Abstract
BACE1 is a promising therapeutic and preventive target for Alzheimer’s disease because it is essential for amyloid deposition. However, the recent demonstration of BACE1 in modulating developmental myelination in both peripheral and central nervous systems raises a concern of its effect on myelin maintenance or remyelination, and inhibition of these processes will potentially be detrimental to the BACE1 inhibitor users who are susceptible to myelination diseases such as adult peripheral nerve injury or multiple sclerosis. In this report, we investigated the role of BACE1 during peripheral nerve remyelination in wild-type (WT) and BACE1-null mice. We show here that genetic deletion of BACE1 affects sciatic nerve remyelination. The impaired remyelination appears to stem from the loss of neuregulin-1 cleavage by BACE1. To demonstrate a direct cleavage of neuregulin-1 by BACE1, we have identified a BACE1 cleavage site that turns out be highly conserved among neuregulin-1 paralogues. Moreover, we show that neuregulin-1 family member neuregulin-3 is also cleavable by BACE1. We hypothesize that the BACE1-cleaved extracellular domain of axonal neuregulin-1, perhaps neuregulin-3 as well, binds to Schwann cell ErbB receptors, which in turn regulate remyelination. Pharmacological inhibition of BACE1 should be carefully monitored to avoid alteration of signaling pathway that regulates remyelination.—Hu, X., He, W., Diaconu, C., Tang, X., Kidd, G. J., Macklin, W. B., Trapp, B. D., Yan, R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves.
Keywords: neuregulin, myelin, nerve crush, α-secretase, γ-secretase
BACE1 is a type I transmembrane aspartyl protease that cleaves the amyloid precursor protein (APP) at the β-secretase site (1,2,3,4). This cleavage precedes and is essential for the γ-secretase to process the membrane-bound β-stub and release β-amyloid peptide (Aβ), a major component of neuritic plaques. The aberrant accumulation of Aβ is widely regarded as a cause of cognitive dysfunction in Alzheimer’s disease (AD) (5); elevated BACE1 activity is indeed found in AD patients (6, 7).
Deletion of BACE1 in mice abolishes the production of Aβ (8,9,10), and partial reduction of BACE1 has dramatic effects on amyloid deposition (11). BACE1 activity inhibited by either specific peptidomimetic inhibitors or siRNA silencers significantly reduces Aβ production and amyloid deposition in animal models (12,13,14). These results and the minor phenotypic changes in BACE1-null mice indicate that BACE1 is a viable drug target for AD therapy. While BACE1-based drugs are under active exploration, it is important to fully understand the physiological roles of BACE1 in vivo. Recent studies have identified reduced myelination in the central and peripheral nervous systems of BACE1-null mice (15, 16). These studies raised the possibility that reduced neuregulin-1 (Nrg-1) cleavage by BACE1 might account for the central nervous system (CNS) and peripheral nervous system (PNS) hypomyelination phenotypes exhibited in BACE1-null mice (15, 16). In support of this hypothesis, AKT phosphorylation, a downstream signaling event in the neuregulin/erbB signaling pathway, is reduced in the brains of BACE1-null mice (16).
Myelination of axons is essential for saltatory conduction of neuronal action potentials. Myelinated axons can be demyelinated as a result of immune-mediated processes, as occurs in multiple sclerosis (MS) in the CNS and Guillain-Barre syndrome in the PNS. Trauma or nerve compression causes PNS demyelination, and the incidence of these injuries increases dramatically after the sixth decade in humans. Recovery from both injury- and immune-mediated demyelination occurs through remyelination, in which Schwann cells (in the PNS) or oligodendrocytes (in the CNS) recapitulate events of developmental myelination and produce new myelin internodes. The finding that BACE1 regulates myelination during development raises the possibility that BACE1 is required for remyelination. Impairing remyelination will have important therapeutic implications if BACE-1 inhibitors are widely used to prevent or delay progression of AD, as the elderly are a population prone to peripheral nerve injuries.
It is noted that genes that regulate myelination during early development are not always required for remyelination in adults. For example, the basic helix-loop-helix (bHLH) transcription factor Olig2 is an essential transcriptional regulator in oligodendrocyte development and specification of motor neurons during vertebrate embryogenesis (17,18,19). However, it is Olig1, the homologue of Olig2, that is required for the maturation of oligodendrocyte progenitors in the adult stage (20). In spatially Olig2-ablated cortices, myelination is disrupted at the progenitor stage (21). Olig1-null mice show subtle alteration in developmental myelination but fail to repair demyelinated lesion in the CNS (20). Therefore, we aimed to investigate whether BACE1 regulates remyelination and performed sciatic nerve-crush experiments that have been widely used to assess peripheral nerve remyelination (22,23,24,25). We found a clear delay in remyelination in BACE1-null mice compared to their WT littermates. Moreover, we found that ectodomain shedding of Nrg-1 is correspondingly decreased with the reduced myelination in BACE1 null mice. The identification of BACE-1 cleavage site in a highly conserved small region juxtamembrane domain of Nrg-1 confirmed that Nrg-1 is a BACE1 substrate. More interestingly, we found that this cleavage site is also conserved in Nrg-3, another member of neuregulin family. The full-length Nrg-3 was indeed elevated in BACE1 null mice, confirming that Nrg-3 is also a substrate of BACE1. Hence, our results suggest that BACE1-cleaved axonal Nrg-1, perhaps Nrg-3 as well, is required for optimal remyelination of peripheral nerve.
MATERIALS AND METHODS
Cell lines and animals
BACE1 stably expressing cell line (HM cell line) was previously generated by expressing HA-tagged BACE1 in HEK-293 cells (26). Human HEK-293 or HM cells were maintained at 37°C in a humidified, 5% CO2 controlled atmosphere in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS, 50 IU/ml penicillin, and 50 μg/ml streptomycin and glutamine. Genotyping and maintenance of BACE1-null mice was as described (16). All experimental protocols were approved by the Animal Care and Use Committee at the Lerner Research Institute in compliance with the guidelines established by the Public Health Service Guide for the Care and Use of Laboratory animals.
