Abstract
Extracellular adenosine has been implicated as an innate antiinflammatory metabolite, particularly during conditions of limited oxygen availability such as ischemia. Because extracellular adenosine generation is primarily produced via phosphohydrolysis from its precursor molecule adenosine-monophosphate (AMP) through the enzyme ecto-5′-nucleotidase (CD73), we examined the contribution of CD73-dependent adenosine production in modulation of intestinal ischemia-reperfusion (IR) injury. Following transcriptional and translational profiling of intestinal tissue that revealed a prominent induction of murine CD73, we next determined the role of CD73 in protection against intestinal IR injury. Interestingly, pharmacological inhibition or targeted gene deletion of CD73 significantly enhanced not only local intestinal injury, but also secondary organ injury, following IR as measured by intestinal and lung myeloperoxidase, aspartate and alanine aminotransferase, interleukin (IL) -1, IL-6, and histological injury. To confirm the role of CD73 in intestinal adenosine production, we measured adenosine tissue levels and found that they were increased with IR injury. In contrast, CD73-deficient (cd73−/−) mice had lower adenosine levels at baseline and no increase with IR injury. Finally, reconstitution of cd73−/− mice or treatment of wild-type mice with soluble 5′-nucleotidase was associated with significantly lower levels of injury. These data reveal a previously unrecognized role of CD73 in attenuating intestinal IR-mediated injury.—Hart, M. L., Henn, M., Köhler, D., Kloor, D., Mittelbronn, M., Gorzolla, I. C., Stahl, G. L., Eltzschig, H. K. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury.
Keywords: CD73, adenosine receptor, hypoxia, HIF, hypoxia-inducible factor
The consequences of depriving an organ of its blood supply have long been recognized as a critical factor in the clinical outcome of stroke, hemorrhagic shock and organ transplantation. Although restoration of blood flow to an ischemic organ is essential to prevent irreversible tissue injury, reperfusion can also augment tissue injury in excess of that produced by ischemia alone by causing destruction of vascular integrity, tissue edema, and disturbances in cellular energy balance (1). Cellular damage after reperfusion of previously viable ischemic tissue is defined as ischemia-reperfusion (IR) injury (1).
Gastrointestinal ischemia-reperfusion generally stems from interruption of blood flow within the superior mesenteric artery or vein and leads to small intestine hypoperfusion (2). Intestinal IR is encountered in a variety of clinical conditions such as hemorrhagic shock, strangulation-obstruction of the intestine, sepsis, vascular surgery, small bowel transplantation, cardiopulmonary bypass, and abdominal aortic surgery, and it can result in multiple organ failure (3). IR of the intestine is a systemic phenomenon that may result in bacterial translocation, endotoxemia, acute respiratory distress syndrome (ARDS) and acute hepatic injury (4, 5). Thus, injury from intestinal IR may cause a local and systemic inflammatory response. As a result, morbidity and mortality associated with intestinal IR are high (∼70%) (2, 4), and specific therapeutic interventions are needed.
It is well known that inflammatory tissue damage is accompanied by accumulation of extracellular adenosine, a naturally occurring antiinflammatory agent (6,7,8,9). Local tissue hypoxia or ischemia in inflamed areas represents one of the most important conditions leading to adenosine release and accumulation (9, 10). At present, the exact source of adenosine during hypoxic or ischemic events is not well defined but likely results from a combination of amplified extracellular phosphohydrolysis of adenine nucleotides via surface ectonucleotidases (11, 12) and decreased adenosine uptake by the surrounding tissues (13). During ischemia, extracellular nucleotides (ATP/ADP) liberated at inflammatory or hypoxic tissue sites from various cells, including PMNs (14), platelets, mast cells, and endothelial cells (6, 7), are metabolized to adenosine via surface expressed ecto-nucleotidases (CD39 and CD73). Ectoapyrase (CD39) converts ATP/ADP to AMP and ecto-5′-nucleotidase (CD73) subsequently converts AMP to adenosine (15).
