Abstract
Emerging evidence suggests that lysophosphatidic acid (LPA) is a physiological regulator of cyclooxygenase-2 (Cox-2) expression. Herein we used ovarian cancer cells as a model to investigate the molecular mechanisms that link the LPA G protein-coupled receptors (GPCRs) to Cox-2 expression. LPA stimulated Cox-2 expression and release of prostaglandins though the LPA1, LPA2, and LPA5 receptors. The effect of LPA involves both transcriptional activation and post-transcriptional enhancement of Cox-2 mRNA stability. The consensus sites for C/EBP in the Cox-2 promoter were essential for transcriptional activation of Cox-2 by LPA. The NF-κB and AP-1 transcription factors commonly involved in inducible Cox-2 expression were dispensable. Dominant-negative C/EPBβ inhibited LPA activation of the Cox-2 promoter and expression. Furthermore, LPA stimulated C/EBPβ phosphorylation and activity through a novel mechanism integrating GPCR signals and a permissive activity from a receptor tyrosine kinase (RTK). This role of RTK was not consistent with LPA activation of C/EBP through transactivation of RTK, as full activation of RTKs with their own agonists only weakly stimulated C/EBP. In addition to the transcriptional activation, the RNA stabilization protein HuR bound to and protected Cox-2 mRNA in LPA-stimulated cells, indicating an active role for HuR in sustaining Cox-2 induction during physiological responses.—Oyesanya, R. A., Lee, Z. P., Wu, J., Chen, J., Song, Y., Mukherjee, A., Dent, P., Kordula, T., Zhou, H., Fang, X. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells.
Keywords: C/EBP, GPCR, HuR, receptor tyrosine kinase
Lysophosphatidic acid (LPA, 1-acyl-sn-glycerol-3-phosphate) is a naturally occurring phospholipid mediator of diverse biological activities (1). It is produced by activated platelets during coagulation and thus is a normal constituent of serum (1, 2). At least five G protein-coupled receptors (GPCRs) of LPA have been identified (3,4,5,6,7). The LPA1/Edg2, LPA2/Edg4, and LPA3/Edg7 receptors are members of the endothelial cell differentiation gene (Edg) family and share 50–57% homology in their amino acid sequences (3,4,5). Recently, LPA4/p2y9/GPR23 of the purinergic receptor family and the related LPA5/GPR92, structurally distant from the Edg LPA receptors, were described (6,7). In addition to these cell surface GPCRs, LPA also been shown to bind and activate the peroxisome proliferator–activated receptor γ (PPARγ), which plays critical roles in controlling fat and energy metabolism (8).
The LPA1–5 receptors couple to multiple G proteins, G12/13, Gi, Gq, and Gs. These G proteins activate diverse signaling pathways including stimulation of phospholipase C and D (9, 10), inhibition of adenylyl cyclase (9), and activation of Ras and the downstream mitogen-activated protein kinases and phosphoinositide 3-kinase (11, 12). Activation of these signaling events downstream of LPA receptors culminates in morphological changes, cell growth, survival, and migration (1, 12, 13). Recently, we and others described that LPA plays a critical role in regulation of gene expression in normal and neoplastic cells (14,15,16,17,18,19,20,21). LPA is a potent modulator of expression of genes involved in the inflammation, angiogenesis, and carcinogenesis such as interleukin 6 (IL-6) (15, 16), interleukin 8 (IL-8) (15), vascular endothelial growth factor (VEGF) (18), urokinase plasminogen activator (19), and cyclooxygenase-2 (Cox-2) (20). LPA may contribute to cancer progression through triggering expression of these target genes, resulting in a more invasive and metastatic microenvironment for tumor cells.
Cyclooxygenases are involved in biosynthesis of prostaglandins (PGEs) from arachidonic acid (AA) (22). Cox-1 is constitutively expressed in most cell types, while Cox-2 is an inducible form, up-regulated by proinflammatory cytokines, stress, and growth factors (22). In addition to the well-established role in inflammation, Cox-2 has been implicated in human carcinogenesis, particularly in cancers of the colon, breast, and skin (22,23,24). Pharmacological suppression of Cox-2 activity with specific inhibitors reduces the number and size of adenomas in patients with familial adenomatous polyposis and prevents colon cancer development (22,23,24). The role of Cox-2 in the development of other types of malignancies, including ovarian cancer, is more controversial. Recent evidence indicates that a majority of ovarian tumors, including serous, endometroid, clear cell, and mucinous carcinomas and borderline tumors, display positive Cox-2 immunoreactivity with ∼70% overall cases showing moderate-to-high levels of expression (20). LPA, a lipid mediator present in ascites of ovarian cancer patients (14), is a potent stimulus of Cox-2 expression in ovarian cancer cell lines (20). Because both expression of LPA receptors and LPA levels are elevated in ovarian cancer (14), the ability of LPA to induce Cox-2 gene expression may reflect a physiological role for LPA in regulation of prostaglandins in ovarian tumor cells in vivo. In addition, genetic deletion of the LPA3 receptor in mice leads to a delayed implantation and defective embryo spacing, associated with reduced uterine expression of Cox-2 mRNA in the LPA3-deficient female mice (25), suggesting that LPA is an endogenous regulator of prostaglandin generation in the uterus crucial to mammalian reproduction.
Despite the prominent role of LPA signaling in regulation of Cox-2 (20, 25, 26), little is known about the LPA receptors, intracellular signaling pathways, and transcription factors involved in the process. The results presented in the current work demonstrate that LPA-induced expression of Cox-2 involves both transcriptional and post-transcriptional regulation. The transcriptional activation of Cox-2 by LPA is mediated primarily by the CCAAT enhancer-binding protein (C/EBP) transcription factor independently of other transcription factors such as NF-κB and AP-1 commonly involved in inducible Cox-2 expression. C/EBP is activated by LPA via a regulatory mechanism integrating LPA GPCR signals and a permissive activity from a receptor tyrosine kinase (RTK). Further, we demonstrated that the transcriptional stimulation is reinforced by post-transcriptional protection of Cox-2 mRNA stability mediated by the RNA binding protein HuR (27), leading to sustained induction of Cox-2 in LPA-treated cells.
MATERIALS AND METHODS
Materials
1-Oleoly (18:1) LPA and sphingosine 1 phosphate (S1P) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Prior to use, these phospholipids were dissolved in PBS containing 0.5% fatty acid-free bovine serum albumin (BSA). BSA, Fugene 6, and protease inhibitor cocktail tablets were purchased from Roche (Indianapolis, IN, USA). The PGE2 EIA kit, NS-398, and AA were purchased from Cayman Co. (Ann Arbor, MI, USA). [3H]-AA and [32P]-dCTP were purchased from Perkin Elmer (Boston, MA, USA) and Amersham Biosciences (Piscataway, NJ, USA), respectively. Plasmid DNA was purified using the endo-free purification kit from Qiagen (Valencia, CA, USA). Luciferase assay reagents were obtained from Promega (Madison, WI, USA). GW9662 and pharmacological inhibitors of MAPKs were from Calbiochem (San Diego, CA, USA). All oligonucleotides and primers were synthesized by Operon Biotechnologies, Inc (Huntsville, AL, USA). Antiphospho CEBPβ and antitubulin α/β antibodies were obtained from Cell Signaling (Danvers, MA, USA). The monoclonal antibodies against Cox-2 and HuR and a polyclonal antibody against Cox-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Insulin, TRIzol, and cell culture medium were obtained from Invitrogen Inc. (Carlsbad, CA, USA). Bovine fetal serum was from Biomeda (Foster City, CA, USA). Insulin-like growth factor I (IGF-I) was from Upstate Biotechnology (Lake Placid, NY, USA). Hepatocyte growth factor (HGF) was from R&D Systems (Minneapolis, MN, USA). Epidermal growth factor (EGF), AG1478, and anti-β-actin monoclonal antibody were obtained from Sigma (St. Louis, MO, USA).
