Abstract
The mechanisms governing transition of the thyroid stimulating hormone (TSH) receptor (TSHR) from basal to active conformations are poorly understood. Considering that constitutively activating mutations (CAMs) and inactivating mutations in each of the extracellular loops (ECLs) trigger only partial TSHR activation or inactivation, respectively, we hypothesized that full signaling occurs via multiple extracellular signal propagation events. Therefore, individual CAMs in the extracellular region were combined to create double and triple mutants. In support of our hypothesis, combinations of mutants in the ECLs are in some cases additive, while in others they are even synergistic, with triple mutant I486A/I568V/V656F exhibiting a 70-fold increase in TSH-independent signaling. The proximity but likely different spatial orientation of the residues of activating and inactivating mutations in each ECL supports a dual functionality to facilitate signal induction and conduction, respectively. This is the first report for G-protein coupled receptors, suggesting that multiple and cooperative signal propagating events at all three ECLs are required for full receptor activation. Our findings provide new insights concerning molecular signal transmission from extracellular domains toward the transmembrane helix bundle of the glycoprotein hormone receptors.—Kleinau, G., Jaeschke, H., Mueller, S., Raaka, B. M., Neumann, S., Paschke, R., Krause, G. Evidence for cooperative signal triggering at the extracellular loops of the TSH receptor.
Keywords: glycoprotein hormone receptors, activation mechanism, signal transduction, endocrinology
The thyroid stimulating hormone receptor (TSHR), luteinizing hormone/choriogonadotropin receptor (LHCGR), and follicle stimulating hormone receptor (FSHR) are members of the glycoprotein hormone receptor (GPHR) subfamily of the G-protein coupling receptor (GPCR) 7 transmembrane helix receptor superfamily (7TMRs; ref. 1). A unique characteristic of the GPHRs is a large N-terminal ectodomain (ECD). The ECD can be subdivided structurally into a leucine-rich repeat hormone binding domain (LRRD) and the hinge region, which connects the LRRD to the transmembrane helix (TMH) 1 of the serpentine domain (SD) (2). The hinge region is composed of cysteine-rich boxes 2 and 3 (C-b2 and C-b3), which are structurally connected via a cysteine-box linker region (C-bl2/3). Cb-2 and C-b3 are disulfide bridged (3). Hormone binding and participating interactions at the LRRD of GPHRs have been experimentally investigated (4,5,6,7,8,9,10,11). The crystal structure of the FSHR LRRD/FSH complex (12) has given detailed insights into the orientation of the hormone docked to the LRRD (13, 14). Furthermore, contacts between the TSHR and activating or inactivating antibodies (15,16,17) have been substantiated by the crystal structure of the TSHR-LRRD in complex with an activating monoclonal autoantibody (18).
Apart from recognition and binding of the hormone or antibodies at the LRRD, it is postulated that interactions between the ECD and the SD are essential for switching from basal to active GPHR conformations. It is assumed that the ECDs of both TSHR and LHCGR are necessary for stabilization of a signaling-competent basal receptor conformation (19,20,21,22,23,24) and that hormone interaction or mutations lead to receptor activation by release of an intramolecular agonist (19, 20). For the TSHR specifically, an additional inverse agonistic influence (silencing effect) of the ECD on the SD in the basal state has been suggested (20, 25,26,27,28). Although the details of involved intramolecular interactions between extracellular components of the ECD and the SD have not been clarified, it is likely that the extracellular loops (ECLs) are molecular key players in these scenarios (20, 24, 28).
In this study, we initially hypothesized that full receptor activation occurs when the external signal is propagated by multiple discrete interactions on the extracellular side, including the hinge region and the ECLs. This hypothesis is based on several published observations concerning GPHRs: 1) the involvement of Tyr385 in the C-terminal tail of the TSHR hinge region in hormone binding and signaling (29,30,31); 2) the existence and functionality of signaling-relevant epitopes in the N- and C-terminal cysteine boxes of the hinge region (32, 33); 3) the recent demonstration that the highly conserved hydrophilic amino acids Glu297 and Glu303 in the N-terminal portion and Asp382 in the C-terminal portion of the hinge region are sensitive for bovine TSH (bTSH) binding and signaling (34); 4) the pathogenicity of an exon 10 deletion in the human LHCGR revealed a significant role for 27 amino acids in the hinge region for hormone-mediated signaling (35, 36); 5) superagonistic GPH analogs of the human hormones (37, 38) are characterized by additional positively charged amino acids located in peripheral loops of the α- and β-subunits; from the FSHR LRRD/FSH complex crystal structure (12) and the expected structural homology in other GPHR/GPH complexes, the modified positions of the superagonists would not participate in the LRRD/hormone complex but rather may interact elsewhere, possibly mediating superagonism by interacting with residues in the hinge region; and 6) single mutations in the ECLs that lead to either constitutive activation or to reductions in TSH-induced signaling mediate almost always partial activation or inhibition (39, 40).
In summary, these observations support the concept that many amino acids in the extracellular regions participate in full GPHR activation and signaling, which presumably result from multiple hormone contacts simultaneously at both the LRRD and the hinge region. Therefore, we postulated that simultaneous modifications of participating extracellular signaling determinants should lead to an amplification of the activation signal compared with single modifications. To test this hypothesis, we combined CAMs in all ECLs (ECL1: I486A; ECL2: I568V; and ECL3: V656F) and a CAM in the N-terminal hinge region (C-b2: S281F) to create double and triple mutations. Our aim was to determine whether additive or synergistic constitutive activation of the Gαs-mediated signaling cascade occurs in double or triple compared to single mutations.
Here we report that both additive and synergistic activation results from combining individual CAMs at the three ECLs and the extracellular hinge region. The strongest influence on combined signaling activity is provided by ECL1. From the observed pattern of close association in ECL2 and ECL3 between the positions of CAMs and of known inactivating mutations that reduce TSH-induced signaling, we were able to identify in the ECL1 a new inactivating mutation and a new CAM. We discuss new aspects of how the signal in the TSHR is distributed from the externally bound hormone toward the TMH bundle to achieve the transition between the basal and active receptor conformations. Our results support a model in which their proximity allows residues at the positions of CAMs and inactivating mutations to function together to transmit the extracellular signal toward the TMHs to convert the TSHR to its active conformation.
MATERIALS AND METHODS
Site-directed mutagenesis
TSHR mutants were constructed by polymerase chain reaction mutagenesis using the human TSHR-pcDNA3.1(−)/hygro as a template, as described previously (41). Mutated TSHR sequences were verified by dideoxy sequencing with dRhodamine Terminator Cycle Sequencing chemistry (ABI Advanced Biotechnologies, Columbia, MD, USA).
