Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lipid that regulates myriad important cellular processes, including growth, survival, cytoskeleton rearrangements, motility, and immunity. Here we report that treatment of Jurkat and U937 leukemia cells with the pan-sphingosine kinase (SphK) inhibitor N,N-dimethylsphingosine to block S1P formation surprisingly caused a large increase in expression of SphK1 concomitant with induction of apoptosis. Another SphK inhibitor, d,l-threo-dihydrosphingosine, also induced apoptosis and produced dramatic increases in SphK1 expression. However, up-regulation of SphK1 was not a specific effect of its inhibition but rather was a consequence of apoptotic stress. The chemotherapeutic drug doxorubicin, a potent inducer of apoptosis in these cells, also stimulated SphK1 expression and activity and promoted S1P secretion. The caspase inhibitor ZVAD reduced not only doxorubicin-induced lethality but also the increased expression of SphK1 and secretion of S1P. Apoptotic cells secrete chemotactic factors to attract phagocytic cells, and we found that S1P potently stimulated chemotaxis of monocytic THP-1 and U937 cells and primary monocytes and macrophages. Collectively, our data suggest that apoptotic cells may up-regulate SphK1 to produce and secrete S1P that serves as a “come-and-get-me” signal for scavenger cells to engulf them in order to prevent necrosis.—Gude, D. R., Alvarez, S. E., Paugh, S. W., Mitra, P., Yu, J., Griffiths, R., Barbour, S. E., Milstien, S., Spiegel, S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal.
Keywords: chemotaxis, leukemia cells, monocytes, secretion
The sphingolipid metabolites ceramide, sphingosine, and sphingosine-1-phosphate (S1P) are now emerging as critical regulators of cell fate (1, 2). Whereas S1P stimulates growth and survival, its precursors, ceramide and sphingosine, are usually proapoptotic, and thus the dynamic balance between these metabolites has been referred to as a “sphingolipid rheostat” that controls cell fate (3). The enzyme that converts sphingosine to S1P, sphingosine kinase (SphK), is a critical regulator of the set point of this rheostat. There are two mammalian SphK isoenzymes, SphK1 and SphK2, which are ubiquitously expressed. Much evidence has linked SphK1 to growth and survival (reviewed in refs. 4,5,6). SphK1 is upregulated in many solid tumors (7), and the common genetic aberration bcr/abl in leukemia has been shown to increase SphK1 expression (8). Moreover, overexpression of SphK1 correlates with chemoresistance and radioresistance of leukemia cells, whereas its down-regulation or inhibition enhances drug-induced lethality (9, 10). Much less is known of the functions of SphK2, although this isoenzyme phosphorylates the prodrug FTY720 (11,12,13), producing an agonist of S1P receptors on lymphocytes that reduces their egress from lymphoid tissues resulting in lymphopenia (14). Moreover, FTY720 produces long-term protection in lymphoproliferative and autoimmune disease models, which also requires its phosphorylation by SphK2, presumably by inducing apoptosis in subsets of cells essential for pathogenesis (15, 16).
N,N-Dimethylsphingosine (DMS), an inhibitor of both SphK1 and SphK2 (17), has been shown to block SphK activity in numerous cell types and induce apoptosis (5). Here we demonstrate that treatment of leukemia cells with DMS not only induces apoptosis but, surprisingly, rather than simply inhibiting SphK1 and SphK2, dramatically up-regulates SphK1 protein expression. Moreover, another apoptotic stimulus, the anticancer drug doxorubicin, commonly used for treatment of leukemia, also markedly increased SphK1 protein expression and activity. This up-regulation resulted in increased secretion of S1P, which in turn is a potent chemoattractant of monocytes and macrophages.
Efficient elimination of cells undergoing apoptosis is crucial for normal tissue homeostasis and for prevention of immune responses. Although much progress has been made in identifying signals generated by apoptotic cells and the mechanisms by which phagocytes recognize and respond to these signals to orchestrate the selective and rapid removal of apoptotic cells (18,19,20,21), little is known of the long-range signals that recruit these cells to the neighborhood of the dying cells. The data presented here suggest that S1P is one of the soluble “come-and-get-me” signals secreted by apoptotic cells to attract phagocytes to come and eat them.
MATERIALS AND METHODS
Reagents
S1P, DMS, d,l-threo-dihydro-sphingosine (DHS), and anti-poly ADP-ribose polymerase (PARP) antibody were from Biomol International (Plymouth Meeting, PA, USA). NuPAGE Novex 10% Bis-Tris gels were from Invitrogen (Carlsbad, CA, USA). [γ-32P]ATP (3000 Ci/mmol) was purchased from Perkin Elmer (Waltham, MA, USA). Z-VAD-FMK (ZVAD) was from EMD Biosciences (San Diego, CA, USA). Fluoroscein isothiocyanate (FITC) -labeled annexin V/propidium iodide (PI) staining kit for apoptosis was from BD Biosciences (San Jose, CA, USA). Rabbit polyclonal antibodies raised against a unique SphK1 peptide sequence (EPPPSWKPQQMPPPEEPL) were described previously (22). Antiserum was purified on a protein A column followed by affinity purification, and its specificity was demonstrated by absorption with the immunogenic peptide, by overexpression, by small interfering RNA (siRNA) down-regulation, and by immunprecipitation (22). The following antibodies were used for immunoblotting: phospho-p44/42 mitogen activated protein (MAP) kinase (Thr202/Tyr204), phospho-p38 MAP kinase (Thr180/Tyr182), and phospho-cJun N-terminal kinase (JNK) (Thr183/Tyr185) (Cell Signaling, Danvers, MA, USA).
