Abstract
Gα12 constitutes, along with Gα13, one of the four families of α subunits of heterotrimeric G proteins. We found that the N terminus of Gα12, but not those of other Gα subunits, contains a predicted mitochondrial targeting sequence. Using confocal microscopy and cell fractionation, we demonstrated that up to 40% of endogenous Gα12 in human umbilical vein endothelial cells colocalize with mitochondrial markers. N-terminal sequence of Gα12 fused to GFP efficiently targeted the fusion protein to mitochondria. Gα12 with mutated mitochondrial targeting sequence was still located in mitochondria, suggesting the existence of additional mechanisms for mitochondrial localization. Lysophosphatidic acid, one of the known stimuli transduced by Gα12/13, inhibited mitochondrial motility, while depletion of endogenous Gα12 increased mitochondrial motility. Gα12Q229L variants uncoupled from RhoGEFs (but not fully functional activated Gα12Q229L) induced transformation of the mitochondrial network into punctate mitochondria and resulted in a loss of mitochondrial membrane potential. All examined Gα12Q229L variants reduced phosphorylation of Bcl-2 at Ser-70, while only mutants unable to bind RhoGEFs also decreased cellular levels of Bcl-2. These Gα12 mutants were also more efficient Hsp90 interactors. These findings are the first demonstration of a heterotrimeric G protein α subunit specifically targeted to mitochondria and involved in the control of mitochondrial morphology and dynamics.—Andreeva, A. V., Kutuzov, M. A., Voyno-Yasenetskaya, T. A. Gα12 is targeted to the mitochondria and affects mitochondrial morphology and motility.
Keywords: heterotrimeric G proteins, Bcl-2, Hsp90, mitochondrial fission, organelle motility
In addition to the vital role mitochondria play in respiration and supplying cells with energy (1, 2), they are also involved in other functions, such as apoptosis (3) or Ca2+ homeostasis (ref. 4 and references therein). Mitochondria are intimately involved in a variety of signaling pathways (5). It has been suggested that in the cells that largely rely on glycolysis for energy production, the main role of mitochondria may be shifted from energy production to signaling (6). In particular, in endothelial cells mitochondria have been implicated in intracellular calcium dynamics and production of nitric oxide and reactive oxygen species (7).
Mitochondria form a reticulum, which has different morphology in different cell types (8). They are motile and also undergo fusion, fission, and branching events, leading to dynamic remodeling of the mitochondrial network (5, 9). Fast mitochondrial movement in animal cells is mediated by microtubule-associated motor proteins, while actin cytoskeleton may be involved in slower short-distance movement and/or anchoring of mitochondria (10,11,12). Basic molecular machinery involved in mitochondrial fission and fusion has been identified (13). A severe reduction in mitochondrial fusion leads to embryonic lethality (14), and several mutations in the components of the fission and fusion machinery have been linked to neurodegenerative disorders (15). Inhibition of mitochondrial fusion is associated with defects in respiration and an increased susceptibility to apoptosis (16).
Heterotrimeric G proteins, consisting of GDP/GTP-binding Gα subunits and Gβγ dimers, transduce signals from hundreds of heptahelical G protein-coupled receptors (GPCRs) (17,18,19) and are also implicated in signaling pathways that do not involve canonical GPCRs (18, 20, 21). Alpha subunit of the G protein G12 (Gα12) constitutes, along with Gα13, one of the four families of heterotrimeric G proteins (22). A variety of functions and interaction partners have been identified for Gα12 and Gα13, some of them overlapping and some specific for either of the two isoforms (23, 24). Here we report that the N-terminal extension of Gα12, but not of Gα13, represents a functional mitochondrial targeting sequence. We found that a substantial proportion of endogenous Gα12, but not other examined Gα subunits, is associated with mitochondria in human umbilical vein endothelial cells (HUVECs) and COS-7 cells. Furthermore, our data indicate that Gα12 is involved in regulation of mitochondrial motility, morphology, and membrane permeability. The canonical paradigm of G protein-coupled signaling implies that heterotrimeric G proteins function in conjunction with their cognate receptors, located at the plasma membrane. Our data support the concept that heterotrimeric G proteins may also have distinct functions at other cellular compartments.
MATERIALS AND METHODS
In silico analyses
The presence of mitochondrial targeting sequences was examined using Mitoprot II 1.0 (http://ihg.gsf.de/ihg/mitoprot.html) and TargetP V1.0 (www.cbs.dtu.dk/services/TargetP). Sequence alignments were performed using ClustalW (www.ebi.ac.uk/clustalw).
Plasmids
Untagged Gα12 constructs were described previously (25). Constructs for EE-tagged Gα12 and Gα12Q229L were from the Guthrie Research Institute (Sayre, PA, USA). Constructs for HA-tagged Gα12 and Gα13 were a generous gift from Silvio Gutkind (National Institute of Dental Research, Bethesda, MD, USA). Myc-tagged Gα12 constructs (26, 27) were kindly provided by Thomas Meigs (University of North Carolina, Asheville, NC, USA). Mutations were introduced using a QuickChange site-directed mutagenesis kit (Stratagene, LA Jolla, CA, USA).
Antibodies
Polyclonal Gα12, Gα13, Gαq, Gβ, Bcl-2, VE-cadherin, GRP-78, monoclonal HA, VE-cadherin, LAMP1, and ZO-1-specific antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The following lots of the Gα12-specific antibody (Sc-409) were used: I150, H052, and D1805. Monoclonal Hsp90- and GM130-specific antibodies were from BD Biosciences (San Jose, CA, USA). Antibodies specific for subunits 2 (monoclonal) and 4 (polyclonal) of cytochrome C oxidase complex IV (COX) were from Molecular Probes (Eugene, OR, USA) and Cell Signaling Technology (Beverly, MA, USA), respectively. Polyclonal c-Jun N-terminal kinase (JNK), phospho-JNK (Thr183/Tyr185), Bcl-2, phospho-Bcl-2 (Ser-70) antibodies were from Cell Signaling Technology. Polyclonal LAMP1 antibody was from Abcam (Cambridge, MA, USA). Anti-Myc tag 4A6 antibody (Upstate Biotechnology, Lake Placid, NY, USA) and 9E10 antibody (Sigma-Aldrich, St. Louis, MO, USA) were used for Western blotting and immunofluorescence microscopy, respectively.
