Abstract
Exercise provides protection against myocardial ischemia-reperfusion (IR) injury. Understanding the mechanisms of this protection may lead to new interventions for the prevention and/or treatment of heart disease. Although presently these mechanisms are not well understood, reports suggest that manganese superoxide dismutase (MnSOD) and calpain may be critical mediators of this protection. We hypothesized that an exercise-induced increase in MnSOD would provide cardioprotection by attenuating IR-induced oxidative modification to critical Ca2+-handling proteins, thereby decreasing calpain-mediated cleavage of these and other proteins attenuating cardiomyocyte death. After IR, myocardial apoptosis and infarct size were significantly reduced in hearts of exercised animals compared with sedentary controls. In addition, exercise prevented IR-induced calpain activation as well as the oxidative modification and calpain-mediated degradation of myocardial Ca2+-handling proteins (L-type Ca2+ channels, phospholamban, and sarcoplasmic/endoplasmic reticulum calcium ATPase). Further, IR-induced activation of proapoptotic proteins was attenuated in exercised animals. Importantly, prevention of the exercise-induced increase in MnSOD activity via antisense oligonucleotides greatly attenuated the cardioprotection conferred by exercise. These results suggest that MnSOD provides cardioprotection by attenuating IR-induced oxidation and calpain-mediated degradation of myocardial Ca2+-handling proteins, thereby preventing myocardial apoptosis and necrosis.—French, J. P., Hamilton, K. L., Quindry, J. C., Lee, Y., Upchurch, P. A., Powers, S. K. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain.
Keywords: ischemia, reperfusion, antioxidant
Cardiovascular disease is the leading cause of death in industrialized countries, with coronary artery disease being the most prevalent manifestation. Coronary artery disease leading to myocardial ischemia and reperfusion (IR) can result in cardiac myocyte death due to both necrosis and apoptosis (1,2,3,4). It follows that developing a cardioprotected phenotype is clearly advantageous and regular bouts of endurance exercise provide consistent protection against IR-induced cardiomyocyte death (5, 6). However, at present, the mechanisms responsible for exercise-induced cardioprotection remain a topic of debate. Evidence indicates that both an increase in the antioxidant enzyme manganese superoxide dismutase (MnSOD), as well as a decrease in the activity of the Ca2+-activated protease calpain, may play an important role in exercise-induced cardioprotection against IR injury (3, 7). Nonetheless, the precise mechanisms of this cardioprotection have not been elucidated and therefore form the rationale for these experiments.
Calpain activation within the myocardium has been directly linked with IR-induced necrotic and apoptotic cardiac myocyte death. Indeed, calpain activation can damage cardiac myocytes via several mechanisms. First, calpain cleaves a host of myocardial proteins, including structural, contractile, and Ca2+-handling proteins, facilitating their degradation by the proteasome (8,9,10). In addition, calpain cleaves and activates the proapoptotic factors BH3 interacting domain death agonist (BID) and caspase-12, stimulating caspase-3 activation and apoptosis (11, 12).
Previous work from our laboratory (3) demonstrated that exercise training attenuated IR-induced calpain activation, resulting in cardioprotection similar to that observed in hearts that received a pharmacological calpain inhibitor. However, the mechanisms of exercise-induced protection against calpain activation are not currently understood. Since calpain is a Ca2+-activated protease, it follows that calpain activation is dependent on the Ca2+ overload that occurs during IR (8). Growing evidence suggests that the increased free radical generation that occurs during IR may exacerbate Ca2+ overload by oxidizing myocardial Ca2+-handling proteins, impairing their function and facilitating their degradation (13, 14).
Based on preliminary experiments and the work of others, we formed the hypothesis that an exercise-induced increase in myocardial MnSOD provides protection against IR injury, in part, by preventing IR-induced oxidative modification of important Ca2+-handling proteins, thereby protecting against calpain activation. To test this hypothesis, we used antisense oligonucleotides directed against MnSOD to prevent exercise-induced increases in myocardial MnSOD activity. This strategy allowed us to investigate the specific effects of increased myocardial MnSOD on IR-induced Ca2+-handling protein oxidation/degradation, calpain activation, and cardiac cell death. This work demonstrates an important mechanistic link between mitochondria-produced reactive oxygen species (ROS), Ca2+-handling proteins, and calpain.
MATERIALS AND METHODS
Animals and experimental design
Male Sprague Dawley rats (3 months; 300–350 g) were used for both the in vivo and ex vivo experiments. For the in vitro experiments, animals were randomly assigned to one of four experimental groups, each of which underwent either sham surgery or myocardial IR: sedentary control (C and C-IR); trained, no treatment (T and T-IR); trained, treated with antisense oligonucleotide against MnSOD (T-AS and T-AS-IR); and trained, treated with a mismatch oligonucleotide (T-M and T-M-IR); sham (n=10/group) or in vivo myocardial IR (n=17/group). For ex vivo experiments, animals were divided into six groups: sedentary control (C), control-perfused (CP), control-IR (C-IR), trained-IR (T-IR), vehicle-IR (V-IR), and calpain-inhibited-IR (CI-IR).
Exercise protocol
Animals assigned to exercise groups were habituated to running by increasing durations of treadmill exercise for 5 days (5, 10, 15, 30, and 45 min/day on days 1–5, respectively). After 2 days of rest, animals then performed 3 consecutive days of treadmill exercise for 60 min/day at 30 m/min with a 0% grade (estimated work rate ∼70% VO2max; ref. 15).
Inhibition of myocardial MnSOD expression
To study the role of MnSOD in exercise-induced cardioprotection, immediately after each exercise session animals were injected (i.p.) with antisense oligonucleotides directed against MnSOD to prevent the increase in MnSOD protein synthesis. Specific details about antisense design, dosage, and mechanisms of action have reported previously (16,17,18).
In vivo IR protocol
The in vivo model of coronary artery ligation has been used successfully by our laboratory and numerous other investigators and has been shown to result in regional myocardial IR (17, 19,20,21,22,23,24,25). In vivo surgeries were performed as described previously (25).