Cell culture
Jurkat and U937 human leukemia cells were cultured and maintained in logarithmic growth phase in RPMI 1640 medium, supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, CellGro, Herndon, VA, USA), 200 U/ml penicillin, 200 μg/ml streptomycin, sodium pyruvate, and l-glutamine (Invitrogen). Acute monocytic leukemia THP-1 cells (TIB-20; American Type Culture Collection, Herndon, VA, USA) were grown in RPMI containing 10% FBS.
Buffy coat preparations were obtained from Virginia Blood Services (Richmond, VA, USA). Thirty milliliters of heparinized human peripheral blood was added to 20 ml of RPMI. Twenty-five milliliters ml of the suspension was layered over 20 ml of lymphocyte separation media (ICN Pharmaceuticals, Costa Mesa, CA, USA) and centrifuged at 400 g for 30 min. Peripheral blood leukocytes (PBLs) were then collected from the interface and washed extensively with RPMI. To obtain adherent monocytes, PBLs (107/well) were cultured on plastic 6-well plates for 1 h in RPMI containing 10% fetal calf serum (FCS) and then washed extensively to remove nonadherent cells. Adherent monocytes were cultured for 7 days in RPMI containing 10% human AB serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and 1000 U/ml of recombinant human macrophage colony stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA) to induce differentiation to macrophages.
Western blotting
Cell suspensions were treated as indicated in figure legends and then centrifuged at 400 g for 8 min. Supernatants were discarded, and cell pellets were washed in PBS and centrifuged again. The resulting cell pellets were lysed in 20 mM Tris-HCl pH 7.4 buffer containing 1 mM EDTA, 0.5 mM 4-deoxypyridoxine, 15 mM NaF, 1 mM 2-mercaptoethanol, 1 mM Na3VO4, 40 mM β-glycerophosphate, 10% glycerol, 1% Triton X-100, and protease inhibitors. Following incubation on ice for 60 min, cell lysates were probe sonicated. Protein concentrations were determined by Coomassie dye binding (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins were separated by 10% SDS-PAGE and then transferred to nitrocellulose. Blots were incubated with primary antibodies (1:1000) overnight in Tris-buffered saline containing 5% nonfat dry milk and 0.1% Tween 20 followed by anti-rabbit horseradish peroxidase-conjugated immunoglobulin G (IgG; 1:10,000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Immunocomplexes were visualized by enhanced chemiluminescence (Pierce Chemical Co., Rockford, IL, USA).
SphK assay
SphK activity was measured in cell lysates in the presence of 5 μM sphingosine essentially as described (23).
S1P secretion
S1P secretion was measured as described previously (24). Briefly, cells were incubated with [3H]sphingosine (1.5 μM, 0.45 μCi) for 10 min. The medium was then removed, cells were washed with cold PBS, and fresh medium was added for the indicated time periods. Alkaline chloroform-methanol extraction was used to extract S1P from the medium and cells (24). In this differential extraction procedure, S1P partitions into the aqueous phase at alkaline pH with high recovery, while sphingosine, as well as ceramide and other sphingolipids, remain in the organic phase. Thin-layer chromatographic analysis of the lipids extracted from the media confirmed that the aqueous phase contained a single major radiolabeled product, [3H]S1P (25). Data are expressed as picomoles S1P released per million cells.
Cell migration
THP-1 cell migration was measured in transwell assays in 24-well plates with polycarbonate filters (8 μm pores; Corning, Lowell, MA, USA). Briefly, cells (106 cells/ml) were added to the upper chamber of each well and incubated at 37°C for 30 min. Chemoattractants were added to the bottom well, and the cells were allowed to migrate for 16 h. The numbers of cells migrating to the bottom well were determined with a Coulter counter model Z1 (Beckman Coulter, Fullerton, CA, USA).
Migration of primary monocytes and macrophages was determined in a modified Boyden chamber using polycarbonate filters (5 μm pores; Poretics, Livermore, CA, USA). Briefly, cells (5×104 cells/well) were added to the upper compartment, while the lower chamber contained either serum-free medium or chemoattractants as indicated. Cells were permitted to migrate for 3 h, after which the nonmigrating cells on the top of the filter were removed mechanically. The migrated cells on the bottom of the filter were then fixed, stained with Diff-Quick, and counted using an ×10 objective.
