistosomula transduced by pLNHX-SmSL-Luc virions (Fig. 1B). Schistosomula were infected by soaking in 1 × 105 to 1 × 106 CFU/ml pLNHX-SmSL-Luc virions and were harvested 5 days postinfection. In addition to the detection of ethidium-stained products of the expected size, the identity of the PCR products was confirmed by Southern hybridization analysis to a labeled pLNHX-SmSL-Luc/KpnI gene probe (Fig. 1B, lanes 1 and 2). The final wash before harvest was investigated by PCR for the presence of contaminating pLNHX-SmSL-Luc plasmids; neither neo- nor luc-specific targets were amplified, indicating absence of residual plasmid carried over in the virion inoculum (Fig. 1B, lanes 4 and 5). Cox I-specific products were amplified (Fig. 1B, lane 3), verifying the integrity of the gDNAs. A direct PCR approach had been employed to detect MLV integrations into chromosomes of Anopheles gambiae cell line MOS-55 exposed, as here, to VSVG pseudotyped MLV virions (34).
Figure 1.
Integration of retroviral provirus into the S. mansoni genome indicated by direct PCR and Southern hybridization analyses of genomic DNA from retrovirus-transduced schistosomes. A) Schematic representation of retroviral construct pLNHX-SmACT-Luc, showing the position of NcoI cleavage sites and also the location of the KpnI fragment employed as the hybridization probe. The retrovirus cassette included the firefly luciferase reporter gene (yellow) driven by the S. mansoni actin 1.1 gene promoter (blue), and flanked by the 5′ and 3′ long terminal inverted repeats of the murine leukemia virus (green). The cassette also included the gene endowing neomycin resistance (red) and the psi motif (gray; involved in packaging the viral DNA). B) Identification of retroviral transgenes in the genome of schistosomes transduced with pseudotyped murine leukemia virus virions, by direct PCR. Top panel: ethidium-stained PCR products resolved in agarose gel. gDNA from schistosomula transduced with pLNHX-SmSL-Luc virions was employed as template for PCR using primers specific for luc (lane 1), neo (lane 2), and cox I, a positive control endogenous schistosome gene (lane 3). Culture medium used as a final wash before harvesting the schistosomula was employed as PCR template with primers specific for luc (lane 4) and neo (lane 5); these served as negative controls to confirm the absence of contaminating pLNHX-SmSL-Luc plasmid potentially present in the virion inoculum. No template gDNA but three primer pairs specific for the luc, neo, and cox I genes were used as negative control (lane 6). Plasmid pLNHX-SmSL-Luc as template and primers specific for luc-positive control (lane 7) or neo (lane 8); nontransduced, control schistosome gDNA and primers specific for cox I were used as positive control (lane 9). Molecular size standards (bp) are shown at the left of the panel; sizes of positive signals for luc (553 bp), neo (383 bp), and cox I (294 bp) are indicated at the right. Bottom panel: autoradiograph of Southern hybridization signals from the PCR products (visualized in left panel) to a radiolabeled transgene probe, a 4.5-kb KpnI fragment of pLNHX-SmSL-Luc spanning the genes encoding neomycin resistance (neo) and firefly luciferase (luc). C) Southern hybridization analysis of genomic DNA from schistosomula transduced by VSVG-pseudotyped retrovirus. Left panel: ethidium-stained gel of genomic DNAs of S. mansoni. Lane 1, NcoI-digested gDNA (6 μg) from control, nonvirus-transduced cercariae; lane 2, NcoI-digested gDNA (6 μg) from schistosomula exposed to VSVG pseudotyped pLNHX-SmACT-Luc virions; lane 3, NcoI-digested plasmid pLNHX-SmACT-Luc (1 μg of plasmid DNA). Values at left are molecular size standards (kb). Right panel: autoradiograph of Southern hybridization signals from the NcoI-digested gDNAs and plasmid DNA from left panel to the radiolabeled transgene probe (5.3-kb KpnI fragment of pLNHX-SmACT-Luc, A).
Southern blot analysis indicated integration of MLV transgenes in the schistosome genome
Because direct PCR analysis of the transduced schistosomes suggested that mobilization of VSVG-MLV was feasible in schistosome tissues (Fig. 1B), a Southern hybridization experiment was undertaken to further investigate integration of MLV into schistosome chromosomes. gDNA isolated from ∼20,000 schistosomula, transduced with pLNHX-SmACT-Luc virions, was digested with NcoI, after which the fragments were resolved by electrophoresis, transferred to nylon, and hybridized to a labeled pLNHX-SmACT-Luc/KpnI gene probe. NcoI cleaves twice within pLNHX-SmACT-Luc, releasing a fragment of ∼1.9 kb that should hybridize to the probe. Hybridization to gDNA from transduced schistosomula was apparent; a robust signal was evident at 1.9 kb (Fig. 1C, lane 2), indicating the presence of numerous copies of the MLV provirus within chromosomes of the schistosome population. Two other strong bands of hybridization, at 1.7 and 2.5 kb were evident, along with minor bands of hybridization from ∼3 to >10 kb in size (Fig. 1C, lane 2). By contrast, no signal was seen in gDNA from untreated (nontransduced) schistosomes (Fig. 1C, lane 1). Signals of variable length larger than 1.9 kb likely represented NcoI fragments of proviral transgene and schistosome chromosomes adjacent to sites of MLV integration. The other two major bands, at ∼1.7 and ∼2.5 kb, indicated the presence of NcoI sites in the schistosome chromosomes near the 5′- or 3′-LTRs of integrated copies of MLV. The pLNHX-SmACT-Luc/KpnI probe extends beyond the 1.9 kb NcoI fragment of pLNHX-SmACT-Luc (Fig. 1A); it would hybridize to all three fragments of an integrated proviral form of the pLNHX-SmACT-Luc construct released by NcoI cleavage of schistosome gDNA: 1) ≥1.6 kb, 2) 1.9 kb, and 3) ≥2.4 kb. This is clearly evident as the three major bands in Fig. 1C, lane 2. Other minor bands of hybridization from 3 to >10 kb in size were apparent, indicating integrations of a number of other copies of the provirus transgene. By contrast, a band of hybridization of ∼6.5 kb, seen strongly in lane 3, was absent from the genomic DNA of the transduced schistosomula (lane 2), demonstrating that the signals apparent in lane 2 represented integration events and not spurious hybridization of any pLNHX-SmACT-Luc vector plasmid that may have contaminated the virion inoculum.