Ex vivo working heart IR protocol
To investigate the effects of calpain inhibition on myocardial Ca2+-handling proteins before and after an IR insult, we selected the in vitro isolated perfused working heart model. Complete details of our isolated working heart preparation as well as the use of the calpain inhibitor CI3 have been described previously (3, 5, 26).
Analysis of antioxidant enzyme activity
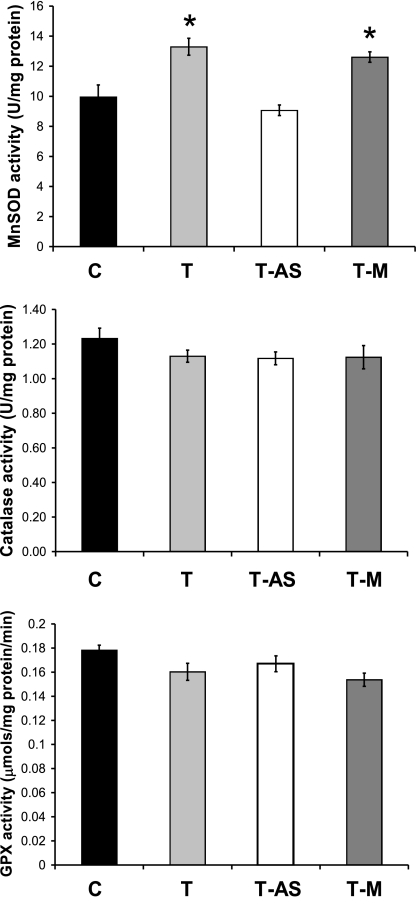
To assess the effect of exercise on myocardial antioxidant capacity, a section of left ventricle from the sham surgery groups was homogenized and assayed for total protein content (27), along with the activities of MnSOD (28), glutathione peroxidase (29), and catalase (30).
Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL)
Apoptotic changes to myonuclear DNA were identified by TUNEL, using a histochemical fluorescent detection kit purchased from Roche Applied Scientific (Nutley, NJ, USA). This procedure has been described in detail previously (31).
Active caspase-3
Active caspase-3 was detected via immunohistochemical analyses of formalin-fixed left ventricular cryosections. To further assess caspase-3 activation, Western blot analyses of the caspase-3 cleaved fragment (120 kDa) of the cytoskeletal protein αII-spectrin (240 kDa) was performed (Sigma, St. Louis, MO, USA; ref. 3).
Measurement of oxidative modification to Ca2+-handling proteins
Immunoprecipitation of Ca2+-handling proteins
Sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA2a), phospholamban (PLB), L-type Ca2+ channels (LTCC), and the Na+/Ca2+ exchanger (NCX) were isolated by immunoprecipitation using the following primary antibodies: SERCA2a (Affinity Bioreagents, Golden, CO, USA), PLB (Upstate, Lake Placid, NY, USA), LTCC (Alomone Laboratories, Jerusalem, Israel), and NCX (Swant, Bellinzona, Switzerland).
Protein carbonyl formation
Proteins, isolated via immunoprecipitation, were examined for carbonyl formation using a commercially available Western blot kit from Chemicon International (Temecula, CA, USA).
4-Hydroxy-2-nonenal formation
Proteins, isolated via immunoprecipitation, were examined for 4-hydroxy-2-nonenal (HNE) formation using a commercially available Western blot kit from Calbiochem (San Diego, CA, USA).
Measurement of intact Ca2+-handling proteins
Western blots were used to determine protein levels of SERCA2a, PLB, LTCC, and NCX. Briefly, proteins were separated using standard SDS-PAGE techniques on 4–20% polyacrylamide gels. Proteins were then transferred to polyvinylidene difluoride membranes and exposed to one of the aforementioned primary antibodies. After primary antibody exposure, an anti-mouse or anti-rabbit 800 (green) or 680 (red) infrared secondary antibody (Li-Cor, Lincoln, NE, USA) was applied for infrared detection. Each blot was then analyzed using an Odyssey infrared imaging system (Li-Cor) and normalized to a Coommassie blue protein stain to verify equal protein loading.
Calpain activity
To assess calpain activity, a calpain-specific cleavage product of the protein αII-spectrin was analyzed as described previously (3).
Measurement of BclII interacting protein (BID), caspase-12, and caspase-3
The truncated and active form of BID (tBID), caspase-12, and caspase-3 proteins were measured via standard Western blotting techniques, as described earlier. The following primary antibodies were used: BID (Santa Cruz Biotech, Santa Cruz, CA, USA), caspase-12 (BioVision, Mountain View, CA, USA), and caspase-3 (AbCam, Cambridge, MA, USA).
Data analyses
All measures were subjected to 1-way ANOVA to determine whether group differences existed. Significant group differences were evaluated via Tukey’s post hoc analyses. Significance was established a priori at P < 0.05.
RESULTS
All animals in exercise groups completed the exercise protocol without incident, with no noticeable difference in exercise performance and with no apparent complications from oligonucleotide. At baseline (pre-IR), no group differences existed in blood pressure, heart rate, or arterial blood gases (i.e., PaO2, PaCO2, and pH). Note that 7 animals died prematurely during the in vivo IR protocol due to ventricular fibrillation that could not be converted to sinus rhythm. Animals dying due to arrhythmia included 3 C, 3 T, 1 T-AS, and 2 T-M. These results are similar to our previous findings, with greater arrhythmia-related death in sedentary groups than in exercised groups (17).
Antisense oligonucleotides prevent exercise-induced increases in MnSOD
To determine the effectiveness of the antisense oligonucleotides, left ventricular MnSOD activity was measured in control and all exercise-trained groups. Compared with C, MnSOD activity was increased in the hearts of the T and T-M groups while MnSOD activity did not differ between T-AS and C animals (Fig. 1). No differences existed in the activities of CuZnSOD (C: 23.60±1.6; T: 24.72±1.8; T-AS: 23.91±2.02; and T-M: 24.54±2.01 U/mg protein, catalase, or glutathione peroxidase). These results confirm our past findings that postexercise treatment with antisense oligonucleotides effectively prevented the exercise-induced increase in MnSOD activity, whereas the relevant mismatch oligonucleotide had no effect on cardiac MnSOD activity (17, 32). It should be noted that, by design, antisense treatment did not abolish myocardial MnSOD activity but, instead, prevented the exercise-induced increase in activity above baseline. In addition, exercise did not have a significant effect on myocardial catalase or glutathione peroxidase activity in any experimental group (Fig. 1).
