Abstract
DYX1C1 was first identified as a candidate gene for dyslexia susceptibility, and its role in controlling neuronal migration during embryogenesis and effect on learning in rodents have been verified. In contrast, genetic association studies have been ambiguous in replicating its effects on dyslexia. To better understand the regulation of DYX1C1 and the possible functional role of genetic variation in the promoter of DYX1C1, we selected three single-nucleotide polymorphisms (SNPs) with predicted functional consequences or suggested associations to dyslexia for detailed study. Electrophoretic mobility shift assays suggested the allele-specific binding of the transcription factors TFII-I (to rs3743205) and Sp1 (to rs16787 and rs12899331) that could be verified by competition assays. In addition, we purified a complex of protein factors binding to the previously suggested dyslexia-related SNP, −3G/A (rs3743205). Three proteins, TFII-I, PARP1, and SFPQ, were unambiguously identified by mass spectrometry and protein sequencing. Two SNPs, rs16787 and rs3743205, showed significant allelic differences in luciferase assays. Our results show that TFII-I, PARP1, and SFPQ proteins, each previously implicated in gene regulation, form a complex controlling transcription of DYX1C1. Furthermore, allelic differences in the promoter or 5′ untranslated region of DYX1C1 may affect factor binding and thus regulation of the gene.—Tapia-Páez, I., Tammimies, K., Massinen, S., Roy. A. L., Kere, J. The complex of TFII-I, PARP1, and SFPQ proteins regulates the DYX1C1 gene implicated in neuronal migration and dyslexia.
Keywords: complex disease, regulation of transcription, learning disability, reading impairment
Reading and writing, like language, are complex human-specific cognitive activities. Dyslexia is a disorder characterized by learning deficits in reading and writing, despite conventional instruction, adequate intelligence and sociocultural opportunity. It is estimated that 5–10% of children will experience significant difficulties in learning to read as a result of dyslexia (1).
Genetic linkage studies, whole-genome linkage scans, and association studies combined with identification of chromosomal translocation breakpoints in dyslexic families have suggested different regions of the genome to be involved in the etiology of dyslexia. Altogether, 9 loci, DYX1–DYX9, on chromosomes 1, 2, 3, 6, 11, 15, 18, and X, have been acknowledged by the Human Gene Nomenclature Committee. Since the publication of DYX1C1 as the first candidate dyslexia gene (2), significant progress has been made in the identification of new candidate genes. In only 3 yr, 5 genes have been associated with dyslexia, namely ROBO1 (3), KIAA0319 (4, 5), DCDC2 (6, 7), C2ORF3, and MRPL19 (8). Interestingly, ROBO1 is an axon and dendrite guidance gene, and studies using in utero RNA interference (RNAi) have shown that Dyx1c1, Kiaa0319, and Dcdc2 are necessary for the normal neuronal migration in mouse and rat neocortex (5, 6, 9). Most recent studies show also that RNAi of Dyx1c1 in rats may cause cortical and hippocampal malformations and impairments in auditory processing and spatial learning (10, 11). The neuroanatomical findings are similar to small neocortical malformations in human dyslexic individuals (12, 13). Little is known about the function of DYX1C1 and mechanisms by which it regulates neuronal migration or dyslexia. The DYX1C1 protein was suggested as a new chaperone or cochaperone in the ubiquitin pathway system that interacts with the U-box protein CHIP (encoded by the STUB1 gene), belonging to a family of ubiquitin-protein ligases (E3s) (14).
The DYX1C1 gene is located near to the region previously pointed in a number of linkage studies (15,16,17), and it was cloned by study of a Finnish family in which the translocation t(2;15)(q11;q21) cosegregated coincidentally with dyslexia (2, 18). Sequence analysis of DYX1C1 cDNA in 20 dyslexic individuals revealed 8 polymorphisms, two of them associated with dyslexia; one of the changes (1249 G/T) introduced a stop codon truncating the protein by 4 amino acids. The second change was in the 5′UTR −3G/A (rs3743205), close to the translation initiation site. The genomic size of the DYX1C1 gene is ∼78 kb, and DYX1C1 contains 10 exons with the start codon in exon 2, encoding a 420-aa protein with a molecular mass of 48 kDa and without major homology to other proteins of known function. However, three tetratricopeptide repeat domains (TPRs) were found toward the C-terminal and a p23 domain toward the N-terminal end, which are thought to play a role in protein-protein interactions (Fig. 1). Cellular localization studies in COS-1 cells suggested that DYX1C1 is a nuclear protein (2), but later it has been found to be present also in the cytoplasm (9).
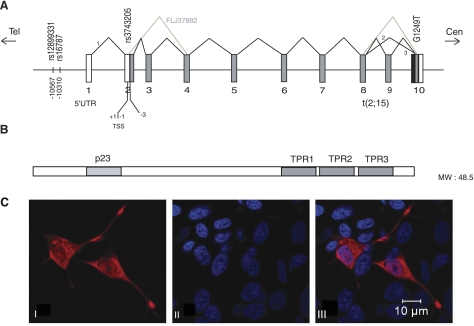
Figure 1.
A) DYX1C1 gene structure and transcripts according to the Genomatix and National Center for Biotechnology Information (NCBI) databases. TSS, translation start site. Numbers 1–10 denote exons. In the top part, 1, 2, 3, and FLJ37882 denote different splice variants of DYX1C1. The SNPs associated to dyslexia are indicated according to their position in the gene. B) Protein structure of DYX1C1. TPR and p23 domains are denoted as dark boxes. C) Cellular localization of DYX1C1 protein: I) SH-SY5Y cells transfected with DYX1C1-V5 fusion construct (2); II) DAPI-stained nuclei (blue); III) merged image from I and II.
A number of attempts have been made to replicate the genetic association of DYX1C1 with dyslexia in other populations, with variable results (4, 19,20,21,22,23). As many observations suggest that regulation, rather than coding changes, may be important for DYX1C1 function in humans, we resequenced the promoter region. In particular, two single-nucleotide polymorphisms (SNPs), rs12899331 and rs16787, in the positions –10567 T/C and –10310 C/A, respectively, were associated with dyslexia (F. Dahdouh et al., personal communication). In the present study, we characterize three possibly functional SNPs in the promoter of DYX1C1 and implicate three transcription factors in the regulation of DYX1C1.