Plasmids
The C/EBPβ, liver-enriched transcriptional activator protein 1 (LAP1), and LAP2 expression vectors were kindly provided by Dr. Linda Sealy (Vanderbilt University School of Medicine, Nashville, TN, USA) (28, 29). The expression of C/EBPβ from these vectors in transfected cells was confirmed by immunoblotting. The dominant-negative form of C/EBPβ, LIP (liver-enriched inhibitory protein), (30) was cloned into pcDNA3.1 by reverse transcriptase-polymerase chain reaction (RT-PCR) amplification of a 444 bp cDNA fragment of C/EBPβ from Caov-3 cells using primers: 5′-GGAATCAAGCTTGGCGCACATGGCGGCG-3′ (forward), and 5′-GCAATACTCGAGCGCTAGCAGTGGCCGGAGG-3′ (reverse). The structure of pcDNA3-LIP was confirmed by automatic sequencing and immunoblotting analysis of expression of the short, truncated form of C/EBPβ (21 kDa) (28) in transfected cells.
Cell culture
The sources of ovarian cancer cell lines used in the study were described previously (15, 17). These cells were cultured in RPMI medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cell lines were frozen at early passages and used for less than 10 wk in continuous culture.
Western blot
Cells were lysed in sodium dodecyl sulfate (SDS) sample buffer or in ice-cold X-100 lysis buffer [1% Triton X-100, 50 mM HEPES (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 100 mM NaF, 10 mM Na PPi, and protease inhibitor cocktail]. Total cellular proteins were resolved by SDS-PAGE, transferred to Immun-Blot membrane [poly(vinylidene difluoride); Bio-Rad, Hercules, CA, USA], and immunoblotted with antibodies following the protocols of the manufacturers. Immunocomplexes were visualized with an enhanced chemiluminescence detection kit from Amersham (Piscataway, NJ, USA) using the horseradish peroxidase-conjugated secondary antibodies (Cell Signaling, Danvers, MA, USA).
Quantitative determination of PGE2 in culture supernatants
Ovarian cancer cell lines were plated in 6-well plates and grown to 60% confluence in complete medium. The cells were starved for 24 h before stimulation with LPA or vehicle for the specified periods of time. The levels of prostaglandin estradiol-17β (PGE2) present in culture supernatants were quantified using the PGE2 EIA kit.
AA release
Ovarian cancer cell lines were plated in 6-well plates and grown to 60% confluence in complete medium. The cells were labeled with 1 μCi [3H] AA/well in 2 ml of serum-free Dulbecco modified Eagle medium (DMEM) for 20 h. The cells were washed 3 times with DMEM and incubated with DMEM containing 0.1% fatty acid-free BSA (DMEM+BSA) for 3 h. The cells were then refed new DMEM+BSA and incubated with LPA or ATP for the indicated periods of time. The cells were dissolved in 2 ml of 0.2 N NaOH overnight. The radioactivity in the supernatants and cells was determined by scintillation spectrometry. The AA release was presented as percentages of the activity present in medium vs. the total labeling in both medium and cells.
Northern blot and mRNA stability assays
Total cellular RNA was extracted from cell lines using the TRIzol reagent following the supplier’s instructions. RNA samples were electrophoresed on agarose gel containing formaldehyde, stained with ethidium bromide, and transferred to N+ hybrid nylon. RNA was immobilized with UV cross-linking, prehybridized, and hybridized to 32P-labeled cDNA probes at 65°C overnight in a hybridization buffer (1% BSA, 0.5 M NaH2PO4, 1 mM EDTA, 7% SDS, 10 μg/ml salmon sperm DNA). The cDNA of the human Cox-2 gene was isolated by RT-PCR amplification from Caov-3 cells using primers: 5′-AGATCATAAGCGAGGGCCAGCTTT-3′ (forward) and 5′-ACTTTCTGTACTGCGGGTGGAACA-3′ (reverse). The 32P-deoxy-CTP-labeled DNA probes were prepared using the High Prime labeling system (Roche). Equal loading of RNA samples was confirmed by rehybridization to the cDNA of 18S rRNA (American Type Culture Collection, Manassas, VA, USA).
To determine Cox-2 mRNA stability, Caov-3 cells were treated with or without 10 μM LPA for 6 h before actinomycin D (5 μg/ml) was added to stop new RNA synthesis. Total cellular RNA was isolated from the cells using TRIzol at 0, 2, 4, and 6 h after addition of actinomycin D. Reverse transcription was performed to synthesize single stranded cDNAs using ThermoScript (Invitrogen). The relative levels of Cox-2 mRNA were quantified by qPCR using the human Cox-2 specific probe and the TaqMan system from Applied Biosystems (Foster City, CA, USA).
siRNA The human LPA1–3 and LPA5 receptor SMARTpool siRNAs and the control siRNAs were obtained from Dharmacon (Lafayette, CO, USA). The human HuR siRNA (sense 5′-GGAUGAGUUACGAA-GCCUGtt-3′ and antisense 5′-CAGGCUUCGUAACUCAUCCtg-3′), Bcl10 siRNAs (Bcl10siRNA1, sense 5′-GGAAAACCCAAAAGGUCUGtt-3′ and antisense 5′-CAGACCUUUUGGGUUUUCCtg-3′; Bcl10 siRNA2, sense 5′-GGUCUGGACACCCUUGUUGtt-3′ and antisense 5′-CAACAAGGGUGUCCAGACCtt-3′; Bcl10 siRNA3, sense 5′-GCAUACUUCUAGGAUAGCUtt-3′ and antisense 5′-AGCUAUCCUAGAAGUAUGCtt-3′), and the nontarget control siRNA (sense 5′-AGUACUGCUUACGAUACGGtt-3′ and antisense 5′-CCGUAUCGUAAGCAGUACUtt-3′) were purchased from Ambion (Austin, TX, USA). The specific siRNA or nontarget control siRNA (2.25 μg) was transfected into ovarian cancer cell lines (1.25×106 cells) with Amaxa nucleofector II (Kit T, Program T32). The transfected cells were cultured in 6-well plates in complete medium. After 48 h, the cells were starved in serum-free RPMI 1640 and stimulated with LPA for 6 or 12 h for Cox-2 induction. RNA was isolated from parallel cultures for RT-qPCR analysis to determine the efficiency of siRNA knockdown.
The reporter vectors and luciferase assays
The proximal sequence (–980 to +15) of the human Cox-2 gene promoter (30) was cloned from the genomic DNA of Caov-3 cells by PCR amplification and inserted into the pGL2-Basic vector (Promega) and verified by automatic sequencing. The 7.2 kb Cox-2 promoter fragment was kindly provided by Dr. Thomas M. McIntyre (Cleveland Clinic Foundation, Cleveland, OH, USA) (30) and were cloned into the pGL2-Basic vector. The C/EBP responsive luciferase vector (pGL2–5xCEBP-TK-Luc) was generated by cloning five repeats of the C/EBP consensus sequence (TTGCGCAATCT) into the NheI and HindIII sites in front of the herpes simplex virus thymidine kinase (TK) gene promoter (–35 to +50) in the pGL2-TK-Luc vector (15). Ovarian cancer cell lines were seeded in 6-well plates and grown to 30–40% confluence before transfection with the luciferase vectors using Fugene 6 (Roche) or TransIT-TKO (Mirus Bio Corp., Madison, WI, USA) according to the instructions of the manufacturers. About 48 h after transfection, the cells were starved for 24–36 h before stimulation with LPA or vehicle for 6 h. Cell extracts were prepared and assayed for luciferase activity using the luciferase assay kit from Promega. The luciferase activity was normalized on the basis of the activity of cotransfected β-galactosidase reporter driven by the cytomegalovirus promoter (pCMVβ-gal).