A few studies suggest that adenosine signaling may modulate tissue protection of the intestine during inflammation. Early studies showed that adenosine applied to the topical surface of the intestine inhibits intestinal IR-induced neutrophil infiltration, oxidative damage, and mucosal destruction in the intestine (16,17,18,19). CD39-deficient mice developed more profound intestinal IR injury and demonstrated increased mortality, and CD39 supplementation inhibited increased vascular permeability associated with IR injury (20). These studies suggest that adenosine modulates intestinal IR injury. However, to date, there is no direct evidence implicating CD73, the “pacemaker” enzyme of extracellular adenosine production, in protection against intestinal IR-induced injury. Therefore, we performed pharmacologic and genetic studies to investigate the role of CD73 in murine intestinal IR injury.
MATERIALS AND METHODS
Mice
Mice deficient in CD73 (cd73−/−) on the C57BL/6 strain have been described previously (12). Cd73+/+ littermates (WT) were matched in age, gender, and weight and used as controls.
Gastrointestinal ischemia-reperfusion injury
All animal protocols were in accordance with the German Law on the Protection of Animals for use of living animals and were approved by the Institutional Animal Care and Use Committee of the University Hospital and the Regierungspräsidium Tübingen. All mice were placed on a temperature-controlled heating table with a rectal thermometer probe attached to a thermal feedback controller to maintain body temperature at 37°C. Cd73−/− or WT mice were anesthetized with isoflurane (The Multi-Station Research Anesthesia System, Harvard Apparatus, March-Hugstetten, Germany). After a midline laparotomy, intestinal ischemia was produced by clamping the superior mesenteric artery for 15 min, followed by unclamping for 3 h of reperfusion as described previously (21,22,23). Sham-operated controls underwent the same surgical procedure but without intestinal IR. In some experiments, WT or cd73−/− mice were treated with a specific inhibitor of ecto-5′-nucleotidase, 5′-[αβ-methylene] diphosphate (APCP; 40 mg/kg/h; Sigma, St. Louis, MO, USA), given via an intracarotid arterial catheter (24) prior to ischemia; the nonmetabolizable adenosine analog 5′-N-ethylcarboxamidoadenosine (NECA, Sigma-Aldrich; dosage of 0.1 mg/kg i.p. plus 0.1 mg/kg s.c.) (12); or dimethyloxallyl glycine (DMOG), a prolyl-4-hydroxylase inhibitor which up-regulates HIF activity (Frontier Scientific, Logan, UT, USA; dosage of 40 or 200 mg/kg i.p. 4 h prior to ischemia). For reconstitution experiments, cd73−/− mice were reconstituted with a bolus of 2 U of soluble 5′-nucleotidase (5′-NT) purified from Crotalus atrox venom (given via an intracarotid arterial catheter; Sigma) (12, 24), followed by 40 U/kg/h intra-arterial (i.a.) infusion during IR. In additional experiments, WT mice were treated with a bolus of 5′-NT (2 U, i.a.), followed by 20 U/kg/h, i.a. during IR. After 3 h of reperfusion, mice were euthanized, and intestinal and lung samples were immediately frozen in liquid nitrogen for further investigations. Blood was collected via cardiac puncture, transferred to a 2-ml tube, and centrifuged (10 min at 3000 rpm); serum was transferred to a new tube and frozen at –80°C.
Interleukin (IL) -1β or IL-6 treatment of T84 and Caco cells
Human intestinal epithelial T84 cells [American Type Culture Collection (ATCC), Rockville, MD, USA] were grown in a 1:1 mixture of Ham’s F12 Medium and Dulbecco’s modified Eagle medium (Life Technologies, Gaithersburg, MD, USA) supplemented with 5% fetal bovine serum (Life Technologies) and 1% antibiotic/antimycotic solution (Sigma). Caco-2 cells (ATCC) were grown in minimal essential medium Eagle (Sigma) with 20% fetal calf serum (Life Technologies), 1% MEM 100× (Sigma), 1% sodium-pyruvate (Sigma), and 1% antibiotic/antimycotic solution (Sigma). Confluent cells were then stimulated for 24 h with 10 ng/ml of human recombinant IL-1β or IL-6 (Promokine, Heidelberg, Germany).