MATERIALS AND METHODS
Preparation of total and nuclear cell extracts
Human neuroblastoma SH-SY5Y cells were grown as described previously (42). At 100% confluence, the cells were washed twice with cold PBS, and nuclear and total extracts were prepared essentially as described previously (43). For nuclear extracts, the cells from 75-mm-diameter dishes were collected by centrifugation and resuspended in approximately 3 times the packed cell volume in hypotonic buffer [10 mM Tris–HCl, pH 7.4; 10 mM NaCl; 6 mM MgCl2; 1 mM dithiothreitol (DTT); 0.4 mM PMSF; 10 mM NaF; 1 mM Na3VO4; and protease inhibitor cocktail] and incubated on ice for 10 min. The cells were lysed in a Dounce homogenizer (pestle B; Wheaton, Millville, NJ, USA), and the nuclei were collected by centrifugation followed by resuspension in 3 vol of high-salt buffer (20 mM HEPES, pH 7.9; 420 mM NaCl; 20% glycerol; 1.5 mM MgCl2; 0.2 mM EDTA; 0.2 mM PMSF; 1 mM DTT; 1 mM Na3VO4; 10 mM NaF; and protein synthesis inhibitors as above). After 30 min at 4°C, the pellets were removed by centrifugation, and supernatants containing the nuclear proteins were stored at −70°C.
For total extracts, cells were harvested in 1 ml RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% Triton X-100; 150 mM NaCl; 5 mM EDTA; 1 mM PMSF; 1 mM Na3VO4; 1 mM DTT; and protease inhibitors) for 30 min (43). Cell lysates were centrifuged at 14,000 g for 15 min, and the resulting supernatants were collected. Protein concentrations of the nuclear and total extracts were measured with the protein assay (Bio-Rad Laboratories, Hercules, CA, USA) with BSA as standard (44).
Cell culture and transfections
Human neuroblastoma SH-SY5Y cells were cultured in modified essential medium (MEM) with Earle’s salts and glutaMAX-I, supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% (v/v) CO2/air. For the transfections used for quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), cells were cultured in phenol red-free MEM medium with 4% of dextran-charcoal-treated serum. Cell culture reagents were obtained from Gibco Invitrogen Corporation (Täby, Sweden). Untransfected cells and cells transfected with empty vectors were used as controls in all the experiments. The transfections were performed either with FuGene6 (Roche, Indianapolis, IN, USA) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), following the recommendations from the manufacturers.
For cellular localization experiments, SH-SY5Y cells were transiently transfected with DYX1C1-V5 fusion construct and stained with mouse anti-V5 antibody (Invitrogen) and Alexa Fluor 555-conjugated anti-mouse-IgG (Invitrogen). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO, USA). Fluorescently labeled samples were mounted with ProLong Gold Antifade Reagent (Invitrogen). The immunofluorescence images were acquired with the Meta LSM 510 confocal imaging system and an Axiovert 200 M Zeiss microscope (Carl Zeiss, Oberkochen, Germany), using an ×63 1.4 numerical aperture objective and multichannel scanning in frame mode.
Electrophoretic mobility shift assay (EMSA)
The EMSA was performed according to standard protocols (45). Binding reactions were performed by preincubating 10 μg of SH-SY5Y of nuclear or total extracts with 0.5 μg of poly (dI-dC) and 1 μl of 100 mM DTT in a 30 μl reaction with a 4× buffer containing 80 mM HEPES (pH 7.6), 2 mM EDTA, 200 mM NaCl, 20% glycerol, 1.2 mg BSA, and 40 mM DTT for 15 min at room temperature. 32P-end-labeled double-stranded probes were used, and the mixture was incubated for 15 min at room temperature. In competition assays before the addition of the probes, unlabeled probes were incubated with a 100- and 200-M excess for 15 min at room temperature. The samples were electrophoresed on 5% nondenaturing polyacrylamide gels in 1× TBE (0.09 M Tris borate, 2 mM EDTA) at 240 V at 4°C for 3 h. The radioactive pattern was visualized by autoradiography using PhosphorImager scanning (Fuji Photo Film Co., Ltd., Stamford, CT, USA). The oligonucleotides used in the EMSA were as follows: rs3743205.G: 5′-CTC CCG TTG CTA CCG GAA TGC CTC TTC AGG-3′, 5′-CCT GAA GAG GCA TTC CGG TAG CAA CGG GAG-3′; rs3743205.A: 5′-CTC CCG TTG CTA CCA GAA TGC CTC TTC AGG-3′, 5′-CCT GAA GAG GCA TTC TGG TAG CAA CGG GAG-3′; rs12899331.T: 5′-AAT ACA CCT GTC AGT CTC CTT CAT GCT TTC-3′, 5′-GAA AGC ATG AAG GAG ACT GAC AGG TGT ATT-3′; rs12899331.C: 5′-AAT ACA CCT GTC AGC CTC CTT CAT GCT TTC-3′, 5′-GAA AGC ATG AAG GAG GCT GAC AGG TGT ATT-3′; rs16787.C: 5′-TCA AAA AAT ACC TAT CGG GCC GGG CGC AGT-3′, 5′-ACT GCG CCC GGC CCG ATA GGT ATT TTT TGA-3′; rs16787.A: 5′-TCA AAA AAT ACC TAT AGG GCC GGG CGC AGT-3′, 5′-ACT GCG CCC GGC CCT ATA GGT ATT TTT TGA-3′. Probes for competition assays were as follows: SRE: 5′-AAT TCT CCT TTA CAC AGG ATG TCC ATA TTA GGA CAT CTC-3′, 5′-GAG ATG TCC TAA TAT GGA CAT CCT GTG TAA AGG AGA ATT-3′; and Sp1 consensus probe (Santa Cruz Biotechnology, Santa Cruz, CA, USA): 5′-ATT CGA TCG GGG CGG GGC GAG C-3′, 5′-G CTC GCC CCG CCC CGA TCG AAT-3′
Western blot analysis
Protein extracts derived from the SH-SY5Y cells were separated on 12% Bis-Tris SDS-PAGE gels (Invitrogen) in NuPAGE N-morpholino propane sulfonic acid (MOPS) sodium dodecyl sulfate (SDS) running buffer and electroblotted to polyvinylidene difluoride (PVDF) Hybond-P transfer membranes (GE Healthcare Bio-Sciences, Little Chalfont, UK). After transfer of proteins, the filters were blocked for unspecific protein binding by 1 h incubation at RT of 5% nonfat dry milk in 1% Tween-PBS followed by incubation of anti-TFII-I antibody (Transduction Laboratories, Lexington, KY, USA; 1:2500). Filters were washed 4 times in Tween-PBS for 15 min and 3 times for 5 min before incubation with ECL anti-mouse horseradish peroxidase (HRP) (GE Healthcare) (1:2×106). Filters were again washed at the same conditions as above, and detection was performed using the ECL Advance Western blotting detection kit (GE Healthcare). Anti-β-actin antibodies (Abcam, Cambridge, UK; 1:5000) were used for normalization.