Deletion and site-directed mutagenesis
The unique AP-1-like site at around –577 was deleted from the pGL2-Cox2–1kb-Luc by restriction digestion with Af1III followed by recirculation of the plasmid. The consensus sequences of NF-κB and C/EBP transcription factors present within –980 to +15 of the Cox-2 promoter in pGL2-Cox2–1kb-Luc were mutated into inactive sequences using site-directed mutagenesis kits from Stratagene (Cedar Creek, TX, USA). The distal NF-κB site GGGGATTCCCTG was changed to CGTCATTCCCTG, and the proximal NF-κB site GGGGACTACCCC mutated to GGTCACTACCCC. The distal C/EBP site GCCTTTCTTAAC was mutated to GCCCCTATTAAC, and the proximal one GGCTTACGCAAT was converted to GACTTACGCTCT. The desired deletion and mutation of these AP-1, NF-κB, and C/EBP binding sites were confirmed by automatic sequencing before the plasmids were used for luciferase assays.
Binding of Cox-2 transcripts with HuR in vitro and in vivo
To assess the interaction of HuR with Cox-2 mRNA, the 3′ untranslated region (3′-UTR) of Cox-2 mRNA (375 bp from nt 1950–2325, NM_000963) was reverse-transcribed and PCR amplified from Caov-3 cells using primers containing the T7 promoter sequences: 5′-TCCTAATACGACTCACTATAGGGAAGTCTAATGATCATATTT-AT-3′ (forward) and 5′-GCTATTTAGGTGACACTATAATCATGGAAGATGCATTG-3′(reverse). The PCR product was purified and utilized as a template for in vitro transcription. The transcripts equivalent to the 3′-UTR of Cox-2 mRNA was synthesized and labeled with biotin-11-CTP by transcription from the T7 promoter using in vitro transcription kit (Promega). Lysates from control and LPA-treated cells were incubated with the biotinylated Cox-2 3′-UTR transcripts in 1× binding buffer [10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 250 mM NaCl, 0.5% Triton X-100] for 30 min at room temperature. The binding complexes were isolated with paramagnetic streptavidin-conjugated Dynabeads (Invitrogen) and washed thoroughly with PBS followed by Western blotting analysis of HuR.
To assess the association of HuR with Cox-2 mRNA endogenously, Caov-3 cells treated with or without LPA were harvested in PBS by scraping from dishes, pelleted, and resuspended in polysome lysis buffer containing 100 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH 7.0), 0.5% Nonidet P-40, 1 mM DTT, 100 U/ml RNaseOUT (Promega), and the complete protease inhibitor cocktail (Roche). For immunoprecipitation, 1500 μg cellular proteins were diluted with 700 μl NT2 buffer [50 mM Tris (pH 7.4), 150 mM NaCl, and 1 mM MgCl2, 0.05% Nonidet P-40, 5% fatty acid-free BSA, 1 mM DTT, 200 U/ml RNaseOUT, and 15 mM EDTA] and incubated for 2 h with 7.5 μg anti-HuR antibody or an IgG1 isotype control antibody. The immunocomplex was incubated for 1 h with protein A Sepharose beads (GE Biosciences) and washed thoroughly with ice-cold NT2 buffer. After digestion of proteins present in the beads with Proteinase K (0.5 mg/ml) at 55°C for 20 min, the bead-free supernatants were extracted with phenol/chloroform and precipitated with 0.3 M NaAc, 150 μg/ml glycogen, and 2.5 volumes of 100% ethanol. The precipitates were dissolved in 15 μl of nuclease-free water. Potential contamination with genomic DNA was removed using the DNA-free™ DNase Treatment & Removal kit (Ambion). Reverse transcription was performed on 5 μl of the samples with the ThermoScript kit (Invitrogen, followed by PCR amplification of a fragment of 481 bp close to the 3′ UTR of Cox-2 mRNA with primers: 5′-TGTTCCACCCGCAGTACAGAAAGT-3′ (forward) and 5′-GCGGGA-AGAACTTGCATTGATGGT-3′ (reverse).
Statistics
All numerical results are presented as means ± sd. The statistical significance of differences was analyzed using Student’s t test, where P < 0.05 was considered statistically significant.
RESULTS
LPA induces Cox-2 protein expression in ovarian cancer and other malignant cells
Several studies have shown that LPA induces expression of Cox-2 in various cell types including renal mesangial cells and colon and ovarian cancer cells (20, 26, 31). However, the molecular mechanism regulating Cox-2 gene expression in response to LPA is poorly understood.
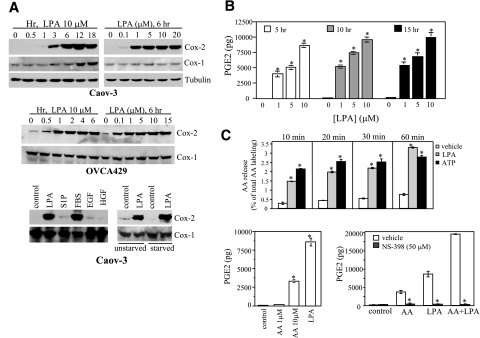
LPA induced robust and sustained expression of Cox-2 protein in several ovarian cancer cell lines examined, with the most striking effect seen in Caov-3 and OVCA-429 cells (Fig. 1A). LPA did not affect Cox-1 expression at early hour points. We observed a slight increase in Cox-1 in Caov-3 cells only after prolonged incubation with LPA for 12–18 h (Fig. 1A), likely reflecting an indirect effect of LPA. LPA also stimulated Cox-2 expression in cell lines of lung cancer and breast cancers (data not shown).
Figure 1.
LPA induces expression of Cox-2 protein and release of PGE2 in ovarian cancer cell lines. A) LPA stimulation of Cox-2 expression in Caov-3 and OVCA429 cells. The ovarian cancer cell lines were starved and stimulated with different concentrations of LPA for the indicated periods of time or 6 h. The cells were lysed in SDS sample buffer and analyzed by immunoblotting for Cox-2. Reblotting for Cox-1, α/β tubulin, or β-actin was included to show equal loading among samples. The effect of LPA (10 μM, 6 h) on Cox-2 expression was also compared with that of S1P (5 μM), EGF (50 ng/ml), FBS (5%), and HGF (20 ng/ml) (bottom left panel). The effect of LPA (10 μM, 6 h) was also compared between serum-starved cells and unstarved cells (bottom right panel). B) PGE2 production in LPA-stimulated cells. Caov-3 cells were treated with the indicated concentrations of LPA for 5, 10, or 15 h before PGE2 levels in culture supernatants were quantified using the PGE2 EIA kits. C) Involvement of AA and Cox-2 enzyme activity in PGE2 production. AA release in Caov-3 cells treated with LPA (10 μM) or ATP (100 μM) for the indicated periods of time was quantified as described in Materials and Methods (top panel). The effect of exogenous AA (1 or 10 μM, 6 h) on PGE2 production in Caov-3 cells was determined and compared with that of LPA (10 μM, 6 h) (bottom left panel). PGE2 production in Caov-3 cells induced by AA (10 μM, 6 h), LPA (10 μM, 6 h), and AA+LPA in the presence of the Cox-2 inhibitor NS398 (50 μM) or vehicle was analyzed and compared (bottom right panel). The results are means ± sd of triplicates, representative of three independent experiments. *P < 0.05.
We chose the most responsive Caov-3 and OVCA-429 cells for further characterization. In both cell lines, the effect of LPA was detectable at submicromolar concentrations and the maximum effect was achieved with 1–10 μM of LPA (Fig. 1A). On treatment with LPA, Cox-2 levels increased significantly within 1 h and peaked at 12 h (Fig. 1A). When Caov-3 cells were cultured in complete medium without starvation, LPA remained capable of stimulating Cox-2 expression (Fig. 1A). However, the basal expression of Cox-2 was higher in unstarved cells and the LPA-mediated induction was weaker compared to that achieved in serum-starved cells (Fig. 1A).
We next compared the effects of LPA, FBS, S1P, and the peptide growth factors EGF, HGF, and IGF-1 on Cox-2 expression in Caov-3 cells (Fig. 1A). FBS could stimulate a prominent increase in Cox-2 protein. LPA is a component of FBS (1, 2, 14) and may account for the ability of FBS to drive Cox-2 expression. Interestingly, EGF, HGF, and S1P only weakly stimulated Cox-2 expression (Fig. 1A), while IGF-1 failed to induce Cox-2 (data not shown). The poor response to S1P suggests that induction of Cox-2 is specifically linked to certain GPCRs and is not a general outcome of GPCR activation.