Sciatic nerve crush
According to established procedures (22), the left sciatic nerves of BACE1−/− and WT control mice were crushed at midthigh 3× (30 s each) with Dumont microforceps (No. 5), the tips of which were cooled in liquid nitrogen after animals were anesthetized with sodium pentobarbital. Then, wounds were carefully sutured shut and animals were allowed to recover. At 5 days, 3 wk and 6 wk after the injury, animals are perfused with glutaraldehyde fixative containing 4% paraformaldehyde and 2.5% glutaraldehyde. The crushed segment was collected (1 mm length from both sides of the crush site) as depicted in Fig. 1A. The distal segment included the region downstream from the crush site; the proximal segment included the region upstream from the crush site. Nerve segments were embedded in plastic resin and examined by electron microscopy. Electron microscopy experiments were performed as described (27). At least three pairs of animals were used at each indicated time point.
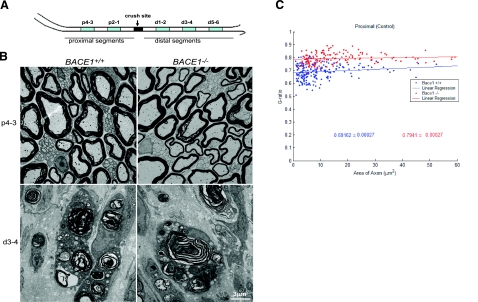
Figure 1.
Sciatic nerve crush causes Wallerian degeneration. A) Sciatic nerves of 2-month-old mice were crushed at the midthigh. Blue segments (1 mm in length) from the distal and proximal ends were examined by electron microscopy. Numbers indicate distance from the crush site. B) Ultrastructure of proximal segments p4–3 and distal segments d3–4 in WT and Bace1-null mouse nerves at 5 days after crush. As in uncrushed nerves, proximal nerve segments are hypomyelinated in BACE1-null mice. In the distal segments, axons have degenerated, and myelin debris is abundant in macrophages in both nerves. Scale bar = 3 μm. C) Scatter plot of g ratio of myelinated fibers in the proximal stump 5 days after crush. Each spot represents the g ratio of one myelinated fiber. The g ratio was calculated by dividing the inner circumference of the axon (without myelin) by the outer circumference of the total fiber (including myelin). Data are presented as mean ± se. Blue: WT mice; red: BACE1-null mice; n = 3; P < 0.001.
Quantification of g ratio
The g ratio of axons from the sciatic nerve of BACE1–null and WT mice was determined as in Michailov et al. (28), and Image J software (NIH Image; National Institutes of Health, Bethesda, MD, USA) was used for digital tracing. The myelinated axon area and circumference were measured by digitally tracing the inner and outer layers of the myelinated fiber. The g ratio was calculated by dividing the inner circumference of the axon (without myelin) by the outer circumference of the total fiber (including myelin). At least three pairs of BACE1–null mice and WT controls were processed for the quantification of g ratios. The g ratio data are displayed as a scatter plot against axon area.
Molecular cloning of Nrg-1
The cDNA encoding full-length human type I and type III Nrg-1 were PCR amplified and inserted into pCDNA3.1-Myc/His vector. Expression of the cDNA was verified by Western blot analysis of lysates from HEK-293 cells transfected with the above constructs. The expressed products were detected by antibodies recognizing either the myc-tag fused at the C terminus or Nrg-1 specific sequences.
BACE1 cleavage site mapping
Soluble recombinant Nrg-1 protein was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The recombinant protein contains the sequence from Met1 to Lys245 and shares the same sequence with our Type I and Type III Nrg-1 expression constructs. The protein (1 μg) was incubated with 1 μg purified BACE1 in a protease assay as described (29). The result suggested that the cleavage site was near the C terminus, and three peptides spanning 220–235, 228–240, and 230–247 were synthesized and HPLC-purified. These peptides were incubated with BACE1 for 24 h at room temperature, and then the reaction mixtures were separated either by HPLC or directly analyzed by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry.
Western blotting and antibodies
For untreated sciatic nerve samples, the entire sciatic nerve was used for protein extraction. For crushed nerves, either proximal or distal stumps were dissected out for protein extraction. The protein extraction buffer contained 20 mM HEPES, pH 7.9; 150 mM NaCl; 10 mM KCl; 0.1 mM sodium vanadate; 1 mM EDTA; 1 mM EGTA; 1.0% nonidet P-40; and 1% Triton X-100. Cell lysates were prepared using the same extraction buffer. Equal amounts of protein (40 μg) were resolved on a NuPAGE Bis–Tris Gel (Invitrogen, Carlsbad, CA, USA) and transferred onto nitrocellulose membranes (Invitrogen). Following protein transfer, the blot was incubated with the specified antibody: Akt and p–Akt (S473) (Cell Signaling Technology, Danvers, MA, USA); MBP (Sternberger Monoclonal Inc., Lutherville, MD, USA); actin (Sigma, St. Louis, MO, USA); myc (Sigma); Neuregulin-1 and neuregulin-3 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
Statistical analysis
Statistical analysis was performed using Microsoft Excel software (Microsoft Corp., Redmond, WA, USA). All data were analyzed for statistical significance using an F test for equal variance, followed by a 2-tailed Student’s t test. Differences were considered significant at values of P < 0.05. All data values are expressed as mean ± se.