Protein purification and protein sequencing
To purify the proteins bound to the EMSA probe, we used the DNA-binding protein purification kit (Roche, Indianapolis, IN, USA). In brief, the rs3743205G probe (double 60-mer) was concatenated by PCR and attached to magnetic beads by ligation to tethered oligonucleotides. The oligonucleotides were bound to the beads by streptavidin-biotin affinity. Total and nuclear extracts from SH-SYS5 cells were incubated with the beads followed by several washing steps. The extractions were performed several times, and the extracts eluted were microdialysed to remove the high concentrations of salts. To monitor the purification and the amount of proteins, the extractions were SDS-PAGE electrophoresed and stained with GelCode SilverSNAP Stain (Pierce Biotechnology, Rockford, IL, USA). The final extracts were further separated into a 10% SDS-PAGE to distinguish them from residual contaminating proteins and stained with GelCode Blue Stain (Pierce Biotechnology). Two bands were isolated and further analyzed by Ingel digestion followed by peptide sequencing. Briefly, stained protein bands were cut from the gel, and the pieces were digested using the MassPREP robotic protein handling system (Waters, Millford, MA, USA); washing was carried out in 50 mM ammonium bicarbonate containing 50% acetonitrile. The protein was reduced (DTT) and alkylated (iodoacetamide) followed by ingel digestion with 0.3 μg trypsin (modified, Promega, Madison, WI, USA) in 50 mM ammonium bicarbonate for 5 h at 40°C. The tryptic peptides were extracted with 1% formic acid/2% acetonitrile, followed by 50% acetonitrile twice. The acetonitrile was evaporated, and the peptide extract was concentrated under a stream of nitrogen to 5–10 μl for injection onto the LC-MS/MS system (CapLC chromatography system and Q-Tof Ultima API mass spectrometer, Waters, Millford, MA, USA). The peptides were separated on a Waters Atlantis C18 column, and the effluent was electrosprayed into the mass spectrometer via a PicoTip emitter (New Objective, Woburn, MA, USA). Data were collected using Data Dependent Acquisition (DDA, Waters) over the mass range 300–2000 m/z. Data analysis was performed using ProteinLynx Global SERVER 2.1 (PLGS 2.1, Waters) software and MassLynx peptide sequence software 4.0 (Waters), combined with use of the BLAST search engine (National Center for Biotechnology Information, Bethesda, MD, USA). Primers for probe rs3743205G, 60-mer: 5′-CTC CCG TTG CTA CCG GAA TGC CTC TTC AGG CTC CCG TTG CTA CCG GAA TGC CTC TTC AGG-3′; 5′-CCT GAA GAG GCA TTC CGG TAG CAA CGG GAG CCT GAA GAG GCA TTC CGG TAG CAA CGG GAG-3′.
Plasmid constructs
The luciferase assay constructs were prepared using the same sequences for the EMSA probes but duplicated to enhance the binding of the factors regulating the luciferase expression (see Fig. 3). Double-stranded probes from both alleles of the rs3743205, rs12899331, and rs16787 (60-mer) were blunt-end cloned into the pGL3 basic and pGL3 promoter vectors (Promega). The pcDNA3-flag-ERa expression construct was a kind gift of Eckard Treuter and the TFII-I Δ and TFII-I β were cloned in mammalian expression vector pEBG, as described previously (46). The cloning of DYX1C1 in pcDNA3.1 has been described previously (2). Full-length cDNA from poly (ADP-ribose) polymerase 1 (PARP1) and splicing factor proline and glutamine-rich (SFPQ) were cloned into pcDNA3.1 expression vector (Invitrogen). To obtain the −3A DYX1C1 construct, the QuickChange II Site-Directed Mutagenesis kit was used according to manufacturer’s instructions (Stratagene, La Jolla, CA, USA).
Figure 2.
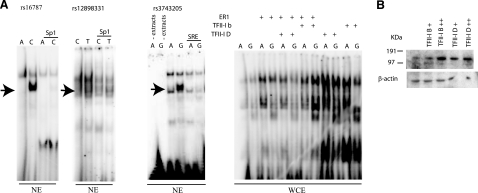
A) EMSA performed by using fragments encompassing the associated polymorphisms as probes. Whole cell extracts (WCE) and nuclear extracts (NE) from neuroblastoma SH-SY5Y cells were used. Arrows show differences in retardation for all three SNPs. An Sp1 consensus (Santa Cruz Biotechnology; sc-2502) was used to compete the binding of factors to rs12899331 and rs16787, as TESS predicted. A probe known to bind to TFII-I was used for competition to TFII-I (SRE; see Materials and Methods). Extracts from cells transiently transfected with TFII-I β, TFII-I Δ, and ER1 were used as positive controls for the binding of the rs3743205. B) Western blot analysis of TFII-I in SH-SY5Y cells. Endogenous levels and transiently transfected cells with 1 μg (+) or 2 μg (++), detection performed with TFII-I antibody (Transduction Laboratories). β-Actin (Santa Cruz Biotechnology) was used as internal loading control.
Figure 3.
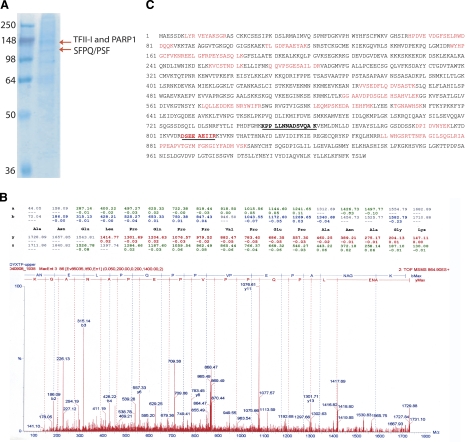
Identification of protein factors. A) PAGE gel showing the two bands isolated with the rs3743205 G probe. Sizes are shown in kDa. B) Proteomics results by ingel digestion MALDI-TOF mass spectrometry for the TFII-I hit (for PARP1 and SFPQ results, see Supplemental Data). C) Amino acid sequence of PARP1. Letters in red denote the hits by Ingel digestion, the residues in bold and underlined are the results from the protein sequencing (for TFII-I and SFPQ results, see Supplemental Data).