Cell culture and transient expression of mutant TSHRs
COS-7 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Paisley, UK) at 37°C in a humidified 5% CO2 incubator. Cells were transiently transfected in 24-well plates (0.5×105 cells/well) with 300 ng DNA/well using the GeneJammer transfection reagent (Stratagene, Amsterdam, the Netherlands).
Fluorescence-activated cell sorter (FACS) analyses
The TSHR cell surface expression level was quantified on a FACS flow cytometer. Transfected cells were detached from the dishes with 1 mM EDTA and 1 mM EGTA in PBS and transferred into Falcon 2054 tubes. Cells were washed once with PBS and then incubated at 4°C for 1 h with a 1:200 dilution of a mouse anti-human TSHR antibody (2C11, 10 mg/L, Serotec, Oxford, UK) in the same buffer. Cells were washed twice and incubated at 4°C for 1 h with a 1:200 dilution of fluorescein-conjugated F(ab′)2 rabbit anti-mouse IgG (Serotec). Before FACS analysis (FACscan Becton-Dickinson, Franklin Lakes, NJ, USA), cells were washed twice and then fixed with 1% paraformaldehyde. Receptor expression was determined by the mean fluorescence intensity. The wild-type TSHR was set at 100%, and the receptor expression of the mutants was calculated according to this. The percentage of signal-positive cells corresponds to transfection efficiency, which was ∼50–60% of viable cells for each mutant.
cAMP accumulation assay
For cAMP assays, cells were grown and transfected in 24-well plates. Forty-eight hours after transfection, cells were incubated in the absence or presence of 100 mU/ml bTSH (Sigma, St. Louis, MO, USA) in serum free medium supplemented with 1 mM isobutylmethylxanthine (Sigma) for 1 h. Reactions were terminated by aspiration of the medium. The cells were washed once with ice-cold PBS and then lysed by addition of 0.1 N HCl. Supernatants were collected and dried. cAMP content of the cell extracts was determined using the cAMP AlphaScree Assay (PerkinElmer Life Sciences, Zaventem, Belgium) according to the manufacturer’s instructions.
Linear regression analysis of constitutive activity as a function of TSHR expression (slopes)
The constitutive activity is expressed as basal cAMP formation as a function of receptor expression determined by FACS. COS-7 cells were transiently transfected in 24-well plates (0.5×105 cells/well) with increasing concentrations of wild-type or mutant TSHR plasmid DNA (50, 100, 150, 200, 250, and 300 ng/well). For determination of the transfected constructs see Fluorescence-Activated Cell Sorter (FACS) Analyses. Basal cAMP formation as a function of receptor expression was analyzed according to Ballesteros et al. (42) using the linear regression module of GraphPad Prism 4 for Windows (GraphPad, San Diego, CA, USA).
Structural bioinformatics and molecular modeling
The homology model of the transmembrane region of the human TSHR was generated based on the X-ray structure of bovine rhodopsin [PDB entry codes: 1F88 (43), 2I35 (44), and 2J4Y (45)]. Reported low root mean square deviation (RMSD) values between backbones of the TMHs of the used rhodopsin structure and the recently solved X-ray structures of the β2-adrenergic receptor ([PDB entry codes: 2R4R (46) and 2RH1 (47)] support the reliability of our TSHR-TMH model. The major structural difference concerns ECL2. Due to 1) the higher similarity in length and amino acid sequence of ECL2 between rhodopsin and TSHR (36.0%) vs. β2-AR and TSHR (27.6%) and 2) an β2-AR-specific additional internal cysteine-bridge, which stabilizes a helical fold in the ECL2 of the β2-AR, we kept the rhodopsin like beta-hairpin structure and location of ECL2 also for the TSHR model. In addition, this rhodopsin-like ECL2 conformation is consistent with results of diverse studies at other GPCRs (48,49,50) and TSHRs (39).
However, several TSHR -specific corrections were made, such as regular helix extensions in TMH2 and TMH5 of TSHR instead of structural bulges in the two helices of rhodopsin caused there specifically by side chains that are not present in TSHR (two consecutive threonines in TMH2 and a proline in TMH5, respectively). Loops were refined by best fit and homology to fragments of other proteins (from PDB). Gaps of missing residues in the loops of the template structure were closed by the Loop Search tool implemented in Sybyl 7.3.5 (Tripos, St. Louis, MO, USA). Conjugate gradient minimizations were performed until convergence at a termination gradient of 0.05 kcal/(mol×Å), and the AMBER 7.0 force field (51) was used. Quality and stability of the model were validated by checking the geometry by PROCHECK (52) and during a molecular dynamics simulation of 2 ns (overall backbone RMSD of 1.8 Å).
RESULTS
Additive and synergistic effects of multiple constitutively activating mutations
The initial hypothesis in this study was that a simultaneous combination of CAMs in the extracellular region of TSHR initiates multiple discrete signal propagation events that lead to an increased constitutive activation compared with individual CAMs. Therefore, we designed and tested single and multiple constitutively active substitutions in C-b2 of the hinge region in the ECD (S281F) and in the extracellular region of the serpentine domain: ECL1 (I486A), ECL2 (I568V), and ECL3 (V656F).
Cell surface expression
The single mutants S281F, I568V, and V656F showed expression levels between 86.3 and 93.5% compared with wild type (Table 1). Only I486A with 67.3% revealed a reduced expression to two-thirds of the wild-type TSHR. Most of the double mutants led to similar or slightly decreased expression patterns when compared with the levels of the single mutations. Although construct S281F/I486A was expressed at 77.1%, the other double mutants with I486A showed a greatly reduced surface expression of 29.2 and 35.5%. Constructs with three substitutions were expressed generally at levels comparable with the double mutants, with the lowest expression of 17.1% exhibited by I486A/I568V/V656F.
TABLE 1.