RESULTS
To determine the role of BACE1 in remyelination, we compared PNS remyelination in BACE1-null mice with their WT littermates. Sciatic nerve crush causes rapid degeneration of axons and myelin sheaths distal to the crush site (30, 31). Normal myelinated axons are absent from the distal stump by 4 days postcrush, and axons from the proximal stump subsequently grow into the distal stump and become remyelinated. Following this established procedure (22,23,24,25), the left sciatic nerves of BACE1-null mice and their WT littermates were crushed at the midthigh and allowed to recover. We examined axonal regrowth and remyelination at defined distances from the crush site, as illustrated in Fig. 1A. At 5 days postcrush, intact myelinated axons were not detected in the distal segments from either WT or BACE1-null mice (Fig. 1B). In both types of mice, the distal stump contained abundant myelin debris in phagocytic macrophages, and Schwann cells were beginning to form bands of Bugner, which facilitate subsequent axonal regeneration (Fig. 1B). In proximal segments, myelinated axons had a normal appearance in the WT mouse while BACE1-null mice exhibited hypomyelination (Fig. 1B), consistent with previous description of uncrushed nerves in BACE1-null mice (15, 16). The g ratio, a parameter indicative of the thickness of myelin sheath relative to the axon diameter, was calculated for quantitative comparison. As expected, the g ratio for BACE-null mice was higher (Fig. 1C)
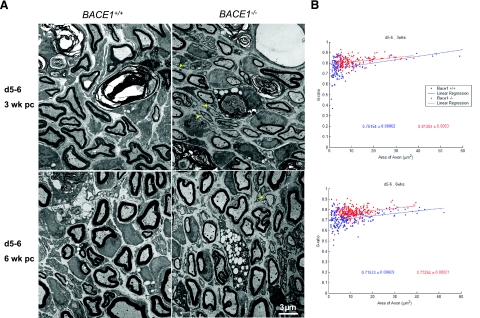
At 3 wk postcrush, occasional myelin debris still remained in the distal segments of both WT and BACE1-null distal nerves (Fig. 2A). In addition, axons had regenerated into both nerves, and many were remyelinated by Schwann cells (Fig. 2A, top panels). Myelin sheath thickness appeared thinner, and axonal diameters were smaller in nerves distal to the crush when compared to noncrushed or proximal sites of crushed nerves. The g ratio of remyelinated axons in the distal stump in both groups of mice (0.76154±0.00062 for WT and 0.81304±0.0003 for BACE1-null mice) was significantly larger than that of the axons in the proximal stump (0.69162±0.00027 for WT and 0.7941±0.00027 for BACE1-null mice); indicating thinner myelin sheath thickness in the distal than in the proximal segments for both sets of mice (Fig. 2A). It should also be noted that the g ratio for axons in the proximal stump was comparable with that seen in untreated normal sciatic nerve of both genotypes (16). The g ratio was larger in BACE1-null mice than in their WT littermates at 3 wk postcrush, indicating that remyelinated myelin sheaths were even thinner in BACE1-null mice (Fig. 2B; n=3, P<0.001).
Figure 2.
Delayed remyelination in BACE1-null peripheral nerve. A) Ultrastructural comparison of WT and BACE-null distal segments (d5–6) 3 and 6 wk after crush. At 3 wk, remyelination is apparent in both nerves. The BACE1-null myelinated fibers have smaller axonal diameters and thinner myelin sheaths. In addition, BACE1-null nerves have significantly more Schwann cells, which form a 1:1 relationship with axons (arrowheads), but have not begun to myelinate. Remyelination increases in both strains of mice between 3 and 6 wk postcrush. Axonal diameters of myelinated fibers appear similar in WT and BACE1-null nerves, but the BACE1-null myelin sheaths are thinner compared with WT fibers. Scale bar = 3 μm. B) Comparison of g ratios establishes that myelinated fibers in BACE1-null nerves are hypomyelinated at both 3 and 6 wk postcrush (n=3; P<0.001). Data are presented as mean ± se. Blue: WT mice; red: BACE1-null mice.
Recovery for 6 wk (Fig. 2A, bottom panels) significantly increased remyelination of regenerated axons, as the g ratio (0.71533±0.00029) in the distal segment of WT mice (Fig. 2B) approached that of myelinated axons in proximal segments (Fig. 1C). Noticeably, the g-ratio of remyelinated axons in the distal stumps of BACE1-null mice was increased relative to myelinated axons in proximal stumps (0.77254±0.00021 vs. 0.7941±0.00027, Fig. 1C), and the reduction in fiber size induced by the crush can account for this small increase in number.
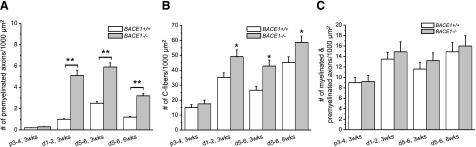
Noticeably, regenerating axons were not remyelinated at the same pace in BACE1 and WT nerves. After attaining a diameter of ∼1 μm, regenerated axons associate 1:1 with Schwann cells prior to remyelination. Quantification of regenerated premyelinated axons in 1:1 association with Schwann cells in electron micrographs detected increased numbers of premyelinated axon/Schwann cell pairs in BACE1-null distal stumps relative to WT distal stumps at 3 wk postcrush; sites more distal to the crush site (d1–2 vs. d5–6) contained more premyelinated axons (Fig. 3A; n=30 micrographs, P<0.01). The number of premyelinated axon/Schwann cell pairs in BACE1 d5–6 segments at 6 wk postcrush was reduced compared to the same segments from 3 wk postcrush (Fig. 3A), demonstrating that regenerated BACE1-null axons continued to remyelinate on prolonged recovery. However, hypomyelination was prominent in BACE1-null nerves at 6 wk postcrush, even though genetic deletion of BACE1 did not significantly affect axonal regeneration because the total number of remyelinated and premyelinated axons were comparable in BACE1-null mice compared to their WT littermates (Fig. 3C). Hence, genetic deletion of BACE1 caused delayed and reduced remyelination.