Figure 1.
Enzymatic antioxidant activities in left ventricular tissue of sham-operated animals. *P < 0.05 vs. C and T-AS groups. Error bars are mean ± se. No significant differences in CuZnSOD existed (P>0.05; data not shown) GPX, glutathione peroxidase.
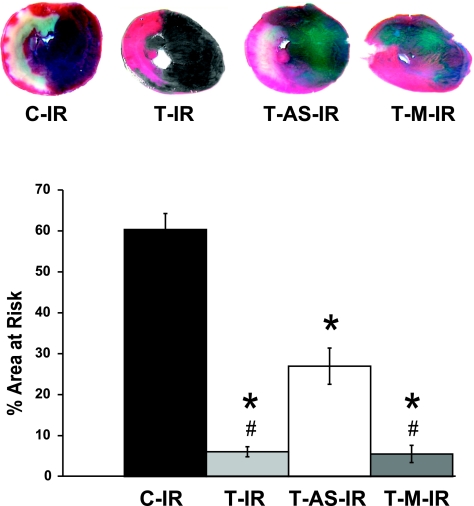
Exercise-induced increases in MnSOD attenuate IR-induced myocardial infarction
Consistent with previous findings (25), our in vivo left coronary artery ligation model resulted in an infarction encompassing 60.3 ± 3.9% of the area at risk for infarction in C-IR animals (Fig. 2). Exercise was associated with a significant decrease in infarct area in all exercised groups regardless of treatment (saline, MnSOD antisense, or mismatch control oligonucleotides). However, when exercise was accompanied by treatment with MnSOD antisense oligonucleotides, protection against infarction was attenuated, as demonstrated by significant differences between T-IR and T-M-IR groups compared with the T-AS-IR group. No differences existed among groups with respect to the area at risk for infarction, represented as a percentage of the total ventricular area (C-IR: 54.0±5.4; T-IR: 52.4±2.5; T-AS-IR: 51.8±1.6; and T-M-IR: 52.4±3.4%). This MnSOD-related protection against myocardial infarction (MI) is consistent with earlier reports from Yamashita et al. (7).
Figure 2.
Exercise-induced MnSOD up-regulation attenuates IR-induced necrosis. Blue areas are those well perfused during in vivo ischemia, red areas indicate myocardium within the area at risk for infarction but still viable based on TTC reactivity, and whitish-yellow areas indicate infarcted tissue. Error bars are mean ± se. *P < 0.05 vs. C-IR group; #P < 0.05 vs. T-AS-IR group.
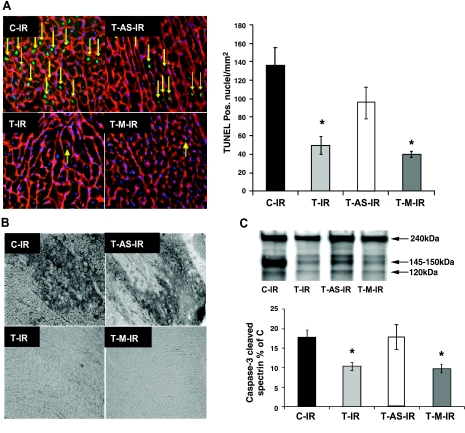
Exercise-induced increases in MnSOD attenuate IR-induced apoptosis
Figure 3A shows representative left ventricular cryosections from TUNEL detection after the IR protocol. Tissue sections were obtained from the area at risk for infarction after 50 min ischemia and 120 min reperfusion in vivo. Compared with C-IR, T-IR and T-M-IR groups had significantly fewer TUNEL-positive myonuclei (green), suggesting fewer apoptotic cardiomyocytes after IR. However, when exercise was accompanied by treatment with MnSOD antisense oligonucleotides (T-AS-IR), protection against these apoptotic nuclear changes was attenuated.
Figure 3.
Exercise-induced MnSOD up-regulation attenuates IR-induced apoptosis. A) Texas Red-labeled laminin, DAPI-labeled nuclei, and FITC-labeled TUNEL-positive nuclei; arrows indicate TUNEL-positive myonuclei. B) Representative sections analyzed immunohistochemically for activated caspase-3. C) Caspase-3 cleaves αII-spectrin to yield a 120-kDa band that was normalized to total αII-spectrin for that sample and expressed as a percentage of C (group not shown). Error bars are mean ± se. *P < 0.05 vs. C-IR and T-AS-IR groups.
To further corroborate the presence of apoptosis in cardiac myocytes, we also used immunohistochemical techniques to detect activated caspase-3 in myocardium from the risk zone after IR (Fig. 3B). These findings were in agreement with TUNEL analyses, with less activated caspase-3 detected in T-IR and T-M-IR compared with C-IR and T-AS-IR. Tissue levels of activated caspase-3 did not differ between T-AS-IR and C-IR.
In addition, we used Western blotting to detect caspase-3-mediated cleavage of the cytoskeletal protein αII-spectrin. When caspase cleaves αII-spectrin, a signature 120 kDa cleavage product is produced and can be detected via immunoblotting. Figure 3C shows the results of this analysis with significantly less caspase cleaved spectrin in T-IR and T-M-IR compared with C-IR and T-AS-IR. Caspase-3-mediated spectrin cleavage in the T-AS-IR group did not differ from C-IR. These findings confirm our immunohistochemstry results indicating that acute exercise is associated with a decrease in IR-induced caspase-3 activation and that this decrease is dependent, at least in part, on MnSOD up-regulation. In total, these results demonstrate that MnSOD up-regulation is a critical component of exercise-mediated cardioprotection against IR-induced apoptosis and necrosis.