Functional characterization of single, double, and triple CAMs in all three ECLs and C-b2 of the TSHR
| Location | Transfected construct | Cell surface expression (% of WT TSHR) | cAMP accumulation (fold over WT TSHR basal)
|
||
|---|---|---|---|---|---|
| Basal | Stimulated | Slope | |||
| WT TSHR | 100 | 1.0 | 11.2 ± 0.4 | 1.0 | |
| pcDNA | 8.1 ± 0.5 | 0.4 ± 0.1 | 0.5 ± 0.1 | — | |
| ECL1 | I486A | 67.3 ± 4.9 | 3.8 ± 0.3 | 10.2 ± 1.1 | 3.9 ± 1.6 |
| ECL2 | I568V | 86.4 ± 3.0 | 2.2 ± 0.3 | 11.7 ± 1.0 | 1.9 ± 0.8 |
| ECL3 | V656F | 93.5 ± 5.7 | 4.1 ± 0.5 | 10.8 ± 0.9 | 4.1 ± 0.9 |
| C-b2 | S281F | 86.3 ± 3.3 | 2.0 ± 0.3 | 12.8 ± 0.2 | 1.7 ± 0.6 |
| ECL1/ECL2 | I486A/I568V | 35.5 ± 2.7 | 4.4 ± 0.5 | 8.1 ± 0.4 | 12.9 ± 4.3 |
| ECL1/ECL3 | I486A/V656F | 29.2 ± 2.5 | 4.1 ± 0.3 | 5.2 ± 0.4 | 16.6 ± 4.8 |
| ECL2/ECL3 | I568V/V656F | 72.9 ± 5.3 | 4.8 ± 0.3 | 10.1 ± 0.6 | 7.8 ± 1.4 |
| C-b2/ECL1 | S281F/I486A | 77.1 ± 3.1 | 4.6 ± 0.8 | 11.5 ± 1.2 | 7.4 ± 0.3 |
| C-b2/ECL2 | S281F/I568V | 78.3 ± 3.9 | 4.8 ± 0.8 | 13.3 ± 0.6 | 2.1 ± 0.3 |
| C-b2/ECL3 | S281F/V656F | 82.9 ± 5.4 | 4.7 ± 0.3 | 13.2 ± 0.6 | 3.3 ± 0.3 |
| ECL1/ECL2/ECL3 | I486A/I568V/V656F | 17.1 ± 0.6 | 4.6 ± 0.8 | 6.4 ± 0.3 | 70.6 ± 9.6 |
| C-b2/ECL1/ECL2 | S281F/I486A/I568V | 35.1 ± 2.7 | 5.0 ± 0.4 | 10.3 ± 1.9 | 16.5 ± 0.3 |
| C-b2/ECL1/ECL3 | S281F/I486A/V656F | 32.9 ± 3.1 | 4.5 ± 0.5 | 8.1 ± 1.5 | 16.1 ± 1.0 |
| C-b2/ECL2/ECL3 | S281F/I568V/V656F | 43.5 ± 5.8 | 4.3 ± 0.7 | 9.0 ± 1.1 | 8.3 ± 0.3 |
COS-7 cells were transfected with the wild-type (WT) TSHR or indicated mutant TSHRs. Because of the basal activity of the WT TSHR, cAMP levels are expressed relative to WT TSHR basal (set at 1). cAMP levels were determined after stimulation with 100 mU/ml bTSH. TSHR cell surface expression was quantified on a FACS flow cytometer. Data are means ± sd of 3 independent experiments, each carried out in duplicate. The pcDNA vector was used as a control.
cAMP accumulation
Constructs were characterized by determination of basal and TSH-induced cAMP production.
Basal activity
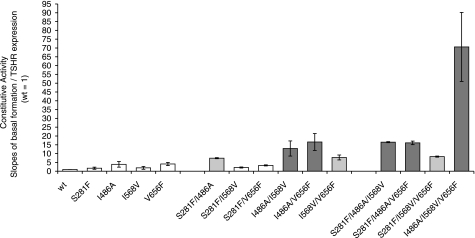
All mutations were constitutively active with basal activities significantly increased compared with the wild-type TSHR (Table 1). To assess the importance of their constitutive activity independently from their cell surface expression, we performed linear regression analyses for each mutant. The slopes of the linear functions (Table 1; Fig. 1) revealed moderate increases for the single mutants and for double mutants S281F/I568V (C-b2/ECL2) and S281F/V656F (C-b2/ECL3) of 1.7 to 4.1 (wild-type TSHR set at 1; Table 1). An additive effect for constitutive activity was observed for mutants I568V/V656F (7.8) and S281F/I486A (7.4), combining CAMs of ECL2/ECL3 and C-b2/ECL1, respectively, and synergistic signal triggering was found for double mutants I486A/I568V (ECL1/ECL2) with a slope of 12.9 and I486A/V656F (ECL1/ECL3) with 16.5. For double-mutated constructs containing S281F (C-b2) but lacking mutation I486A (ECL1), the observed basal signaling was less than additive. The mutant with all three ECL CAMs (I486A/I568V/V656F) showed the strongest synergistic enhancement with a slope of 70.6. The triple mutants including S281F in combination with I486A had synergistic slope values comparable with those of the double mutants I486A/I568V and I486A/V656F. Combining CAMs of the C-b2, ECL2, and ECL3 shown in triple mutant S281F/I568V/V656F led to an additive increase of activity to 8.3. The CAM of ECL1 always contributed the strongest increase of basal activity in combined mutants.
Figure 1.
Slopes of basal activity. Hormone-independent signaling: slopes of basal cAMP formation (expression normalized data). Double and triple combinations of single CAMs between hinge region, C-b2 (S281F), and within the ECL1 (I486A), ECL2 (I568V), and ECL3 (V656F) are shown. Increasing additive (light gray) and synergistic (dark gray) effects of cAMP accumulation by multiple CAMs are observed for double and triple mutants of the ECLs. The triple mutant of all 3 ECL-CAMs creates the strongest synergistic increase of basal activity. All combined mutants exhibiting synergistic activity included the ECL1 CAM (I468A).
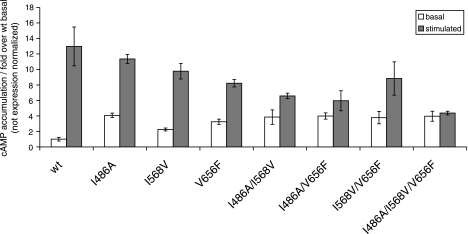
TSH-stimulated cAMP accumulation decreases progressively from single to double and again to triple mutants of the ECLs (Fig. 2) and shows reverse correlations to the increasing slopes of basal activity (Fig. 1). Finally, TSH does not activate the triple ECL-CAM with the strongest synergistic basal activation further.
Figure 2.
Additional hormone activation of multiple constitutively activating mutants. Hormone-stimulated signaling is decreased in a stepwise fashion in multiple CAMs. The triple CAM at the ECLs is not further activated by TSH, suggesting an activation state approaching full signaling. This is supported by the result that the double mutant displaying only an additive increase in constitutive activity is more responsive to activation by TSH than those showing synergistic constitutive activation.
Newly identified constitutively activating and partially inactivating mutations for TSH-mediated signaling support the importance of ECL1 for TSHR function
Characterization of individual amino acids in the ECLs is a prerequisite for understanding the functionalities of the ECLs, particularly regarding their possible roles in signal transmission between the ECD and SD. The amino acids of ECL2 and ECL3 of the TSHR were mutated and characterized previously by diverse side-chain substitutions (39, 40, 53). By combining single CAMs of the ECLs in double and triple mutants, we identified that ECL1 might be a key player in synergistic signal triggering in the extracellular region (Table 1). The amino acids comprising ECL1 have been characterized only partially in previous mutational studies (41). Thus, based on our observation of the high importance of ECL1, we reasoned that other amino acids in ECL1 apart from the known signaling-sensitive positions Ile486 and Tyr481 could be involved in signaling. Therefore, we designed and tested mutations S479A, E480A, N483A, D487A, W488A, W488F, W488L, Q489A, and T490A by functional assays (Table 2).