Cell culture and transfection
Transient transfection of COS-7 cells was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. HUVECs, obtained from Clonetics Corp. (San Diego, CA, USA), were grown and transfected as described previously (28).
Gα12 knockdown was performed as described previously (28) using the Gα12 siGENOME SMARTpool reagent from Dharmacon Research Inc. (Lafayette, CO, USA). Control siRNA was from Santa Cruz Biotechnology (29).
Isolation of mitochondria, hypotonic rupture, and Proteinase K treatment were performed (30).
Western blotting and quantitation of ECL data were performed as described previously (31). To determine proportion of Gα12 targeted to mitochondria, signal intensity in the mitochondrial fractions (P10) was normalized to the sum of the signals in P10 and S10 fractions. To quantitate the extent of phosphorylation of JNK and Bcl-2, signal intensity for phosphorylated forms of these proteins (detected by corresponding phospho-specific antibodies) were normalized to their total content (detected by antibodies recognizing JNK and Bcl-2 irrespectively of their phosphorylation state) in the same sample. Total cellular levels of Bcl-2 were determined by normalization to β-actin.
Immunoprecipitation from mitochondrial fractions
Mitochondrial pellets were lysed in 20 mM HEPES (pH 7.5), 5 mM MgCl2, 0.5% Nonidet P-40 (Fisher Scientific, Pittsburgh, PA, USA), 1:200 dilution of proteinase inhibitor cocktail (Sigma-Aldrich); incubated on ice for 15 min; and cleared by centrifugation. Extracts were precleaned by incubation at 4°C for 30 min with 20 μl protein A/G agarose beads (Santa Cruz Biotechnology). Supernatants were supplemented with 3 μg c-Myc antibody and 20 μl protein A/G agarose beads and rotated overnight at 4°C. Beads were pelleted and washed 3 times with 0.5 ml lysis buffer.
Confocal microscopy
HUVECs cultured on gelatin-coated coverslips were fixed with 3.7% paraformaldehyde, followed by permeabilization in 0.5% Triton X-100. Cells were incubated with primary antibodies, followed by incubation with appropriate secondary antibodies, using Tris-buffered saline containing bovine serum albumin as a blocking buffer. To visualize mitochondria in living cells, MitoTracker Deep Red 633 or Red CMXRos (Molecular Probes/Invitrogen) was added to the medium (200 nM) and the cells were incubated at 37°C for 1 h. Images were acquired using an LSM 510 confocal microscope (Zeiss, Oberkochen, Germany), equipped with an ×63 water-immersion objective with appropriate filter sets and processed using LSM Browser (Zeiss) and Photoshop (Adobe, San Jose, CA, USA). For time-lapse microscopy, stacks of images were recorded using the Zeiss time series module at time intervals of 7–9 s.
Quantitative analysis of mitochondrial motility
Image analysis was performed using ImageJ software (http://rsb.info.nih.gov/ij/), supplemented with SpotTracker plugin (32). For small round mitochondria, coordinates of their centers were used. For tubular mitochondria, their ends were tracked. In the latter case, manual tracking was used throughout the image sequence. The velocity data were recalculated from pixel per frame into micrometers per second using Excel (Microsoft, Redmond, WA, USA). Threshold for the fast movements was set at 0.1 μm/s.
RESULTS
Gα12 is located in mitochondria
Using algorithms predicting intracellular protein targeting, we found that Gα12 shows a remarkably high probability of being targeted to mitochondria (Fig. 1A). Therefore, we examined whether endogenous Gα12 would colocalize with MitoTracker, a dye that accumulates in mitochondria with active respiration. Polyclonal Gα12-specific antibodies from three different lots all yielded mitochondrial staining in HUVECs (Fig. 1B), although they differed in their ability to recognize other cellular structures, in particular the nucleus. These findings indicated that a considerable proportion of endogenous Gα12 is associated with mitochondria.
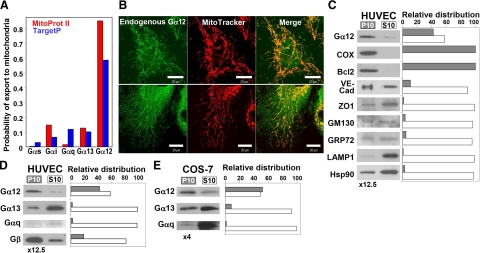
Figure 1.
Endogenous Gα12 is localized to mitochondria in HUVECs and COS-7 cells. A) Prediction of mitochondrial targeting of different Gα subunits using Mitoprot II (red) and TargetP (blue). UniProt accession numbers: Gαs, P63092; Gαi1, P63096; Gαq, P50148; Gα12, Q03113; Gα13, Q14344. B) Colocalization of endogenous Gα12 with MitoTracker in HUVECs. Living cells were incubated with MitoTracker, fixed, and immunostained with Gα12-specific antibodies (top and bottom panels: lots H052 and I150, respectively). Scale bars = 20 μm. C) Distribution of Gα12 and organelle markers in the mitochondrial fraction (P10) and the fraction containing other organelles except nuclei (S10). P10 was resuspended in 1/12.5 of initial volume, and equal volumes of P10 and S10 were loaded on the gel. The following marker proteins were examined: COX and Bcl-2 (mitochondria), VE-cadherin, and zonula occludens-1 (ZO1) (plasma membrane/microsomal fraction), GM130 (Golgi apparatus), GRP72 (endoplasmic reticulum), LAMP1 (lysosomes), as well as Hsp90 as a representative of proteins mainly present in the cytoplasm. D, E) Distribution of subunits of different heterotrimeric G proteins in HUVECs and in COS-7 cells, respectively. In E, P10 was resuspended in 1/4 of initial volume. Experiments shown were repeated twice with similar results.