Retrotransposon-anchored PCR retrieved MLV transgene integrations
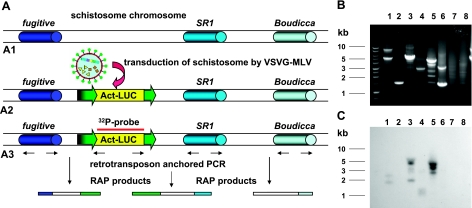
Similar preparations of gDNA isolated from schistosomula transduced with pLNHX-SmACT-Luc virions were employed as templates for an anchored PCR-based approach that we term RAP (11). In brief, RAP employs a primer specific for the luc transgene from the pLNHX-SmACT-Luc cassette in tandem with second primer specific for one of the endogenous retrotransposons known to be present at high copy number in the genome of natural populations of S. mansoni. Specifically, the retrotransposon-specific primers targeted the SR1, SR2, Boudicca, fugitive and SMα elements. Figure 2A provides a schematic representation of the RAP protocol. We analyzed the patterns of the resulting PCR products in ethidium-stained gels (representative example shown in Fig. 2B) and by Southern hybridization analysis of the RAP products to a 32P-labeled luciferase gene probe (Fig. 2C). The patterns of hybridization indicated the presence of amplicons representing integration events of MLV into S. mansoni chromosomes. Subsequently, we cloned a number of these Southern hybridization-positive PCR bands into pCR-XL-TOPO, including fragments amplified with retrotransposon SR1 paired with luciferase transgene-specific primers (lane 5, Fig. 2C) and fragments amplified with retrotransposon fugitive paired with luciferase transgene-specific primers (lane 3, Fig. 2C). Only one round of RAP was required to amplify apparent integration junctions.
Figure 2.
A) Schematic representation of the RAP technique, designed to recover integration junctions between integrated retroviral provirus and endogenous mobile genetic elements resident within the S. mansoni genome. A1) Schematic depiction of endogenous retrotransposons within the schistosome chromosomes; numerous copies of SR1, Boudicca, and the fugitive have been described interspersed throughout the S. mansoni genome (28, 30, 31). A2) Schematic representation of the integration of the MLV retrovirus into schistosome chromosomes after transduction of cultured schistosomes by VSVG-pseudotyped MLV virions. A3) Schematic depiction of the RAP technique used to investigate transgene integrations. The position of the probe used in Southern hybridizations is indicated. B) Ethidium-stained gels revealing RAP products amplified from gDNA extracted from schistosomula transduced with VSVG pseudotyped pLNHX-SmACT-Luc virions; the PCR products were amplified using primers specific for the endogenous schistosome retrotransposons and the luciferase transgene. C) Southern hybridization of labeled retroviral transgene gene probe to the RAP products shown in B. Lane 1, RAP products amplified with luciferase left- and Boudicca forward-directed primers; lane 2, RAP products from luciferase left- and fugitive forward-directed specific primers; lane 3, RAP products from luciferase left- and fugitive reverse-directed specific primers; lane 4, RAP products from luciferase left- and SR1 forward-specific primers; lane 5, RAP products from luciferase left- and SR1 reverse-specific primers; lane 6, RAP products from luciferase left- and SR2 forward-specific primers; lane 7, RAP products from luciferase left- and SR2 reverse-specific primers; lane 8, RAP products from luciferase left- and SMα forward-specific primers. Values at left are molecular size standards (kb).
Widespread retrovirus integrations into schistosome genome
We investigated ∼100 positive clones (from libraries of RAP products in pCR-XL-TOPO screened with a luciferase gene probe) by restriction digest pattern analysis. Approximately 30 distinct patterns were identified, and clones representative of each of the patterns were sequenced. From these sequences, we confirmed 16 discrete integrations of MLV into schistosome chromosomes (Table 1). The sequences of these transgene integration sites have been assigned the GenBank accession numbers ET202011–ET202026. The accession numbers include annotations of the genomic locations of the transgenes as determined by Blast analyses of the NCBI (www.ncbi.nlm.nih.gov/BLAST) and Sanger Institute (www.sanger.ac.uk/cgi-bin/blast/submitblast/s_mansoni/omni) databases. In brief, MLV proviral transgenes were found in the vicinity of endogenous schistosome retrotransposons SR2, fugitive, Perere-2, and Saci-4 (35) (e.g., ET202011, ET202012, ET202018), and also near other repetitive sequences, including the Sm7 satellite sequences and the Sh122 repetitive sequence (ET202021, ET202016). MLV provirus transgenes were also located near to or within the introns of protein-encoding genes, including those encoding immunophilin, zinc finger protein Sma-Zic, glutathione-S-transferase, cathepsin D, and hypoxanthine-guanine phosphoribosyl transferase (e.g., ET202013, ET202024, ET202015) (Table 1). The transgene integration junction sequences definitively documented somatic transgenesis of the S. mansoni genome by VSVG-pseudotyped MLV retrovirus, and indicated a widespread distribution of MLV provirus integrations throughout the schistosome genome.
TABLE 1.