TABLE 2.
Phenotype characteristics of mutations in ECL1 and transition regions to TMH2 and TMH3
| Transfected construct | Cell surface expression (% of WT TSHR) | cAMP accumulation (fold over WT TSHR basal)
|
||
|---|---|---|---|---|
| Basal | Stimulated | Slope | ||
| WT TSHR | 100 | 1.0 | 11.9 ± 0.8 | 1.0 |
| pcDNA | 7.3 ± 0.5 | 0.4 ± 0.1 | 0.5 ± 0.1 | — |
| S479A | 79.2 ± 0.9 | 0.4 ± 0.1 | 11.8 ± 0.7 | — |
| E480A | 78.5 ± 2.2 | 0.5 ± 0.1 | 9.9 ± 0.4 | — |
| N483A | 89.5 ± 3.2 | 0.4 ± 0.1 | 13.0 ± 1.2 | — |
| D487A | 94.3 ± 4.5 | 0.6 ± 0.1 | 11.4 ± 2.4 | — |
| W488A | 11.0 ± 0.3 | 0.5 ± 0.1 | 0.5 ± 0.1 | — |
| W488F | 14.0 ± 0.8 | 0.6 ± 0.1 | 1.3 ± 0.2 | — |
| W488L | 12.0 ± 0.4 | 0.5 ± 0.1 | 0.6 ± 0.1 | — |
| Q489A | 51.9 ± 1.7 | 0.6 ± 0.1 | 4.0 ± 0.5 | — |
| T490A | 85.1 ± 2.4 | 2.0 ± 0.3 | 12.9 ± 2.2 | 1.9 ± 0.7 |
Seven amino acids within ECL1 of the TSHR were characterized by alanine mutations. The side chain of Trp488 was also substituted to Phe and Leu. COS-7 cells were transfected with the WT TSHR or indicated mutated TSHRs. cAMP levels were determined after stimulation with 100 mU/ml bTSH. TSHR cell surface expression was quantified on a FACS flow cytometer. Data are means ± sd of 3 independent experiments, each carried out in duplicate. The pcDNA vector was used as a control.
Cell surface expression
FACS analyses revealed that the alanine mutations of Asp487, Gln-489, and Thr490 show a cell surface expression in the range of 50–90% compared with wild-type TSHR (set at 100%; Table 2). The alanine substitution at Trp488 showed an impaired cell surface expression of 11%. We conclude that the drastic substitution of a hydrophobic, bulky, and aromatic side chain (Trp488) with a small hydrophobic, nonaromatic amino acid (W488A) cannot maintain the fold-stabilizing function of the tryptophan. The importance of a tryptophan at this position is further supported by the poor expression levels of a leucine (hydrophobic and large) and phenylalanine (hydrophobic and aromatic) mutation (12–14%, respectively). In conclusion, the tryptophan is strictly necessary at position 488 in ECL1 of the TSHR.
cAMP accumulation
The various mutations generally displayed phenotypes that affected basal and/or stimulated cAMP production relative to wild-type TSHR (Table 2). Most strikingly, mutant Q489A displayed a strongly decreased cAMP accumulation after TSH treatment, while T490A displayed increased basal Gαs-mediated cAMP signaling with a slope of 1.9 and TSH-stimulated cAMP production similar to wild-type TSHR (Table 2). In summary, we identified at ECL1 both a new CAM T490A and a new partially inactivating mutation Q489A for TSH-mediated signaling (Table 2; Fig. 3).
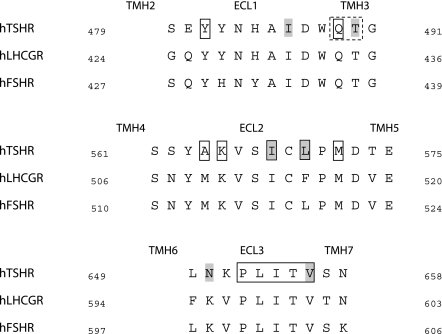
Figure 3.
Colocalization of constitutively activating and inactivating mutations within the ECLs of the glycoprotein hormone receptors. Sequence alignment of the 3 human ECLs of the glycoprotein hormone receptors shows colocalization of positions of CAMs (gray background) and inactivating mutations (boxed) at the TSHR. In support of the assumed necessity of this colocalization, we identified new signaling-sensitive positions in the ECL1 (dashed box) in addition to previously published mutations: a new partially inactive mutation (Q489A) for TSH induced signaling as well as a CAM (T490A).
DISCUSSION
For GPCRs in general and for GPHRs in particular, details of the signal transduction process are fragmentary or poorly understood, including the detailed changes in intramolecular contacts between the ECD and SD that characterize the basal and hormone-activated conformations. Moreover, it is not understood why the constitutive activation of GPHRs as well as a decreased TSH-mediated signaling caused by single mutations in the extracellular region is almost only partial. We have shown in this study for the first time experimentally that simultaneous modifications at the ECD and ECLs by CAMs led to additive or synergistic increases in TSHR signaling compared with single CAMs. Based on this new observation and previously published findings, we discuss details of extracellular signaling mechanisms and suggest new intra- and intermolecular interactions critical for TSHR function.
The ECLs are extracellular portions of the SD that connect the TMHs (ECL1: TMH2-TMH3; ECL2: TMH4–5; and ECL3: TMH6-TMH7). Several CAMs at specific positions in the ECLs have been reported [ECL1: I486M and I486F (54); ECL2: I568T, I568V, I568M, and I568F (39, 53, 54); and ECL3: N650Y and V656F (55, 56) (Figs. 3and 4)]. In general, CAMs in GPCRs cause a shift from the off state (or basal) conformation to an activated state either by disrupting interactions of the off state or by facilitating new interactions stabilizing an on state (57). Considering both options, a reductional conclusion can be drawn that the wild-type residues of CAMs are signaling-sensitive positions and facilitate the signal induction process.
Figure 4.
Backbone of the molecular SD model and location of signaling-sensitive amino acids in the ECLs. Homology model of the TSHR SD. Close up view of the ECLs that connect the TMHs (ECL1, magenta; ECL2, orange; and ECL3, yellow). The proximity of positions of both inactivating mutations for TSH-mediated signaling (red balls, Cα atom) and positions of CAMs (green balls, Cα atom) reflects the inherent dual functionality in each ECL to facilitate induction and conduction of signaling. Note that positions of inactivating residues (red) are spatially clustered and commonly more exposed to the extracellular regions and thus more available for interactions with the hinge region, whereas those of CAMs (green) are rather constraining the loop conformations.