LPA induces PGE2 production and AA release
We examined whether LPA-induced Cox-2 enzyme is functionally active, contributing to biosynthesis and release of PGE2. As shown in Fig. 1B, LPA treatment strongly increased PGE2 levels in culture supernatants in a dose-dependent manner.
LPA treatment led to significant release of AA (Fig. 1C), supplying substrate for production of PGE2 in LPA-stimulated cells. Further, addition of exogenous AA (10 μM) to unstimulated Caov-3 cells also resulted in significant PGE2 generation (Fig. 1C), suggesting the cells could utilize the basal Cox enzymes to synthesize PGE2 when the substrate becomes available. We also observed a synergism between LPA treatment and exogenous AA in stimulation of PGE2 production (Fig. 1C). However, these effects of LPA, AA, and LPA plus AA on PGE2 generation were all highly sensitive to the Cox-2 enzyme inhibitor NS-398, which did not affect LPA-induced Cox-2 protein expression (data not shown). These results suggest that LPA activates both AA release and Cox-2 expression. The two processes cooperate to up-regulate PGE2 levels in LPA-treated ovarian cancer cells.
The LPA1, LPA2, and LPA5 receptors mediate LPA-induced Cox-2 expression
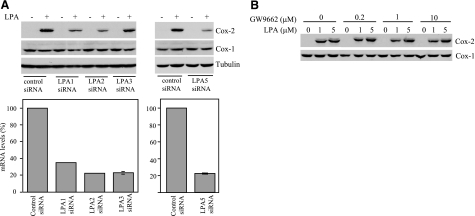
To identify the LPA receptors responsible for Cox-2 induction, we assessed expression of LPA receptors in ovarian cancer cell lines by RT-PCR. Caov-3 and OVCA-429 expressed mRNAs of the LPA1–3 receptors and the newly identified LPA5 receptor (data not shown). The RT-PCR product of LPA4 mRNA was not detected in Caov-3 cells and, therefore, was not further assessed for its role in Cox-2 induction. We utilized small-interfering RNA (siRNA) to down-regulate expression of each of the expressed LPA receptors in Caov-3 cells (Fig. 2A). The expression of LPA1–3 and LPA5 mRNAs was decreased by 60–80%, as determined by RT-qPCR (Fig. 2A, bottom panel). Down-regulation of LPA1, LPA2, or LPA5 caused a significant suppression of LPA-induced Cox-2 expression (Fig. 2A, top panel). In contrast, down-regulation of LPA3 did not affect LPA-dependent induction of Cox-2 (Fig. 2A).
Figure 2.
LPA stimulates Cox-2 expression through LPA1, LPA2, and LPA5 but not LPA3 or PPARγ. A) Involvement of LPA1 and LPA2 in LPA induction of Cox-2. Each of the LPA1–3 and LPA5 receptors in Caov-3 cells was down-regulated by siRNA. Expression of LPA receptor mRNAs in the knockdown cells was determined by RT-qPCR and compared with that in control siRNA-treated cells, which was defined as 100% (bottom panels). The outcome of the LPA receptor knockdown was assessed by immunoblotting analysis of Cox-2 expression induced by LPA (10 μM, 6 h) (top panels). B) LPA induction of Cox-2 independent of the PPARγ receptor. Caov-3 cells were stimulated with 1 or 5 μM LPA for 6 h in the presence of indicated concentrations of the PPARγ antagonist GW9662. GW9662 was added 45 min before LPA. Similar results were obtained from three independent experiments.
PPARγ can stimulate Cox-2 expression through activating the PPARγ binding sites located in the human Cox-2 gene promoter (30). To address the possibility that LPA may stimulate Cox-2 expression via PPARγ, we treated Caov-3 cells with LPA in the presence of the PPARγ antagonist GW9662 (30). GW9662 had little effect, if any, on LPA-afforded Cox-2 expression (Fig. 2B, bottom panel), indicating that LPA induces Cox-2 expression through a PPARγ-independent pathway.
The effect of LPA involves both transcriptional activation and post-transcriptional enhancement of Cox-2 mRNA stability
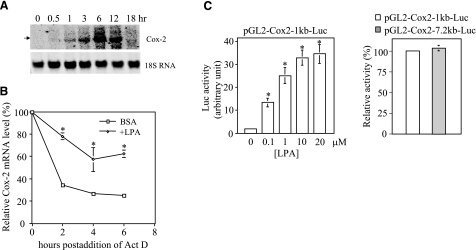
Cox-2 is one of the immediate early response genes and its induction usually declines rapidly within 1–4 h (32, 33). However, LPA-mediated Cox-2 protein expression lasted much longer in ovarian cancer cells and was detectable at high levels 12–18 h after addition of LPA (Fig. 1A). Consistent with this, Cox-2 mRNA levels reached the maximum at 6 h and remained highly elevated at 12 h (Fig. 3A). The sustained induction of Cox-2 mRNA by LPA suggests that Cox-2 mRNA could be stabilized in LPA-treated cells. To examine this possibility, we compared the decay kinetics of Cox-2 transcripts in Caov-3 cells treated with or without LPA. As shown in Fig. 3B, Cox-2 transcripts in control cells degraded quickly after actinomycin D was added to halt new RNA synthesis. In contrast, pretreatment with LPA led to significant stabilization of Cox-2 mRNA as compared to untreated control cells (Fig. 3B).
Figure 3.
Effect of LPA on Cox-2 expression involves both transcriptional activation and post-transcriptional enhancement of Cox-2 mRNA stability. A) Analysis of the steady-state levels of Cox-2 mRNA. Caov-3 cells were stimulated with 10 μM LPA for the indicated periods of time. Total cellular RNA was extracted and analyzed by Northern blotting. The membrane was reprobed for 18S RNA to show equal loading among samples. B) Measurement of Cox-2 mRNA stability. Caov-3 cells were treated for 6 h with LPA (10 μM) or BSA (control) before addition of actinomycin D (Act D, 5 μg/ml). Total RNA was isolated from the cells at 0, 2, 4, and 6 h after addition of Act D. The relative Cox-2 mRNA levels were determined by RT-qPCR and plotted as a function of hours postaddition of Act D. The values at 0 h were defined as 100%, with other time points presented as relative percentages. C) Reporter analysis of the Cox-2 gene promoter. Caov-3 cells transfected with pGL2-Cox2–1kb-Luc containing the –980 to +15 fragment of the Cox-2 promoter were stimulated with LPA at the indicated concentrations for 6 h and assayed for luciferase activity (left panel). LPA-induced luciferase activity from Caov-3 cells transfected with pGL2-Cox2–1kb-Luc (defined as 100%) was compared with the activity from the cells transfected with pGL2-Cox2–7.2bk-Luc (right panel). All numeric results are means ± sd of triplicates, representative of three independent experiments. *P < 0.05.
To determine whether LPA induction of Cox-2 mRNA was initiated from transcriptional activation, we cloned an ∼1 kb proximal fragment (–980 to +15) of the human Cox-2 gene promoter and evaluated its ability to drive transcription of the luciferase reporter in response to LPA. LPA stimulated 5- to 20-fold increases in luciferase activity in Caov-3 (Fig. 3C) and OVCA-429 (data not shown). In addition, we compared the response of this reporter with that of a luciferase vector containing 7.2 kb 5′ flanking region (pGL2-Cox2–7.2kb-Luc) (30). Similar ranges of LPA-stimulated luciferase activity were obtained from each of the two plasmids, suggesting that essential LPA-regulatory elements are located within the 1 kb sequences of the Cox-2 promoter (Fig. 3C).
LPA induces transcriptional activation of Cox-2 via C/EBP
To identify the transcription factors driving Cox-2 expression, we analyzed the Cox-2 gene promoter by deletion and mutation of the regulatory cis elements. Within the 1 kb region that responded well to LPA are numerous transcription factor binding sites, including those for AP-1, NF-κB, and C/EBP (Fig. 4A). Deletion of the unique AP-1-like site at around –577 did not attenuate the response to LPA as determined by luciferase assays (Fig. 4A). In agreement with this, ectopic expression of TAM67, a dominant-negative form of c-Jun (34), did not inhibit LPA-induced Cox-2 (data not shown).