Identification of insertion site loci of MLV transgene integrations into schistosome chromosomes, including length of sequenced RAP product, GenBank accession numbers of the insertion junctions, and accession numbers of target S. mansoni genes at the insertion sites
| Clone
|
Accession number
|
RAP primer
|
Insert size (bp)
|
Target schistosome gene at provirus integration site
|
|
|---|---|---|---|---|---|
| Identity | Accession | ||||
| P2–40
|
ET202011 | SR1 R/LUC left | 4118 | Perere-2 and SR1non-LTR retrotransposons | BN000793.1; U66337.1 |
| P4–36 | ET202014 | SR1 R/LUC left | 4415 | Perere-2; zinc finger protein Sma-Zic; intron of GST gene | BN000793.1; AB231864.1; D87493.1 |
| P5–13 | ET202015 | SR1 R/LUC left | 5392 | Perere-2 retrotransposon; HGPRT gene, exon 1–5; immunophilin gene | BN000793.1; X13531.1; 42969.1 |
| P3–30 | ET202012 | SR1 R/LUC left | 5158 | Perere-2 non-LTR retrotransposon | BN000793.1 |
| P7–48 | ET202017 | SR1 R/LUC left | 3147 | Perere retrotransposon; S. japonicum SJCHGC06882 protein mRNA; Boudiccaretrotransposon mRNA | BN000793.1; AY810086.1; BK004066.1 |
| P6–47 | ET202016 | SR1 R/LUC left | 4132 | Perere-2 non-LTR retrotransposon; S. haematobium Sh122 repetitive sequence | BN000793.1; DQ831682.1 |
| P10–6 | ET202018 | SR1 R/LUC left | 5229 | Perere-2 non-LTR retrotransposon; Saci-4 retrotransposon | BN000793.1; BN000802.1 |
| P4–1 | ET202013 | SR1 R/LUC left | 6291 | Perere-2retrotransposon; S. japonicum SJCHGC01035 protein mRNA; cathepsin D gene; SR2 retrotransposon; S. japonicumSJCHGC06937 protein mRNA | BN000793.1; AY815954.1; AY309267.1; AF025676.1; AY809979.1 |
| P11–7 | ET202019 | FUG R/LUC left | 2732 | Retrotransposon fugitive | BK005226.1 |
| P14–33 | ET202022 | FUG R/LUC left | 4108 | Retrotransposon fugitive | BK005226.1 |
| P16–49 | ET202024 | FUG R/LUC left | 5185 | Retrotransposon fugitive; match to Sanger schistosome contigs but matched sequence remains unidentified | BK005226.1; 10398d11.p1k; 2677c12.p1k |
| P20–12 | ET202025 | FUG R/LUC left | 4662 | Retrotransposon fugitive | BK005226.1 |
| P12–11 | ET202020 | FUG R/LUC left | 3843 | retrotransposons fugitive and Boudicca | BK005226.1; AY308024.1 |
| P14–7 | ET202021 | FUG R/LUC left | 4173 | Retrotransposons fugitiveand SR2; zinc finger protein Sma-Zic gene; Sm7 satellite | BK005226.1; AF025672.2; AB231864.1; AF036744.1 |
| P20–39 | ET202026 | FUG R/LUC left | 5442 | Retrotransposon fugitive | BK005226.1 |
| P14–41 | ET202023 | FUG R/LUC left | 5994 | Retrotransposon fugitive; S. japonicum retrotransposon SjR2; intron of GST gene; zinc finger protein Sma-Zic | BK005226.1; AF412214.1; D87492.1; AB231864.1 |
Intact and mutated provirus transgenes
As illustrated in Table 1, 16 integration junctions were recovered using the RAP method and sequenced. The RAP targeted endogenous retrotransposons and the luciferase transgene. Eight integration junctions were recovered using the SR1 retrotransposon anchor and 8 using the fugitive retrotransposon anchor, in both cases paired to a transgene-specific primer directed in the antisense direction on the luciferase gene. Of the 16 integration junctions—and although we have only recovered and sequenced the 5′ region (left hand side) of the provirus as a consequence of the RAP method—7 of the 16 included the complete (intact) MLV provirus. These included the intact 5′-LTR and downstream sequences (including the psi encapsidation signal, neomycin resistance gene, actin gene promoter and luciferase reporter gene). By contrast, 9 of the 16 (56%) were mutated MLV provirus forms, where the 5′-LTR and some adjacent downstream residues of the provirus had been deleted. In most of the mutated proviral transgenes, the entire 5′-LTR, the psi encapsidation signal and about one-third of the neo gene were deleted. The remainder of neo, the actin gene promoter, and luciferase reporter gene were integrated into the schistosome genome. More specifically, of the 8 integrations recovered with the SR1 anchor, 3 were intact, while 5 were mutated. For the 8 integrations recovered with the fugitive primer, 4 were intact and 4 were mutated. Schematic representations of these intact and mutated proviral transgenes are shown in Fig. 3.
Figure 3.
Identification of target site motif at sites of integration of MLV into schistosome chromosomes. For all 16 integration junctions covered by RAP, BLAST analysis identified the specific or approximate integration junction by determining schistosome genomic sequences on one side and MLV LTR and adjacent transgenes on the other. Twenty-five residues of the schistosome genome sequence and 25 residues of the MLV sequence are presented to the left and the right, respectively, of the red vertical line, which indicates the integration site. Schematic representations of the structure of specific integration events of the provirus are presented at right. In some, the complete 5′-LTR and adjacent residues were recovered; in others, deletion of the MLV LTR was evident. Red vertical lines indicate the integration junction of MLV into the schistosome chromosomes. Dotted lines depict regions where the provirus has been deleted. Because of the nature of the retrotransposon-anchored PCR used to locate the MLV integrations, only the 5′ region of the provirus was available for analysis; 3′ regions of the MLV transgene are depicted as gray boxes, although we have not actually cloned and sequenced these regions. The gGATcc-like motif identified at the integration site is highlighted by red, boldface font. Clone numbers are provided at the left.
Integration target motif, gGATcc
At each of the 16 integration junctions, the motif gGATcc, or a close variant thereof, occurred at the site of integration. As shown in Fig. 3, the gGATcc-like motif was located adjacent to the 5′ terminus of the MLV. In the 7 intact of 16 integrations, this motif was flanked by the terminal residues of the intact 5′-LTR of MLV, TTTGAAAGA … . Moreover, the gGATcc-like motif was present at the site of integration of the 9 of 16 insertions, which were 5′-LTR truncated. It appeared that gGATcc or perhaps the central trinucleotide core of this motif, GAT, or even the central dinucleotide, AT, was a preferred target for MLV integration. In addition, there was a T residue flanking the gGATcc motif in all of the 16 MLV integrations, i.e., T was the terminal 5′-residue of the intact or mutated MLV proviral transgene. Because we isolated only one side of the MLV provirus, we do not yet know whether target site duplications had occurred at both the 5′ and 3′ termini of the integration; target site duplications of 4–6 bp are characteristic of retrovirus integrations into mammalian chromosomes (36).