Annexin V/PI assays for apoptosis
Cells were stained with annexin V-FITC and PI and then evaluated for apoptosis by flow cytometry according to the manufacturer’s protocol (BD Biosciences). Briefly, 106 cells were washed twice with PBS and stained with 5 μl of annexin V and 5 μl of PI (50 μg/ml) in buffer containing 10 mM HEPES (pH 7.4), 140 mM NaOH, and 2.5 mM CaCl2 for 15 min at room temperature in the dark. Apoptotic cells were identified using a Coulter Epics-XL-MCL cytofluorometer with the EXPO32 Flow Cytometry analytic program (Beckman Coulter). Cells in the lower right quadrant correspond to early apoptotic cells (annexin V-positive), whereas those in the upper right quadrant correspond to late apoptotic cells (annexin V- and PI-positive).
RESULTS
SphK inhibitors induce apoptosis and up-regulate expression of SphK1 in Jurkat T lymphocytes and monocyte-like U937 cells
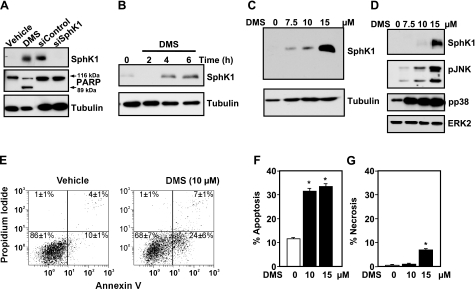
It was previously shown that apoptotic stimuli such as the DNA damaging agent actinomycin D and tumor necrosis factor alpha (TNF-α) induce proteolysis of SphK1 in MOLT-4 leukemia cells and MCF-7 breast cancer cells, respectively (26, 27). Surprisingly, treatment of Jurkat cells for 6 h with the potent SphK inhibitor DMS, which has previously been shown to induce activation of executioner caspase-3 in these cells as well as cleavage of PARP, hallmarks of apoptosis (28, 29), dramatically increased rather than decreased levels of a protein with the same apparent MW as SphK1 that was detected by immunoblotting with anti-SphK1 antibody (Fig. 1A). Because endogenous levels of SphK1 are very low and barely detectable in Jurkat cells, lysates from M2 melanoma cells, which express high levels of SphK1, transfected with control siRNA or siRNA targeting SphK1, which reduced SphK1 protein by more than 80%, were included to demonstrate the specificity of the anti-SphK1 antibody (Fig. 1A). SphK1 expression was clearly evident as early as 4 h after treatment (Fig. 1B); it was detected with a concentration of DMS as low as 7.5 μM and increased dramatically with higher concentrations (Fig. 1C). In contrast, no increase in expression of SphK2 was detected even after treatment with 15 μM DMS. In agreement with previous studies (28, 29), concentrations of DMS that greatly enhanced expression of SphK1 also induced apoptosis, as determined by PARP cleavage (Fig. 1A); increased externalization of phosphatidylserine, as determined by annexin V staining (Fig. 1E–G); and stimulated the stress-activated protein kinases JNK (p46 and p54) and p38 (Fig. 1D).
Figure 1.
SphK inhibitor DMS induces SphK1 expression. A–D) Jurkat T cells were treated without or with 15 μM DMS for 6 h (A) or the indicated time (B) or with the indicated concentration of DMS for 8 h (C) or 4 h (D). Equal amounts of cell lysates (20 μg) were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. Blots were stripped and reprobed with anti-tubulin antibody (A–C) or with anti-extracellular regulated kinase (ERK) 2 (D) as loading controls. Blots were probed with anti-PARP antibody as a marker of apoptosis (A); arrows indicate p116 full-length PARP and its p89 fragment; lysates (20 μg) from M2 melanoma cells transfected with scrambled siRNA or siRNA targeted to SphK1 were included as controls to indicate the molecular weight of SphK1. Cell lysates were also immunoblotted with pJNK and pp38 antibodies (D). E–G) Jurkat T cells were treated with vehicle or the indicated concentrations of DMS for 6 h, then stained with annexin V/PI, and apoptosis was determined by flow cytometry, as described in Materials and Methods. The percentages in the bottom right quadrants (E) correspond to early apoptotic cells (annexin V-positive), whereas percentages in the top right quadrants correspond to late apoptotic cells (annexin V- and PI-positive). Apoptotic cells are annexin V-positive (F), whereas necrotic cells are PI-positive only (G). Similar results were obtained in four independent experiments. *P < 0.01.
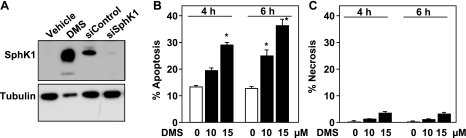
It was important to examine whether DMS also increased SphK1 levels in other types of cells. To this end, U937 monocytic cells, which are sensitive to apoptosis induced by DMS (29, 30), were utilized. As with Jurkat T cells, DMS also increased SphK1 levels in U937 cells (Fig. 2A) and, in agreement with previous studies (29, 30), induced apoptosis (Fig. 2B). It should be noted that the extensive apoptosis induced by DMS also was accompanied by a reduction in levels of tubulin, a known caspase substrate whose cleavage has previously been implicated in apoptosis-associated events in mammalian and worm systems (31, 32).