Exercise-induced increases in MnSOD attenuates Ca2+-handling protein oxidation
One possible mechanism of exercise/MnSOD-induced protection against MI and apoptotic cell death is through the maintenance of Ca2+-handling protein function. To test this postulate, oxidative modification of the Ca2+-handling proteins SERCA2a, PLB, NCX, and LTCC was measured via detection of carbonyl and HNE formation. Increases in carbonyl formation were observed in SERCA2a, PLB, and LTCC but not NCX after IR and were attenuated by exercise (T-IR and T-M-IR; Fig. 4A). In addition, increases in HNE formation were observed after IR in isolated SERCA2a and PLB proteins but not NCX or LTCC (no HNE formation was observed on the LTCC in any of the experimental groups). Similar to carbonyl formation, HNE was attenuated in T-IR and T-M-IR hearts (Fig. 4B). Importantly, protection against both carbonyl and HNE formation was significantly decreased in animals treated with MnSOD antisense (T-AS-IR). This demonstrates that MnSOD provides protection against the oxidative modification of myocardial Ca2+-handling proteins during IR.
Figure 4.
Exercise-induced MnSOD up-regulation attenuates IR-induced oxidative modification of critical myocardial Ca2+-handling proteins. Oxidative modification to critical Ca2+-handling proteins: A) carbonyl formation; B) HNE formation. Error bars are mean ± se. *P < 0.05 vs. C group; #P < 0.05 vs. T-AS-IR group.
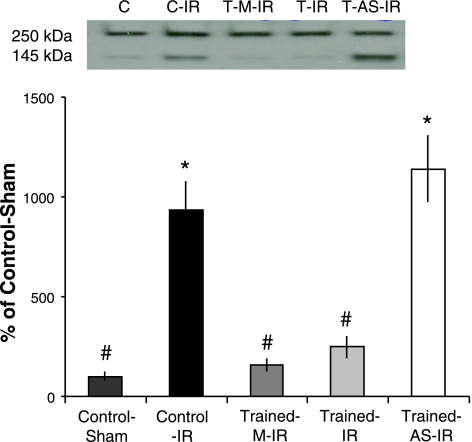
Exercise-induced increases in MnSOD attenuate IR-induced calpain activation
Exercise almost completely attenuated IR-induced calpain activation (Fig. 5). However, when the exercise-induced increases in MnSOD were prevented via antisense oligonucteotides (T-AS-IR), protection against calpain activation was lost. This suggests that MnSOD plays a critical role in preventing IR-induced calpain activation. In addition, this finding adds to earlier work from our laboratory that first documented exercise-induced regulation of calpain activity, suggesting that increased MnSOD is an essential component of exercise-induced prevention of calpain activation (3).
Figure 5.
Exercise-induced MnSOD up-regulation prevents IR-induced calpain activation. Western blotting for calpain-cleaved αII-spectrin (an in vivo calpain substrate). Representative blots display intact (250 kDa) and calpain-cleaved αII-spectrin (145 kDa). Values below reflect the ratio of 145-kDa cleavage product to 250-kDa intact protein, expressed as percentage of C. Values are mean ± se. *P < 0.05 vs. C group; #P < 0.05 vs. T-AS-IR group.
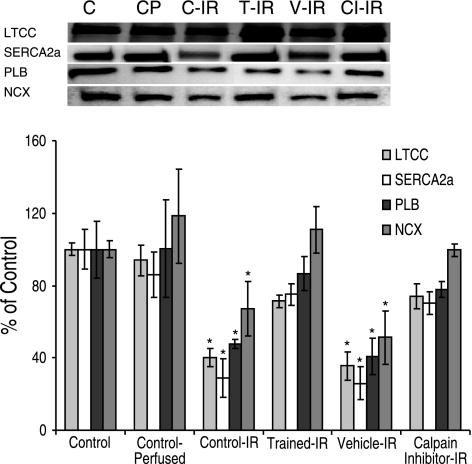
Calpain degrades critical Ca2+-handling proteins during IR
To confirm that the loss of Ca2+-handling proteins after IR was due to calpain activation, calpain was inhibited using a selective, irreversible inhibitor (CI3; Calbiochem). These experiments were performed using an isolated working heart model of IR. This model was used because of its ability to selectively inhibit myocardial calpain activity without the negative effects of global inhibition in vivo (33). The calpain inhibitor was added to the perfusion buffer ∼30 min before the 25 min IR insult. After IR, a significant reduction in the amount of intact Ca2+-handling proteins was observed (LTCC, SERCA, PLB, and NCX; Fig. 6). The degradation of these proteins was attenuated by calpain inhibition (CI-IR) as well as exercise training (T-IR). These data suggest that calpain is responsible for the IR-induced degradation of these proteins.
Figure 6.
Calpain inhibition prevents IR-induced degradation of critical myocardial Ca2+-handling proteins. Western blotting for intact Ca2+-handling proteins. Values are means ± se. *P < 0.05 vs. CP group.