Figure 3.
Premyelinated axon/Schwann cell pairs and Remak C fibers are increased in BACE1-null mice. A, B) The numbers of premyelinated axons (A) and C fibers (B) are significantly increased in electron micrographs of BACE1-null distal stumps at 3 and 6 wk postcrush. C) The combined number of remyelinated and premyelinated axons, however, is similar in WT and BACE1-null mice. The increased axonal density in the distal stump is due to the smaller size of remyelinated axons (n=3; *P<0.05; **P<0.01).
To our surprise, we found that the numbers of unmyelinated Remak C fibers were significantly increased in the distal stumps of BACE1-null mice (Fig. 3B). Although the reason for this alteration is unclear, altered C-fiber bundling has been described in BACE1-null nerves (15) and may contribute to the higher pain sensitivity found in BACE1-null mice when compared to their WT littermates (16).
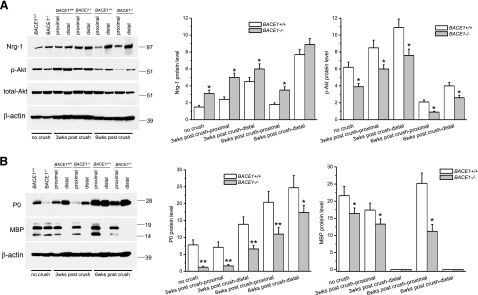
To determine the molecular alterations attributed to the above morphological changes, we examined potential involvement of the Nrg signaling pathway. The level of axonal Nrg-1 type III β1 isoform has been demonstrated to regulate peripheral myelin sheath thickness (28, 32), and increased binding of the neuronal Nrg-1 to ErbB receptors on Schwann cells transduces signals to Schwann cells to induce expression of myelin proteins and ensheathment of axons (33, 34). We previously proposed that BACE1 regulates myelin sheath thickness by cleaving axonal Nrg-1 (15, 16). This hypothesis was supported by significant increases in levels of full-length Nrg-1 in BACE1-null mice compared to their WT littermates (15, 16). In sciatic nerves of BACE1-null mice prior to the crush, the level of Nrg-1 type III protein was indeed increased (Fig. 4). Three or 6 wk after crush, increased full-length Nrg-1 protein persisted in proximal nerve segments of BACE1-null mice when compared to their WT littermate controls (Fig. 4A). Noticeably, total levels of Nrg-1 in both BACE1-null and WT distal stumps appeared increased compared to the proximal stumps. This observation is consistent with the previous report that expression of Nrg-1 and its putative receptor ErbB2/ErbB3 is induced in response to axonal regeneration and myelination (35).
Figure 4.
Altered full-length Nrg-1, Akt phosphorylation, and myelin proteins in BACE1-null mice. A) Western blot analysis of the indicated segments dissected out from either the untreated or crushed sciatic nerves. Equal amounts of protein from each sample were loaded for Western blot analysis of Nrg-1 and Akt expression; β-actin served as loading control. The levels of Nrg-1 and phosphorylated Akt were quantified by normalizing their levels relative to β-actin (n=3; *P<0.05). B) Western blot analysis of the same samples as in A, using antibodies specific to P0 and MBP. Plots show their levels relative to β-actin (n=3; *P<0.05; **P<0.01).
Nrg-1 is known to influence PI-3 kinase activity and the phosphorylation state of Akt (16, 32). The increased expression of Nrg-1 in regenerating distal stumps clearly affects the levels of the signaling molecule Akt. The total level of Akt was slightly reduced in distal stumps when compared to proximal stumps from both BACE1-null and WT mice (Fig. 4A). However, phosphorylated Akt (Fig. 4A) was significantly reduced in both proximal and distal stumps of BACE1-null mice. Overall, the reduced levels of phosphorylated Akt were correlated with the state of myelination, and they were consistent with the hypomyelination states in BACE1-null mice (16, 32, 36).
Altered Schwann cell signal transduction will affect the expression of myelin proteins. The levels of myelin proteins (P0) and myelin basic protein (MBP) were lower in BACE1-null mice compared to their WT littermates before the crush (Fig. 4B). As expected, MBP levels in the distal stumps were also lower than in the proximal stumps in both WT and BACE1-null mice, and the reduction was more obvious in the BACE1-null mice (Fig. 4B). Consistent with previous reports (37, 38), P0 levels in BACE1-null and WT distal stumps were increased at 3 to 6 wk postcrush when compared to proximal stumps. Why remyelinating internodes have a higher density of P0 than myelinated internodes is not known. Nevertheless, the increase was more obvious in the WT nerves than in the BACE1-null nerves (Fig. 4B). Together, our results indicate that the levels of myelin proteins correlated with the observed relatively thicker myelin sheath in WT regenerated sciatic nerves than in BACE1 sciatic nerves during remyelination.