Figure 2.
DMS increases expression of SphK1 in U937 cells. U937 cells were treated with vehicle or with 15 μM DMS for 24 h (A) or with the indicated concentrations of DMS for 4 or 6 h (B, C). A) Cells were lysed and equal amounts of protein (60 μg) were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. Blots were stripped and reprobed with anti-tubulin antibody as loading controls. Lysates from M2 melanoma cells transfected with scrambled siRNA or siRNA targeted to SphK1 were included as controls to indicate the molecular weight of SphK1. B, C) Cells were stained with annexin V/PI. Apoptotic cells are annexin V-positive (B), whereas necrotic cells are PI-positive only (C). Similar results were obtained in four independent experiments. *P < 0.05.
We next examined whether increased expression of SphK1 was also induced by another SphK inhibitor. DHS, a commonly used SphK inhibitor (3, 33), also markedly increased levels of SphK1 (Fig. 3A). To further confirm that the observed band was indeed SphK1, lysates from cells treated with DHS were immunoprecipitated with antibody directed against SphK1. Western blotting revealed a single band with the predicted molecular weight of SphK1 (Fig. 3B). In contrast, SphK1 was absent when the same cell lysate was immunoprecipitated with nonspecific IgG. DHS also induced more than 80% apoptosis without significantly increasing necrotic cell death (Fig. 3C, D). Annexin V/PI analysis can distinguish between early (annexin V-positive) and late (both annexin V- and PI-positive) apoptotic cells. As shown in Fig. 3E, F, after treatment with DHS, the majority of the cells were early apoptotic, and a very small percentage were necrotic (PI-positive only).
Figure 3.
Another SphK inhibitor, DHS, increases expression of SphK1 and induces apoptosis in Jurkat cells. A) Jurkat cells were treated for 18 h without or with 10 or 20 μM DHS. Equal amounts of cell lysates were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. Blots were stripped and reprobed with anti-tubulin antibody as loading controls. B) Cell lysates (50 μg) from DHS-treated Jurkat cells were immunoprecipitated with either anti-SphK1 antibody or rabbit IgG. Equal amounts of each immunoprecipitate were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. C–F) Jurkat cells were treated for 6 h without or with the indicated concentrations of DHS, then stained with annexin V/PI. Apoptotic cells are annexin V-positive (C), whereas necrotic cells are PI-positive only (D). Representative results are shown in E and F. The percentages in the bottom right quadrants correspond to early apoptotic cells (annexin V-positive), whereas percentages in the top right quadrants correspond to late apoptotic cells (annexin V- and PI-positive). Similar results were obtained in four independent experiments. *P < 0.01.
The chemotherapeutic drug doxorubicin stimulates SphK1 expression and promotes S1P secretion
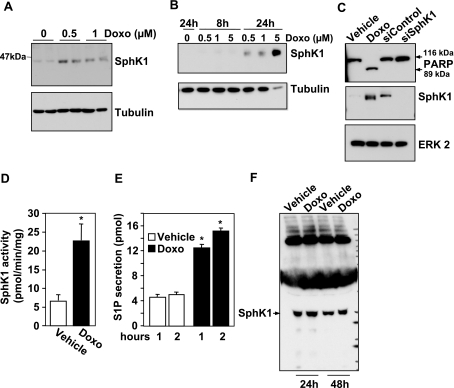
It was next important to determine whether enhanced expression of SphK1 was a specific response to SphK inhibitors or a consequence of apoptosis induced by these compounds. To this end, Jurkat T cells were treated with the chemotherapeutic drug doxorubicin, which is known to cause apoptosis of these cells (9, 34). Doxorubicin induced a dose-dependent increase in SphK1 expression (Fig. 4A). Similarly, SphK1 expression was increased in U937 cells after 24 h of exposure to doxorubicin, accompanied by a reduction in tubulin levels (Fig. 4B). Moreover, in agreement with previous studies (9, 34), treatment with 0.5 μM doxorubicin for 24 h also induced significant cleavage of PARP (Fig. 4C), an indicator of caspase activation associated with apoptosis. Over this same treatment period, SphK1 activity in doxorubicin-treated cells increased more than 3-fold (Fig. 4D). Increased SphK1 activity was further supported by the observation that following 24 h doxorubicin treatment, secretion of S1P was increased more than 3-fold (Fig. 4E). Because it has been suggested that SphK1 can be secreted from some types of cells (35), including U937 cells (36), we examined the possibility that S1P might be produced extracellularly from SphK1 released from apoptotic or dead cells. However, although some SphK1 could be detected in the medium of Jurkat T cells, this level was not increased even after prolonged treatment with doxorubicin. Collectively, these data suggest that doxorubicin, which induces apoptosis of leukemia cells, enhances cellular levels of SphK1, which in turn increases production and secretion of S1P.
Figure 4.