Figure 4.
LPA induces transcriptional activation of Cox-2 through C/EBP independently of AP-1 or NF-κB. A) Deletion and mutational analysis of the Cox-2 promoter. The unique AP-1-like, two NF-κB, and two C/EBP binding sites were deleted or point mutated as detailed in Materials and Methods. Caov-3 cells transfected with the wild-type or mutant constructs were treated with LPA for 6 h and assayed for luciferase activity. B) Requirement of Bcl10-dependent NF-κB activation for LPA-induced production of IL-8 but not that of Cox-2. The control and Bcl10 siRNA-treated Caov-3 cells were stimulated for 6 h with 10 μM LPA or vehicle. Cox-2 expression in cell lysates and IL-8 concentrations in culture supernatants were determined by immunoblotting and ELISA analysis, respectively. C) Inhibition of LPA-induced activation of the Cox-2 promoter and Cox-2 expression by the dominant-negative LIP. Caov-3 cells transfected with pGL2-Cox2–1kb-Luc along with pcDNA3 or pCDNA3-LIP were treated for 6 h with LPA (10 μM) and assayed for luciferase activity (left panel). Caov-3 cells were transfected with pcDNA3-LIP or pcDNA3 using Amaxa nucleofector, stimulated for 6 h with LPA (10 μM) and analyzed by immunoblotting for Cox-2 and C/EBPβ (right panel). The values beneath each lane represent relative intensities (%) quantified by densitometry with Cox-2 induced by LPA in pcDNA3-transfected cells defined as 100%. Data are representative of three independent experiments.
Similarly, mutation of the two NF-κB consensus sites did not interfere with the responsiveness of the promoter to LPA (Fig. 4A). Recent studies suggest that LPA and other GPCR agonists stimulate NF-κB activation through the CARMA3/Bcl10/Malt1 signalosome, a process similar to antigen receptor-mediated NF-κB activation in lymphocytes (35, 36). To confirm the mutagenesis results, we down-regulated Bcl10 expression with three individual siRNAs. Each significantly reduced Bcl10 expression. However, none of these siRNAs could prevent LPA-mediated Cox-2 expression (Fig. 4B). In contrast, the Bcl10 siRNAs markedly inhibited LPA-stimulated IL-8 production, a response fully dependent on NF-κB activation as we described previously (15). The results suggest that in sharp contrast to many other Cox-2 inducers, LPA stimulated Cox-2 expression independently of NF-κB although LPA potently induced NF-κB DNA-binding and the transcriptional activity in these cells (data not shown).
We next targeted the two C/EBP consensus motifs present in the 1 kb fragment of the Cox-2 promoter. Although mutation of the individual site only slightly decreased the promoter activity, simultaneous mutation of the two C/EBP sites resulted in almost complete loss of LPA-induced luciferarse activity (Fig. 4A), suggesting that these C/EBP-binding sites are essential for the transcriptional activation of Cox-2 by LPA. In further support of this, coexpression of LIP, a dominant-negative, truncated form of C/EBPβ (29), inhibited LPA-induced luciferase activity by 60% in cells transfected with pGL-2-Cox2–1kb-Luc (Fig. 4C, left panel). LIP also suppressed LPA-induced Cox-2 protein expression when transiently transfected and expressed in Caov-3 cells (Fig. 4C, right panel).
C/EBPβ, a major isoform of the C/EBP family, was previously reported to be overexpressed in ovarian cancers (37). To assess the effect of overexpression of C/EBPβ on activity of the Cox-2 promoter, we transfected Caov-3 cells with pGL2-Cox2–1kb-Luc along with the C/EBPβ LAP1, LAP2, or a control vector. Expression of the C/EBPβ LAP1 or LAP2 was not sufficient to induce activation of luciferase activity from pGL2-Cox2–1kb-Luc (data not shown). The observation suggests that LPA-induced Cox-2 expression requires C/EBP activation rather than mere changes in C/EBP protein levels.
LPA stimulates phosphorylation and activity of C/EBP through a regulatory mechanism integrating GPCR signals and a permissive activity of RTK
To confirm that LPA stimulation triggers activation of C/EBP, we examined whether LPA regulates phosphorylation of C/EBPβ, one major form of post-transcriptional modifications associated with activation of the transcription factor. Treatment of ovarian cancer cell lines with LPA induced rapid C/EBPβ phosphorylation (Fig. 5A, left panel). To further substantiate the activation of C/EBP by LPA, we constructed a C/EBP-responsive luciferase vector (pGL2–5xCEBP-TK-Luc) in which 5 copies of the C/EBP consensus sequence were linked to the basic TK promoter. LPA consistently stimulated 5- to 10-fold increases in luciferase activity in pGL2–5xCEBP-TK-Luc-transfected Caov-3 (Fig. 5A, right panel) or OVCA-429 cells (data not shown) but not from the cells transfected with the backbone vector lacking the C/EBP responsive sites (pGL2-TK-Luc). These results indicate that LPA stimulates CEBPβ phosphorylation and C/EBP transcriptional activity.
Figure 5.
LPA induces phosphorylation and activation of C/EBP through a novel mechanism integrating LPA GPCR signals and a permissive activity from RTK. A) Stimulation of C/EBPβ phosphorylation and C/EBP transcriptional activity by LPA. The C/EBPβ phosphorylation in Caov-3 cells challenged with LPA (10 μM) for the indicated periods of time (min) was analyzed by immunoblotting using a C/EBPβ phospho-specific antibody (left panel). Caov-3 cells transfected with pGL2–5xCEBP-TK-Luc were stimulated for 6 h with LPA (10 μM) and assayed for luciferase activity (right panel). B) Comparison of the ability of LPA, EGF, HGF, and IGF to activate C/EBP and the Cox-2 promoter. Caov-3 cells transfected with pGL2–5xCEBP-TK-Luc (left panel) or with pGL2-Cox2–1kb-Luc (right panel) were stimulated for 6 h with LPA (10 μM), HGF (20 ng/ml), EGF (50 ng/ml), or IGF (50 ng/ml) and assayed for the basal and agonist-stimulated luciferase activity. C) Permissive role of EGFR or another RTK in LPA-induced C/EBPβ phosphorylation and activation. Phosphorylation of C/EBPβ in Caov-3 cells treated with HGF (20 ng/ml), LPA (10 μM), or LPA (10 μM)+HGF (20 ng/ml) in the presence of AG1478 (1 μM) or vehicle was analyzed by immunoblotting (top panel). Caov-3 cells transfected with the C/EBP-responsive pGL2–5xCEBP-TK-Luc were stimulated as in B and assayed for luciferase activity (bottom panel). D) Similar role of RTK in LPA-induced Cox-2 expression. Caov-3 cells were stimulated for 6 h with LPA (10 μM) or with LPA (10 μM)+HGF (20 ng/ml) in the presence of AG1478 (1 μM) or vehicle, and analyzed by immunoblotting for Cox-2 expression. In all tests where inhibition of EGFR was desired, AG1478 was added 45 min before addition of LPA. Results are representative of three independent assays. Numeric data are means ± sd of triplicates. *P < 0.05, AG1478-treated vs. untreated cells.
We next asked how LPA stimulates CEBP activation resulting in transcriptional up-regulation of Cox-2. Compared to LPA, agonists of receptor tyrosine kinases (RTKs) including EGF, HGF, and IGF only weakly induced Cox-2 expression in Caov-3 cells, as shown in Fig. 1A. Similarly, these RTK agonists were weak stimuli of C/EBP transcriptional activity, as indicated by luciferase assays with the C/EBP-responsive construct pGL2–5xCEBP-TK-Luc (Fig. 5B). Thus C/EBP seems to be a rate-limiting regulator of Cox-2, preferentially activated by GPCRs rather than RTKs. However, LPA-induced C/EBP phosphorylation and activation were sensitive to the EGFR inhibitor AG1478 (Fig. 5C). Down-regulation of EGFR expression with siRNA had a similar inhibitory effect on C/EBP (data not shown), suggesting that EGFR activity, albeit insufficient on its own to trigger strong C/EBP activation, was required for LPA GPCR signaling to C/EBP.