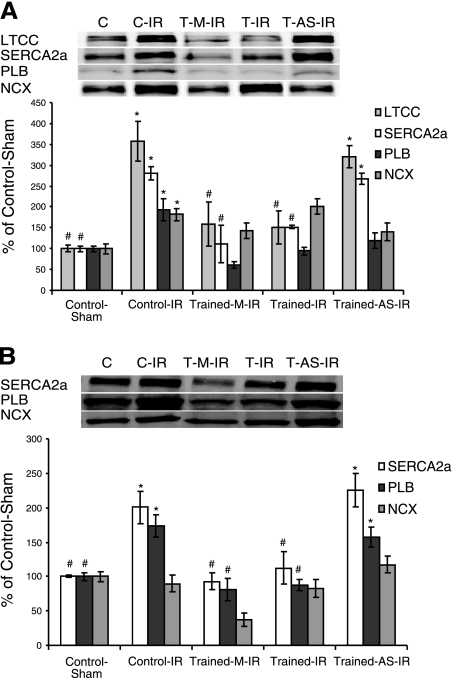
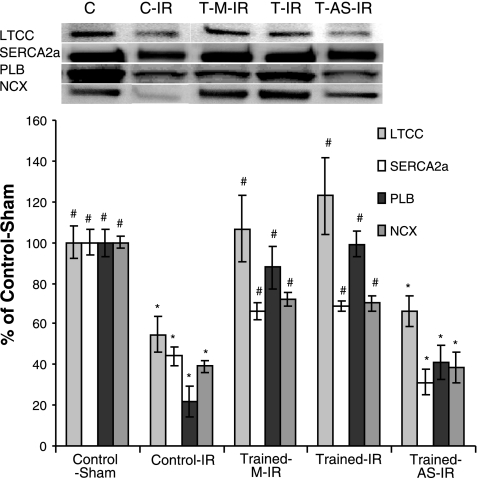
Exercise-induced increases in MnSOD attenuate degradation of Ca2+-handling proteins
Degradation of critical Ca2+-handling proteins during IR has been well characterized and is thought to contribute to cytosolic Ca2+ overload and IR injury (14, 34). Our data support these findings, demonstrating a loss of SERCA2a, PLB, LTCC, and NCX after IR (Fig. 7). Importantly, our data demonstrate that exercise related increases in MnSOD provide protection against the degradation of these Ca2+-handling proteins. Specifically, degradation of all four proteins measured was attenuated in exercised animals (T-IR and T-M-IR) but not in exercised animals that received injections containing antisense oligonucleotide against MnSOD (T-AS-IR). When these findings were combined with the calpain inhibition experiments described earlier, the logical conclusion is that exercise-induced increases in MnSOD attenuate the degradation of these proteins by preventing their oxidation.
Figure 7.
Exercise-induced MnSOD up-regulation attenuates the IR-induced degradation of myocardial Ca2+-handling proteins. Western blotting for intact Ca2+-handling proteins: LTCC, SERCA2a, PLB, and NCX. Values are mean ± se. *P < 0.05 vs. C group; #P < 0.05 vs. T-AS-IR group.
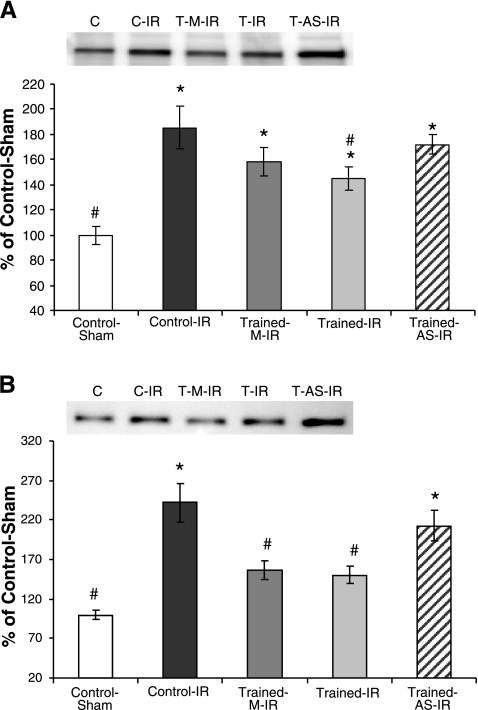
Exercise-induced increases in MnSOD attenuate caspase-12 and BID activation
Calpain has previously been shown to contribute to apoptosis by cleaving and activating caspase-12, facilitating apoptosis via the endoplasmic reticulum stress pathway. In addition, calpain has been shown to cleave and activate the proapoptotic factor BID, facilitating mitochondrial-induced apoptosis (12, 35). To determine the contribution of calpain activation to IR-induced apoptosis, cleaved caspase-12 and BID were assessed via Western blotting (Fig. 8). Both active caspase-12 and BID protein were decreased in exercised animals (T-IR and T-M-IR) after IR. Importantly, protection against IR-induced caspase-12 and BID activation was lost in animals treated with MnSOD antisense oligonucleotide (T-AS-IR).
Figure 8.
Exercise-induced MnSOD up-regulation attenuates IR-induced caspase-12 and Bid activation. A) Western blots for active caspase-12 protein. B) Western blots for active Bid protein. Values are mean ± se. *P < 0.05 vs. C group; #P < 0.05 vs. T-AS-IR group.
DISCUSSION
Overview of principle findings
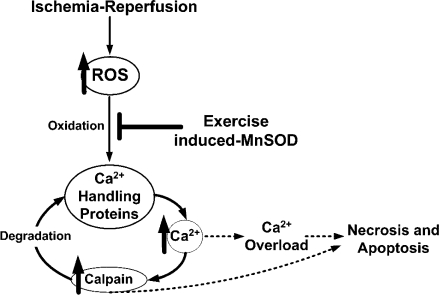
It is well established that successive bouts of endurance exercise promote a cardiac phenotype that is protected from IR injury. However, a detailed understanding of the mechanisms responsible for exercise-induced myocardial protection against IR injury remains evasive. Nonetheless, two potential mediators of exercise-induced cardioprotection are an increased expression of the mitochondrial antioxidant enzyme, MnSOD and a decrease in calpain activation. Although exercise increases myocardial MnSOD activity and provides cardioprotection, the downstream mechanisms by which increased MnSOD activity contributes to cardioprotection are unknown. To address this issue, we tested the hypothesis that exercise-induced increases in myocardial MnSOD provide protection against IR injury, in part, by attenuating IR-induced oxidative modification of important Ca2+-handling proteins and preventing subsequent calpain activation. Our results support this hypothesis and reveal that exercise-induced increases in myocardial MnSOD activity attenuate the oxidation and degradation of Ca2+-handling proteins, preventing calpain activation during IR. Importantly, these events are associated with both protection against MI as well as cardiomyocyte apoptosis after an IR insult. Figure 9 illustrates our hypothesized sequence of events underlying how exercise-induced increases in myocardial MnSOD can contribute to cardioprotection via protection of Ca2+-handling proteins and prevention of calpain activation. These findings not only contribute to the understanding of the mechanisms of exercise-induced cardioprotection but also provide important mechanistic insight regarding the interaction between superoxide generated by the mitochondria, oxidation of Ca2+-handling proteins, and activation of calpain during IR. A detailed discussion of these and related issues follows.
Figure 9.