We next analyzed partitioning of Gα12 and various organelle markers upon fractionation of HUVECs using differential centrifugation. After removal of cell debris and nuclei, HUVEC lysates were separated into the crude mitochondrial fraction (10,000 g pellet, P10) and 10,000 g supernatant (S10), containing plasma membrane and the microsomal fraction, as well as cytosolic proteins. Approximately 40% of Gα12 was associated with the mitochondrial fraction (Fig. 1C). As expected, the two mitochondrial markers, COX and Bcl-2, were found exclusively in P10; whereas markers for cytosolic proteins, plasma membrane, Golgi apparatus, endoplasmic reticulum and lysosomes were detected mainly in the S10 fraction, indicating only minimal contamination of the mitochondrial fraction by other organelles (Fig. 1C). Comparison of partitioning of Gα12, Gα13, Gαq, and Gβ showed that only small amounts of Gα13 and Gαq were present in the P10 fraction, while ∼20% of Gβ was mitochondrial (Fig. 1D).
We also examined partitioning of Gα12 in COS-7 (Fig. 1E) and NIH 3T3 cells (not shown), where ∼50% and 15–20% of total Gα12, respectively, was in the mitochondrial fraction, while neither Gα13 nor Gαq were enriched in mitochondria.
Gα12 is located mostly on the mitochondrial surface
We next investigated the location of endogenous Gα12 in HUVEC mitochondria using Proteinase K protection assay with or without hypotonic disruption of the outer membrane. Both Gα12 and COX were resistant to hypotonic treatment, while Gα13 was almost completely removed (Fig. 2A). In contrast, proteinase K treatment removed >80% of the mitochondrial Gα12. Outer membrane disruption had no effect on the efficiency of Proteinase K for both Gα12 and COX (Fig. 2A). Upon solubilization of mitochondria in 1% Triton X-100, Gα12 was completely digested by proteinase K (data not shown). These results indicate that Gα12 is located on the mitochondrial surface, although a small fraction of Gα12 may translocate into the matrix or be protected in a labile detergent-sensitive protein complex.
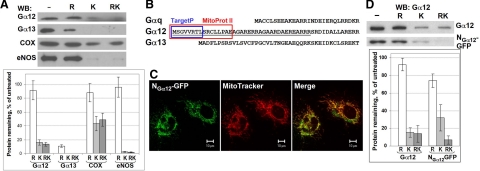
Figure 2.
N terminus of Gα12 targets proteins to the mitochondrial surface. A) Mitochondrial fraction of HUVECs was subjected to hypotonic rupture (R) and/or treated with Proteinase K (K) as indicated. The effect of these treatments on Gα12, Gα13, COX, and eNOS was assessed by Western blotting with corresponding antibodies. Data were normalized to the protein content in untreated aliquots. Data shown are the means of two replicates; error bars show values obtained in each replicate. B) Sequences in Gα12 predicted by Mitoprot II (red) and TargetP (blue) to function as signal peptide for mitochondrial targeting. UniProt accession numbers: Gαq, P21279; Gα12, P27600; Gα13, P27601. C) Mitochondrial targeting of NGα12-GFP (GFP fused to the N terminus of Gα12, underlined in B). Twenty-four hours after transfection, living HUVECs were incubated with MitoTracker and examined using confocal microscopy. D) N terminus of Gα12 does not mediate GFP translocation into mitochondrial matrix. Mitochondrial fraction obtained from COS-7 cells (either nontransfected or transfected with NGα12-GFP) was subjected to Proteinase K protection assay as in A. Endogenous Gα12 and NGα12-GFP were detected by Western blotting with Gα12-specific antibody. Data are means of 3 replicates; error bars indicate sd.
We also compared the sensitivities of Gα12 and endothelial nitric oxide synthase (eNOS) to proteolysis. Like Gα12, eNOS is a hydrophilic protein with lipid modifications and is anchored on the outer mitochondrial membrane in HUVECs (30). Similar to Gα12, and in line with published observations (30), eNOS was unaffected by hypotonic treatment, but efficiently cleaved by proteinase K (Fig. 2A). These data corroborate the conclusion that mitochondrial Gα12 is mostly located on the outer surface.
N terminus of Gα12 is sufficient for mitochondrial targeting
The N-terminal region of Gα12 may determine specificity of Gα12 interactions (29, 33). Both MitoProt II and Target P detected potential mitochondrial targeting signals in the N terminus of Gα12 (Fig. 2B). We fused 37 N-terminal amino acids of Gα12 to GFP and examined intracellular location of the fusion protein (designated as NGα12-GFP). Clear colocalization of NGα12-GFP with MitoTracker was observed (Fig. 2C). This demonstrated that the N terminus of Gα12 is sufficient for mitochondrial targeting.
We next examined whether the N terminus of Gα12 would be sufficient for GFP translocation into the mitochondrial matrix. NGα12-GFP behaved qualitatively similar to endogenous Gα12 in the Proteinase K protection assay (Fig. 2D). These data indicate that the N terminus of Gα12 is sufficient for mitochondrial targeting, yet it is unable to efficiently mediate Gα12 and NGα12-GFP translocation into the mitochondrial matrix.
Since Gα12 is palmitoylated at Cys11 (34), we tested whether this modification might prevent translocation into the matrix. However, NGα12-GFP-C11A mutant behaved similarly to NGα12-GFP with intact Cys11 in the Proteinase K protection assay (data not shown). Thus, the role of palmitoylation as a factor that precludes translocation into mitochondrial matrix can be excluded.
Depletion of endogenous Gα12 by siRNA increases mitochondrial motility
Lysophosphatidic acid (LPA) inhibits mitochondrial motility and affects mitochondrial morphology in CV-1 cells via Rho (12). Since Rho is the major effector of G12/13, we examined whether LPA would affect mitochondrial motility and morphology in HUVECs. In line with the observations for CV-1 cells (12), mitochondria in HUVECs exhibited clearly distinguishable nearly stationary (0–0.05 μm/s) or motile (>0.1 μm/s) states. LPA significantly reduced the frequency of the motile state (Fig. 3A–C) (12).
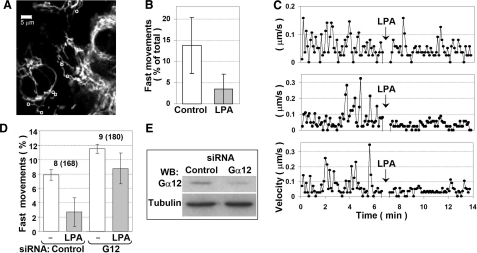
Figure 3.