To further explore this novel mode of crosstalk between the two receptor types, we examined whether the requirement of EGFR in the process could be relieved by activation of another RTK such as c-Met. Treatment of Caov-3 cells with HGF alone only slightly activated C/EBP phosphorylation (Fig. 5C) and C/EBP transcriptional activity (Fig. 5B). However, when EGFR was inhibited by AG1478, LPA was fully capable of stimulating C/EBP phosphorylation and transcriptional activity if the cells were costimulated with HGF to activate c-Met. HGF also reversed the inhibitory effect of AG1478 on LPA-induced Cox-2 expression (Fig. 5D), further confirming the essential role of C/EBP in transcriptional activation of Cox-2. Taken together, these results demonstrate that RTK, not necessarily EGFR, provides an obligatory activity that acts in concert with LPA GPCR signaling to activate C/EBP and the subsequent Cox-2 expression.
The mRNA-binding protein HuR associates with and stabilizes Cox-2 mRNA in LPA-treated cells
Several regulatory mechanisms control mRNA stability under different physiological and pathophysiological conditions (38,39,40,41). One of such regulations involves the RNA-binding protein HuR that associates with AUUUA repeats present in the 3′ UTR of mRNAs encoding cytokines and angiogenic factors (27, 40, 41).
Two major Cox-2 transcripts (2.8 and 4.6 kb) are derived from alternative polyadenylation of the Cox-2 gene (41, 42). In ovarian cancer cells, LPA induced the 4.6 kb transcript, which contains a long 3′-UTR (41, 42). The 2.8 kb transcript lacking the 3′-UTR was not induced at detectable levels by LPA (Fig. 3A). Importantly, multiple AUUUA repeats are present within the 3′-UTR of the 4.6 kb Cox-2 transcript (40, 41). Moreover, HuR is highly expressed in primary ovarian cancers and ovarian cancer cell lines (43). These observations prompted us to examine whether HuR participates in protection of Cox-2 mRNA stability, contributing to the persistent induction of Cox-2 observed in LPA-stimulated cells.
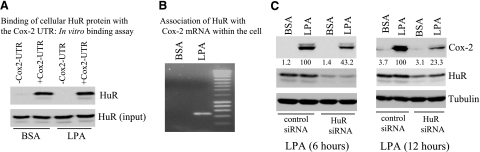
We first examined whether HuR physically binds to Cox-2 mRNA in vitro. To this end, the 3′ UTR of Cox-2 mRNA was synthesized and labeled with biotin by in vitro transcription. As demonstrated in Fig. 6A, the Cox-2 3′-UTR could bind with HuR protein from lysates of Caov-3 cells. Of note, incubation of 90 μg cellular protein with 1.5 μg Cox-2 3′-UTR brought down amounts of HuR similar to those present in 30 μg cell lysates (Fig. 6A). Therefore, approximately one-third of the total HuR protein formed complex with the exogenous Cox-2 3′-UTR, demonstrating that HuR has a strong binding affinity for the Cox-2 3′-UTR at least in in vitro binding assays.
Figure 6.
HuR binds to Cox-2 mRNA, contributing to the sustained induction of Cox-2 by LPA. A) Binding of HuR with the 3′UTR of Cox-2 mRNA in vitro. In vitro transcribed, biotinylated 3′UTR of the Cox-2 mRNA (1.5 μg) was incubated with cellular protein (90 μg) from Caov-3 cells treated with BSA or LPA (10 μM, 6 h). Amounts of HuR pulled down by the Cox-2 3′UTR were analyzed by immunoblotting with an anti-HuR antibody. Cell lysates (30 μg) were included to serve as inputs. B) Association of HuR protein with Cox-2 mRNA within the cell. HuR was immunoprecipitated from Caov-3 cells treated for 6 h with BSA (control) or LPA (10 μM). The mRNAs present in the HuR immunocomplex were extracted, reverse-transcribed, and amplified by PCR using Cox-2 specific primers as detailed in Materials and Methods. C) Effect of HuR knockdown on LPA-induced Cox-2 expression. The control and HuR down-regulated Caov-3 cells were stimulated for 6 h (left panel) or 12 h (right panel) with 10 μM LPA. Cox-2, Cox-1 and HuR levels in these cells were analyzed with immunoblotting. The values beneath each lane represent relative intensities (%) quantified by densitometry, with Cox-2 induced by LPA in control siRNA-treated cells defined as 100%. Similar results were obtained from two independent assays.
To confirm that the HuR-Cox-2 mRNA association occurs within the cell, we immunoprecipitated HuR from Caov-3 cells treated with LPA or BSA for 6 h. The immunocomplexes were then subjected to reverse transcription followed by PCR amplification of a 481 bp fragment of Cox-2 cDNA. As shown in Fig. 6B, the fragment was detected in immunoprecipitates of the cells treated with LPA but not in those of control cells. It was also absent from immunoprecipitates of LPA-treated cells when a control antibody was used to replace the specific HuR antibody (data not shown). HuR, therefore, indeed formed a complex with Cox-2 mRNA in LPA-treated cells. The lack of Cox-2 mRNA in the immunoprecipitates of control cells suggests that the interaction between HuR and Cox-2 mRNA may not be constitutive. It could be regulated through activation of HuR protein or rely on LPA-dependent increases in Cox-2 mRNA abundance.
To determine the functional significance of HuR binding to Cox-2 mRNA, we assessed the effect of down-regulation of HuR on LPA-induced Cox-2 expression. Knockdown of HuR expression resulted in a significant decrease in LPA-induced Cox-2 as shown in Fig. 6C. Stronger suppression of Cox-2 expression was observed in cells treated with LPA for 12 h compared to the cells treated with LPA for 6 h. The observation suggests that HuR-mediated stabilization of Cox-2 mRNA contributes more significantly to Cox-2 expression when transcriptional activation becomes less active after prolonged incubation with LPA.
DISCUSSION
In the present study, we demonstrated that LPA induces robust and sustained expression of Cox-2 in ovarian cancer cells through both transcriptional and post-transcriptional mechanisms, mediated by the C/EBP transcription factor and the mRNA stability protein HuR, respectively. The identification of C/EBP as a primary transcription factor responsible for LPA-induced Cox-2 expression is somewhat surprising, as C/EBP has not been previously linked to any biological actions of LPA. Other transcription factors activated by LPA and commonly involved in inducible Cox-2 expression, such as NF-κB and AP-1, are instead dispensable for LPA-induced Cox-2. Previous studies of GPCR regulation of Cox-2 in different cell systems have led to diverse mechanisms involving multiple transcription factors (44, 45). A predominant and specific role of C/EBP in transcriptional activation of Cox-2 via GPCR signaling has not been previously described and likely represents a general mechanism regulating Cox-2 expression by LPA and other GPCR agonists in different cell types. It is also a novel and interesting observation that, in ovarian cancer cells, the C/EBP transcriptional activity is preferentially stimulated by LPA compared to the RTK agonists EGF, HGF, and IGF.
Because C/EBPβ expression is elevated in primary ovarian cancers and in ovarian cancer cell lines (37), the isotype likely plays a major role in mediating transcriptional activation of Cox-2 in response to LPA. C/EBPβ has been shown to be a key transcription factor in regulation of Cox-2 expression by aspirin and salicylate (46). Furthermore, expression of other isotypes of C/EBP, such as C/EBPα and C/EBPδ, is limited or undetectable in ovarian cancer cell lines (data not shown). We did not observe activation of the Cox-2 promoter by means of transfection of exogenous C/EBPβ, suggesting that overexpression of C/EBPβ protein is insufficient to confer the transcriptional activation of Cox-2. The luciferase reporter analysis established that LPA treatment stimulates C/EBP transcriptional activity. Multiple post-translational modifications are associated with C/EBP activation, including phosphorylation (47), acetylation (48), and sumoylation (28, 49). Treatment with LPA resulted in prominent phosphorylation of C/EBPβ. It is not known whether LPA-dependent activation of C/EBP is also regulated by acetylation and sumoylation in addition to phosphorylation.