Transgenes transcriptionally active
We employed reverse transcription PCR to investigate transcription activity of the transgene reporter genes. We analyzed total RNA from groups of schistosomula exposed to 105 or 106 CFU/ml virions. More specifically, ∼1000 schistosomula were cultured with 20,000 or 200,000 pLNHX-SmSL-Luc virions, which correlated to 20 or 200 virions/schistosomulum.
Schistosomula were infected 1 or 6 days after cercarial transformation by soaking in pLNHX-SmSL-Luc virions, and harvested 2 to 5 days later. Transcripts encoding neo and luc were detected (Fig. 4; lanes 1 and 4, luc; lanes 2 and 5, neo) in both inoculum size treatment groups, although there was no obvious difference in intensity of the signals. Cox I was included as a positive control for the integrity of the RNA (Fig. 4, lanes 3 and 6). A negative control without cDNA gave no product (Fig. 4, lane 7). Additional control reactions were carried out employing RNA rather than cDNA as the template to ensure the absence of gDNAs; the results were negative, indicating the total RNA preparations were free of contaminating gDNA (not shown). Identities of the neo and luc products were confirmed by Southern hybridization to a labeled ∼4.5 kb KpnI fragment of pLNHX-SmSL-Luc. The presence of these transcripts indicated that the MLV 5′-LTR and the SmSL promoter were active in the transduced schistosomes since the 5′-LTR drives transcription of neo and SmSL was expected to drive transcription of luc (Fig. 4).
Figure 4.
RT-PCR demonstrates reporter gene expression from S. mansoni schistosomula transduced by VSVG pseudotyped MLV virions. A) Endpoint PCR using reverse-transcribed total RNA/cDNA. B) Southern hybridization of products from A to a radiolabeled 4.5-kb KpnI fragment of pLNHX-SmSL-Luc. Lane 1, RNA/cDNA and luciferase specific primers; lane 2, RNA/cDNA- and neo-specific primers; lane 3, RNA/cDNA- and cox I (positive control; endogenous schistosome gene) -specific primers; lane 4, RNA/cDNA and luciferase-specific primers; lane 5, RNA/cDNA and neo-specific primers; lane 6, RNA/cDNA and cox I (positive control) -specific primers; lane 7, negative control that included the three pairs of primers specific for luc, neo, and cox I but without template RNA/cDNA; lane 8, positive control using plasmid of pLNHX-SmSL-Luc as the template and two primer pairs specific for luc and neo; lane 9, positive control that included genomic DNA from S. mansoni and primers specific for cox I. The schistosomula analyzed in lanes 1, 2, and 3 had been transduced with 10× more virions than those in lanes 4, 5, and 6. Values at left are molecular size standards (bp); sizes of RT-PCR products are indicated.
Luciferase confirms productive transduction of schistosomes and developmental activity of actin gene promoter
In addition to transgene transcription, we investigated reporter luciferase enzyme activity in tissues of the transduced schistosomes. Initially, adult worms perfused from mice 2 to 3 wk previously were exposed to pLNHX-SmSL-Luc or pLNHX-SmACT-Luc virions (8×103 and 8×104 CFU/ml, respectively) for 1 day. Four to 6 days later, soluble extracts of transduced worms were examined for luciferase activity. Luciferase was detected in the adult worms transduced with pLNHX-SmACT-Luc virions, in worms that had been in culture either for 2 (Fig. 5A, lane 2; 6.0 RLU/μg) or 3 (Fig. 5A, lane 4; 8.8 RLU/μg) weeks after perfusion from mice. By contrast, far less luciferase was detected in the worms transduced with the pLNHX-SmSL-Luc virions (Fig. 5A, lanes 1 and 3, 0.6 and 0.6 RLU/μg). These findings indicated that the S. mansoni actin gene promoter was more active than the SmSL promoter in driving luciferase from proviral MLV transgenes (Fig. 5A).
Figure 5.
Reporter luciferase activity in cultured S. mansoni schistosomula and mixed-sex adult worms following transduction with VSVG-pseudotyped murine leukemia virus virions. A) Luciferase activity (RLU/μg soluble protein) in soluble extracts of mixed-sex adult schistosomes that were exposed to VSVG-pseudotyped pLNHX-SmACT-Luc or VSVG-pseudotyped pLNHX-SmSL-Luc virions for 24 h and harvested 4 to 6 days later. Columns: worms exposed to 1) pLNHX-SmSL-Luc virions after 15 days in culture; 2) pLNHX-SmACT-Luc virions after 15 days in culture; 3) pLNHX-SmSL-Luc virions after 21 days in culture; and 4) pLNHX-SmACT-Luc virions after 21 days in culture. B) Luciferase activity in soluble extracts of schistosomula cultured for increasing times after mechanical transformation of schistosomula from cercariae. Columns: 1) control schistosomula not exposed to virus; 2–6) schistosomula at 1, 5, 10, 15, and 20 days after transformation from cercariae, respectively, exposed to VSVG-pseudotyped pLNHX-SmACT-Luc virions for 24 h and harvested 3 days later. C) Anti-luciferase antibody staining of schistosomula of increasing age of culture in vitro after mechanical transformation from cercariae. Somula transformed 0, 1, 5, 10, 15, and 20 days previously were exposed to VSVG-pseudotyped pLNHX-SmACT-Luc virions. At 3 days after transduction with retrovirus virions, the worms were fixed with formalin, permeabilized with detergent, probed with goat anti-luciferase antiserum, and visualized with donkey anti-goat conjugated to Alexa Fluor 594. Top panels: epifluorescence images; bottom panels: bright field images. Control schistosomula were cultured for 20 days but not exposed to retrovirus (extreme right panels). Scale bar = 100 μm.