LPA reduces (A–C) and Gα12 depletion (D–E) increases mitochondrial motility in HUVECs. A–C) HUVECs were starved for 24 h and incubated with MitoTracker to visualize mitochondria. A) The first frame of the recorded image sequence. Movement of 27 individual mitochondria was tracked under control conditions. Those that showed fast movement (exceeding the threshold of 0.1 μm/s) were also tracked after LPA addition (7 mitochondria marked with circles). Scale bar = 5 μm. B) Percentage of fast movements during entire recording before (control) and after (LPA) addition of LPA. Data are means ± sd; P = 0.011, paired Student’s t test. C) Representative plots of velocity vs. time for 3 individual mitochondria. Arrows indicate LPA addition. D–E) HUVECs were transfected with Gα12 siRNA duplexes or control siRNA. Mitochondrial movement was tracked 24 h after transfection for 6 min before and for additional 6 min after LPA addition. Mitochondrial movement was quantified as the percentage of mitochondria moving faster than 0.1 μm/s at any time point during recording. D) Quantification data shown are the means of two replicates (two separate slides), with error bars showing values obtained in each replicate. Data indicate total number of cells and number of individual mitochondria (in parentheses). The extent of Gα12 depletion was assessed by Western blotting using α-tubulin as a loading control (E).
To assess the possible role of Gα12 in mitochondrial motility, we depleted Gα12 using siRNA. Gα12 depletion increased the percentage of motile mitochondria (i.e., those that exceeded the 0.1 μm/s threshold during the time of recording) (Fig. 3D). In line with our previously published results (28), an ∼2- to 3-fold decrease in Gα12 levels could be achieved in HUVECs (Fig. 3E).
We also tested the effect of LPA in the cells with partially depleted Gα12 as compared with the cells transfected with control siRNA. Although the inhibition of mitochondrial motility by LPA was still observable in these cells, it was weaker than in the cells with endogenous level of expression of Gα12, which is in line with a contribution of Gα12 (Fig. 3D).
Effects of Gα12 mutants on mitochondrial morphology
Gα12 depletion did not result in noticeable changes in mitochondrial morphology. We examined whether mitochondrial morphology would be affected by the fully functional activated Gα12Q229L and Gα12Q229L unable to bind p115RhoGEF and related RhoGEFs (Gα12Δp115) and therefore unable to stimulate Rho (26). Expression of Gα12Q229L did not result in any major alterations in mitochondrial morphology (Fig. 4A), although in some experiments cells expressing this construct exhibited slightly more compact mitochondrial network (data not shown), and the overall amount of MitoTracker-labeled mitochondria was slightly decreased as compared to surrounding untransfected cells (Fig. 4B).
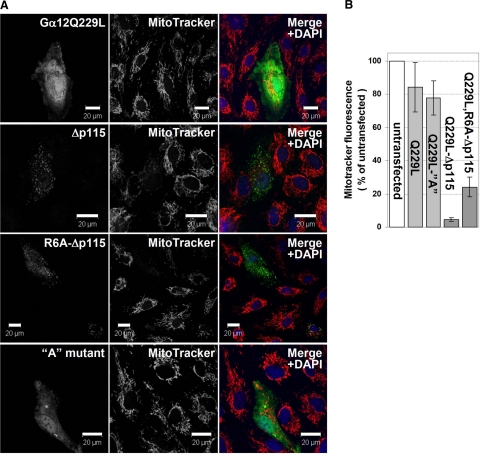
Figure 4.
Gα12 mutants unable to activate Rho lead to mitochondrial fragmentation and to an increased mitochondrial permeability. HUVECs were transfected with the Myc-tagged Gα12Q226L, Gα12Q229L-Δp115RhoGEF, Gα12Q229L,R6A-Δp115RhoGEF, and Gα12Q229L A (with residues 4–8 replaced with a NAAIR sequence; ref. 27; constructs as indicated). A) 24 h after transfection, cells were incubated with MitoTracker, fixed, and immunostained with Myc-specific antibody. Scale bars = 20 μM. B) MitoTracker-labeled mitochondria in transfected and surrounding untransfected cells were quantified using ImageJ. Fifteen transfected cells for each Gα12 mutant were analyzed. Error bars indicate sd.
In contrast, Gα12Δp115 induced a complete fragmentation of fluorescent tubular mitochondrial network into punctate structures, apparently unconnected to each other (Fig. 4A). In addition, MitoTracker fluorescence was strongly decreased (Fig. 4), indicating an impairment of respiration. The onset of this phenotype could apparently be triggered by the levels of Gα12Δp115 not yet detectable by immunofluorescence microscopy, since very few cells with detectable Gα12Δp115 expression had any remaining tubular mitochondria.
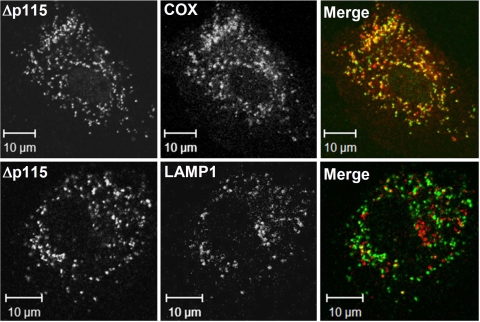
A considerable proportion of Gα12Δp115-containing structures was positive for COX, while almost none were positive for LAMP1, a marker for late endosomes and lysosomes (Fig. 5). These data argue against an immediate link between mitochondrial fragmentation observed in the presence of Gα12Δp115 and mitochondrial degradation. However, it cannot be excluded that in the cells where mitochondria are directed for degradation, Myc-Gα12 (which would be easily accessible on the mitochondrial surface) is already degraded, in which case we would not see such cells as transfected. It should be noted that in some G12Δp115-expressing cells (especially when higher amounts of Gα12 DNA were used for transfection) COX staining was barely detectable, while it was normal in surrounding nontransfected cells (data not shown).
Figure 5.
Punctate structures induced by Rho-uncoupled Gα12Q229L are positive for a mitochondrial (COX) rather than late endosomal/lysosomal (LAMP1) marker. Experiment was performed as in Fig. 4, and cells were stained with Myc-specific antibody (Δp115) and polyclonal antibodies against COX and LAMP1 as indicated.