Doxorubicin increases SphK1 expression, S1P secretion, and apoptosis. Jurkat cells were treated with the indicated concentrations of doxorubicin (A) or with 0.5 μM (C–F) for 24 h. U937 cells were treated with the indicated concentrations of doxorubicin for 8 or 24 h (B). A, B) Equal amounts of cell lysates were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. Blots were stripped and reprobed with anti-tubulin antibody as a loading control. C) Blots were also probed with anti-PARP antibody. Arrows indicate p116 full length PARP and its p89 fragment. Blots were stripped and reprobed with anti-ERK2 as a loading control. Lysates from M2 melanoma cells transfected with scrambled siRNA or siRNA targeted to SphK1 (20 μg) were included as controls to indicate the molecular weight of SphK1. D) SphK1 activity was determined in lysates from Jurkat cells treated with vehicle or doxorubicin (0.5 μM) for 24 h. E) Jurkat cells were treated with vehicle (open bars) or doxorubicin (0.5 μM, solid bars) for 24 h, labeled for 10 min with [3H]sphingosine, and washed extensively, and [3H]S1P secretion was determined after 1 or 2 h, as described in Materials and Methods. F) Jurkat cells were treated with vehicle or 0.5 μM doxorubicin (Doxo) for 24 or 48 h. The medium was collected and concentrated, and proteins were analyzed by immunoblotting with anti-SphK1 antibody. Similar results were obtained in two independent experiments. *P < 0.01.
Inhibition of apoptosis diminishes induction of SphK1 and S1P secretion
To determine whether the increase of SphK1 induced by doxorubicin is a result of apoptosis, we used the caspase inhibitor ZVAD. As expected, pretreatment of Jurkat cells with ZVAD completely attenuated PARP cleavage (Fig. 5A) induced by doxorubicin and markedly reduced apoptosis by almost 50%, as determined by annexin V/PI staining (Fig. 5B). Importantly, this caspase inhibitor also significantly diminished up-regulation of SphK1 expression in response to doxorubicin at both 24 and 48 h (Fig. 5A). Moreover, S1P secretion induced by doxorubicin was also prevented by pretreatment with ZVAD (Fig. 5D). Interestingly, induction of SphK1 or PARP cleavage is more sensitive to inhibition of caspases by ZVAD than apoptosis (compare Fig. 5A, B), suggesting that apoptosis induced by doxorubicin proceeds by both caspase-dependent and independent pathways. Taken together, these results indicate that apoptosis triggers the increase of SphK1 expression, which in turn is responsible for the generation of S1P and its secretion.
Figure 5.
Inhibition of apoptosis suppresses doxorubicin-induced SphK1 expression and S1P secretion. A) Jurkat cells were pretreated for 15 min with 40 μM ZVAD and then treated for the indicated time with 0.5 μM doxorubicin. Equal amounts of cell lysate proteins were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody or with anti-PARP antibody. Arrows indicate p116 full length PARP and its p89 fragment. Blots were stripped and reprobed with anti-tubulin as a loading control. Lysates from cells transfected with scrambled siRNA or siRNA targeted to SphK1 (20 μg) were included as controls to indicate the molecular weight of SphK1. B–D) Jurkat cells were pretreated for 15 min with 40 μM ZVAD and then treated with 0.5 μM doxorubicin for 24 h. Cells were stained with annexin V/PI, and apoptosis (B) or necrosis (C) was determined by flow cytometry, as described in Materials and Methods. Cells (106) were labeled for 10 min with [3H]sphingosine, washed extensively, and [3H]S1P secretion was determined at 1 and 2 h (D), as described in Materials and Methods. Similar results were obtained in two independent experiments. *P < 0.01 vs. vehicle; **P < 0.01 vs. samples without ZVAD treatment.
S1P released from apoptotic cells is a monocyte attraction factor
Efficient engulfment of apoptotic cells by monocytes and macrophages is crucial for normal tissue homeostasis and for regulation of immune responses. Long-range attraction signals from dying cells might be a prerequisite for their removal (20). Indeed, it has been demonstrated that apoptotic cells secrete chemotactic factors, in a caspase-dependent manner, to attract phagocytic cells (20, 37, 38). Because previous studies suggest that apoptotic cells induce migration of monocytic cells and primary macrophages by release of lipid attraction signals (37), we wondered whether S1P might be one of these signals. To examine this possibility, we evaluated the migratory responses of human monocytic cells to S1P. S1P was a very potent chemoattractant for U937 cells, a human lymphoma cell line displaying many monocytic characteristics (Fig. 6A). A concentration of S1P as low as 10 nM significantly increased chemotaxis of U937 cells (Fig. 6A) as well as THP-1 human monocytic cells (Fig. 6B). In agreement with other reports (22, 39,40,41), S1P-induced migratory responses were bell-shaped, and the cells were less responsive to higher concentrations of S1P. The optimal concentration of S1P stimulated migration of THP-1 cells to nearly the same extent as monocyte chemoattractant protein-1 (MCP-1/CCL2). Importantly, chemotaxis of primary human monocytes was also markedly enhanced by S1P (Fig. 6C). A concentration of S1P as low as 1 nM induced chemotaxis, and maximal chemotactic activity was observed at 10 nM. Moreover, chemotaxis of primary macrophages was also maximally stimulated by 1 nM S1P (Fig. 6D), which is within the same range as the association constants for binding to the S1P receptors (42). Collectively, these results suggest that S1P, which can be secreted from dying cells, is a potent chemoattractant for phagocytic cells.