Proposed mechanism of exercise-induced increases in MnSOD leading to cardioprotection.
Exercise attenuates IR-induced apoptosis and MI via increases in MnSOD
Our results confirm earlier work demonstrating that an exercise-induced increase in cardiac MnSOD is essential for exercise-induced protection against MI (7). In addition, we report, for the first time, that increased myocardial MnSOD activity plays a key role in mediating protection against IR-induced calpain activation, Ca2+-handling protein degradation, and cardiomyocyte apoptosis. Collectively, these findings support the concept that MnSOD is a critical component of exercise-mediated protection against IR-induced cardiacmyocyte death.
Exercise retards IR-induced oxidation and degradation of Ca2+-handling proteins via increases in MnSOD
To investigate the mechanisms of cardioprotection conferred by exercise-induced increases in MnSOD activity, we examined the oxidation and degradation of several critical Ca2+-handling proteins. We postulated that the IR-induced oxidation of Ca2+-handling proteins would result in Ca2+ overload and calpain activation that could contribute to myocardial cell death.
Our results support the notion that superoxide generated within the mitochondria can exacerbate IR-induced calpain activation and contribute to the oxidative modification and degradation of myocardial Ca2+-handling proteins. The mitochondrial electron transport chain has indeed been identified as a major contributor to IR-induced ROS production. A significant body of evidence also exists supporting the idea that ROS contribute to Ca2+-handling protein dysfunction (13). Conversely, decreases in the oxidative modification to a broad range of cellular components have been demonstrated with increased myocardial levels of MnSOD overexpression (7, 36,37,38,39). However, the current experiments are the first to directly link exercise-induced increases in MnSOD with a decrease in the oxidation of myocardial Ca2+-handling proteins after an IR insult.
Supporting our data, functional impairment in several of the Ca2+-handling proteins evaluated in this study have been reported after ROS exposure (40). In particular, ROS generated during IR have been shown to interact with various sarcoplasmic reticulum (SR) proteins, causing SR dysfunction and damage (41, 42). For example, SERCA and its regulatory protein PLB play a critical role in reuptake of cytosolic Ca2+ into the SR. This activity is inhibited in a concentration-dependent manner on exposure to ROS (43). Further, both hydrogen peroxide and superoxide promote oxidative damage to SERCA resulting in a diminished uptake of Ca2+ into the SR. Insufficient removal of cytosolic Ca2+ across the plasma membrane via NCX activity is another potential impairment resulting from oxidant damage to Ca2+-handling proteins, as evidence suggests that NCX is susceptible to ROS-mediated oxidative damage (44, 45).
In addition, exposure to superoxide radicals results in decreased cardiomyocyte LTCC activity and this effect can be prevented by treatment with SOD (46,47,48). However, preservation of LTCC activity should lead to an increased cytosolic Ca2+ pool (49). Nonetheless, it is also well known that activation of “prosurvival” pathways involving protein kinase C (PKC) and ecto-5′-nucleotidase, for example, requires an elevation in intracellular Ca2+. In fact, it has been shown that transient exposures to Ca2+ limit myocardial infarction through activation of PKC and, subsequently, ecto-5′-nucleotidase (50). Activation and mitochondrial translocation of activated PKC, in turn, are known to participate in the mitochondrial ATP-dependent potassium channel mediated cardioprotection (51). Hence, it is possible that by preventing oxidation to Ca2+-handling proteins, exercise preserves normal cellular Ca2+handling such that protective pathways dependent on modest increases in cytosolic Ca2+ are activated, and yet the cells are protected against cytosolic Ca2+ overload leading to activation of proteolytic pathways. This is an interesting hypothesis and worthy of future investigation.
Compounding the problem of oxidation-induced dysfunction of critical Ca2+-handling proteins is the possibility that oxidation increases their susceptibility to proteolytic degradation. We observed a decrease in Ca2+-handling proteins (SERCA2a, PLB, NCX, and LTCC) after IR, which was attenuated by exercise. This protection was reduced by treatment with MnSOD antisense, suggesting that MnSOD plays a critical role in preventing Ca2+-handling protein degradation during IR. This observation is not surprising considering that several studies have demonstrated that oxidative modification predisposes many cellular proteins for proteolytic breakdown. For example, when oxidized, the sodium/potassium exchanger and the ryanodine receptor are increasingly prone to calpain-mediated degradation (34, 52).
Exercise prevents IR-induced calpain activation via increases in MnSOD
We have previously reported that exercise attenuates IR-induced calpain activation in the heart and that inhibition of calpain activity protects against IR-mediated cardiac injury (3). In the current study, we demonstrate again that exercise attenuates IR-induced calpain activation and that protection against calpain activation is lost when the exercise-induced increase in MnSOD is prevented by antisense treatment. This suggests that increased MnSOD activity contributes to the exercise-related decrease in calpain activation during IR. Since calpain is activated in the presence of Ca2+, one probable explanation is that increased MnSOD activity decreases IR-induced Ca2+ overload and calpain activation by preventing oxidative modification of myocardial Ca2+-handling proteins. Moreover, we demonstrate that calpain facilitates the degradation of LTCC, SERCA2a, PLB, and NCX during IR, ex vivo (Fig. 6). Hence, it appears that calpain activation can further exacerbate the IR-induced Ca2+ overload in the heart by degrading Ca2+-handling proteins that are critical in maintaining cellular Ca2+ homeostasis.
Exercise-induced increases in cardiac MnSOD activity prevent caspase-3, caspase-12, and BID activation after IR
Ca2+ overload promotes mitochondrial cytochrome c release and permeability transition pore opening, allowing the release of apoptotic factors into the cytosol (53). Through activation of calpain, Ca2+ may also promote apoptosis via proteolytic activation of caspase-12, an endoplastic reticulum membrane bound caspase that promotes apoptosis via the activation of caspase-3 (53). Moreover, activation of BID by calpain-mediated cleavage can also lead to mitochondria directed apoptosis (12, 53). Specifically, tBID can translocate to the mitochondria where it triggers cytochrome c release (11, 12). In the current experiments, myocardial IR resulted in increased activation of caspase-3, caspase-12, and BID along with an increased number of apoptotic myonuclei (i.e., TUNEL-positive nuclei). Importantly, all of these proapoptotic mediators and the number of TUNEL-positive nuclei were significantly attenuated by exercise-induced increases in myocardial MnSOD activity. Indeed, when exercise was coupled with MnSOD antisense treatment, the exercise-induced protection against IR-induced apoptosis was dampened. To our knowledge, these are the first data to directly demonstrate the protective effects of MnSOD against IR-induced apoptosis. Moreover, these findings are consistent with the postulate that exercise-induced MnSOD attenuates apoptosis, at least in part, by decreasing IR associated calpain activation.