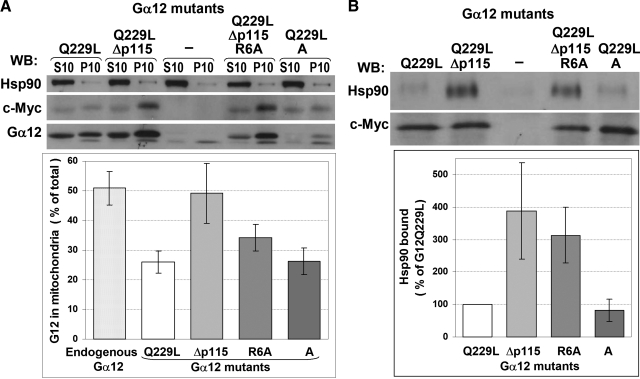
Western blot analysis showed that the fully functional Gα12Q229L was targeted to mitochondria less efficiently than endogenous Gα12 (∼50% and ∼25% of total content, respectively), while the proportion of the Δp115 mutant found in the mitochondrial fraction (∼50%) was similar to that of endogenous Gα12 (Fig. 6A).
Figure 6.
Mitochondrial targeting of Gα12Q229L mutants and their interaction with Hsp90. A) COS-7 cells were transfected with either pcDNA3.1-Myc-His6, or Gα12Q229L-Myc constructs as indicated. Cells were harvested 48 h after transfection and fractionated into mitochondrial pellet (P10) and supernatant (S10). Fractions were probed by Western blotting for the presence of Hsp90 and Gα12 (the latter detected using either c-Myc or Gα12 antibody; note that mutations in the R6A and A constructs are within the epitope of Gα12 antibody and are likely to affect its ability to recognize respective proteins). B) Mitochondrial fractions were solubilized in 0.5% Nonidet P-40; their amounts were adjusted to contain similar amounts of Myc-tagged Gα12; and immunoprecipitation was performed using c-Myc antibody. The presence of Myc-tagged Gα12 and Hsp90 in immunoprecipitates was detected using respective antibodies. Bottom panel shows quantification of the Western blotting data for Hsp90 binding, normalized to c-Myc signal in respective samples. ECL quantification data are means of two independent experiments; error bars indicate values obtained in each experiment.
Since Gα12Δp115 had dramatic effects on mitochondrial morphology and membrane potential, we attempted to create its variant with compromised mitochondrial targeting. Availability of such mutant would permit testing whether mitochondrial targeting of Gα12 is required for the observed effects. Using MitoProt II and TargetP algorithms, we found that mutating Arg6 would be most efficient in reducing the probability of mitochondrial localization. We introduced an R6A mutation into Gα12Δp115 and examined mitochondrial targeting of this protein. Surprisingly, the R6A mutant was still targeted to mitochondria, although somewhat less efficiently than Gα12QΔp115 with intact Arg6 (Fig. 6A). We also examined the “A” mutant of Gα12Q229L (27), which has amino acids 4–8 replaced by a NAAIR sequence and is predicted to have an almost zero probability of being targeted to mitochondria. However, the A mutant showed mitochondrial targeting similar to that of Gα12Q229L with intact N terminus (Fig. 6A). Like Gα12Δp115, the R6A mutant induced fragmentation of mitochondria and the loss of membrane potential as evidenced by the loss of MitoTracker staining, although the phenotype was somewhat less severe as compared to Gα12Δp115 with intact N terminus (Fig. 4B). The A mutant (which has intact site of interaction with RhoGEFs) behaved similarly to the fully functional Ga12Q229L (Fig. 4).
Observations that fully functional mutationally activated Gα12 is targeted to mitochondria less efficiently than endogenous Gα12 suggested that activation state of Gα12 may affect its mitochondrial localization. To assess whether activation of endogenous Gα12 would result in its redistribution from mitochondria, we isolated mitochondrial fraction in the absence and in the presence of AlF4−. No difference could be detected in the distribution of endogenous Gα12 (data not shown), indicating that Gα12 does not easily diffuse from mitochondria upon activation.
Gα12Q229L mutants unable to bind p115RhoGEF interact with Hsp90 more efficiently
Hsp90 is a major interactive partner of Gα12, but not Gα13 (35). Hsp90 interacts with Gα12 independently of the activation state of the latter or may be recruited to Cα12-containing signaling complexes upon Gα12 activation (35, 36). Since Hsp90 is known to be associated with mitochondria (37,38,39) and was also reported to target Gα12 to the lipid rafts (40), we hypothesized that interaction with Hsp90 may provide an additional mechanism that allows mitochondrial localization of Gα12 despite compromised mitochondrial targeting sequence. We examined whether the Gα12 mutants used in the above experiments would still be able to interact with Hsp90. Mitochondrial fractions from COS-7 cells transfected with Myc-tagged Gα12 constructs or empty vector were subjected to immunoprecipitation with Myc tag-specific antibody. Only traces of Hsp90 could be detected in the material immunoprecipitated from cells transfected with empty vector, while Hsp90 was clearly present in the samples from Gα12Q229L-expressing cells (Fig. 6B). Hsp90 interacted similarly with Gα12Q229L and the A mutant, while its interaction with both mutants unable to bind p115RhoGEF was dramatically increased (Fig. 6B). Similar results were obtained when immunoprecipitation was performed from S10 fractions (data not shown). These results indicate that Gα12 does interact with mitochondria-associated Hsp90, and that the inability of Gα12 to interact with p115RhoGEF correlates with enhanced Hsp90 binding.
Effects of Gα12Q229L mutants on JNK and Bcl-2
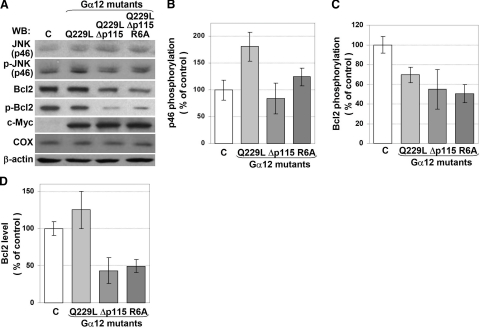
Gα12Δp115 is unable to stimulate JNK in adenocarcinoma cells (26). In COS-7 cells, Rho-uncoupled Gα12 mutants also failed to stimulate p46 JNK phosphorylation, while Gα12Q229L stimulated p46 phosphorylation by almost 2-fold (Fig. 7A, B); no effect of Gα12Q229L on p54 JNK could be detected (data not shown).