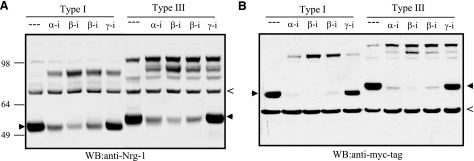
Although reduced cleavage of Nrg-1 by BACE1 is suggested to affect developmental myelination BACE1-null mice (15, 16) and remyelination in this study, it was unclear whether BACE1 directly cleaves Nrg-1. To address this, we cloned full-length type-I Nrg-1 (70–90 kDa) and type-III β1 Nrg-1 (90–110 kDa), two potential substrates of BACE1, and added a myc tag at their C terminus. Expression of these two constructs in HEK-293 cells, which naturally express α-, β-, and γ-secretases, was analyzed in the presence or absence of various protease inhibitors. Both isoforms of Nrg-1 were, indeed, cleavable by BACE1 because the specific BACE1 inhibitor IV, which is a potent inhibitor of BACE1 (39, 40), reduced the levels of BACE1 cleaved Nrg-1 C-terminal fragments (Fig. 5A, B; arrowheads). Correspondingly, both full-length Nrg-1 isoforms were increased after protease inhibition. This was demonstrated using both antibodies specific to Nrg-1 (Fig. 5A) and to the myc tag (Fig. 5B). Consistent with previous observations (32, 41), Nrg-1 isoforms were also cleaved by α-secretase-like membrane-bound metalloprotease, as treatment with specific metalloprotease inhibitor GM6001 also reduced levels of cleaved Nrg-1 products and increased full-length Nrg-1 (Fig. 5A, B). By contrast, inhibition of γ-secretase caused significant increase of the cleaved C-terminal fragments, consistent with the secondary γ-secretase cleavage of the membrane-bound Nrg-1 C-terminal fragments generated after either BACE1 or metalloprotease cleavage (42). As with the γ-secretase-cleaved fragment of APP, the small γ-secretase-cleaved fragment of Nrg-1 was not readily detected in this assay. These results support the hypothesis that both type I and III Nrg-1 are cleaved by α-, β-, and γ-secretases, activities reminiscent of the cleavage of APP by these secretases.
Figure 5.
The cleavage of Nrg-1 by BACE1. Type I and III Nrg-1 were transiently expressed in HEK-293 cells in the absence or presence of ADAM protease inhibitor GM6001 (α-I; 1 μM), BACE1 inhibitor IV (β-I; 1 μM left; 4 μM right), or γ-secretase inhibitor L-685485 (γ-I; 5 μM). Anti-Nrg-1 detected both full-length and the processed C-terminal Nrg-1 (Α). Both Nrg-1 isoforms have an myc tag at their C terminus, and monoclonal myc antibody was also used to detect the expressed products (B). Arrowheads indicate the altered cleavage product. Both antibodies detected nonspecifically reacted proteins in all samples, indicated with the symbol <. Although both BACE1 and ADAM protease inhibitors reduce the levels of C-terminal fragments, the addition of γ-secretase inhibitor increases the intensity of the ADAM- or BACE1-cleaved C-terminal fragment, as expected.
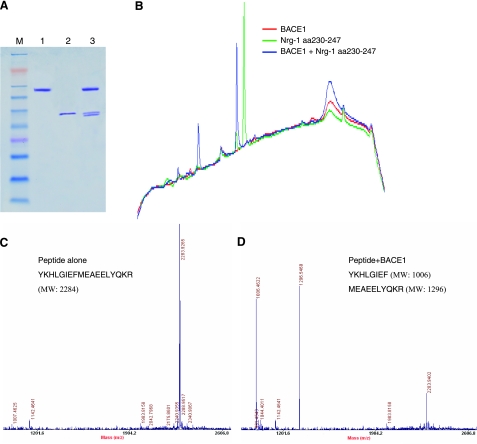
To help map the cleavage site of Nrg-1 by BACE1, we developed an in vitro enzymatic assay using purified BACE1 enzyme and recombinant human Nrg-1 protein spanning the entire extracellular domain. A BACE1 cleaved product migrated in proximity to the parental protein (Fig. 6A), suggesting a BACE1 cleavage site near the C terminus, i.e., near the juxtamembrane region. Further in vitro enzymatic assays using BACE1 and selected synthetic peptides identified a cleaved peptide spanning residues 230–247 (Fig. 6B). MALDI-TOF mass spectrometry analysis of the cleaved product identified a BACE1-mediated Nrg-1 cleavage site between the F–M residues of peptide YKHLGIEFMEAEELYQKR (Fig. 6C, D). The presence of negative-charged residues at both P2′ site and P2 cleavage subsites was unexpected based on the predicted optimal BACE1 cleavage sequences (43, 44). However, the cleavage subsite of WT-APP by BACE1 is also less optimal, and interaction of BACE1 with its substrates on the membrane likely influences optimal cleavage (26). When residues FM were mutated to KK, the same-length mutant peptide was not cleavable by BACE1 (data not shown), confirming that Nrg-1 is cleavable by BACE1.
Figure 6.
Mapping the cleavage site of Nrg-1 by BACE1. A) In vitro cleavage of recombinant Nrg-1 was performed in a reaction containing purified BACE1 and recombinant Nrg-1 protein spanning residues 1–246. Each reaction mix contains BACE1 (lane 1), recombinant Nrg-1 protein (lane 2), or both proteins (lane 3). Recombinant Nrg-1 was cleaved by BACE1, producing a second band migrating slightly faster than full-length Nrg-1t. B) BACE1 cleavage of an Nrg-1 peptide spanning residues 230–247. The in vitro reaction of BACE1 and Nrg-1 aa 230–247 proceeded at room temperature for 24 h, after which the reaction mixture was separated by reverse-phase HPLC. Individual color traces indicate reaction conditions. Two new peaks are present in the sample containing both BACE1 and the peptide (blue), indicating cleavage of the peptide by BACE1. C, D) The indicated peptide spanning Nrg-1 residues 230–247 was incubated with purified BACE1 in a protease reaction overnight, and the mixture was analyzed by MALDI-TOF mass spectrometry analysis. The molecular weights of peptides in the reaction mix were determined before (C) and after (D) enzymatic cleavage. The molecular weight of the original peptide is 2284. The molecular weights of cleaved products are 1006 and 1296, respectively, matching the hydrolyzed product as indicated with the corresponding sequences.
As demonstrated, Nrg-1 was directly cleaved by BACE1 at a juxtamembrane site. The sequences surrounding the BACE1 cleavage site are identical not only between Nrg-1 type I and III β1 isoforms but also among vertebrate species (Fig. 7A). The released soluble Nrg-1 N-terminal domain possesses both EGF- and/or Ig-like domains that are important for its signal transduction, and this soluble fragment may provide the instructive signal that regulates myelination and remyelination (36).