CONCLUSIONS
These experiments support the hypothesis that an exercise-induced increase in myocardial MnSOD activity plays an important role in mediating exercise-induced protection against cardiac myocyte apoptosis and infarction. Antisense oligonucleotides directed against MnSOD prevented the exercise-induced increase in MnSOD activity, attenuating exercise-induced protection against cardiomyocyte death by ∼40–50%. Further, this work provides the first evidence that exercise-induced increases in MnSOD contribute to protection against oxidation of critical Ca2+-handling proteins, calpain activation, calpain-mediated degradation of Ca2+-handling proteins, and apoptosis (via activation of caspase-12, BID, and caspase-3).
Importantly, our experiments reveal that the protection conferred by exercise was not completely lost when increases in MnSOD were prevented with the administration of antisense oligonucleotides. This suggests that although exercise-induced increases in myocardial MnSOD are a critical component that contribute to cardioprotection, increases in MnSOD alone are not solely responsible for exercise-induced cardioprotection. Future studies should focus on identifying mechanisms working in concert with those described here.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute grant R01 067855–01, awarded to S.K.P., and American Heart Association grant 0415135B, awarded to J.P.F.
References
- Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. 2005;38:73–79. doi: 10.1016/j.yjmcc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Baines C P, Molkentin J D. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- French J P, Quindry J C, Falk D J, Staib J L, Lee Y, Wang K K, Powers S K. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H128–H136. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- Regula K M, Kirshenbaum L A. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol. 2005;38:3–13. doi: 10.1016/j.yjmcc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lennon S L, Quindry J, Hamilton K L, French J, Staib J, Mehta J L, Powers S K. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96:1299–1305. doi: 10.1152/japplphysiol.00920.2003. [DOI] [PubMed] [Google Scholar]
- Taylor R P, Ciccolo J T, Starnes J W. Effect of exercise training on the ability of the rat heart to tolerate hydrogen peroxide. Cardiovasc Res. 2003;58:575–581. doi: 10.1016/s0008-6363(03)00285-2. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll D E, Thompson V F, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Trumbeckaite S, Neuhof C, Zierz S, Gellerich F N. Calpain inhibitor (BSF 409425) diminishes ischemia/reperfusion-induced damage of rabbit heart mitochondria. Biochem Pharmacol. 2003;65:911–916. doi: 10.1016/s0006-2952(02)01610-6. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Hagihara H, Ohga Y, Nakajima-Takenaka C, Murata K Y, Taniguchi S, Takaki M. Calpain inhibitor-1 protects the rat heart from ischemia-reperfusion injury: analysis by mechanical work and energetics. Am J Physiol Heart Circ Physiol. 2005;288:H1690–H1698. doi: 10.1152/ajpheart.00666.2004. [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed J C, Gottlieb R A. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- Chen M, Won D J, Krajewski S, Gottlieb R A. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- Kim S J, Kudej R K, Yatani A, Kim Y K, Takagi G, Honda R, Colantonio D A, Van Eyk J E, Vatner D E, Rasmusson R L, Vatner S F. A novel mechanism for myocardial stunning involving impaired Ca(2+) handling. Circ Res. 2001;89:831–837. doi: 10.1161/hh2101.098547. [DOI] [PubMed] [Google Scholar]
- Zima A V, Blatter L A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Lawler J M, Powers S K, Hammeren J, Martin A D. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc. 1993;25:1259–1264. [PubMed] [Google Scholar]
- Thierry A R, Vives E, Richard J P, Prevot P, Martinand-Mari C, Robbins I, Lebleu B. Cellular uptake and intracellular fate of antisense oligonucleotides. Curr Opin Mol Ther. 2003;5:133–138. [PubMed] [Google Scholar]
- Hamilton K L, Quindry J C, French J P, Staib J, Hughes J, Mehta J L, Powers S K. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med. 2004;37:1360–1368. doi: 10.1016/j.freeradbiomed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Lebedeva I, Stein C A. Antisense oligonucleotides: promise and reality. Annu Rev Pharmacol Toxicol. 2001;41:403–419. doi: 10.1146/annurev.pharmtox.41.1.403. [DOI] [PubMed] [Google Scholar]
- Coombes J S, Powers S K, Demirel H A, Hamilton K L, Jessup J, Vincent H K, Shanely R A. Vitamin E deficiency fails to affect myocardial performance during in vivo ischemia-reperfusion. Int J Vitam Nutr Res. 2000;70:293–300. doi: 10.1024/0300-9831.70.6.293. [DOI] [PubMed] [Google Scholar]
- Coombes J S, Powers S K, Demirel H A, Jessup J, Vincent H K, Hamilton K L, Naito H, Shanely R A, Sen C K, Packer L, Ji L L. Effect of combined supplementation with vitamin E and α-lipoic acid on myocardial performance during in vivo ischaemia-reperfusion. Acta Physiol Scand. 2000;169:261–269. doi: 10.1046/j.1365-201x.2000.00740.x. [DOI] [PubMed] [Google Scholar]
- Coombes J S, Powers S K, Hamilton K L, Demirel H A, Shanely R A, Zergeroglu M A, Sen C K, Packer L, Ji L L. Improved cardiac performance after ischemia in aged rats supplemented with vitamin E and alpha-lipoic acid. Am J Physiol Renal Physiol. 2000;279:R2149–R2155. doi: 10.1152/ajpregu.2000.279.6.R2149. [DOI] [PubMed] [Google Scholar]