In pursuit of the molecular mechanism linking the LPA receptors to C/EBP activation, we observed that LPA-induced C/EBP activation and Cox-2 expression involves an obligatory activity from EGFR. Transactivation of EGFR has been proposed as a mechanism to mediate many biological actions of LPA in numerous studies (50, 51). In contrast, the possibility for involvement of a parallel RTK signal in biological responses to LPA or other GPCR agonists has been rarely studied (52). In our experiments, EGF and other RTK agonists only weakly stimulated C/EBP activity and Cox-2 expression compared to LPA (Figs. 1Aand 5B), allowing us to distinguish the input of GPCR from that of RTK in these cells. Based on the differential abilities of LPA and EGF to activate C/EBP, it is hard to imagine that transactivation of EGFR could trigger robust activation of C/EBP and Cox-2 expression in LPA-stimulated cells. It is more likely that optimal activation of C/EBP relies on combinatorial signaling components from LPA GPCRs and EGFR. The EGFR signal may feed in at some point downstream of GPCRs. Furthermore, our results indicate that activities of other RTKs, not necessarily EGFR, could cooperate with the LPA GPCRs in regulation of C/EBP activity and Cox-2 expression. EGFR is usually recognized to serve such a role probably because it is more universally expressed and exhibits higher activity than other RTKs, particularly in cancer cells (53). The high EGFR activity present in malignant cells may be necessary for appropriate GPCR signaling.
LPA-induced Cox-2 gene expression is mediated by LPA1, LPA2, and LPA5 receptors independent of LPA3 and LPA4. The LPA1 receptor is the most commonly expressed subtype present in both normal and cancerous tissues (14). The LPA2 receptor subtype is abnormally overexpressed in ovarian cancers and other human malignancies (14, 54, 55). It mediates LPA-dependent cytokine production in ovarian and breast cancer cells (15, 17). Similarly, Hu et al. (18) reported that LPA2 expression correlates with the ability of LPA to induce VEGF expression in ovarian cancer cells (18). The importance of LPA2 in modulation of gene expression is further highlighted by the observation that transgenic expression of LPA2 driven by an ovary-selective promoter led to the production of higher levels of VEGF and uPA mRNA and proteins in ovaries of transgenic mice (56). In addition to the role in modulation of gene expression, LPA2 may also regulate cell motility (57, 58). Involvement of LPA5 in LPA-induced Cox-2 expression is an interesting observation, because the biological functions of this new LPA receptor are totally unknown (7). Requirement of LPA1, LPA2, and LPA5 in LPA induction of Cox-2 suggests that these LPA receptors could each contribute to the response to LPA. Alternatively, the effect of LPA may depend on combined functions of these receptors. A recent study suggests that these LPA receptors can crosstalk to each other through forming heterodimers with signaling properties likely different from their homodimers (59). In addition, the LPA3 receptor does not seem to be a mediator of Cox-2 induction in ovarian cancer cells, although this receptor is highly expressed in most ovarian cancer cell lines (14). The result is inconsistent with the critical role of LPA3 in the uterine Cox-2 expression, as suggested by studies of LPA3 knockout mice (25), indicating that different LPA receptor subtypes can mediate Cox-2 expression, depending on the cellular context.
The proximal region of the 3′-UTR of COX-2 mRNA contains highly conserved AU-rich elements that have potential to interact with multiple mRNA binding proteins, including β-catenin, TIAR, AUF1, HuR, hnTIA-1, and hnRNP (38,39,40,41). In the current study, we focused on the role of HuR, a member of the ELAV (embryonic lethal abnormal vision) family of mRNA-binding proteins (27). HuR overexpression is associated with increased levels of Cox-2 protein in cancers of the colon, stomach, breast, and ovary (37, 60,61,62). However, few studies have provided direct evidence that HuR plays a causal role in Cox-2 overexpression in malignant cells. Our results indicate that HuR physically binds to the Cox-2 3′-UTR and protects Cox-2 mRNA stability. This protein-mRNA association contributes significantly to the sustained induction of Cox-2 by LPA. HuR thus provides a positive feedback to Cox-2 induction during physiological responses to LPA and probably other environmental stimuli.
Acknowledgments
We thank Dr. T. M. McIntyre (Cleveland Clinic Foundation) for the 7.2 kb promoter DNA of the human Cox-2 gene and Dr. L. Sealy (Vanderbilt University) for providing the C/EBPβ LAP1 and LAP2 expression vectors. This work was supported in part by U.S. National Institutes of Health (NIH)/National Cancer Institute grant CA102196 (X.F.), U.S. Department of Defense career development award W81XWH0410103 (X.F.), the Massey Cancer Center pilot project grant (X.F.), and NIH grant P30 CA16059 to the Massey Cancer Center of Virginia Commonwealth University.
References
- Moolenaar W H. Development of our current understanding of bioactive lysophospholipids. Ann N Y Acad Sci. 2000;905:1–10. doi: 10.1111/j.1749-6632.2000.tb06532.x. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, Jalink K, Fahrenfort I, Moolenaar W H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J H, Weiner J A, Post S R, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Bleu T, Hallmark O G, Goetzl E J. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Lee C W, Rivera R, Gardell S, Dubin A E, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- McIntyre T M, Pontsler A V, Silva A R, St. Hilaire A, Xu Y, Hinshaw J C, Zimmerman G A, Hama K, Aoki J, Arai H, Prestwich G D. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E J, Groenink A, Jalink K, Eichholtz T, Moolenaar W H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- Van der Bend R L, de Widt J, van Corven E J, Moolenaar W H, van Blitterswijk W J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992;285:235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L R, Marshall C J. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993;268:20717–20720. [PubMed] [Google Scholar]
- Moolenaar W H, Kranenburg O, Postma F R, Zondag G C. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- Mills G B, Moolenaar W H. The emerging role of lysophosphatidic acid in Cancer. Nature Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- Fang X, Schummer M, Mao M, Yu S, Tabassam F H, Swaby R, Hasegawa Y, Tanyi J L, LaPushin R, Eder A, Jaffe R, Erickson J, Mills G B. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu S, Bast R C, Liu S, Xu H J, Hu S X, LaPushin R, Claret F X, Aggarwal B B, Lu Y, Mills G B. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- Sivashanmugam P, Tang L, Daaka Y. Interleukin 6 mediates the lysophosphatidic acid-regulated cross-talk between stromal and epithelial prostate cancer cells. J Biol Chem. 2004;279:21154–21159. doi: 10.1074/jbc.M313776200. [DOI] [PubMed] [Google Scholar]
- Lee Z, Swaby R F, Liang Y, Yu S, Liu S, Lu K H, Bast R C, Mills G B, Fang X. Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res. 2006;66:2740–2748. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- Hu Y L, Tee M K, Goetzl E J, Auersperg N, Mills G B, Ferrara N, Jaffe R B. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- Pustilnik T B, Estrella V, Wiener J R, Mao M, Eder A, Watt M A, Bast R C, Mills G B. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5:3704–3710. [PubMed] [Google Scholar]
- Symowicz J, Adley B P, Woo M M, Auersperg N, Hudson L G, Stack M S. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005;65:2234–2242. doi: 10.1158/0008.5472.CAN-04-2781. [DOI] [PubMed] [Google Scholar]