N-terminal portions and ECLs of the TSHR
Structural interaction and dual functional character
It has been reported that CAMs in the ECLs of the TSHR are only functional in the presence of the ECD (20). This finding suggests that CAM-mediated signaling in the extracellular region is dependent on contacting portions of the N-terminal ECD with the ECLs. In particular, most likely the hinge region as portion of the ECD is involved in these interactions (20, 23, 32, 58; Fig. 5A). Interestingly, deletion of the whole ECD causes a significant but moderate increase in constitutive signaling of the TSHR (20), but this increase could not be observed in comparable deletion constructs at the LHCGR (19, 24). Regardless of this difference between these two GPHRs, an important dual role for the hinge region is to facilitate the formation of an activation competent conformation and to mediate the interplay between the ECD and SD during receptor activation (20,21,22, 24). Furthermore, a direct interaction of TSH with the ECLs of the TSHR SD domain is not known so far as well as not required in this functional-structural model (1).
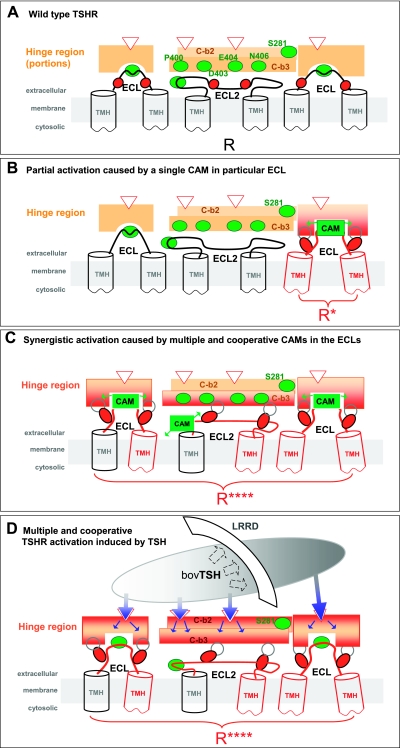
Figure 5.
Scenarios for signaling mechanisms in the extracellular region of the TSHR. A) The three ECLs are covered in the basal partially active wild-type receptor (R) by portions of the hinge region (orange boxes) of the ECD. In the hinge region, several contact points for receptor-TSH binding are known (e.g., Glu297, Glu303, Asp382, and Tyr385; red open arrowheads). C-b2 (light orange) and C-b3 (orange) are located in structural proximity to each other and to the ECLs. They function most probably as an interface between the ECD and the TMH bundle. Positions of observed CAMs (green balls) are located in the hinge region [e.g., at Ser-281 (C-b2), Pro400, Asp403, Glu404, Asn406 (C-b3)] and in the ECLs (ECL1: Ile486 and Thr490; ECL2: Ile568 and Leu570; and ECL3: Val656 and Asn650). B) Partial activation by an extracellular single CAM (green box and arrows) causes a local and limited conformational change at the hinge region (red-orange) enabling proximal positions of inactivating mutations (red ovals) to facilitate local active conformations. This local event affects only those TMHs (red) that are related to the particular CAM location. Due to the absence of cooperative effects between all ECLs in each of these single mutations, the signal is neither fully transduced nor fully blocked. C) Synergistic signal triggering caused by simultaneous modifications (CAMs, green boxes) at all 3 ECLs promotes multiple and cooperative conformational effects both in the ECLs and via the hinge-region (red-orange) to amplify signal propagation at the extracellular side of the TSHR. CAM combinations in the 3 ECLs achieved cooperative changes essential for fully active conformation (R****) via multiple TMHs (red). D) New scenario of multiple extracellular signal distribution at the TSHR. Hormone-induced activation by multiple contacts happens not only to the LRRD, (dashed arrows) but also simultaneously (blue arrow) to several sensitive amino acids (arrowheads) located in the hinge region (see A). Hormone binding is proposed to induce multiple activations at the hinge region to convert it to an active conformation. At the interface between the hinge region and ECLs, new multiple contacts (gray open circles) occur at residues of inactivating mutations within the ECLs (red ovals; see also Fig. 4). The cooperative effect of defined determinants (Fig. 4) at all ECLs amplifies the signal synergistically via a widespread propagation of multiple conformational changes toward the connected TMH bundle (red). Together, these combined events lead to the fully active conformation (R****).
The semiquantitative analysis of mutant phenotype functionalities using the mutant resource for GPHRs (www.fmp-berlin.de/SSFA; ref. 2) revealed that for ECL2 and ECL3 in proximity to CAMs, two or more inactivating mutations for TSH-mediated signaling could always be observed (Fig. 3). Assuming this observation might reflect an appropriate colocalization of signaling-sensitive determinants, we predicted additional signaling-sensitive amino acids within ECL1 in the neighborhood of a known CAM. Indeed, we identified a new partially inactivating mutation and a new CAM in ECL1 by mutagenesis studies (Table 2). The proximity of inactivating and constitutively activating mutations within each ECL of the TSHR is similar to results reported previously for epitopes of the hinge region (23) and supports the critical dual functionality of ECLs as well as the ability of ECD to promote induction and conduction of receptor activation (Figs. 4and 5B).
This is consistent with the homology model of the SD of TSHR in which inactivating residues within ECLs are positioned in exposed locations for possible interactions with the hinge region (Fig. 4). Therefore, our data support a structural and functional interplay between the ectodomain, especially the hinge region, and ECLs in the TSHR (1, 58). In contrast to inactivating positions, residues of CAMs within the ECLs of our structural model are more buried and likely constrain the loop conformation. Positions of inactivating and activating mutations are in proximity by sequence but differently clustered in molecular space and likely thus different in function. The functional interrelations between positions causing signal induction and conduction are also indicated by previous studies of double mutations combining constitutively activating and inactivating mutations (22) that revealed a dominant effect of inactivating mutations. In this case, the mutation can no longer form a constitutively active conformation, since activation is prevented by the lack of an appropriate side chain needed to establish an active conformation.
Cooperative effects of the ECLs lead to synergistic amplification of the signal
The level of constitutive activity for single, double, and triple CAMs in the ECLs reveals a stepwise and finally synergistic enhancement in cAMP signaling (Fig. 1). The finding that the CAM combinations in all three ECLs of the TSHR led to strong synergistic signaling supports the collective importance of each ECL for switching the receptor from the basal to the activated conformation (Fig. 5C). The strongest contribution is provided by ECL1. The stepwise decrease in TSH activation of most double CAM mutations and the absence of TSH activation in the triple ECL-CAM mutation suggest an activation state approaching full signaling (Fig. 2). This conclusion is supported by the result that I568V/V656F with only an additive increase in constitutive activity is more activated by TSH than multiple CAMs showing synergistic constitutive activation. In addition, the triple ECL-CAM exhibits much lower cell surface expression than wild-type TSHR, further suggesting that the lower absolute activity of this receptor might reflect a fully active conformation. These results support the suggestion that for the triple ECL-CAM, multiple signal triggering events are conducted through the TMHs to yield a fully or near fully active receptor (Figs. 2and 5C).