Real-time qRT-PCR
Total RNA was extracted from SH-SY5Y cells at 6, 12, and 24 h after transient transfection, using the RNAeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. For the cDNA synthesis, 0.5 μg of total RNA in a volume of 20 μl was used as template, with RNase H treatment using the Superscript III RT-PCR kit (Invitrogen). For qRT-PCR, TaqMan gene expression assays were used to quantitate DYX1C1 (Hs00608519_m1) and TFII-I (Hs. Hs01073660_m1) (Applied Biosystem, Foster City, CA, USA). PARP1, SFPQ, and HPRT mRNA were quantitated using Power SYBR Green PCR Master Mix (Applied Biosystems). Relative expression of the different gene transcripts was calculated by the delta-delta-Ct (DDCt) method and converted to the relative expression ratio (2–DDCt). All data were normalized to the endogenous reference gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression. The following primers were used: PARP1-F 5′-CCCAAAGGAATTCCG AGAAA-3′, PARP1-R 5′-TCCTTTTTGGTGCTGATG-3′; SFPQ-F 5′-GGGCTGT TGTAATAGTGGATGA-3′, SFPQ-R 5′-CCAAAGACTTCCATCGCTGA-3′; HPRT-F 5′-TCAGGCAGTATAATCCAAAGATGGT-3′, HPRT-R 5′-AGTCTGGCTTATATCC AACACTTCG-3′.
Luciferase reporter assays
The neuroblastoma SH-SY5Y cells were seeded in 6-well plates at 90% confluence (2.5×106/ml). After 24 h, the cells were cotransfected with 1 μg or 500 ng of plasmid containing the variations (see luciferase reporter constructs in pGL3) and 50 ng of the pRLTK vector containing the herpes simplex thymidine kinase promoter linked to a constitutively expressing renilla luciferase reporter gene (Promega). The pGL3 empty vectors were used as negative controls and GFP plasmid was used as positive control for transfections. At 24 h after transfection, the cells were harvested, proteins were extracted, and luciferase activity was determined using the Dual-Luciferase® Reporter Assay System (Promega) and a Tecan Infinite luminescence reader (Tecan AG, Männedorf, Switzerland). All data were normalized to renilla luciferase. The statistical significance of the experiments was measured by Student’s t test with a threshold of P ≤ 0.05.
Bioinformatics
For the analysis of transcription binding sites, the transcription element search system (TESS; http://www.cbil.upenn.edu/tess/) and the Genomatix package (http://www.genomatix.de) were used. For the gene coexpression data, the TMM (http://microarray.cpmc.columbia.edu/cgi-bin/find-links.cgi) and Gemma databases (http://bioinformatics.ubc.ca/gemma) were used.
RESULTS
Cellular localization of DYX1C1 protein in SH-SY5Y cells
We used the neuroblastoma cell line SH-SY5Y, which is a widely used cell line for studying neuronal biochemistry and a good system for dyslexia research, as the cells resemble neurons in culture and are genetically stable. We first determined the cellular localization of the DYX1C1 protein in the neuroblastoma SH-SY5Y cell line by transient transfections with expression constructs. The DYX1C1-V5 fusion construct (2) was stained with monoclonal anti-V5 and Alexa Fluor 555-conjugated anti-mouse-IgG. The nuclei were stained with DAPI. The staining showed protein localization in both cytoplasm and nucleus (Fig. 1).
Prediction of DYX1C1 cis-regulatory elements and allele-specific differential retardation in electrophoretic mobility shift assays
We chose three promoter SNPs as particularly interesting for characterization because they appeared to form a haplotype associated with dyslexia (F. Dahdouh et al., personal communication). Comparison of TESS analyses of both alleles for the three SNPs, −3G/A (rs3743205), −10567T/C (rs12899331), and −10310C/A (rs16787), suggested that these polymorphisms may alter the binding affinity for several transcription factors. The sequences containing the −3A polymorphism but not the −3G allele were predicted to bind the TFII-I and Elk1 transcription factors. The −10567T/C and −10310C/A polymorphisms were predicted to have many differences in binding affinity, including the Sp1 transcription factor family.
As the factors predicted by TESS are nuclear factors, we used nuclear extracts from the SH-SY5Y cells to test by EMSA. We synthesized double-stranded DNA probes containing the variations and used these to screen for differences in protein factor binding by EMSA. We observed allele-specific differential retardation for each of the three SNPs, rs3743205, rs12899331, and rs16787 (Fig. 2A).
As TFII-I had been suggested to bind to the −3A probe by TESS, we tested the binding of TFII-I to our probe by overexpressing two isoforms of TFII-I (Δ and β) in SH-SY5Y cell culture and used these extracts in EMSAs. We detected enhanced binding affinity of both alleles −3G and −3A in these extracts, confirming the true binding of TFII-I to this site (Fig. 3A). Others have shown that TFII-I regulates gene expression of some certain responsive genes in the presence of ERα (24); therefore, we tested to enrich the SH-SY5Y cell extracts with ERα and could observe an increase in the binding affinity in total cell extracts. We also tested to overexpress ERα together with TFII-IΔ and TFII-Iβ. Here we observed a decrease of the TFII-IΔ binding to both A and G probes, whereas in the case of TFII-Iβ it enhanced the binding to the G probe and decreased binding to the A probe (Fig. 2A).
In addition, we performed competition assays. We used probes from the well-studied c-fos promoter, where the serum response element (SRE) is known to bind TFII-I, and the sequence of this consensus is unrelated to our 30-bp probe (25). We observed a reduction of TFII-I binding to the labeled −3A and −3G probes. To compete for the Sp1 binding site, we used a commercially available consensus probe (see Materials and Methods). The competition assays confirmed the predicted factors in all cases.