Given the superiority of the actin promoter in driving luciferase activity in the adult worms, we investigated transduction of blood-stage schistosomula with pLNHX-SmACT-Luc virions. Furthermore, because Pearce and colleagues (25) had demonstrated developmental regulation of the actin gene promoter, we investigated the influence of the age of the developing schistosomulum on transgene activity. Schistosomula that had been maintained in culture for 1, 5, 10, 15, and 20 days after mechanical transformation from cercariae were exposed to ∼40 pLNHX-SmACT-Luc virions per schistosomulum, after which transgene reporter activity was investigated using two procedures. First, 3 days after virus transduction, soluble extracts of schistosomulum tissue were prepared and analyzed for luciferase activity. Elevated activity was evident in the 10- and 15-day-old schistosomula, 64.9 and 50.9 RLU/μg, respectively (Fig. 5B, lanes 4 and 5). By contrast, only minimal luciferase activity was recorded from the 1-, 5- and 20-day-old schistosomula, 1.7, 1.6, and 1.5 RLU/μg, respectively (Fig. 5B, lanes 2, 3, and 6). Second, an immunostaining technique was deployed using an antiluciferase specific antibody and an Alexa Fluor 594 labeled secondary antibody to monitor transgene expression in similarly aged groups of schistosomula. We visualized the presence of luciferase in each age group of developing schistosomes, i.e., in 1-, 5-, 10-, 15- and 20-day-old schistosomes (Fig. 5C); the percentages of the populations positive for luciferase were 8.7% (day 1), 18.3% (day 5), 23.3% (day 10), 20.7% (day 15), and 11% (day 20). Further, on days 10 and 15, schistosomula were generally brighter. Control 20-day-old schistosomula, which were not exposed to virions but were processed through the same immunostaining process, showed no or minimal (11.3%) Alexa Fluor 594 fluorescence (Fig. 5C). Together, the markedly elevated luciferase enzyme activities, the stronger immunofluorescence, and the larger proportion of the population immunostaining for luciferase in 10- and 15-day-old schistosomula suggested that there was an authentic influence of schistosomulum developmental age on the activity of the S. mansoni actin gene promoter.
DISCUSSION
MLV is a simple C-type gammaretrovirus pathogen of mice (37), which has been developed over the past two decades as a tool for forward genetics studies of model organisms, including the zebrafish (2, 38), and as a vector for human gene therapy (39, 40). In addition, earlier studies have indicated that MLV pseudotyped with the glycoprotein of VSV could facilitate transgenesis of a diverse array of other model and pathogenic eukaryotic species, including surf clams, oysters, mosquitoes, and amoebae (34, 41,42,43). In a preliminary report, we suggested that VSVG-pseudotyped MLV could infect developmental stages of the human blood fluke S. mansoni (24). Here, we have now definitely confirmed that VSVG-MLV can transduce blood-stage forms of S. mansoni, that integration of the provirus efficiently occurs soon after exposure of the worms to even small numbers of virions, that integration may exhibit an unusual genome target site preference, and that reporter provirus transgene activities can be driven in schistosome cells by the retrovirus LTR or endogenous schistosome gene promoters.
Direct PCR analysis and Southern hybridization indicated that MLV proviral transgenes had integrated into the schistosome chromosomes. Subsequently, we employed an anchored PCR protocol to search for MLV provirus integrations, a technique termed RAP, which uses direct PCR targeting nondigested template DNA (11). RAP is similar to the Alu-PCR procedure that has been employed to locate adenovirus within human chromosomes (44). Luciferase gene probe hybridization to RAP products indicated the presence of MLV transgenes in the vicinity of endogenous schistosome retrotransposons. Nucleotide sequence analysis of integration junctions verified insertion of MLV provirus at disparate sites in schistosome chromosomes. At a more global level, this outcome confirmed the utility of VSVG-MLV for transgenesis of schistosomes. Characterization of MLV proviral integrations into schistosome chromosomes also indicates there is no evolutionary block to at least some of the molecular steps of the MLV life cycle in organisms as distant as flatworms and mammals. It has been suggested that blocks to key steps in the developmental cycle likely would limit the utility of MLV in lower vertebrates and invertebrates (45).
We isolated 16 discrete integration events. Because RAP was used to recover these integrations, all of the events characterized here were localized to the vicinity of the endogenous retrotransposons SR1 and fugitive employed as RAP anchors. Both of these retrotransposons occur at high copy number in the schistosome genome and are presumed to be interspersed (28, 31). Nonetheless, we were able to employ the schistosome genomic sequence flanking the integration sites to attempt to identify the target sites of these integration events, using BLAST analysis of the draft S. mansoni genome. MLV proviral transgenes were found near endogenous schistosome retrotransposons and other repetitive sequences and nearby or within the introns of protein-encoding genes. In mammalian cells, MLV displays moderate target specificity, in particular for the transcription start sites of protein-encoding genes, CpG islands and the 5′ ends of genes, including the first intron. Other retroviruses exhibit other preferences; HIV-1 targets transcriptionally active regions of RNA polymerase II transcribed genes, ASLV weakly targets active genes and HTLV-1 does not specifically target transcription units and transcription start sites (2, 46,47,48).
About one-half of the MLV proviral transgenes recovered displayed an intact 5′-LTR, whereas others were mutated by deletions of the 5′-LTR and adjacent genes. Deletions of 5′-LTR regions of proviral retroviruses are known to occur, with the deletion generally occurring before integration of the provirus (36, 49). Nonetheless, all 16 of the proviral transgenes were likely to display transcriptionally active reporter genes because the actin gene promoter and firefly luciferase coding regions were intact in all of them. Hallmarks of integration into mammalian chromosomes include duplication of 4–6 bp of host chromosome flanking the 5′- and 3′-LTRs of the retrovirus. We have been unable to establish whether this duplication also occurs in schistosome chromosome integrations because we have so far only examined one side of the transgene. In addition, usually there is deletion of 2 bp from the terminus of the 5′- and 3′-LTRs at integration of MLV and other retroviruses into mammalian chromosomes (50). This deletion did not occur in schistosome cells since sequence analysis of the 7 intact proviral transgenes demonstrated the presence of the entire 5′-LTR. In this regard, integration of MLV into schistosome chromosomes diverged from the mammalian paradigm. There is minimal information available on the integration of MLV into chromosomes of other invertebrates. However, MLV integration into mosquito chromosomes resulted in the characteristic 2 bp deletion, and moreover, no target site specificity was apparent (34).