Following from this interpretation for single CAMs that lead to partial receptor activation, we suggest a local and limited event affecting the TMHs adjacent to the CAM location (Fig. 5B). The hypotheses of simultaneous effects at different structural locations in the extracellular portion for full signaling activity might also help us to understand the special phenotype of specific CAMs with drastic alterations. Such single CAMs S281L and I486F at known signaling-sensitive positions in the extracellular region are reported to activate the TSHR strongly or nearly TSH like (20, 22, 41, 59). It is suggested for the LHCGR (60, 61) as well as for the TSHR (ref. 32; also see Supplemental Data) that the amino acid S281 (TSHR S281 and LHCGR S277) is spatially located centrally between the ECLs in the structural vicinity of the ECLs. Proximity and a tight spatial packing between S281/ECL1/ECL2 are suggested and confirmed experimentally (60, 61). Fragments of the hinge region most likely function together with portions of the ECLs as a structural entity. Therefore, certain drastic side-chain alterations in bulk and shape (e.g., S281L) at these signaling-sensitive locations cause a very strong sterical/conformational influence on the neighboring structural fragments (such as the ECLs). This is mainly due to the tightly packed environment. It finally leads to multiple simultaneous signaling effects via several signal conductors.
The finding of multiple activation-signal propagation may also reveal new information about the phenotype of partially inactivating mutations for TSH-mediated signaling. Most likely, the wild-type amino acids at the positions of inactivating mutants facilitate active conformations for the signal conduction process. A large number of mutational experiments at the TSHR show that hormone-induced Gαs-mediated signaling is only partially interrupted by mutations in the ECLs (Figs. 3and 4; ref. 2). As discussed above for single CAMs, we also propose a local interruption of a multicomponent signaling process for the partial inactivation of TSH-mediated signaling by inactivating mutations. Thus, for each of these single-point mutations, signaling is neither fully blocked nor fully activated (Fig. 5B).
New scenario of extracellular activation mechanism for glycoprotein hormone receptors
Our data of cooperative and synergistic receptor activation by multiple CAMs also support a similar extracellular mechanism for TSH-mediated activation of the TSHR as follows (Fig. 5D). Hormone-induced activation is initiated by multiple contacts between hormones and the LRRD and simultaneously also between hormones and the hinge region, subsequently inducing the hinge region to an activating conformation. Accordingly, the hinge region transmits the signal to the ECLs, likely via several trigger points at the positions of inactivating mutations within the ECLs (open black ovals, Fig. 5D; red balls, Fig. 4). Thereafter, the cooperative effect of defined determinants at all ECLs amplifies the signal synergistically via a widespread propagation of multiple conformational changes toward the connected TMH bundle. Interestingly, such cooperative effects on the extracellular side of the TSHR correspond to the mirror image of previously described cooperative effects of three modified peripheral loops in superactive (binding and signaling) TSH analogs (62).
A functional interrelation between extracellular components and the SD in different activity states is not restricted to the GPHRs. For example, the N-terminal tail of the melanocortin-4 GPCR functions as a tethered agonist by interactions with the ECLs (63). Furthermore, the rhodopsin N-terminal domain makes contacts with the ECLs and an interaction with ECL3 was shown to be functionally important (64). Critical contributions of N-terminal extracellular domains in agonist binding and activation for family B receptors of GPCRs, like secretin, vasoactive intestinal polypeptide, and corticotropin releasing factor receptors were shown (65).
Taken together, we report here the synergistic activation caused by multiple CAMs in the extracellular region of the TSHR, representing a cooperative role for the ECLs in ligand-independent and most probably ligand-dependent signaling for a GPCR. The proximity of activating and inactivating mutations but different spatial orientations of their wild-type side chains within each ECL supports a dual functionality to promote signal induction and conduction, respectively. Since the identified critical residues are highly conserved among the glycoprotein hormone receptors, the current study also reveals implications for the homologous gonadotrophic receptors (FSHR and LHCGR) and potentially also for the leucine-rich repeat containing GPCR.
Acknowledgments
We thank S. Fiedler and E. Bösenberg for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (KR 1273/1–1 and PA 423 /12–1); by a Formel-1 grant (NML Formel.1–98) of the Medical Faculty, University of Leipzig; and by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
References
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kleinau G, Brehm M, Wiedemann U, Labudde D, Leser U, Krause G. Implications for molecular mechanisms of glycoprotein hormone receptors using a new sequence-structure-function analysis resource. Mol Endocrinol. 2007;21:574–580. doi: 10.1210/me.2006-0309. [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk G D, Jaume J C, McLachlan S M. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- Braun T, Schofield P R, Sprengel R. Amino-terminal leucine-rich repeats in gonadotropin receptors determine hormone selectivity. EMBO J. 1991;10:1885–1890. doi: 10.1002/j.1460-2075.1991.tb07714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick N, Huang J, Puett D, Isaacs N W, Lapthorn A J. Determination of residues important in hormone binding to the extracellular domain of the luteinizing hormone/chorionic gonadotropin receptor by site-directed mutagenesis and modeling. Mol Endocrinol. 1996;10:1147–1159. doi: 10.1210/mend.10.9.8885249. [DOI] [PubMed] [Google Scholar]
- Bhowmick N, Narayan P, Puett D. Identification of ionizable amino acid residues on the extracellular domain of the lutropin receptor involved in ligand binding. Endocrinology. 1999;140:4558–4563. doi: 10.1210/endo.140.10.7077. [DOI] [PubMed] [Google Scholar]
- Vischer H F, Granneman J C, Noordam M J, Mosselman S, Bogerd J. Ligand selectivity of gonadotropin receptors. Role of the beta-strands of extracellular leucine-rich repeats 3 and 6 of the human luteinizing hormone receptor. J Biol Chem. 2003;278:15505–15513. doi: 10.1074/jbc.M300634200. [DOI] [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22:2692–2703. doi: 10.1093/emboj/cdg260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galet C, Ascoli M. The differential binding affinities of the luteinizing hormone (LH)/choriogonadotropin receptor for LH and choriogonadotropin are dictated by different extracellular domain residues. Mol Endocrinol. 2005;19:1263–1276. doi: 10.1210/me.2004-0410. [DOI] [PubMed] [Google Scholar]