Identification of additional proteins retarded in EMSA: DYX1C1 trans-acting proteins
To further characterize the proteins bound differentially to the DYX1C1 promoter in EMSA, we performed a series of protein extraction experiments. By using a concatenated probe corresponding to the −3G allele that showed differential binding of proteins in the EMSA (Fig. 2A), we enriched proteins that were resolved in SDS-PAGE. We used silver staining of gels to monitor the protein purification. The concatenated and biotinylated probe was bound to avidin-coated magnetic beads, and the beads were incubated with total cellular protein and nuclear extracts from the neuroblastoma cell line SH-SY5Y. PAGE resolution of the protein extracts revealed four bands, and we chose to proceed with further analysis of the two strongest and largest molecular weight bands by ingel digestion followed by matrix-assisted laser desorpton/ionization-time of flight mass spectrometry (MALDI-TOF-MS) and protein sequencing by the liquid chromatography mass spectrometry (LC-MS/MS) system (Fig. 3). We identified three trans-acting proteins that bind to the −3G allele in the 5′UTR of the DYX1C1 gene. Proteins isolated from the upper band were identified as TFII-I and PARP1 and from the lower band as SFPQ/PSF (Fig. 3A).
Effect of TFII-I, PARP1, and SFPQ in the regulation of DYX1C1 in the neuroblastoma cell line SH-SY5Y
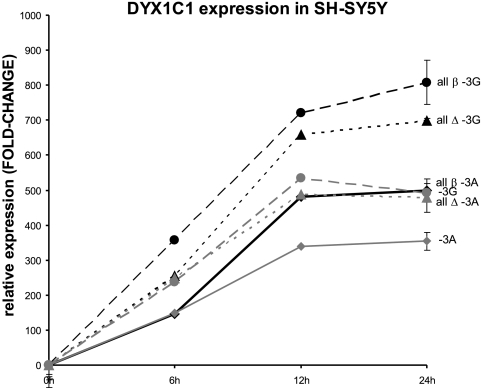
To study the effect of the three protein factors TFII-I, PARP, and SFPQ that bind to DNA around rs3743205 in the transcriptional regulation of DYX1C1, we transiently transfected expression constructs of DYX1C1 (−3G or −3A), PARP1, SFPQ, and TFII-I (Δ or β) to SH-SY5Y cells. Using a DYX1C1 TaqMan assay, we measured the expression levels of both −3G and −3A alleles of DYX1C1. Cell lysates were prepared at 6, 12, and 24 h after transient transfection (Fig. 4). The levels of the −3G allele were significantly higher than the levels of −3A allele, independent of which variant of TFII-I was overexpressed (Δ or β) in combination with the PARP1 and SFPQ. We observed in three independent assays that the levels of the −3G allele are significantly higher than the levels of the −3A allele. We also confirmed the genotype of rs3743205 in the SH-SY5Y cells as −3G allele. The overexpression of each of the factors was confirmed in each experiment by qRT-PCR (data not shown).
Figure 4.
Effects of overexpression of PARP1, TFII-I, and SFPQ in DYX1C1 by qRT-PCR. We transiently transfected PARP1, TFII-I (both β and Δ), SFPQ, and DYX1C1 (both alleles −3G and −3A) to SH-SY5Y cells and detected the levels of DYX1C1 expression by a TaqMan assay. The x-axis values indicate time after transfection. All β, all Δ, −3G, and −3A denote transfections with all the constructs and the β or Δ variant for TFII-I and the −3G or −3A variant alleles for DYX1C1 construct, respectively. Error bars shown for the 24 h experiments indicate sem.
Functional verification of the effects of allele-specific binding differences
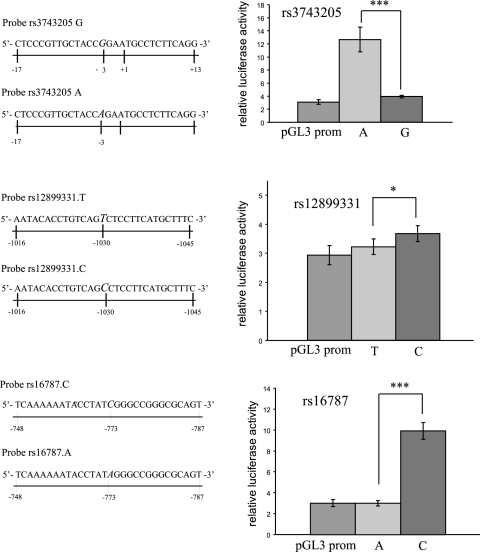
To further verify the effects of the DYX1C1 5′UTR and promoter polymorphisms and to confirm the roles of the transcription factors, we prepared constructs using a tandem repeat of the EMSA probes (Fig. 5) cloned into the pGL3 basic and pGL3 promoter vectors (Promega). The inserts were made separately for both alleles of the investigated SNPs. These constructs were transiently transfected into SH-SY5Y. Probes cloned into pGL3 promoter vector containing the upstream SV40 promoter yielded significant differences in luciferase activity with all three SNPs: for rs16787 and rs3743205, P < 0.001; and for rs12899331, P < 0.05 (Fig. 5).
Figure 5.
Luciferase reporter assay. We used constructs containing the rs3743205, rs12899331, and rs16787 SNPs in pGL3 promoter vectors (Promega). Left panels: numbers below each bar indicate the positions of the sequence in the DYX1C1, where +1 is the first base of the translation start site. 5′ to 3′ sequences of one copy of the inserts of each construct and each allele are shown; in the experiments, two copies were inserted to enhance the luciferase levels. Right panels: diagrams show relative luciferase activity values from three independent experiments; each experiment was performed in triplicate. pGL3 prom, luciferase values from the transfected empty vector. The constructs were transiently transfected to SH-SY5Y cells. Threshold of significance is P ≤ 0.05. *P < 0.005; ***P < 0.001.
DISCUSSION
In only a few years, several candidate genes for dyslexia susceptibility have been identified by analysis of rare chromosome translocations coinciding with dyslexia and genetic association studies. As in many complex disorders, genetic replication studies have so far resulted in ambiguous and conflicting results for each of the genes. However, functional studies have surprisingly implicated the same fundamental neurobiological process for each of the three genes DYX1C1, DCDC2, and KIAA0319, namely control of neuronal migration during embryonal development (5, 6, 9). The accumulated information may well suggest that each of the implicated genes can increase the risk for dyslexia if its function is compromised, even though genetic association studies may be underpowered or true population differences may exist, explaining the ambiguous results. As in many other examples, even rare genetic defects may unveil an important pathogenic pathway, and therefore these poorly characterized genes deserve further functional studies.