Figure 7.
Effects of Gα12Q229L mutants on JNK and Bcl-2. COS-7 cells were transfected with either pcDNA3.1-Myc-His6 or Gα12Q229L-Myc constructs as indicated. A) The presence and phosphorylation levels of JNK (p46) and Bcl-2 (phosphorylated at Ser-70), as well as Myc-tagged Gα12 and COX (as a mitochondrial marker) were detected by Western blotting using respective antibodies (c, control). The diagrams show quantification of the ECL data shown in A: effects of Gα12Q229L-Myc mutants on phosphorylation state of JNK (p46) (B), or Bcl-2 (C), or on the cellular levels of Bcl-2 (D). Data are means of 3 replicates; error bars indicate sd. The experiment was repeated twice (3 replicates in each experiment) with similar results.
Since both mutants unable to stimulate p115RhoGEF and JNK led to an apparent loss of mitochondrial membrane potential in our experiments, and since Bcl-2 is known to be phosphorylated by JNK (41,42,43) and to be involved in regulation of mitochondrial membrane potential (44), we examined whether Gα12 would affect phosphorylation and cellular levels of Bcl-2. All three Gα12 variants reduced Bcl-2 phosphorylation (Fig. 7 A, C), and the Rho-uncoupled mutants were slightly more efficient than Gα12Q229L (although the observed difference was not statistically significant). In contrast, Gα12Δp115 and the R6A mutant, but not Gα12Q229L, considerably reduced cellular levels of Bcl-2 (Fig. 7A, D). These observations indicate a correlation between the ability of Gα12 mutants to impair mitochondrial membrane potential and their ability to down-regulate Bcl-2, a protein that plays a role in maintaining this potential.
DISCUSSION
Here we demonstrated, for the first time, that Gα12 is likely to be the only Gα subunit specifically targeted to mitochondria. Up to 40% is associated with mitochondria. Our data suggest that Gα12 is involved in the regulation of mitochondrial morphology and motility.
Mitochondrial targeting of Gα12
Possible association of Gα subunits with mitochondria has been suggested (45,46,47), yet neither the mechanisms of mitochondrial association nor proportion of Gα subunits located in mitochondria have been addressed.
Unlike other Gα, Gα12 exhibits remarkably high probability of mitochondrial targeting. Mitochondrial localization of endogenous Gα12 was confirmed by confocal microscopy and cell fractionation. Our data suggest that most of mitochondrial Gα12 is on the cytoplasmic surface, although ∼20% is protected from proteolysis and thus may be imported. Since Gα12 carrying mutations in this sequence was still targeted to mitochondria, it seems likely that other proteins may promote Gα12 targeting to mitochondria. One such candidate protein may be Hsp90, which was reported to play a role in Gα12 targeting to the lipid rafts (40). Hsp90 is also present in mitochodnria, where it is involved in protein import (38, 39) and regulation of Bcl-2 function (37). Our data show that Gα12 does interact with Hsp90 in mitochondria.
Role of Gα12 in regulation of mitochondrial motility
Mitochondria are mobile organelles whose motility depends on the microtubules and actin cytoskeleton (12, 48, 49). siRNA-mediated depletion of Gα12 increases mitochondrial motility, suggesting that endogenous Gα12 inhibits mitochondrial movement. Actin cytoskeleton is known to hinder mitochondrial movements. Since Gα12/13 positively regulate actin polymerization through the Rho pathway (23), it seems plausible that Gα12-dependent regulation of mitochondrial motility may be mediated by changes in actin cytoskeleton. Gα12 is also known to activate JNK in a Rho-dependent manner (50). Recently it has been shown that active JNK phosphorylates kinesin-1 heavy chains and inhibits kinesin-1 microtubule-binding activity (51). Since mitochondrial movement depends on microtubule-associated motors dynein and kinesin, it is tempting to speculate that the Gα12-Rho-JNK-kinesin axis may be responsible for the observed regulation.
Role of Gα12 in regulation of mitochondrial morphology
While Gα12Q229L induced only subtle (if any) changes in the mitochondrial network, RhoGEF-uncoupled Gα12 mutants resulted in a complete fragmentation of the tubular mitochondrial network, concomitant with the loss of mitochondrial membrane potential. It should be noted that RhoGEF-uncoupled Gα12 can still interact with other partners, such as PP2A (27) and Hsp90 (present study). It seems possible that normal mitochondrial morphology requires a balance between Rho activity and some other Gα12-regulated pathways. Our data suggest that such pathways likely converge on Bcl-2. Bcl-2 plays an antiapoptotic role and inhibits mitochondrial fragmentation and membrane permeabilization (44, 52). Ser-70 phosphorylation enhances or is even required for Bcl-2 function (53, 54). Ser-70 in Bcl-2 can be phosphorylated by several kinases, including JNK (42, 43), and can be dephosphorylated by PP2A (55), both of which can be stimulated by Gα12 (50, 56). Decreased levels of Bcl-2 Ser-70 phosphorylation in the cells expressing all tested Gα12 mutants indicate that Gα12-dependent stimulation of Bcl-2 dephosphorylation prevails under our experimental conditions. A clear difference between Gα12Q229L and the Δp115 mutants observed in our experiments was that only the latter considerably decreased the levels of endogenous Bcl-2. Down-regulation of Bcl-2 might underlie the loss of mitochondrial membrane potential and mitochondrial fragmentation.
While this manuscript was under revision, a study was published that establishes a link between activation of Gα12 and degradation of Bcl-2 in MDCK and HEK293 cells via Gα12-dependent regulation PP2A, JNK, and monomeric G proteins (57). Although the details of the pathways involved and their balance apparently differ in our experimental system and in those studied by Yanamadala and co-workers (57) (since they observed Bcl-2 down-regulation by fully functional Gα12Q229L, while we only observed this phenomenon with Rho-uncoupled Gα12Q229L), it appears from these data that Gα12 may be involved in regulation of Bcl-2 in a variety of cells.
We found that mitochondrial Gα12 is associated with Hsp90, which is in line with Hsp90 role in protein targeting to mitochondria (38, 39). We found that Δp115 mutants interact with Hsp90 more efficiently than Gα12Q229L. Enhanced binding of Gα12Δp115 to Hsp90 might interfere with interaction of the latter with Bcl-2 and affect its functionality, since Bcl-2 function is dependent on its association with HSP90β (37).