Figure 6.
S1P is a potent chemoattractant for monocytic cell lines and primary monocytes. Vehicle or S1P was added at increasing concentrations (1, 10, 100, and 1000 nM; solid bars) directly to the lower chamber, and transmigration of U937 cells (A) and THP-1 monocytic cells (B) was determined as described in Materials and Methods. CCL2 (50 ng/ml; shaded bar) was used as a positive control. Primary monocytes (C) or macrophages (D) were allowed to migrate toward vehicle, increasing concentrations of S1P (1, 10, 100, and 1000 nM; solid bars), or 10% FCS (shaded bar) in modified Boyden chambers. Data are expressed as number of migrating cells per field and are means ± sd of triplicate determinations. Similar results were obtained in two independent experiments. *P < 0.001.
DISCUSSION
Ample evidence links expression of SphK1 and formation of S1P to cell growth and survival (4, 6, 43). Consistent with this evidence, a substantial decrease of SphK1 levels was detected during apoptosis of human T cell Molt-4 leukemia cells induced by the DNA-damaging agent actinomycin D (26). Moreover, prolonged treatment of MCF-7 human breast cancer cells with TNF-α also induced a decrease in SphK1 protein, which was dependent on the lysosomal pathway of apoptosis and specifically on cathepsin B (27). Unexpectedly, we observed that apoptotic stimuli, including the anticancer drug doxorubicin, markedly increased expression of SphK1, and formation of S1P and its secretion, in Jurkat T cells and U937 monocytic cells. In agreement, recent studies demonstrated that apoptotic Jurkat T cells or MCF-7 cells exposed to staurosporin released S1P, which was quantified by mass spectroscopy (44, 45). Consistent with these studies, we found that blockage of the apoptotic process by a general caspase inhibitor prevented S1P secretion and suppressed the up-regulation of SphK1 by doxorubicin in Jurkat cells.
An important question raised by our study is that of the function of up-regulation of SphK1 and secretion of S1P from apoptotic cells. Efficient clearance of apoptotic cells by phagocytes, mainly macrophages, is essential for development, homeostasis, and response to injury and is crucial to prevent secondary necrosis and inflammation due to leakage of cytotoxic or antigenic cell contents. Although much has been learned of the recognition of “eat me” signals of apoptotic cells by macrophages and of the engulfment process, much less is known of the long range “come and get me” signals that attract macrophages to the vicinity of dying cells. However, “eat me” signals alone, such as phosphatidylserine exposed on the surfaces of dying cells, may not be sufficient for efficient clearance of apoptotic cells, because macrophages are not normally in the neighborhood of apoptotic cells (18, 20, 21). Recently, it has been suggested that apoptotic cells secrete a soluble lipid factor in order to attract macrophages to begin the elimination process (37). That study suggested that the most likely mediator was lysophosphatidylcholine, produced in apoptotic cells by caspase 3-mediated cleavage and activation of calcium-independent phospholipase A2. More recently, it was suggested that this attraction was mediated by the phagocyte receptor G2A (46), whereas others have demonstrated that G2A is a proton-sensing GPCR (47). However, it should be pointed out that only high concentrations of lysophosphatidylcholine (ca. 20 μM) induced chemotaxis of THP-1 macrophages (37, 46), and there is no indication that it is indeed secreted by apoptotic cells. In contrast, it is now clear that apoptotic cells secrete S1P (the present study and refs. 44, 45), and, importantly, we have found that low-nanomolar concentrations of S1P are chemotactic for macrophage cell lines, including THP-1 cells, as well as primary human monocytes and macrophages. Moreover, S1P markedly induced macrophage cytoskeletal rearrangements, which are critical for forward cell movement (48). It is also possible that S1P could facilitate migration in conjunction with other molecules derived from the apoptotic cells (e.g., lysophosphatidylcholine, interleukin-8, or MCP-1/CCL2) (37, 49). Collectively, our study suggests that apoptotic Jurkat T and U937 cells increase SphK1 levels to produce and secrete S1P as a “come and get me” signal. In support of this notion, it has been demonstrated that S1P1 receptor activation is important for macrophage homing and their accumulation in lymph nodes (50). Of interest, genetic studies in Caenorhabditis elegans identified two evolutionarily conserved pathways that function upstream of the Rho family GTPase CED-10, a homologue of mammalian Rac1, a key regulator of the actin cytoskeleton during phagocytosis of apoptotic cells (reviewed in ref. 21). Thus, it is tempting to speculate that recruitment signal gradients sensed by G protein-coupled S1P1 receptors known to be linked to activation of Rac (1, 51) might regulate phagocyte migration toward apoptotic cells.