Whereas preferences are exhibited by different retroviral species in relation to target site selection, a target site primary sequence motif is unapparent at integration of MLV, HIV-1, and other retroviruses into mammalian chromosomes. Indeed, primary sequence does not determine target selection. By contrast, remarkably, all 16 of 16 MLV integrations into schistosome chromosomes were located at a gGATcc-like motif. The core trinucleotide GAT or dinucleotide AT may be a recognition site for integration, in similar fashion to recognition sites exhibited by transposons, e.g., TTAA by piggyBac, TA(T/A)TA by Tn7, and TA by mariner elements (51,52,53). In any event, the apparent target sequence specificity suggested that the MLV integrase may operate with schistosome cellular cofactors, and if so, the integrase-cofactor complex may endow target site specificity on MLV integration. It is also possible that the viral integrase is not functional in schistosome cells and that schistosome DNA repair or other enzymes, rather than viral integrase, insert the proviral transgene, endowing the target site specificity on MLV proviral integrations into schistosome chromosomes.
Reporter gene silencing of MLV provirus transgenes is known in some contexts (37, 54); however, the actin promoter drove reporter luciferase activity, and the 5′-LTR drove transcription of neo. These findings indicated, at least in somatic cells, that reporter gene silencing was not occurring and might not be an impediment to MLV-mediated functional genomics of schistosomes. Given that the virions employed here were replication incompetent, we anticipate that only those schistosome cells that were transduced with MLV virions would have expressed the proviral transgenes. The tissue- and organ-specific localization of these cells is not yet known, as we have yet to undertake detailed immunolocalization studies of transduced schistosomes. However, it is likely that surface- and/or gut-located cells were transduced. Exogenous double-stranded RNA is taken in through the gut of blood stage schistosomes, rather than traversing the surface (10). The schistosome tegument (surface) is covered by a unique lipid bilayer (55). Whether pseudotyped virions can cross this barrier awaits elucidation. Nonetheless, VSVG pseudotyped MLV virions clearly can efficiently transduce schistosomes and so the resolution of the transduction pathway, across the tegument or through the gut, will likely also provide insights into the nature of the schistosome surface and the barrier presented by the enigmatic surface double lipid bilayer (21).
In overview, the present findings demonstrated VSVG-pseudotyped MLV mediated transduction of blood stages of S. mansoni, chromosomal integration of retroviral transgenes, transgene activity, and somatic transgenesis. It has been suggested that evolutionary blocks would constrain the utility of MLV in nonmammalian taxa (45). By contrast, we conclude now that essential post-transduction steps of the MLV retrovirus developmental cycle that were postulated to be disabled or absent in nonmammalian species are clearly functional in schistosome cells, including presumably the activity of retroviral reverse transcriptase and integrase, and the assembly of the preintegration complex. Indeed, we consider that comparison of host cell accessory factors for retroviral activity in cells as phylogenetically distant as mammals and schistosomes could inform our understanding of the molecular pathogenesis of retroviral infection of human and other vertebrate cells. The atypical target site preference displayed by MLV in schistosome chromosomes provides a phenomenon where comparisons would likely be informative. Finally, given that there is an intact RNAi pathway in schistosomes (10) and that RNAi induced visible and lethal phenotypes can be achieved (56, 57), it may be feasible to establish lines of transgenic schistosomes carrying hairpin gene constructs in order to advance functional genomics approaches for these parasites. Also, given that MLV integrated nearby or within protein-encoding genes, insertional mutagenesis screens and gene trapping are additional avenues that can be addressed in the future if heritable lines of transgenic schistosomes can be established.
Acknowledgments
Schistosome-infected snails and mice were supplied by Dr. Fred A. Lewis (Biomedical Research Institute, Rockville, MD, USA) through U.S. National Institutes of Health (NIH) contract NO155270. We gratefully acknowledge support from the NIH, award number RO1 AI072773. The research was also supported, in part by the Ellison Medical Foundation (ID-IA-0037–02). P.J.B. was a recipient of a Scholar Award in Molecular Parasitology from the Burroughs Wellcome Fund.