Figure 7.
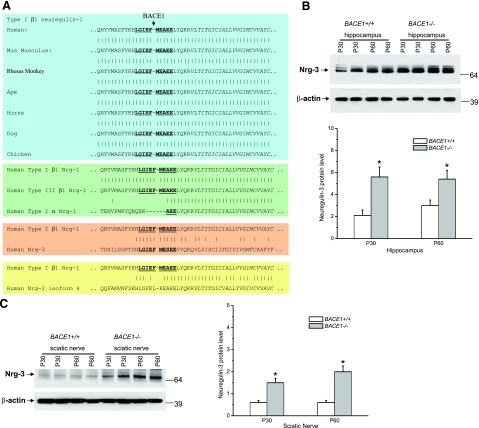
Highly conserved BACE1cleavage site among Nrg proteins. A) The cleavage site of type I β1 Nrg-1 by BACE1 is highly conserved among vertebrate paralogues. The same cleave site is also identical between type I and III β1 Nrg-1 spliced variants but not with type I α1 Nrg-1 protein. Note that a stretch of 11 residues spanning the BACE1 cleavage site is also conserved between type I Nrg-1 and Nrg-3, whereas the conservation is reduced in Nrg-2. B, C) Western blot analysis of protein extracts from mouse hippocampus (B) or sciatic nerves (C) using antibody specific to neuregulin-3. Two pairs of P30 and P60 old mice were used for protein extractions; results are from three independent experiments. Plots show their levels relative to β-actin (n=3; *P<0.05).
Further sequence comparison revealed that the BACE1 cleavage site is also present in neuregulin-3 (Nrg-3), but not in Nrg-2 and Nrg-4. Surprisingly, only the 11 residues evenly spanning the cleavage are completely identical between Nrg-1 and Nrg-3; the remaining stretches of surrounding sequences are not identical (Fig. 7A). This interesting feature suggests that Nrg-3 is likely a BACE1 substrate. To determine whether Nrg-3 is cleavable by BACE1, we examined hippocampal and sciatic nerve lysates by Western blot analysis and found that the levels of full-length Nrg-3 were significantly elevated in BACE1 null mice (Fig. 7B, C). Hence, we show here that both Nrg-1 and Nrg-3 can be directly processed by BACE1.
DISCUSSION
Remyelination in the peripheral nervous system is especially relevant in the context of BACE1-targeted AD therapeutics, as demyelination/remyelination of distal peripheral nerves is a common age-related event. We have now provided evidence that BACE1 regulates not only myelin sheath thickness during development but also remyelination in the adult. Prior to the sciatic nerve crush, myelin sheath is thinner and the g ratio is higher in BACE-null mice than in WT control mice, consistent with the previous report (15, 16). After sciatic nerve crush, the stumps distal to the crush site initiate sequential cellular changes known as Wallerian degeneration, including axonal degeneration and myelin breakdown, and the debris is phagocytosed by Schwann cells and macrophages (45). Coincident with axonal degeneration, normally quiescent Schwann cells dedifferentiate and proliferate, an event critically important for the promotion of axonal regeneration. Remyelination is initiated when the regenerating axons enter the band of Bungner and make contact with Schwann cells. In this study, we found that the remyelination in the distal stumps of BACE1 null mice was delayed, as demonstrated by an increased number of unmyelinated axons, reduced thickness of myelin sheath, and a higher g ratio.
Expression of many genes has been altered in association with depriving Schwann cells of contact with axons in the injured axonal stumps to allow regrowth of axons and their remyelination in the distal stumps (46). We have focused our study on Nrg and demonstrated that total levels of Nrg-1 are higher in the distal stumps than in the proximal stumps. It should be noted that part of the increased concentration of Nrg-1 comes from Schwann cells. It has been demonstrated that the induced expression of various Nrg-1 isoforms and their receptor ErbB2/3 by Schwann cells in the crushed distal stumps is increased, and this increased expression is needed for Schwann cell proliferation (35). If the increased level of Nrg-1 expression from Schwann cells is similar in two types of mice, we can assume that BACE1 may mainly affect axonal Nrg-1, and only the axonal Nrg-1 regulates myelination, as previously demonstrated (33).
Type III Nrg-1 is the only identified axonal Nrg-1 isoform demonstrated to provide an instructive signal for sciatic nerve myelination (33). However, our data showed that both type I and type III Nrg-1 are cleavable by BACE1. Whether the soluble BACE1 cleaved product of type I Nrg-1 will play a role in regulating Schwann cell differentiation and myelination deserves to be further investigated. In addition, we found that a stretch of 11 residues spanning the BACE1 cleavage site is identical between Nrg-1 and Nrg-3, but not in Nrg-2 (Fig. 7A), and further confirmed that Nrg-3 is a BACE1 substrate as well. Nrg-3 possesses a similar structural organization to Nrg-1 and also binds to ErbB receptor (47). Genetic studies have suggested that the activity of ErbB4 may also be regulated in the CNS by a ligand distinct from NRG1. Nrg-3/ErbB4 pathway is found to regulate survival of oligodendrocytes (48). Thus, our results suggest that genetic deletion of BACE1 may also alter signaling initiated from Nrg-3. It will be necessary to test whether Nrg-3 can complement the function of Nrg-1 in myelination when Nrg-1 is knocked out in an animal.