We undertook this study to understand better the regulation of DYX1C1 and to search for explanations for genetic association results. We have adopted a working hypothesis that even slightly altered expression of DYX1C1, rather than coding changes, may influence the risk of dyslexia. Of note, dyslexia was associated with haploinsufficiency of DYX1C1, cosegregating with a chromosome translocation that disrupted one copy of the gene, whereas the other copy remained intact (2).
Our results showed that polymorphisms in the promoter and 5′ UTR of DYX1C1 are functional. Furthermore, for the DNA segment, including the rs3743205 (−3G/A) polymorphism, we have identified a complex of three proteins that may be highly relevant in the context of brain development and that have not previously been reported to act together in gene regulation. These three factors are discussed in more detail below.
TFII-I is a 120 kDa protein, initially characterized for its binding to initiator sites of various promoters functioning as a basal transcription factor. Later, it was shown that it could also interact with upstream regulatory factors, including USF1 and c-myc (26). TFII-I has been suggested to play a role in signal transduction as well as in transcription initiation. Interestingly, TFII-I maps to 7q11.3, and deletions of this region are associated with the neurodevelopmental Williams-Beuren syndrome (WBS) in humans. WBS has multisystemic manifestations, including supravalvular aortic stenosis, hypercalcemia in infancy, mild to moderate mental retardation, cognitive defects, and characteristic facial features. WBS has also been associated with dyslexia (27). In addition, TFII-I is expressed in a variety of tissues, with the highest expression in the brain.
PARP1 is an intracellular protease that plays an important role in the development of neuronal cell death, apoptosis, and necrosis and in the sequelae of cerebral ischemia. PARP1 activation is involved in a number of pathological conditions, including ischemic/reperfusion injury, traumatic brain injury, Parkinson’s disease, and Alzheimer’s disease. PARP1 has also been implicated in the regulation of gene expression through modification of transcription factors (modifying histones to alter chromatin structure) or functioning as part of enhancer/promoter binding complexes in conjunction with other DNA binding factors and coactivators (28). Interestingly, PARP1 has been shown to be involved in learning and long-term memory in the sea slug Aplysia californica (29) and in rats (30). Working-memory deficits are characteristic in some dyslexic individuals (31)
The SFPQ or polypyrimidine tract-binding (PTB) protein-associated splicing factor (PSF) is a nuclear factor initially identified as a splicing factor but later shown to have multifunctional characteristics in a variety of nuclear processes (32). PSF contains both DNA-binding domain and RNA-binding domain, making it able to bind both DNA and RNA. Studies in mouse suggest that PSF could be involved in the control of neuronal-specific splicing events occurring at particular stages of neuronal differentiation and maturation (33).
Both TFII-I and PARP1 can regulate gene expression. TFII-I often activates transcription but it has also been shown that TFII-I can down-regulate some estrogen-responsive genes by interacting with ERα (24). Our EMSA results suggested that these factors trans-activate the wild-type allele −3G, but when the variation −3A was present, the binding of the factors was weaker (Fig. 3). Combined with information that our luciferase reporter assays gave significant differences between the alleles, it is possible that TFII-I acted as a repressor in this context. When the A allele was present and the binding in EMSA was weaker, the expression of the −3A allele was higher (Fig. 5). However, we have tested the direct effect of the three factors identified above by overexpressing them together with the two alleles of (−3G/A) DYX1C1. We show a direct effect of TFII-I, PARP1, and SFPQ in the regulation of DYX1C1 and also show that the effect is allele dependent (Fig. 4).
Transcriptional regulation is a complex phenomenon in which many proteins may play roles in gene regulation, and the direction of the effect may depend on balance of the tested factors and additional cellular factors. At least TFII-I may act either as enhancer or suppressor depending on the context of other factors.
As for SFPQ/PSF, splicing and transcription are coupled nuclear processes, and PSF also has the ability to bind to DNA. Therefore, PSF should also be considered an interesting candidate molecule in the regulation of the DYX1C1 gene. Interestingly, the SFPQ gene lies in a region that has been linked to speech sound disorder, language impairment, and dyslexia on chromosome 1p34-p36 by several groups (34,35,36,37). Recently, it has been shown that sfpq in zebra fish is expressed in all tissues, but the strongest expression is in the brain during neuronal development, suggesting its importance for neuronal development. Interestingly, a mutant for sfpq in zebra fish, whitesnake (38), shows abnormal shape of midbrain and hindbrain.
Finally, using bioinformatic tools to explore the coexpression of genes based on microarray data by TMM and Gemma, we could confirm coexpression between TFII-I, SFPQ, and PARP1. This finding supports the concept that these pairs of proteins might have coregulatory roles on target genes.
Microarray studies of gene-expression differences in human and nonhuman primate brains have shown an excess of genes with higher expression levels in the brain of humans compared to that of chimpanzees (39, 40). These differences were observed exclusively in the brain and not in other tissues, such as liver or heart (41). These results suggested that the genetic differences are specific to the brain and are likely associated with our more complex cognitive functions. Interestingly, when genes with the most consistent expression changes in human brain were listed, the TFII-I gene appeared at the top of the list, with an average fold change of 2.5–4.2 (41). Elucidation and understanding of the biology behind this gene and the other genes related to dyslexia will open new pathways in the study of brain functions.
Supplementary Material
Acknowledgments
We thank Angel Cedazo-Minguez (Department of Neurobiology, Care Sciences and Society, Karolinska Institute, Stockholm, Sweden) for kindly providing the SH-SY5Y cells, and Ingegerd Fransson and Anssi Riekki for excellent technical support. I.T.-P. is supported by the Swedish Brain Foundation (Hjärnfonden). The ERα expression construct was kindly provided by Eckardt Treuter (Department of Biosciences and Nutrition, Karolinska Institute, Stockholm, Sweden). Ingel digestion, peptide extraction, LC-MS/MS, and database searches for protein identification were performed at the Protein Analysis Center, Karolinska Institutet. A.L.R. is supported by grants from the U.S. National Institutes of Health (HD046034). Work in J.K.’s laboratory is supported by the Swedish Royal Bank Tercentennial Foundation, the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Sigrid Jusélius Foundation, the Academy of Finland, and the Folkhälsan Institute of Genetics.