In conclusion, we have identified Gα12 as the only α subunit of a heterotrimeric G protein that is specifically targeted to mitochondria and provide evidence for a previously unrecognized role of Gα12 in regulation of mitochondrial motility and morphology. Several important questions remain to be answered. First, it remains to be shown which of the observed effects require mitochondrial targeting of Gα12. Since the R6A mutation moderately decreases the efficiency of mitochondrial targeting and also slightly decreases severity of the phenotype induced by Rho-uncoupled Gα12Q229L, it seems plausible that mitochondrial fragmentation and the loss of potential require mitochondrial association of Gα12. Another question concerns the additional mechanisms that allow mitochondrial association of Gα12 despite mutations in its mitochondrial targeting sequence. Although our preliminary data indicate that activation of Gα12 does not lead to its release from mitochondria, in order to understand possible mechanisms of regulation of mitochondrial Gα12 it would be essential to investigate whether its mitochondrial association is reversible. It would also be important to precisely dissect the molecular mechanisms that link Rho-uncoupled Gα12 and Bcl-2 degradation.
In a classical model of G protein-coupled signaling, heterotrimeric G proteins are supposed to function at or in the vicinity of the plasma membrane, where perception of the stimuli by their cognate heptahelical receptors occurs, and where they initiate signaling that reaches different cellular compartments through cascades of intermediate signaling events. However, a number of recent studies indicate that heterotrimeric G proteins may themselves rapidly shuttle between the plasma membrane and intracellular membranes (58) or may even play distinct roles in some organelles, such as the nucleus (59, 60). Our data lend further support to the emerging concept that hetertrimeric G proteins may have distinct functions at cellular locations distant from the plasma membrane.
Acknowledgments
We are grateful to Silvio Gutkind (National Institute of Dental and Cranial Research, National Institutes of Health) and Thomas Meigs (University of North Carolina, Asheville) for the constructs provided. This work was supported by U.S. National Institutes of Health grants GM56159, GM65160, and HL06078 and by a grant from the American Heart Association (to T.V.Y.). T.V.Y. is an Established Investigator of the American Heart Association.
References
- DiMauro S, Schon E A. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- Schultz B E, Chan S I. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct. 2001;30:23–65. doi: 10.1146/annurev.biophys.30.1.23. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze A, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P J, Saxton W M. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H M, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Quintero M, Colombo S L, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S M, Duchen M R. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- Collins T J, Bootman M D. Mitochondria are morphologically heterogeneous within cells. J Exp Biol. 2003;206:1993–2000. doi: 10.1242/jeb.00244. [DOI] [PubMed] [Google Scholar]
- Yaffe M P. Dynamic mitochondria. Nat Cell Biol. 1999;1:E149–150. doi: 10.1038/14101. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- Ligon L A, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Minin A A, Kulik A V, Gyoeva F K, Li Y, Goshima G, Gelfand V I. Regulation of mitochondria distribution by RhoA and formins. J Cell Sci. 2006;119:659–670. doi: 10.1242/jcs.02762. [DOI] [PubMed] [Google Scholar]
- Chan D C. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer S A, Ewald A J, Griffin E E, Fraser S E, Chan D C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan D C. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14:R283–289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Pinloche S, Dumuis A. G protein-coupled receptors: dominant players in cell-cell communication. Int Rev Cytol. 2002;212:63–132. doi: 10.1016/s0074-7696(01)12004-8. [DOI] [PubMed] [Google Scholar]
- Landry Y, Niederhoffer N, Sick E, Gies J P. Heptahelical and other G-protein-coupled receptors (GPCRs) signaling. Curr Med Chem. 2006;13:51–63. [PubMed] [Google Scholar]
- Offermanns S. G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol. 2003;83:101–130. doi: 10.1016/s0079-6107(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Cismowski M J. Non-receptor activators of heterotrimeric G-protein signaling (AGS proteins) Semin Cell Dev Biol. 2006;17:334–344. doi: 10.1016/j.semcdb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Patel T B. Single transmembrane spanning heterotrimeric g protein-coupled receptors and their signaling cascades. Pharmacol Rev. 2004;56:371–385. doi: 10.1124/pr.56.3.4. [DOI] [PubMed] [Google Scholar]
- Strathmann M P, Simon M I. G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc Natl Acad Sci U S A. 1991;88:5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo N A, Manning D R. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Kurose H. Galpha12 and Galpha13 as key regulatory mediator in signal transduction. Life Sci. 2003;74:155–161. doi: 10.1016/j.lfs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya T, Conklin B R, Gilbert R L, Hooley R, Bourne H R, Barber D L. G alpha 13 stimulates Na-H exchange. J Biol Chem. 1994;269:4721–4724. [PubMed] [Google Scholar]
- Meigs T E, Juneja J, DeMarco C T, Stemmle L N, Kaplan D D, Casey P J. Selective uncoupling of G alpha 12 from Rho-mediated signaling. J Biol Chem. 2005;280:18049–18055. doi: 10.1074/jbc.M500445200. [DOI] [PubMed] [Google Scholar]
- Zhu D, Tate R I, Ruediger R, Meigs T E, Denker B M. Domains necessary for Galpha12 binding and stimulation of protein phosphatase-2A (PP2A): is Galpha12 a novel regulatory subunit of PP2A? Mol Pharmacol. 2007;71:1268–1276. doi: 10.1124/mol.106.033555. [DOI] [PubMed] [Google Scholar]
- Andreeva A V, Vaiskunaite R, Kutuzov M A, Profirovic J, Skidgel R A, Voyno-Yasenetskaya T. Novel mechanisms of G protein-dependent regulation of endothelial nitric-oxide synthase. Mol Pharmacol. 2006;69:975–982. doi: 10.1124/mol.105.018846. [DOI] [PubMed] [Google Scholar]