References
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Wang D, Jao L E, Zheng N, Dolan K, Ivey J, Zonies S, Wu X, Wu K, Yang H, Meng Q, Zhu Z, Zhang B, Lin S, Burgess S M. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc Natl Acad Sci U S A. 2007;104:12428–12433. doi: 10.1073/pnas.0705502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu B, Shoue D A, Fraser M J, Jr, Adams J H. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proc Natl Acad Sci U S A. 2005;102:16391–16396. doi: 10.1073/pnas.0504679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli M T, Li C, Rasgon J L, Jacobs-Lorena M. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci U S A. 2007;104:5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M H, Coates C J, George A L., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Grevelding C G. Transgenic flatworms. Maule A G, Marks N J, editors. Wallingford, UK: CABI; Parasitic Flatworms. Molecular Biology, Biochemistry, Immunology and Physiology. 2006:149–173. [Google Scholar]
- Li X, Massey H C, Jr, Nolan T J, Schad G A, Kraus K, Sundaram M, Lok J B. Successful transgenesis of the parasitic nematode Strongyloides stercoralis requires endogenous non-coding control elements. Int J Parasitol. 2006;36:671–679. doi: 10.1016/j.ijpara.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Grant W N, Skinner S J, Newton-Howes J, Grant K, Shuttleworth G, Heath D D, Shoemaker C B. Heritable transgenesis of Parastrongyloides trichosuri: a nematode parasite of mammals. Int J Parasitol. 2006;36:475–483. doi: 10.1016/j.ijpara.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kalinna B H, Brindley P J. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends Parasitol. 2007;23:197–204. doi: 10.1016/j.pt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Krautz-Peterson G, Radwanska M, Ndegwa D, Shoemaker C B, Skelly P J. Optimizing gene suppression in schistosomes using RNA interference. Mol Biochem Parasitol. 2007;153:194–202. doi: 10.1016/j.molbiopara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Morales M E, Mann V H, Kines K J, Gobert G N, Fraser M J, Jr, Kalinna B H, Correnti J M, Pearce E J, Brindley P J. piggyBac transposon mediated transgenesis of the human blood fluke, Schistosoma mansoni. FASEB J. 2007;21:3479–3489. doi: 10.1096/fj.07-8726com. [DOI] [PubMed] [Google Scholar]
- 12.Spiliotis, M., Lechner, S., Tappe, D., Scheller, C., Krohne, G., and Brehm, K. (2008) Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int. J. Parasitol. doi:10.1016/j.ijpara.2007.4.002 [DOI] [PubMed] [Google Scholar]
- Brindley P J, Pearce E J. Genetic manipulation of schistosomes. Int J Parasitol. 2007;37:465–473. doi: 10.1016/j.ijpara.2006.12.012. [DOI] [PubMed] [Google Scholar]
- King C H, Dickman K, Tisch D J. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Hotez P J, Molyneux D H, Stillwaggon E, Bentwich Z, Kumaresan J. Neglected tropical diseases and HIV/AIDS. Lancet. 2006;368:1865–1866. doi: 10.1016/S0140-6736(06)69765-1. [DOI] [PubMed] [Google Scholar]
- Cioli D, Botros S S, Wheatcroft-Francklow K, Mbaye A, Southgate V, Tchuente L A, Pica-Mattoccia L, Troiani A R, El-Din S H, Sabra A N, Albin J, Engels D, Doenhoff M J. Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int J Parasitol. 2004;34:979–987. doi: 10.1016/j.ijpara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tran M H, Pearson M S, Bethony J M, Smyth D J, Jones M K, Duke M, Don T A, McManus D P, Correa-Oliveira R, Loukas A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- Hu W, Brindley P J, McManus D P, Feng Z, Han Z G. Schistosome transcriptomes: new insights into the parasite and schistosomiasis. Trends Mol Med. 2004;10:217–225. doi: 10.1016/j.molmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Haas B J, Berriman M, Hirai H, Cerqueira G G, Loverde P T, El-Sayed N M. Schistosoma mansoni genome: closing in on a final gene set. Exp Parasitol. 2007;117:225–228. doi: 10.1016/j.exppara.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Liu F, Lu J, Hu W, Wang S Y, Cui S J, Chi M, Yan Q, Wang X R, Song H D, Xu X N, Wang J J, Zhang X L, Zhang X, Wang Z Q, Xue C L, Brindley P J, McManus D P, Yang P Y, Feng Z, Chen Z, Han Z G. New perspectives on host-parasite interplay by comparative transcriptomic and proteomic analyses of Schistosoma japonicum [Online] PLoS Pathog. 2006;2:e29. doi: 10.1371/journal.ppat.0020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi S, Wilson R A. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- Lazdins J K, Stein M J, David J R, Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982;53:39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Basch P F. Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J Parasitol. 1981;67:179–185. [PubMed] [Google Scholar]
- Kines K J, Mann V H, Morales M E, Shelby B D, Kalinna B H, Gobert G N, Chirgwin S R, Brindley P J. Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus. Exp Parasitol. 2006;112:209–220. doi: 10.1016/j.exppara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Correnti J M, Jung E, Freitas T C, Pearce E J. Transfection of Schistosoma mansoni by electroporation and the description of a new promoter sequence for transgene expression. Int J Parasitol. 2007;37:1107–1115. doi: 10.1016/j.ijpara.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley P J, Laha T, McManus D P, Loukas A. Mobile genetic elements colonizing the genomes of metazoan parasites. Trends Parasitol. 2003;19:79–87. doi: 10.1016/s1471-4922(02)00061-2. [DOI] [PubMed] [Google Scholar]
- Drew A C, Brindley P J. A retrotransposon of the non-long terminal repeat class from the human blood fluke Schistosoma mansoni. Similarities to the chicken-repeat-1-like elements of vertebrates. Mol Biol Evol. 1997;14:602–610. doi: 10.1093/oxfordjournals.molbev.a025799. [DOI] [PubMed] [Google Scholar]
- Drew A C, Minchella D J, King L T, Rollinson D, Brindley P J. SR2 elements, non-long terminal repeat retrotransposons of the RTE-1 lineage from the human blood fluke Schistosoma mansoni. Mol Biol Evol. 1999;16:1256–1269. doi: 10.1093/oxfordjournals.molbev.a026216. [DOI] [PubMed] [Google Scholar]