In addition to its potent effects on migration of monocytic cells, S1P released from apoptotic cells might also have other functions. Cells might increase SphK1 and S1P, prosurvival mediators, in a desperate final attempt to prevent cell death but be incapable of overcoming the much stronger push toward death. It is tempting to speculate that there is a point of no return, past which the cell cannot escape death by accumulating S1P and instead exports it. Here we suggest that the function of this S1P is to attract phagocytic cells (monocytes and macrophages) for cell debris clearance, but other roles are also possible. In this regard, S1P present in apoptotic cell-conditioned medium promoted macrophage survival, thus protecting these cells in their apoptotic neighborhood (44). This proposed function of S1P induction as a means of apoptosis escape is supported by recent findings that low doses of DMS in vivo actually increase SphK activity in mouse heart tissue, and in fact lead to overall cardioprotection (52). Of note, although DMS inhibits rat heart SphK1, it stimulates SphK2 (53). In this regard, SphK2 has been shown to be the isozyme form that is responsible for release of S1P from apoptotic Jurkat cells treated with staurosporin (44). Thus, it is possible that, depending on the apoptotic stimuli, different SphK isozymes contribute to secretion of S1P from apoptotic cells.
Recently, it was suggested that apoptotic tumor cell-derived S1P contributes to macrophage polarization toward a tumor-associated macrophage (TAM) M2 phenotype (45) that then contributes to the tumor cell’s ability to subvert antitumor immunity and inflammatory mechanisms (54). In contrast to M1-activated macrophages, TAMs have a reduced capacity to produce proinflammatory mediators such as TNF-α and instead release immunosuppressive molecules, including TGF-β and PGE2. Apoptotic cell-derived S1P also promotes cyclooxygenase-2 mRNA stabilization and protein expression in macrophages, a prerequisite for PGE2 formation (55). Many studies have led to the suggestion that TAMs, which are a major component of the leukocyte infiltrate of tumors, may play a role in promoting tumor progression by supporting tumor cell survival and growth (56). Although our results indicate that S1P release by apoptotic cells can provide a functional “come and get me” signal to circulating phagocytes, this S1P might also contribute to macrophage polarization and survival (44, 45). These actions of S1P might have implications for a variety of human diseases besides cancer, including autoimmunity and inflammation.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants R37 GM043880 and R01 CA61774 to S.S. S.M. was supported by the National Institute of Mental Health Intramural Research Program.
References
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun Y A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek P G, Coso O A, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Taha T A, Hannun Y A, Obeid L M. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J. Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Curr Pharm Des. 2006;12:4625–4635. doi: 10.2174/138161206779010422. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- French K J, Schrecengost R S, Lee B D, Zhuang Y, Smith S N, Eberly J L, Yun J K, Smith C D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- Li Q F, Huang W R, Duan H F, Wang H, Wu C T, Wang L S. Sphingosine kinase-1 mediates BCR/ABL-induced upregulation of Mcl-1 in chronic myeloid leukemia cells. Oncogene. 2007;26:7904–7908. doi: 10.1038/sj.onc.1210587. [DOI] [PubMed] [Google Scholar]
- Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, Cuvillier O. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20:95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- Baran Y, Salas A, Senkal C E, Gunduz U, Bielawski J, Obeid L M, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- Paugh S W, Payne S G, Barbour S E, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the imunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik J H, Wu M T, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits VEGF-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Don A S, Martinez-Lamenca C, Webb W R, Proia R L, Roberts E, Rosen H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogs. J Biol Chem. 2007;282:15833–15842. doi: 10.1074/jbc.M609124200. [DOI] [PubMed] [Google Scholar]
- Neviani P, Santhanam R, Oaks J J, Eiring A M, Notari M, Blaser B W, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker B J, Cortes J, Marcucci G, Chen C S, Verrills N M, Roy D C, Caligiuri M A, Bloomfield C D, Byrd J C, Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall L C, Van Brocklyn J R, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Grimsley C, Ravichandran K S. Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–656. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Ravichandran K S. “Recruitment signals” from apoptotic cells: invitation to a quiet meal. Cell. 2003;113:817–820. doi: 10.1016/s0092-8674(03)00471-9. [DOI] [PubMed] [Google Scholar]
- Kinchen J M, Ravichandran K S. Journey to the grave: signaling events regulating removal of apoptotic cells. J Cell Sci. 2007;120:2143–2149. doi: 10.1242/jcs.03463. [DOI] [PubMed] [Google Scholar]