Axonal Nrg-1/ErbB signal is shown to regulate expression of MBP and P0 (36). Interestingly, expression of P0 and MBP appeared to be differentially regulated in the proximal and distal stumps of crushed nerves. Compared to WT proximal stumps, levels of both MBP and P0 were reduced in the BACE1-null proximal stumps. This reduction correlated with the elevated levels of full-length Nrg-1 in the proximal stumps, supporting the role of BACE-1 cleaved NRG-1 in myelin maintenance following distal site insult. P0 protein concentrations in distal stumps were increased in both lines, but to a lesser degree in BACE1-null mice. In contrast to P0, MBP levels in the distal stumps where dramatically reduced in both lines of mice, suggesting that P0 and MBP expression is differentially regulated during remyelination and that MBP expression in remyelinating Schwann cells may not be simply regulated by axonal Nrg-1. Glial Nrg-1 isoforms can actually down-regulate expression of MBP (49), implying a complexity in MBP gene expression from different sources or possibly spliced variants of Nrg-1. The altered ratio of P0/MBP in crushed distal stumps is unlikely to affect myelin formation or function, as complete elimination of MBP in the spontaneous myelin mutant mouse shiverer has no effect on PNS myelination or myelinated peripheral nerve function (50). In contrast, the CNS of shiverer mice is severely hypomyelinated, and MBP plays an essential role in maintaining the major dense line of CNS but not PNS myelin. It has been suggested that the cytoplasmic terminal of P0 structurally maintains the major dense lines of PNS myelin, which is altered in P0-null mice (51).
In summary, we have shown that genetic deletion of BACE1 reduces myelination and can alter phosphorylation of Schwann cell Akt via reduced signaling from Nrg-1/ErbB2/ErbB3. Specifically, reduced BACE1 cleavage of axonal Nrg1 at amino acids F-M (aa 237–238) is likely to limit both myelination and remyelination. Although therapeutic approaches for AD focus on reducing BACE1 cleavage of APP, our data clearly demonstrate that this might have a negative effect on myelination/remyelination. However, this effect is likely small because loss of BACE1 delays remyelination rather than prevents remyelination. Furthermore, since the cleavage subsites in Nrg-1 and APP are quite different, it may be possible to identify BACE1 inhibitors that selectively inhibit APP cleavage. Pharmacological inhibition of BACE1 is an important target for AD therapy and should be monitored to minimize alteration of signaling pathways initiated from Nrg-1.
Acknowledgments
We thank M. Yin (Electron Microscopy Facility at the Cleveland Clinic Foundation) for her assistance in electron microscopic analysis, and Satya Yadav and Weizhen Sheng for HPLC and MALDI-TOF mass spectrometer analyses. We also thank Drs. Qi Shi, Xiangdong Zhou, and Marguerite Prior for the discussion during this study. This study was supported by a grant from the U.S. National Institutes of Health to R.Y. (AG025493) and was partially supported by the National Multiple Sclerosis Society. The authors declare no conflicts of interests.
References
- Vassar R, Bennett B D, Babu-Khan S, Kahn S, Mendiaz E A, Denis P, Teplow D B, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski M A, Biere A L, Curran E, Burgess T, Louis J C, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski M J, Shuck M E, Miao H, Tory M C, Pauley A M, Brashier J R, Stratman N C, Mathews W R, Buhl A E, Carter D B, Tomasselli A G, Parodi L A, Heinrikson R L, Gurney M E. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett D R, Tew D G, Meek T D, Chapman C, Gloger I S, Murphy K E, Southan C D, Ryan D M, Smith T S, Simmons D L, Walsh F S, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson J P, Barbour R, Basi G S, Caccavello R, Davis D, Doan M, Dovey H F, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari S M, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Selkoe D J. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;62:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- Yang L B, Lindholm K, Yan R, Citron M, Xia W, Yang X L, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Rosene D L, Moss M B, Raju S, Hyman B T, Irizarry M C. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt D R, Price D L, Wong P C. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett B D, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis J C, Yan Q, Richards W G, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Roberds S L, Anderson J, Basi G, Bienkowski M J, Branstetter D G, Chen K S, Freedman S B, Frigon N L, Games D, Hu K, Johnson-Wood K, Kappenman K E, Kawabe T T, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols N F, Power M, Robertson D W, Schenk D, Schoor M, Shopp G M, Shuck M E, Sinha S, Svensson K A, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- McConlogue L, Buttini M, Anderson J P, Brigham E F, Chen K S, Freedman S B, Games D, Johnson-Wood K, Lee M, Zeller M, Liu W, Motter R, Sinha S. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- Chang W P, Koelsch G, Wong S, Downs D, Da H, Weerasena V, Gordon B, Devasamudram T, Bilcer G, Ghosh A K, Tang J. In vivo inhibition of Abeta production by memapsin 2 (beta-secretase) inhibitors. J Neurochem. 2004;89:1409–1416. doi: 10.1111/j.1471-4159.2004.02452.x. [DOI] [PubMed] [Google Scholar]