References
- Yule W, Rutter M, Berger M, Thompson J. Over- and under-achievement in reading: distribution in the general population. Br J Educ Psychol. 1974;44:1–12. doi: 10.1111/j.2044-8279.1974.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma B, Lyytinen H, Muller K, Kaaranen M, Lindsberg P J, Hannula-Jouppi K, Kere J. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc Natl Acad Sci U S A. 2003;100:11553–11558. doi: 10.1073/pnas.1833911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, Kere J. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N A, Hill G, van den Bree M, Harold D, Moskvina V, Green E K, Owen M J, Williams J, O'Donovan M C. No support for association between dyslexia susceptibility 1 candidate 1 and developmental dyslexia. Mol Psychiatry. 2005;10:237–238. doi: 10.1038/sj.mp.4001596. [DOI] [PubMed] [Google Scholar]
- Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating B J, Taylor J M, Hacking D F, Scerri T, Francks C, Richardson A J, Wade-Martins R, Stein J F, Knight J C, Copp A J, LoTurco J, Monaco A P. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK, Pennington BF, DeFries JC, Gelernter J, O'Reilly-Pol T, Somlo S, Skudlarski P, Shaywitz S E, Shaywitz B A, Marchione K, Wang Y, Paramasivam M, LoTurco J J, Page G P, Gruen J R. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci U S A. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Anthoni H, Dahdouh F, Konig I R, Hillmer A M, Kluck N, Manthey M, Plume E, Warnke A, Remschmidt H, Hulsmann J, Cichon S, Lindgren C M, Propping P, Zucchelli M, Ziegler A, Peyrard-Janvid M, Schulte-Korne G, Nothen M M, Kere J. Strong genetic evidence of DCDC2 as a susceptibility gene for dyslexia. Am J Hum Genet. 2006;78:52–62. doi: 10.1086/498992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthoni H, Zucchelli M, Matsson H, Muller-Myhsok B, Fransson I, Schumacher J, Massinen S, Onkamo P, Warnke A, Griesemann H, Hoffmann P, Nopola-Hemmi J, Lyytinen H, Schulte-Korne G, Kere J, Nothen M M, Peyrard-Janvid M. A locus on 2p12 containing the co-regulated MRPL19 and C2ORF3 genes is associated to dyslexia. Hum Mol Genet. 2007;16:667–677. doi: 10.1093/hmg/ddm009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Paramasivam M, Thomas A, Bai J, Kaminen-Ahola N, Kere J, Voskuil J, Rosen G D, Galaburda A M, Loturco J J. DYX1C1 functions in neuronal migration in developing neocortex. Neuroscience. 2006;143:515–522. doi: 10.1016/j.neuroscience.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Threlkeld S W, McClure M M, Bai J, Wang Y, Loturco J J, Rosen G D, Fitch R H. Developmental disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1. Brain Res Bull. 2007;71:508–514. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G D, Bai J, Wang Y, Fiondella C G, Threlkeld S W, Loturco J J, Galaburda A M. Disruption of neuronal migration by RNAi of Dyx1c1 Results in neocortical and hippocampal malformations. Cereb Cortex. 2007;17:2562–2572. doi: 10.1093/cercor/bhl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A M, Kemper T L. Cytoarchitectonic abnormalities in developmental dyslexia: a case study. Ann Neurol. 1979;6:94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- Galaburda A M, Sherman G F, Rosen G D, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Matsumoto M, Yada M, Nakayama K I. Interaction of U-box-type ubiquitin-protein ligases (E3s) with molecular chaperones. Genes Cells. 2004;9:533–548. doi: 10.1111/j.1356-9597.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- Grigorenko E L, Wood F B, Meyer M S, Hart L A, Speed W C, Shuster A, Pauls D L. Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. Am J Hum Genet. 1997;60:27–39. [PMC free article] [PubMed] [Google Scholar]
- Schulte-Korne G, Grimm T, Nothen M M, Muller-Myhsok B, Cichon S, Vogt I R, Propping P, Remschmidt H. Evidence for linkage of spelling disability to chromosome 15. Am J Hum Genet. 1998;63:279–282. doi: 10.1086/301919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D W, Robinson L, Turic D, Duke M, Webb V, Milham C, Hopkin E, Pound K, Fernando S, Easton M, Hamshere M, Williams N, McGuffin P, Stevenson J, Krawczak M, Owen M J, O'Donovan M C, Williams J. Family-based association mapping provides evidence for a gene for reading disability on chromosome 15q. Hum Mol Genet. 2000;9:843–848. doi: 10.1093/hmg/9.5.843. [DOI] [PubMed] [Google Scholar]
- Nopola-Hemmi J, Taipale M, Haltia T, Lehesjoki A E, Voutilainen A, Kere J. Two translocations of chromosome 15q associated with dyslexia. J Med Genet. 2000;37:771–775. doi: 10.1136/jmg.37.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg KG, Couto J M, Feng Y, Anderson B, Cate-Carter T D, Macciardi F, Tannock R, Lovett M W, Humphries T W, Barr C L. Support for EKN1 as the susceptibility locus for dyslexia on 15q21. Mol Psychiatry. 2004;9:1111–1121. doi: 10.1038/sj.mp.4001543. [DOI] [PubMed] [Google Scholar]
- Scerri T S, Fisher S E, Francks C, MacPhie I L, Paracchini S, Richardson A J, Stein J F, Monaco A P. Putative functional alleles of DYX1C1 are not associated with dyslexia susceptibility in a large sample of sibling pairs from the UK. J Med Genet. 2004;41:853–857. doi: 10.1136/jmg.2004.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini G, Bravaccio C, Calamoneri F, Donatella Cocuzza M, Fiorillo P, Gagliano A, Mazzone D, del Giudice EM, Scuccimarra G, Militerni R, Pascotto A. No evidence for association between dyslexia and DYX1C1 functional variants in a group of children and adolescents from Southern Italy. J Mol Neurosci. 2005;27:311–314. doi: 10.1385/jmn:27:3:311. [DOI] [PubMed] [Google Scholar]