- Hait N C, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration towards epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rosenfeldt H M, Bektas M, Wang F, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 1 Induces G12/13-mediated stress fiber formation yet promotes growth and survival independent of G protein coupled receptors. J Biol Chem. 2003;278:46452–46460. doi: 10.1074/jbc.M308749200. [DOI] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian C A, Payne S G, Beaven M A, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Payne S G, Milstien S, Spiegel S. A rapid and sensitive method to measure secretion of sphingosine-1-phosphate. Methods Enzymol. 2007;434:257–264. doi: 10.1016/S0076-6879(07)34014-7. [DOI] [PubMed] [Google Scholar]
- Taha T A, Osta W, Kozhaya L, Bielawski J, Johnson K R, Gillanders W E, Dbaibo G S, Hannun Y A, Obeid L M. Down-regulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53. J Biol Chem. 2004;279:20546–20554. doi: 10.1074/jbc.M401259200. [DOI] [PubMed] [Google Scholar]
- Taha T A, Kitatani K, Bielawski J, Cho W, Hannun Y A, Obeid L M. Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J Biol Chem. 2005;280:17196–17202. doi: 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent fas-induced apoptosis of type II jurkat T cells. J Biol Chem. 2000;275:15691–15700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- Sweeney E A, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, Igarashi Y. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer. 1996;66:358–366. doi: 10.1002/(SICI)1097-0215(19960503)66:3<358::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Adrain C, Duriez P J, Brumatti G, Delivani P, Martin S J. The cytotoxic lymphocyte protease, granzyme B, targets the cytoskeleton and perturbs microtubule polymerization dynamics. J Biol Chem. 2006;281:8118–8125. doi: 10.1074/jbc.M509361200. [DOI] [PubMed] [Google Scholar]
- Taylor R C, Brumatti G, Ito S, Hengartner M O, Derry W B, Martin S J. Establishing a blueprint for CED-3-dependent killing through identification of multiple substrates for this protease. J Biol Chem. 2007;282:15011–15021. doi: 10.1074/jbc.M611051200. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Wu C H, Gordon J, Rastegar M, Ogretmen B, Safa A R. Proteinase-3, a serine protease which mediates doxorubicin-induced apoptosis in the HL-60 leukemia cell line, is downregulated in its doxorubicin-resistant variant. Oncogene. 2002;21:5160–5174. doi: 10.1038/sj.onc.1205639. [DOI] [PubMed] [Google Scholar]
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae S S, Steffansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme: Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Hammad S M, Taha T A, Nareika A, Johnson K R, Lopes-Virella M F, Obeid L M. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins Other Lipid Mediat. 2006;79:126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober S M, Xiao Y J, Blumenthal S G, Lindemann R K, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth I B, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Truman L A, Ogden C A, Howie S E, Gregory C D. Macrophage chemotaxis to apoptotic Burkitt’s lymphoma cells in vitro: role of CD14 and CD36. Immunobiology. 2004;209:21–30. doi: 10.1016/j.imbio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Wang F, Van Brocklyn J R, Hobson J P, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G(12/13)-Rho and G(i) pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P S, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia R L, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Hait N C, Oskeritzian C A, Paugh S W, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Weigert A, Johann A M, von Knethen A, Schmidt H, Geisslinger G, Brune B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- Weigert A, Tzieply N, von Knethen A, Johann A M, Schmidt H, Geisslinger G, Brune B. Tumor cell apoptosis polarizes macrophages: role of sphingosine-1-phosphate. Mol Biol Cell. 2007;8:3810–3819. doi: 10.1091/mbc.E06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter C, Waibel M, Radu C G, Yang L V, Witte O N, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic ‘find-me’ signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- Murakami N, Yokomizo T, Okuno T, Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- Treede I, Braun A, Sparla R, Kuhnel M, Giese T, Turner J R, Anes E, Kulaksiz H, Fullekrug J, Stremmel W, Griffiths G, Ehehalt R. Anti-inflammatory effects of phosphatidylcholine. J Biol Chem. 2007;282:27155–27164. doi: 10.1074/jbc.M704408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub F J, Han D K, Liles W C, Adams L D, Coats S A, Ramachandran R K, Seifert R A, Schwartz S M, Bowen-Pope D F. Fas/FADD-mediated activation of a specific program of inflammatory gene expression in vascular smooth muscle cells. Nat Med. 2000;6:79079–79076. doi: 10.1038/77521. [DOI] [PubMed] [Google Scholar]
- Singer I I, Tian M, Wickham L A, Lin J, Matheravidathu S S, Forrest M J, Mandala S, Quackenbush E J. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- Hla T, Lee M J, Ancellin N, Paik J H, Kluk M J. Lysophospholipids-receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- Jin Z Q, Karliner J S. Low dose N,N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCepsilon dependent mechanism. Cardiovasc Res. 2006;71:725–734. doi: 10.1016/j.cardiores.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Vessey D A, Kelley M, Zhang J, Li L, Tao R, Karliner J S. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Johann A M, Weigert A, Eberhardt W, Kuhn A M, Barra V, von Knethen A, Pfeilschifter J M, Brune B. Apoptotic cell-derived sphingosine-1-phosphate promotes HuR-dependent cyclooxygenase-2 mRNA stabilization and protein expression. J Immunol. 2008;180:1239–1248. doi: 10.4049/jimmunol.180.2.1239. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard J W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]