- Singer O, Marr R A, Rockenstein E, Crews L, Coufal N G, Gage F H, Verma I M, Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- Kao S C, Krichevsky A M, Kosik K S, Tsai L H. BACE1 suppression by RNA interference in primary cortical neurons. J Biol Chem. 2004;279:1942–1949. doi: 10.1074/jbc.M309219200. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt A N, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Hu X, Hicks C W, He W, Wong P, Macklin W B, Trapp B D, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson D J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Lu Q R, Sun T, Zhu Z, Ma N, Garcia M, Stiles C D, Rowitch D H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Arnett H A, Fancy S P, Alberta J A, Zhao C, Plant S R, Kaing S, Raine C S, Rowitch D H, Franklin R J, Stiles C D. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Yue T, Xian K, Hurlock E, Xin M, Kernie S G, Parada L F, Lu Q R. A critical role for dorsal progenitors in cortical myelination. J Neurosci. 2006;26:1275–1280. doi: 10.1523/JNEUROSCI.4717-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Risling M, Carlstedt T, Lendahl U, Timmusk T, Metsis M, Yamamoto Y, Ibanez C F. Targeted expression of a multifunctional chimeric neurotrophin in the lesioned sciatic nerve accelerates regeneration of sensory and motor axons. Proc Natl Acad Sci U S A. 1998;95:5269–5274. doi: 10.1073/pnas.95.9.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Akpinar P, Murray S, Strickland S. Fibrin is a regulator of Schwann cell migration after sciatic nerve injury in mice. Neurosci Lett. 2003;338:185–188. doi: 10.1016/s0304-3940(02)01387-3. [DOI] [PubMed] [Google Scholar]
- Smith G M, Rabinovsky E D, McManaman J L, Shine H D. Temporal and spatial expression of ciliary neurotrophic factor after peripheral nerve injury. Exp Neurol. 1993;121:239–247. doi: 10.1006/exnr.1993.1091. [DOI] [PubMed] [Google Scholar]
- Haney C A, Sahenk Z, Li C, Lemmon V P, Roder J, Trapp B D. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol. 1999;146:1173–1184. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer’s beta-secretase (BACE1) determines its late Golgi localization and access to beta-amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- Yin X, Baek R C, Kirschner D A, Peterson A, Fujii Y, Nave K A, Macklin W B, Trapp B D. Evolution of a neuroprotective function of central nervous system myelin. J Cell Biol. 2006;172:469–478. doi: 10.1083/jcb.200509174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov G V, Sereda M W, Brinkmann B G, Fischer T M, Haug B, Birchmeier C, Role L, Lai C, Schwab M H, Nave K A. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- He W, Lu Y, Qahwash I, Hu X Y, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J A, Engle W K. Structural and histochemical changes in the axons following nerve crush. Arch Neurol. 1968;19:598–612. doi: 10.1001/archneur.1968.00480060068009. [DOI] [PubMed] [Google Scholar]
- Schroder J M. Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res. 1972;45:49–65. doi: 10.1016/0006-8993(72)90215-6. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper R M, Loeb J A, Shrager P, Chao M V, Falls D L, Role L, Salzer J L. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K A, Salzer J L. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lemke G. Neuregulin-1 and myelination. Sci STKE. 2006;2006:e11. doi: 10.1126/stke.3252006pe11. [DOI] [PubMed] [Google Scholar]
- Carroll S L, Miller M L, Frohnert P W, Kim S S, Corbett J A. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3 is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Velardez M O, Warot X, Yu Z X, Miller S J, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc A C, Poduslo J F. Axonal modulation of myelin gene expression in the peripheral nerve. J Neurosci Res. 1990;26:317–326. doi: 10.1002/jnr.490260308. [DOI] [PubMed] [Google Scholar]
- Wong E, Mizisin A P, Garrett R S, Miller A L, Powell H C. Changes in aldose reductase after crush injury of normal rat sciatic nerve. J Neurochem. 1992;58:2212–2220. doi: 10.1111/j.1471-4159.1992.tb10966.x. [DOI] [PubMed] [Google Scholar]
- Brady S F, Singh S, Crouthamel M C, Holloway M K, Coburn C A, Garsky V M, Bogusky M, Pennington M W, Vacca J P, Hazuda D, Lai M T. Rational design and synthesis of selective BACE-1 inhibitors. Bioorg Med Chem Lett. 2004;14:601–604. doi: 10.1016/j.bmcl.2003.11.061. [DOI] [PubMed] [Google Scholar]
- Pietrak B L, Crouthamel M C, Tugusheva K, Lineberger J E, Xu M, Dimuzio J M, Steele T, Espeseth A S, Stachel S J, Coburn C A, Graham S L, Vacca J P, Shi X P, Simon A J, Hazuda D J, Lai M T. Biochemical and cell-based assays for characterization of BACE-1 inhibitors. Anal Biochem. 2005;342:144–151. doi: 10.1016/j.ab.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Sisodia S S, George-Hyslop P H. Gamma-secretase, Notch Abeta and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Tomasselli A G, Qahwash I, Emmons T L, Lu Y, Leone J W, Lull J M, Fok K F, Bannow C A, Smith C W, Bienkowski M J, Heinrikson R L, Yan R. Employing a superior BACE1 cleavage sequence to probe cellular APP processing. J Neurochem. 2003;84:1006–1017. doi: 10.1046/j.1471-4159.2003.01597.x. [DOI] [PubMed] [Google Scholar]
- Turner R T, III, Loy J A, Nguyen C, Devasamudram T, Ghosh A K, Koelsch G, Tang J. Specificity of memapsin 1 and its implications on the design of memapsin 2 (beta-secretase) inhibitor selectivity. Biochemistry. 2002;41:8742–8746. doi: 10.1021/bi025926t. [DOI] [PubMed] [Google Scholar]
- Webster H D. Transient, focal accumulation of axonal mitochondria during the early stages of wallerian degeneration. J Cell Biol. 1962;12:361–383. doi: 10.1083/jcb.12.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J W, Keynes R J. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Zhang D, Sliwkowski M X, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski P J. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci U S A. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteron C, Ferrer-Montiel A, Cabedo H. Characterization of a neural-specific splicing form of the human neuregulin 3 gene involved in oligodendrocyte survival. J Cell Sci. 2006;119:898–909. doi: 10.1242/jcs.02799. [DOI] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks M J, Bedell-Hogan D, Marchionni M A, Salzer J L. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–1299. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Peripheral myelin in the mouse mutant Shiverer. J Comp Neurol. 1980;193:729–739. doi: 10.1002/cne.901930310. [DOI] [PubMed] [Google Scholar]
- Martini R, Mohajeri M H, Kasper S, Giese K P, Schachner M. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J Neurosci. 1995;15:4488–4495. doi: 10.1523/JNEUROSCI.15-06-04488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]