- Muehlich S, Schneider N, Hinkmann F, Garlichs C D, Goppelt-Struebe M. Induction of connective tissue growth factor (CTGF) in human endothelial cells by lysophosphatidic acid, sphingosine-1-phosphate, and platelets. Atherosclerosis. 2004;175:261–268. doi: 10.1016/j.atherosclerosis.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Liao Z, Mason K A, Milas L. Cyclo-oxygenase-2 and its inhibition in cancer: is there a role? Drugs. 2007;67:821–845. doi: 10.2165/00003495-200767060-00001. [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk J E, Kargman S L, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Torrance C J, Jackson P E, Montgomery E, Kinzler K W, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani C M. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–1028. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos J J, Anliker B, Inoue A, Skinner M K, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H. Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol. 2005;11:5638–5643. doi: 10.3748/wjg.v11.i36.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X C, Steitz J A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton E M, Sealy L. Modification of CCAAT/enhancer-binding protein-beta by the small ubiquitin-like modifier (SUMO) family members, SUMO-2 and SUMO-3. J Biol Chem. 2003;278:33416–33421. doi: 10.1074/jbc.M305680200. [DOI] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Pontsler A V, St Hilaire A, Marathe G K, Zimmerman G A, McIntyre T M. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem. 2002;277:13029–13036. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- Hahn A, Barth H, Kress M, Mertens P R, Goppelt-Struebe M. Role of Rac and Cdc42 in lysophosphatidic acid-mediated cyclo-oxygenase-2 gene expression. Biochem J. 2002;362:33–40. doi: 10.1042/0264-6021:3620033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W L, DeWitt D L, Garavito R M. Cyclooxygenases: structural, cellular, and molecular biology Annu. Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Chen L C, Chen B K, Chang J M, Chang W C. Essential role of c-Jun induction and coactivator p300 in epidermal growth factor-induced gene expression of cyclooxygenase-2 in human epidermoid carcinoma A431 cells. Biochim Biophys Acta. 2004;1683:38–48. doi: 10.1016/j.bbalip.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Brown P H, Alani R, Preis L H, Szabo E, Birrer M J. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–886. [PubMed] [Google Scholar]
- Wang D, You Y, Lin P C, Xue L, Morris S W, Zeng H, Wen R, Lin X. Bcl10 plays a critical role in NF-kappaB activation induced by G protein-coupled receptors. Proc Natl Acad Sci U S A. 2007;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister-Lucas L M, Ruland J, Siu K, Jin X, Gu S, Kim D S, Kuffa P, Kohrt D, Mak T W, Nunez G, Lucas P C. CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci U S A. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundfeldt K, Ivarsson K, Carlsson M, Enerback S, Janson P O, Brannstrom M, Hedin L. The expression of CCAAT/enhancer binding protein (C/EBP) in the human ovary in vivo: specific increase in C/EBPbeta during epithelial tumour progression. Br J Cancer. 1999;79:1240–1248. doi: 10.1038/sj.bjc.6690199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cok S J, Acton S J, Morrison A R. The proximal region of the 3′-untranslated region of cyclooxygenase-2 is recognized by a multimeric protein complex containing HuR, TIA-1, TIAR, and the heterogeneous nuclear ribonucleoprotein U. J Biol Chem. 2003;278:36157–36162. doi: 10.1074/jbc.M302547200. [DOI] [PubMed] [Google Scholar]
- Lee H K, Jeong S. Beta-Catenin stabilizes cyclooxygenase-2 mRNA by interacting with AU-rich elements of 3′-UTR. Nucleic Acids Res. 2006;34:5705–5714. doi: 10.1093/nar/gkl698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J L, Sully G, Clark A R, Saklatvala J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Jang B C, Wu M T, Paik J H, Furneaux H, Hla T. The RNA-binding protein HuR regulates the expression of cyclooxygenase-2. J Biol Chem. 2003;278:25227–25233. doi: 10.1074/jbc.M301813200. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem J. 1996;318:325–331. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Weichert W, Pest S, Koch I, Licht D, Kobel M, Reles A, Sehouli J, Dietel M, Hauptmann S. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res. 2004;64:189–195. doi: 10.1158/0008-5472.can-03-1987. [DOI] [PubMed] [Google Scholar]
- Ki S H, Choi M J, Lee C H, Kim S G. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J Biol Chem. 2007;282:1938–1947. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- Nie M, Pang L, Inoue H, Knox A J. Transcriptional regulation of cyclooxygenase 2 by bradykinin and interleukin-1beta in human airway smooth muscle cells: involvement of different promoter elements, transcription factors, and histone h4 acetylation. Mol Cell Biol. 2003;23:9233–9244. doi: 10.1128/MCB.23.24.9233-9244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K K, Liou J Y, Cieslik K. Transcriptional control of COX-2 via C/EBPbeta. Arterioscler Thromb Vasc Biol. 2005;25:679–685. doi: 10.1161/01.ATV.0000157899.35660.61. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci U S A. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesena T I, Cardinaux J L, Kwok R, Schwartz J. CCAAT/enhancer-binding protein (C/EBP) beta is acetylated at multiple lysines: acetylation of C/EBPbeta at lysine 39 modulates its ability to activate transcription. J Biol Chem. 2007;282:956–967. doi: 10.1074/jbc.M511451200. [DOI] [PubMed] [Google Scholar]
- Kim J, Cantwell C A, Johnson P F, Pfarr C M, Williams S C. Transcriptional activity of CCAAT/enhancer-binding proteins is controlled by a conserved inhibitory domain that is a target for sumoylation. J Biol Chem. 2002;277:38037–38044. doi: 10.1074/jbc.M207235200. [DOI] [PubMed] [Google Scholar]
- Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cunnick J M. Trans-regulation of epidermal growth factor receptor by lysophosphatidic acid and G protein-coupled receptors. Biochim Biophys Acta. 2002;1582:100–106. doi: 10.1016/s1388-1981(02)00143-9. [DOI] [PubMed] [Google Scholar]
- Rubio I, Rennert K, Wittig U, Wetzker R. Ras activation in response to lysophosphatidic acid requires a permissive input from the epidermal growth factor receptor. Biochem J. 2003;376:571–576. doi: 10.1042/BJ20031410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon D S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Schulte K M, Beyer A, Kohrer K, Oberhauser S, Roher H D. Lysophosphatidic acid, a novel lipid growth factor for human thyroid cells: over-expression of the high-affinity receptor edg4 in differentiated thyroid cancer. Int J Cancer. 2001;92:249–256. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1166>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Shida D, Sako A, Ishikawa M, Hama K, Aoki J, Arai H, Nagawa H. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 2004;6:R640–R646. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M C, Lee H Y, Yeh C C, Kong Y, Zaloudek C J, Goetzl E J. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2) Oncogene. 2004;23:122–129. doi: 10.1038/sj.onc.1206986. [DOI] [PubMed] [Google Scholar]
- Xu J, Lai Y J, Lin W C, Lin F T. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J Biol Chem. 2004;279:10459–10468. doi: 10.1074/jbc.M311891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S Y, Schinkmann K A, Avraham S. RAFTK/Pyk2 mediates LPA-induced PC12 cell migration. Cell Signal. 2006;18:1063–1071. doi: 10.1016/j.cellsig.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Zaslavsky A, Singh L S, Tan H, Ding H, Liang Z, Xu Y. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim Biophys Acta. 2006;1761:1200–1212. doi: 10.1016/j.bbalip.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Denkert C, Koch I, von Keyserlingk N, Noske A, Niesporek S, Dietel M, Weichert W. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol. 2006;19:1261–1269. doi: 10.1038/modpathol.3800645. [DOI] [PubMed] [Google Scholar]
- Denkert C, Weichert W, Winzer K J, Muller B M, Noske A, Niesporek S, Kristiansen G, Guski H, Dietel M, Hauptmann S. Expression of the ELAV-like protein HuR is associated with higher tumor grade and increased cyclooxygenase-2 expression in human breast carcinoma. Clin Cancer Res. 2004;10:5580–5586. doi: 10.1158/1078-0432.CCR-04-0070. [DOI] [PubMed] [Google Scholar]
- Erkinheimo T L, Lassus H, Sivula A, Sengupta S, Furneaux H, Hla T, Haglund C, Butzow R, Ristimaki A. Cytoplasmic HuR expression correlates with poor outcome and with cyclooxygenase 2 expression in serous ovarian carcinoma. Cancer Res. 2003;63:7591–7594. [PubMed] [Google Scholar]