- Demirel H A, Powers S K, Zergeroglu M A, Shanely R A, Hamilton K, Coombes J, Naito H. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–2212. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- Hamilton K L, Powers S K, Sugiura T, Kim S, Lennon S, Tumer N, Mehta J L. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281:H1346–H1352. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- Powers S K, Demirel H A, Vincent H K, Coombes J S, Naito H, Hamilton K L, Shanely R A, Jessup J. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol Renal Physiol. 1998;275:R1468–R1477. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- Hamilton K L, Staib J L, Phillips T, Hess A, Lennon S L, Powers S K. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med. 2003;34:800–809. doi: 10.1016/s0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- Lennon S L, Quindry J C, French J P, Kim S, Mehta J L, Powers S K. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol Scand. 2004;182:161–169. doi: 10.1111/j.1365-201X.2004.01346.x. [DOI] [PubMed] [Google Scholar]
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- McCord J M, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- Flohe L, Gunzler W A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Quindry J, French J, Hamilton K, Lee Y, Mehta J L, Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005;40:416–425. doi: 10.1016/j.exger.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lennon S L, Quindry J C, Hamilton K L, French J P, Hughes J, Mehta J L, Powers S K. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287:H975–H980. doi: 10.1152/ajpheart.01208.2003. [DOI] [PubMed] [Google Scholar]
- Galvez A S, Diwan A, Odley A M, Hahn H S, Osinska H, Melendez J G, Robbins J, Lynch R A, Marreez Y, Dorn G W., 2nd Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- Wu Y, Hamilton S L. Functional interactions of cytoplasmic domains of the skeletal muscle Ca2+ release channel. Trends Cardiovasc Med. 1998;8:312–319. doi: 10.1016/s1050-1738(98)00023-1. [DOI] [PubMed] [Google Scholar]
- Bajaj G, Sharma R K. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun. 2006;345:1558–1564. doi: 10.1016/j.bbrc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- Adlam V J, Harrison J C, Porteous C M, James A M, Smith R A, Murphy M P, Sammut I A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z, Szanto A, Gallyas F, Jr, Hideg K, Sumegi B, Varbiro G. A novel SOD-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free Radic Biol Med. 2006;41:835–848. doi: 10.1016/j.freeradbiomed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Jin Z Q, Zhou H Z, Cecchini G, Gray M O, Karliner J S. MnSOD in mouse heart: acute responses to ischemic preconditioning and ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;288:H2986–H2994. doi: 10.1152/ajpheart.01144.2004. [DOI] [PubMed] [Google Scholar]
- Chen Z, Siu B, Ho Y S, Vincent R, Chua C C, Hamdy R C, Chua B H. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- Kourie J I. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- Kim M S, Akera T. O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol Heart Circ Physiol. 1987;252:H252–H257. doi: 10.1152/ajpheart.1987.252.2.H252. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Beamish R E, Dhalla N S. Depression of heart sarcolemmal Ca2+-pump activity by oxygen free radicals. Am J Physiol Heart Circ Physiol. 1989;256:H368–H374. doi: 10.1152/ajpheart.1989.256.2.H368. [DOI] [PubMed] [Google Scholar]
- Suzuki Y J, Ford G D. Superoxide stimulates IP3-induced Ca2+ release from vascular smooth muscle sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 1992;262:H114–H116. doi: 10.1152/ajpheart.1992.262.1.H114. [DOI] [PubMed] [Google Scholar]
- Coetzee W A, Ichikawa H, Hearse D J. Oxidant stress inhibits Na-Ca-exchange current in cardiac myocytes: mediation by sulfhydryl groups? Am J Physiol Heart Circ Physiol. 1994;266:H909–H919. doi: 10.1152/ajpheart.1994.266.3.H909. [DOI] [PubMed] [Google Scholar]
- Kato M, Kako K J. Na+/Ca2+ exchange of isolated sarcolemmal membrane: effects of insulin, oxidants and insulin deficiency. Mol Cell Biochem. 1988;83:15–25. doi: 10.1007/BF00223194. [DOI] [PubMed] [Google Scholar]
- Tokube K, Kiyosue T, Arita M. Effects of hydroxyl radicals on KATP channels in guinea-pig ventricular myocytes. Plügers Arch. 1998;437:155–157. doi: 10.1007/s004240050760. [DOI] [PubMed] [Google Scholar]
- Guerra L, Cerbai E, Gessi S, Borea P A, Mugelli A. The effect of oxygen free radicals on calcium current and dihydropyridine binding sites in guinea-pig ventricular myocytes. Br J Pharmacol. 1996;118:1278–1284. doi: 10.1111/j.1476-5381.1996.tb15534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee W A, Opie L H. Effects of oxygen free radicals on isolated cardiac myocytes from guinea-pig ventricle: electrophysiological studies. J Mol Cell Cardiol. 1992;24:651–663. doi: 10.1016/0022-2828(92)91049-b. [DOI] [PubMed] [Google Scholar]
- Striessnig J. Pharmacology, structure and function of cardiac L-type Ca(2+) channels. Cell Physiol Biochem. 1999;9:242–269. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- Node K, Kitakaze M, Sato H, Minamino T, Komamura K, Shinozaki Y, Mori H, Hori M. Role of intracellular Ca2+ in activation of protein kinase C during ischemic preconditioning. Circulation. 1997;96:1257–1265. doi: 10.1161/01.cir.96.4.1257. [DOI] [PubMed] [Google Scholar]
- Kim H D. Infarct size-limiting effect of calcium preconditioning in rabbit hearts. J Korean Med Sci. 2003;18:337–343. doi: 10.3346/jkms.2003.18.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarjova N, Ho C, Mellgren R L, Askari A, Huang W H. Different sensitivities of native and oxidized forms of Na+/K(+)-ATPase to intracellular proteinases. Biochim Biophys Acta. 1994;1192:125–131. doi: 10.1016/0005-2736(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Logue S E, Gustafsson A B, Samali A, Gottlieb R A. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol. 2005;38:21–33. doi: 10.1016/j.yjmcc.2004.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.