- Marino C, Citterio A, Giorda R, Facoetti A, Menozzi G, Vanzin L, Lorusso M L, Nobile M, Molteni M. Association of short-term memory with a variant within DYX1C1 in developmental dyslexia. Genes Brain Behav. 2007;6:640–646. doi: 10.1111/j.1601-183X.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- Marino C, Giorda R, Luisa Lorusso M, Vanzin L, Salandi N, Nobile M, Citterio A, Beri S, Crespi V, Battaglia M, Molteni M. A family-based association study does not support DYX1C1 on 15q21.3 as a candidate gene in developmental dyslexia. Eur J Hum Genet. 2005;13:491–499. doi: 10.1038/sj.ejhg.5201356. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Azuma M, Tsuboi Y, Kabe Y, Yamaguchi Y, Wada T, Watanabe H, Handa H. TFII-I down-regulates a subset of estrogen-responsive genes through its interaction with an initiator element and estrogen receptor alpha. Genes Cells. 2006;11:373–381. doi: 10.1111/j.1365-2443.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- Kim D W, Cheriyath V, Roy A L, Cochran B H. TFII-I enhances activation of the c-fos promoter through interactions with upstream elements. Mol Cell Biol. 1998;18:3310–3320. doi: 10.1128/mcb.18.6.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A L. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene. 2001;274:1–13. doi: 10.1016/s0378-1119(01)00625-4. [DOI] [PubMed] [Google Scholar]
- Temple C M. Deep dyslexia in Williams syndrome. J Neurolinguistics. 2003;16:457–488. [Google Scholar]
- Kraus W L, Lis J T. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M, Visochek L, Katzoff A, Levitan D, Susswein A J, Klein R, Valbrun M, Schwartz J H. Long-term memory requires polyADP-ribosylation. Science. 2004;304:1820–1822. doi: 10.1126/science.1096775. [DOI] [PubMed] [Google Scholar]
- Hatip-Al-Khatib I, Iwasaki K, Chung E H, Egashira N, Mishima K, Fujiwara M. Inhibition of poly (ADP-ribose) polymerase and caspase-3, but not caspase-1, prevents apoptosis and improves spatial memory of rats with twice-repeated cerebral ischemia. Life Sci. 2004;75:1967–1978. doi: 10.1016/j.lfs.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Grigorenko E L. The first candidate gene for dyslexia: Turning the page of a new chapter of research. Proc Natl Acad Sci U S A. 2003;100:11190–11192. doi: 10.1073/pnas.2134926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shav-Tal Y, Zipori D. PSF and p54(nrb)/NonO–multi-functional nuclear proteins. FEBS Lett. 2002;531:109–114. doi: 10.1016/s0014-5793(02)03447-6. [DOI] [PubMed] [Google Scholar]
- Chanas-Sacre G, Mazy-Servais C, Wattiez R, Pirard S, Rogister B, Patton J G, Belachew S, Malgrange B, Moonen G, Leprince P. Identification of PSF, the polypyrimidine tract-binding protein-associated splicing factor, as a developmentally regulated neuronal protein. J Neurosci Res. 1999;57:62–73. doi: 10.1002/(SICI)1097-4547(19990701)57:1<62::AID-JNR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tzenova J, Kaplan B J, Petryshen T L, Field L L. Confirmation of a dyslexia susceptibility locus on chromosome 1p34–p36 in a set of 100 Canadian families. Am J Med Genet B Neuropsychiatr Genet. 2004;127:117–124. doi: 10.1002/ajmg.b.20139. [DOI] [PubMed] [Google Scholar]
- Grigorenko E L, Wood F B, Meyer M S, Pauls J E, Hart L A, Pauls D L. Linkage studies suggest a possible locus for developmental dyslexia on chromosome 1p. Am J Med Genet. 2001;105:120–129. [PubMed] [Google Scholar]
- Miscimarra L, Stein C, Millard C, Kluge A, Cartier K, Freebairn L, Hansen A, Shriberg L, Taylor H G, Lewis B, Iyengar S K. Further evidence of pleiotropy influencing speech and language: analysis of the DYX8 region. Hum Hered. 2007;63:47–58. doi: 10.1159/000098727. [DOI] [PubMed] [Google Scholar]
- Rabin M, Wen X L, Hepburn M, Lubs H A, Feldman E, Duara R. Suggestive linkage of developmental dyslexia to chromosome 1p34–p36. Lancet. 1993;342:178. doi: 10.1016/0140-6736(93)91384-x. [DOI] [PubMed] [Google Scholar]
- Lowery L A, Rubin J, Sive H. Whitesnake/sfpq is required for cell survival and neuronal development in the zebrafish. Dev Dyn. 2007;236:1347–1357. doi: 10.1002/dvdy.21132. [DOI] [PubMed] [Google Scholar]
- Caceres M, Lachuer J, Zapala M A, Redmond J C, Kudo L, Geschwind D H, Lockhart D J, Preuss T M, Barlow C. Elevated gene expression levels distinguish human from non-human primate brains. Proc Natl Acad Sci U S A. 2003;100:13030–13035. doi: 10.1073/pnas.2135499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard W, Khaitovich P, Klose J, Zollner S, Heissig F, Giavalisco P, Nieselt-Struwe K, Muchmore E, Varki A, Ravid R, Doxiadis G M, Bontrop R E, Paabo S. Intra- and interspecific variation in primate gene expression patterns. Science. 2002;296:340–343. doi: 10.1126/science.1068996. [DOI] [PubMed] [Google Scholar]
- Preuss T M, Caceres M, Oldham M C, Geschwind D H. Human brain evolution: insights from microarrays. Nat Rev Genet. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- Akterin S, Cowburn R F, Miranda-Vizuete A, Jimenez A, Bogdanovic N, Winblad B, Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- Rico-Bautista E, Negrin-Martinez C, Novoa-Mogollon J, Fernandez-Perez L, Flores-Morales A. Downregulation of the growth hormone-induced Janus kinase 2/signal transducer and activator of transcription 5 signaling pathway requires an intact actin cytoskeleton. Exp Cell Res. 2004;294:269–280. doi: 10.1016/j.yexcr.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Long Island, NY, USA: Cold Spring Harbor Laboratory Press; Molecular CloningA Laboratory Manual. 2001 [Google Scholar]
- Cheriyath V, Novina C D, Roy A L. TFII-I regulates Vbeta promoter activity through an initiator element. Mol Cell Biol. 1998;18:4444–4454. doi: 10.1128/mcb.18.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.