- Andreeva A V, Kutuzov M A, Vaiskunaite R, Profirovic J, Meigs T E, Predescu S, Malik A B, Voyno-Yasenetskaya T. G alpha12 interaction with alphaSNAP induces VE-cadherin localization at endothelial junctions and regulates barrier function. J Biol Chem. 2005;280:30376–30383. doi: 10.1074/jbc.M502844200. [DOI] [PubMed] [Google Scholar]
- Gao S, Chen J, Brodsky S V, Huang H, Adler S, Lee J H, Dhadwal N, Cohen-Gould L, Gross S S, Goligorsky M S. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem. 2004;279:15968–15974. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- Kutuzov M A, Andreeva A V, Voyno-Yasenetskaya T A. Regulation of apoptosis signal-regulating kinase 1 (ASK1) by polyamine levels via protein phosphatase 5. J Biol Chem. 2005;280:25388–25395. doi: 10.1074/jbc.M413202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage D, Neumann F R, Hediger F, Gasser S M, Unser M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans Image Process. 2005;14:1372–1383. doi: 10.1109/tip.2005.852787. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Negishi M. N-terminal short sequences of alpha subunits of the G12 family determine selective coupling to receptors. J Biol Chem. 2003;278:14936–14939. doi: 10.1074/jbc.M301409200. [DOI] [PubMed] [Google Scholar]
- Ponimaskin E, Harteneck C, Schultz G, Schmidt M F. A cysteine-11 to serine mutant of G alpha12 impairs activation through the thrombin receptor. FEBS Lett. 1998;429:370–374. doi: 10.1016/s0014-5793(98)00638-3. [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, Kozasa T, Voyno-Yasenetskaya T A. Interaction between the G alpha subunit of heterotrimeric G(12) protein and Hsp90 is required for G alpha(12) signaling. J Biol Chem. 2001;276:46088–46093. doi: 10.1074/jbc.M108711200. [DOI] [PubMed] [Google Scholar]
- Sabath E, Negoro H, Beaudry S, Paniagua M, Angelow S, Shah J, Grammatikakis N, Yu A S, Denker B M. G{alpha}12 regulates protein interactions within the MDCK cell tight junction and inhibits tight-junction assembly. J Cell Sci. 2008;121:118–124. doi: 10.1242/jcs.014878. [DOI] [PubMed] [Google Scholar]
- Cohen-Saidon C, Carmi I, Keren A, Razin E. Antiapoptotic function of Bcl-2 in mast cells is dependent on its association with heat shock protein 90beta. Blood. 2006;107:1413–1420. doi: 10.1182/blood-2005-07-2648. [DOI] [PubMed] [Google Scholar]
- Fan A C, Bhangoo M K, Young J C. Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J Biol Chem. 2006;281:33313–33324. doi: 10.1074/jbc.M605250200. [DOI] [PubMed] [Google Scholar]
- Young J C, Hoogenraad N J, Hartl F U. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- Waheed A A, Jones T L. Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J Biol Chem. 2002;277:32409–32412. doi: 10.1074/jbc.C200383200. [DOI] [PubMed] [Google Scholar]
- Brichese L, Cazettes G, Valette A. JNK is associated with Bcl-2 and PP1 in mitochondria: paclitaxel induces its activation and its association with the phosphorylated form of Bcl-2. Cell Cycle. 2004;3:1312–1319. doi: 10.4161/cc.3.10.1166. [DOI] [PubMed] [Google Scholar]
- Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May W S., Jr Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer S J. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J C, Arnoult D, Youle R J. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Cortese J D. Stimulation of rat liver mitochondrial fusion by an outer membrane-derived aluminum fluoride-sensitive protein fraction. Exp Cell Res. 1998;240:122–133. doi: 10.1006/excr.1998.4004. [DOI] [PubMed] [Google Scholar]
- Kuyznierewicz I, Thomson M. GTP-binding proteins G(salpha), G(ialpha), and Ran identified in mitochondria of human placenta. Cell Biol Int. 2002;26:99–108. doi: 10.1006/cbir.2001.0823. [DOI] [PubMed] [Google Scholar]
- Rezaul K, Wu L, Mayya V, Hwang S I, Han D. A systematic characterization of mitochondrial proteome from human T leukemia cells. Mol Cell Proteomics. 2005;4:169–181. doi: 10.1074/mcp.M400115-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M, Sgourdas G, Bonder E M. Disassembly of actin filaments leads to increased rate and frequency of mitochondrial movement along microtubules. Cell Motil Cytoskeleton. 1998;40:368–378. doi: 10.1002/(SICI)1097-0169(1998)40:4<368::AID-CM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Kaziro Y, Itoh H. The Src family tyrosine kinase is involved in Rho-dependent activation of c-Jun N-terminal kinase by Galpha12. Oncogene. 1999;18:4425–4434. doi: 10.1038/sj.onc.1202832. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady S T. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor R C, Duriez P J, Martin S J. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Blagosklonny M V. Unwinding the loop of Bcl-2 phosphorylation. Leukemia. 2001;15:869–874. doi: 10.1038/sj.leu.2402134. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, May W S., Jr Mono- and multisite phosphorylation enhances Bcl2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci U S A. 2004;101:153–158. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvolo P P, Clark W, Mumby M, Gao F, May W S. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- Zhu D, Kosik K S, Meigs T E, Yanamadala V, Denker B M. Galpha12 directly interacts with PP2A: evidence FOR Galpha12-stimulated PP2A phosphatase activity and dephosphorylation of microtubule-associated protein, tau. J Biol Chem. 2004;279:54983–54986. doi: 10.1074/jbc.C400508200. [DOI] [PubMed] [Google Scholar]
- Yanamadala V, Negoro H, Gunaratnam L, Kong T Q, Denker B M. G alpha(12) stimulates apoptosis in epithelial cells through JNK1-mediated Bcl-2 degradation and up-regulation of I kappa B alpha. J Biol Chem. 2007;282:24352–24363. doi: 10.1074/jbc.M702804200. [DOI] [PubMed] [Google Scholar]
- Chisari M, Saini D K, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem. 2007;282:24092–24098. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampoelz B, Knoblich J A. Heterotrimeric G proteins: new tricks for an old dog. Cell. 2004;119:453–456. doi: 10.1016/j.cell.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Mir F, Gould R M, Le Breton G C. Characterization of thromboxane A2 receptor signaling in developing rat oligodendrocytes: nuclear receptor localization and stimulation of myelin basic protein expression. J Neurosci Res. 2006;84:1402–1414. doi: 10.1002/jnr.21061. [DOI] [PubMed] [Google Scholar]