- Lin W, Bernard M P, Cao D, Myers R V, Kerrigan J E, Moyle W R. Follitropin receptors contain cryptic ligand binding sites. Mol Cell Endocrinol. 2007;260–262:83–92. doi: 10.1016/j.mce.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd J. Ligand-selective determinants in gonadotropin receptors. Mol Cell Endocrinol. 2007;260–262:144–152. doi: 10.1016/j.mce.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Fan Q R, Hendrickson W A. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:203–204. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q R, Hendrickson W A. Assembly and structural characterization of an authentic complex between human follicle stimulating hormone and a hormone-binding ectodomain of its receptor. Mol Cell Endocrinol. 2007;260–262:73–82. doi: 10.1016/j.mce.2005.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudermann T, Nurwakagari P, Ben-Menahem D. Hormone binding to the follicle-stimulating hormone receptor–crystal clear! Exp Clin Endocrinol Diabetes. 2005;113:245–247. doi: 10.1055/s-2005-865679. [DOI] [PubMed] [Google Scholar]
- Sanders J, Bolton J, Sanders P, Jeffreys J, Nakatake N, Richards T, Evans M, Kiddie A, Summerhayes S, Roberts E, Miguel R N, Furmaniak J, Smith B R. Effects of TSH receptor mutations on binding and biological activity of monoclonal antibodies and TSH. Thyroid. 2006;16:1195–1206. doi: 10.1089/thy.2006.16.1195. [DOI] [PubMed] [Google Scholar]
- Sanders J, Miguel R N, Bolton J, Bhardwaja A, Sanders P, Nakatake N, Evans M, Furmaniak J, Smith B R. Molecular interactions between the TSH receptor and a thyroid-stimulating monoclonal autoantibody. Thyroid. 2007;17:699–706. doi: 10.1089/thy.2007.0041. [DOI] [PubMed] [Google Scholar]
- Morgenthaler N G, Ho S C, Minich W B. Stimulating and blocking thyroid-stimulating hormone (TSH) receptor autoantibodies from patients with Graves’ disease and autoimmune hypothyroidism have very similar concentration, TSH receptor affinity, and binding sites. J Clin Endocrinol Metab. 2007;92:1058–1065. doi: 10.1210/jc.2006-2213. [DOI] [PubMed] [Google Scholar]
- Sanders J, Chirgadze D Y, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel R N, Blundell T L, Furmaniak J, Smith B R. Crystal structure of the TSH Receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17:395–410. doi: 10.1089/thy.2007.0034. [DOI] [PubMed] [Google Scholar]
- Sangkuhl K, Schulz A, Schultz G, Schoneberg T. Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J Biol Chem. 2002;277:47748–47755. doi: 10.1074/jbc.M203491200. [DOI] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V, Ho S C, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16:736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- Karges B, Gidenne S, Aumas C, Haddad F, Kelly P A, Milgrom E, de Roux N. Zero-length cross-linking reveals that tight interactions between the extracellular and transmembrane domains of the LH receptor persist during receptor activation. Mol Endocrinol. 2005;19:2086–2098. doi: 10.1210/me.2004-0378. [DOI] [PubMed] [Google Scholar]
- Neumann S, Claus M, Paschke R. Interactions between the extracellular domain and the extracellular loops as well as the 6th transmembrane domain are necessary for TSH receptor activation. Eur J Endocrinol. 2005;152:625–634. doi: 10.1530/eje.1.01891. [DOI] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Neumann S, Krause G, Paschke R. Significance of ectodomain cysteine boxes 2 and 3 for the activation mechanism of the thyroid-stimulating hormone receptor. J Biol Chem. 2006;281:31638–31646. doi: 10.1074/jbc.M604770200. [DOI] [PubMed] [Google Scholar]
- Nurwakagari P, Breit A, Hess C, Salman-Livny H, Ben-Menahem D, Gudermann T. A conformational contribution of the luteinizing hormone-receptor ectodomain to receptor activation. J Mol Endocrinol. 2007;38:259–275. doi: 10.1677/jme.1.02160. [DOI] [PubMed] [Google Scholar]
- Zhang M L, Sugawa H, Kosugi S, Mori T. Constitutive activation of the thyrotropin receptor by deletion of a portion of the extracellular domain. Biochem Biophys Res Commun. 1995;211:205–210. doi: 10.1006/bbrc.1995.1797. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tong K P, Fremont V, Chen J, Narayan P, Puett D, Weintraub B D, Szkudlinski M W. The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology. 2000;141:3514–3517. doi: 10.1210/endo.141.9.7790. [DOI] [PubMed] [Google Scholar]
- Ho S C, Van Sande J, Lefort A, Vassart G, Costagliola S. Effects of mutations involving the highly conserved S281HCC motif in the extracellular domain of the thyrotropin (TSH) receptor on TSH binding and constitutive activity. Endocrinology. 2001;142:2760–2777. doi: 10.1210/endo.142.7.8246. [DOI] [PubMed] [Google Scholar]
- Ho S C, Goh S S, Su Q, Khoo D H. Cysteine 390 mutation of the TSH receptor modulates its ectodomain as an inverse agonist on the serpentine domain with decrease in basal constitutive activity. Mol Cell Endocrinol. 2005;245:158–168. doi: 10.1016/j.mce.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ban T, Akamizu T, Kohn L D. Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J Biol Chem. 1991;266:19413–19418. [PubMed] [Google Scholar]
- Costagliola S, Panneels V, Bonomi M, Koch J, Many M C, Smits G, Vassart G. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002;21:504–513. doi: 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi M, Busnelli M, Persani L, Vassart G, Costagliola S. Structural differences in the “hinge region” of the glycoprotein-hormone receptors: evidences from the sulfated tyrosine residues. Mol Endocrinol. 2006;20:3351–3363. doi: 10.1210/me.2005-0521. [DOI] [PubMed] [Google Scholar]
- Kleinau G, Jaschke H, Neumann S, Lattig J, Paschke R, Krause G. Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem. 2004;279:51590–51600. doi: 10.1074/jbc.M404748200. [DOI] [PubMed] [Google Scholar]
- Chen C R, Chazenbalk G D, McLachlan S M, Rapoport B. Evidence that the C terminus of the A subunit suppresses thyrotropin receptor constitutive activity. Endocrinology, 2003;144:3821–3827. doi: 10.1210/en.2003-0430. [DOI] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jäschke H, Neumann S, Krause G, Paschke R. Significance of the Hinge region of the TSHR for receptor activation and hormone binding. Exp Clin Endocrinol Diabetes. 2007;115:33. [Google Scholar]
- Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J Clin Endocrinol Metab. 2000;85:2281–2286. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- Mueller T, Gromoll J, Simoni M. Absence of exon 10 of the human luteinizing hormone (LH) receptor impairs LH, but not human chorionic gonadotropin action. J Clin Endocrinol Metab. 2003;88:2242–2249. doi: 10.1210/jc.2002-021946. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M W, Teh N G, Grossmann M, Tropea J E, Weintraub B D. Engineering human glycoprotein hormone superactive analogues. Nat Biotechnol. 1996;14:1257–1263. doi: 10.1038/nbt1096-1257. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M W, Fremont V, Ronin C, Weintraub B D. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- Kleinau G, Claus M, Jaeschke H, Mueller S, Neumann S, Paschke R, Krause G. Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid stimulating hormone receptor. J Biol Chem. 2007;282:518–525. doi: 10.1074/jbc.M606176200. [DOI] [PubMed] [Google Scholar]
- Claus M, Jaeschke H, Kleinau G, Neumann S, Krause G, Paschke R. A hydrophobic cluster in the center of the third extracellular loop is important for thyrotropin receptor signaling. Endocrinology. 2005;146:5197–5203. doi: 10.1210/en.2005-0713. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Neumann S, Kleinau G, Mueller S, Claus M, Krause G, Paschke R. An aromatic environment in the vicinity of serine281 is a structural requirement for TSH receptor function. Endocrinology. 2006;147:1753–1760. doi: 10.1210/en.2005-1138. [DOI] [PubMed] [Google Scholar]
- Ballesteros J A, Jensen A D, Liapakis G, Rasmussen S G, Shi L, Gether U, Javitch J A. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke C A, Motoshima H, Fox B A, Le Trong I, Teller D C, Okada T, Stenkamp R E, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Salom D, Lodowski D T, Stenkamp R E, Trong I L, Golczak M, Jastrzebska B, Harris T, Ballesteros J A, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian D D, Schertler G F. Crystal structure of a thermally stable rhodopsin mutant. J Mol Biol. 2007;372:1179–1188. doi: 10.1016/j.jmb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S G, Choi H J, Rosenbaum D M, Kobilka T S, Thian F S, Edwards P C, Burghammer M, Ratnala V R, Sanishvili R, Fischetti R F, Schertler G F, Weis W I, Kobilka B K. Crystal structure of the human beta(2) adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum D M, Hanson M A, Rasmussen S G, Thian F S, Kobilka T S, Choi H J, Kuhn P, Weis W I, Kobilka B K, Stevens R C. High-resolution crystal structure of an engineered human β2-adrenergic G protein coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Trkola A, Lin S W, Nagashima K A, Kajumo F, Zhao L, Olson W C, Wu L, Mackay C R, Allaway G P, Sakmar T P, Moore J P, Maddon P J. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol. 1998;72:279–285. doi: 10.1128/jvi.72.1.279-285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Durell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- Aarons E J, Beddows S, Willingham T, Wu L, Koup R A. Adaptation to blockade of human immunodeficiency virus type 1 entry imposed by the anti-CCR5 monoclonal antibody 2D7. Virology. 2001;287:382–390. doi: 10.1006/viro.2001.1046. [DOI] [PubMed] [Google Scholar]
- Case D A, Pearlman D A, Caldwell J W, Cheatham T E, III, Wang J, Ross W S, Simmerling C L, Darden T A, Merz K M, Stanton R V, Cheng A L, Vincent J J, Crowley M, Tsui V, Gohlke H, Radmer R J, Duan Y, Pitera J, Massova I, Seibel G L, Singh U C, Weiner P K, Kollman P A. San Francisco, CA, USA: University of California; AMBER 7. 2002 [Google Scholar]
- Laskowski R A, Moss D S, Thornton J M. Main-chain bond lengths and bond angles in protein structures. J Mol Biol. 1993;231:1049–1067. doi: 10.1006/jmbi.1993.1351. [DOI] [PubMed] [Google Scholar]
- Claus M, Maier J, Paschke R, Kujat C, Stumvoll M, Fuhrer D. Novel thyrotropin receptor germline mutation (Ile568Val) in a Saxonian family with hereditary nonautoimmune hyperthyroidism. Thyroid. 2005b;15:1089–1094. doi: 10.1089/thy.2005.15.1089. [DOI] [PubMed] [Google Scholar]
- Parma J, Van Sande J, Swillens S, Tonacchera M, Dumont J, Vassart G. Somatic mutations causing constitutive activity of the thyrotropin receptor are the major cause of hyperfunctioning thyroid adenomas: identification of additional mutations activating both the cyclic adenosine 3′,5′-monophosphate and inositol phosphate-Ca2+ cascades. Mol Endocrinol. 1995;9:725–733. doi: 10.1210/mend.9.6.8592518. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Van Sande J, Cetani F, Swillens S, Schvartz C, Winiszewski P, Portmann L, Dumont J E, Vassart G, Parma J. Functional characteristics of three new germline mutations of the thyrotropin receptor gene causing autosomal dominant toxic thyroid hyperplasia. J Clin Endocrinol Metab. 1996;81:547–554. doi: 10.1210/jcem.81.2.8636266. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Holzapfel H P, Wonerow P, Scherbaum W A, Paschke R. Somatic mutations in the thyrotropin receptor gene and not in the Gs alpha protein gene in 31 toxic thyroid nodules. J Clin Endocrinol Metab. 1997;82:3885–3891. doi: 10.1210/jcem.82.11.4382. [DOI] [PubMed] [Google Scholar]
- Parnot C, Miserey-Lenkei S, Bardin S, Corvoi P, Clauser E. Lessons from the constitutively active mutants of G-protein coupled receptors. Trends Endocrin Metabol. 2002;13:8336–8343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- Rapoport B, McLachlan S M. The thyrotropin receptor in Graves’ disease. Thyroid. 2007;17:911–922. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Govaerts C, Lefort A, Van Sande J, Costagliola S, Pardo L, Vassart G. A conserved Asn in TM7 of the thyrotropin receptor is a common requirement for activation by both mutations and its natural agonist. FEBS Lett. 2002;517:195–200. doi: 10.1016/s0014-5793(02)02620-0. [DOI] [PubMed] [Google Scholar]
- Nishi S, Nakabayashi K, Kobilka B, Hsueh A J. The ectodomain of the luteinizing hormone receptor interacts with exoloop 2 to constrain the transmembrane region: studies using chimeric human and fly receptors. J Biol Chem. 2002;277:3958–3964. doi: 10.1074/jbc.M109617200. [DOI] [PubMed] [Google Scholar]
- Nakabayashi K, Kudo M, Hsueh A J, Maruo T. Activation of the luteinizing hormone receptor in the extracellular domain. Mol Cell Endocrinol. 2003;202:139–144. doi: 10.1016/s0303-7207(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Leitolf H, Tong K P, Grossmann M, Weintraub B D, Szkudlinski M W. Bioengineering of human thyrotropin superactive analogs by site-directed “lysine-scanning” mutagenesis. Cooperative effects between peripheral loops. J Biol Chem. 2000;275:27457–27465. doi: 10.1074/jbc.M003707200. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin B R, Vaisse C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson Z, Wheatley M. The third extracellular loop of G-protein-coupled receptors: more than just a linker between two important transmembrane helices. Biochem Soc Trans. 2004;32:1048–1050. doi: 10.1042/BST0321048. [DOI] [PubMed] [Google Scholar]
- Holtmann M H, Hadac E M, Miller L J. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. Studies of chimeric receptors. J Biol Chem. 1995;270:14394–14398. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]