- Copeland C S, Brindley P J, Heyers O, Michael S F, Johnston D A, Williams D L, Ivens A C, Kalinna B H. Boudicca, a retrovirus-like long terminal repeat retrotransposon from the genome of the human blood fluke Schistosoma mansoni. J Virol. 2003;77:6153–6166. doi: 10.1128/JVI.77.11.6153-6166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha T, Loukas A, Smyth D J, Copeland C S, Brindley P J. The fugitive LTR retrotransposon from the genome of the human blood fluke, Schistosoma mansoni. Int J Parasitol. 2004;34:1365–1375. doi: 10.1016/j.ijpara.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Laha T, Kewgrai N, Loukas A, Brindley P J. Characterization of SR3 reveals abundance of non-LTR retrotransposons of the RTE clade in the genome of the human blood fluke, Schistosoma mansoni. BMC Genomics. 2005;6:154. doi: 10.1186/1471-2164-6-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotila L D, Hirai H, Rekosh D M, Lo Verde P T. A retroposon-like short repetitive DNA element in the genome of the human blood fluke, Schistosoma mansoni. Chromosoma. 1989;97:421–428. doi: 10.1007/BF00295025. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Beeman R W, Shike H, Besansky N J, Mukabayire O, Higgs S, James A A, Burns J C. Pantropic retroviral vectors integrate and express in cells of the malaria mosquito, Anopheles gambiae. Proc Natl Acad Sci U S A. 1996;93:6181–6185. doi: 10.1073/pnas.93.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco R, Machado A A, Bisson-Filho A W, Verjovski-Almeida S. Identification of 18 new transcribed retrotransposons in Schistosoma mansoni. Biochem Biophys Res Commun. 2005;333:230–240. doi: 10.1016/j.bbrc.2005.05.080. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Yasunaga J, Taniguchi Y, Tamiya S, Nakahata T, Matsuoka M. Preferential selection of human T-cell leukemia virus type 1 provirus lacking the 5′ long terminal repeat during oncogenesis. J Virol. 2007;81:5714–5723. doi: 10.1128/JVI.02511-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksakova I A, Romanish M T, Gagnier L, Dunn C A, van de Lagemaat L N, Mager D L. Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line [Online] PLoS Genet. 2006;2:e2. doi: 10.1371/journal.pgen.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubbu S, Balciunas D, Amsterdam A, Ekker S C. Insertional mutagenesis strategies in zebrafish. Genome Biol. 2007;8:S9. doi: 10.1186/gb-2007-8-s1-s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa R W, Blesch A. Murine and HIV-based retroviral vectors for in vitro and in vivo gene transfer. Methods Mol Med. 2006;129:241–254. doi: 10.1385/1-59745-213-0:241. [DOI] [PubMed] [Google Scholar]
- Vu H N, Ramsey J D, Pack D W. Engineering of a stable retroviral gene delivery vector by directed evolution. Mol Ther. 2008;16:308–314. doi: 10.1038/sj.mt.6300350. [DOI] [PubMed] [Google Scholar]
- Lu J K, Chen T T, Allen S K, Matsubara T, Burns J C. Production of transgenic dwarf surfclams, Mulinia lateralis, with pantropic retroviral vectors. Proc Natl Acad Sci U S A. 1996;93:3482–3486. doi: 10.1073/pnas.93.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulo V, Cadoret J P, Shike H, Shimizu C, Miyanohara A, Burns J C. Infection of cultured embryo cells of the pacific oyster, Crassostrea gigas, by pantropic retroviral vectors. In Vitro Cell Dev Biol Anim. 2000;36:395–399. doi: 10.1290/1071-2690(2000)036<0395:IOCECO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Que X, Kim D, Alagon A, Hirata K, Shike H, Shimizu C, Gonzalez A, Burns J C, Reed S L. Pantropic retroviral vectors mediate gene transfer and expression in Entamoeba histolytica. Mol Biochem Parasitol. 1999;99:237–245. doi: 10.1016/s0166-6851(99)00021-3. [DOI] [PubMed] [Google Scholar]
- Wu P, Phillips M I, Bui J, Terwilliger E F. Adeno-associated virus vector-mediated transgene integration into neurons and other nondividing cell targets. J Virol. 1998;72:5919–5926. doi: 10.1128/jvi.72.7.5919-5926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks C, Miller A D. Many nonmammalian cells exhibit postentry blocks to transduction by gammaretroviruses pseudotyped with various viral envelopes, including vesicular stomatitis virus G glycoprotein. J Virol. 2001;75:6375–6383. doi: 10.1128/JVI.75.14.6375-6383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Lobelle-Rich P A, Puetter A, Levy L S. Substitution of feline leukemia virus long terminal repeat sequences into murine leukemia virus alters the pattern of insertional activation and identifies new common insertion sites. J Virol. 2005;79:57–66. doi: 10.1128/JVI.79.1.57-66.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R S, Beitzel B F, Schroder A R, Shinn P, Chen H, Berry C C, Ecker J R, Bushman F D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences [Online] PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D, Crise B, Li Y, Princler G, Lum N, Stewart C, McGrath C F, Hughes S H, Munroe D J, Wu X. Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses. J Virol. 2007;81:6731–6741. doi: 10.1128/JVI.02752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya S, Matsuoka M, Etoh K, Watanabe T, Kamihira S, Yamaguchi K, Takatsuki K. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood. 1996;88:3065–3073. [PubMed] [Google Scholar]
- Shoemaker C, Hoffman J, Goff S P, Baltimore D. Intramolecular integration within Moloney murine leukemia virus DNA. J Virol. 1981;40:164–172. doi: 10.1128/jvi.40.1.164-172.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler A M. Use of the piggyBac transposon for germ-line transformation of insects. Insect Biochem Mol Biol. 2002;32:1211–1220. doi: 10.1016/s0965-1748(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Seringhaus M, Biery M C, Sarnovsky R J, Umansky L, Piccirillo S, Heidtman M, Cheung K H, Dobry C J, Gerstein M B, Craig N L, Snyder M. Large-scale mutagenesis of the yeast genome using a Tn7-derived multipurpose transposon. Genome Res. 2004;14:1975–1986. doi: 10.1101/gr.2875304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe D J, Grant T E, Robertson H M. Factors affecting transposition of the Himar1 mariner transposon in vitro. Genetics. 1998;149:179–187. doi: 10.1093/genetics/149.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Otsu M, Nagasawa T, Nakauchi H, Onodera M. Potent vaccine therapy with dendritic cells genetically modified by the gene-silencing-resistant retroviral vector GCDNsap. Mol Ther. 2006;13:301–309. doi: 10.1016/j.ymthe.2005.09.021. [DOI] [PubMed] [Google Scholar]
- McLaren D J, Hockley D J. Blood flukes have a double outer membrane. Nature. 1977;269:147–149. doi: 10.1038/269147a0. [DOI] [PubMed] [Google Scholar]
- Correnti J M, Brindley P J, Pearce E J. Long-term suppression of cathepsin B levels by RNA interference retards schistosome growth. Mol Biochem Parasitol. 2005;143:209–215. doi: 10.1016/j.molbiopara.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Morales M E, Rinaldi G, Gobert G N, Kines K J, Tort J F, Brindley P J. RNA interference of Schistosoma mansoni cathepsin D, the apical enzyme of the hemoglobin proteolysis cascade. Mol Biochem Parasitol. 2008;157:160–168. doi: 10.1016/j.molbiopara.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]