Real-time RT-PCR
To examine the influence of intestinal IR on CD73 transcript levels, C57BL/6 mice underwent intestinal ischemia followed by reperfusion. Mice were euthanized at indicated time points, and mucosal scrapings were performed. Total RNA was isolated using the total RNA isolation NucleoSpin RNA II Kit according to the manufacturer’s instructions (Macherey & Nagel, Düren, Germany). cDNA synthesis was performed using reverse transcription according to the manufacturer’s instructions (i-script Kit; Bio-Rad Laboratories, Munich, Germany). The primer sets for the PCR reaction contained 1 μM sense and 1 μM antisense with SYBR Green I (Molecular Probes, New Brunswick, NJ, USA). Primer sequences for murine CD73 were (sense/antisense, respectively) 5′-CAAATCCCACACAACCACTG-3′ and 5′-TGCTCACTTGGTCACAGGAC-3′. Murine β-actin sense primer, 5′-ACATTGGCATGGCTTTGTTT-3′ and antisense primer, 5′-GTTTGCTCCAACCAACTGCT-3 were used to control for the starting template. Levels and fold change in mRNA were determined as described previously (27).
Western blot analysis
The Western blot analysis technique was used to examine total CD73 protein level. Briefly, protein extracts were solubilized in reducing Laemmli sample buffer and heated to 70°C for 10 min. Samples were resolved on a 10% polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were blocked at 4°C overnight in 5% nonfat dry milk (Applichem, Cheshire, CT, USA) in Tris-buffered saline with Tween (TBS-T). The membranes were incubated with 4 μl/ml CD73 rabbit polyclonal antibody raised against the C terminus (Abgent, San Diego, CA, USA) for 2 h at room temperature, followed by 5-min washes in TBS-T. The membranes were then incubated with 1:2000 goat anti-rabbit horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Danvers, MA, USA). The wash was repeated, and proteins were detected by enhanced chemiluminescence. To ensure equal loading, membranes were detected for β-actin. Blots were stripped for 15 min at room temperature in stripping buffer (Pierce, Rockford, IL, USA) and washed and blocked as mentioned above. Membranes were incubated with β-actin rabbit monoclonal antibody (dilution 1:1000) (Cell Signaling) for 2 h at room temperature followed by 5 min washes in TBS-T. The membranes were then incubated with 1:2000 goat anti-rabbit HRP (Santa Cruz Biotechnology). The wash was repeated, and proteins were detected by enhanced chemiluminescence.
Myeloperoxidase (MPO) activity
MPO activity, an index of neutrophil infiltration, was measured in intestinal and lung homogenates as described previously (21,22,23, 25).
Alanine (ALT) and aspartate (AST) aminotransferase serum activity
Serum ALT and AST levels were measured using a microtiter plate adaptation of a commercially available kit (Teco Diagnostics, Anaheim, CA, USA) (26).
IL-1 and IL-6 serum activity
Serum cytokine activity was measured using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA).
Histological sections
Mice were euthanized at the indicated time points and intestine was removed and fixed in Tissue-Tek (Sakura Finetek Europe, Zoeterwoude, The Netherlands). Cryostat sections (5 μm) were mounted on glass slides, air dried, and postfixed in acetone/methanol (1:1) for 10 min at room temperature. Sections were stained with hematoxylin and eosin (H&E) and the extent of the mucosal damage was quantified, as described previously using this model (23) using a semiquantitative grading system adapted from Chiu et al. (28). Injury was classified using the following 5-point scale, in which a numerical score was assigned based on the degree of mucosal and submucosal damage: 0 = normal; 1 = development of apical subepithelial space; 2–3 = epithelial lifting; 4 = cellular infiltration; and 5 = disintegration of lamina propria, hemorrhage, and ulceration.
Adenosine measurements
Jejunum from mice was removed and immediately snap-frozen with clamps that were precooled to the temperature of liquid nitrogen within a time lag of 3 to 5 s. The frozen jejunum was pulverized under liquid nitrogen, and the tissue protein was precipitated with ice-cold 0.6 N perchloric acid. Tissue adenosine levels were determined as described previously (29).
RESULTS
Intestinal CD73 is induced by IR
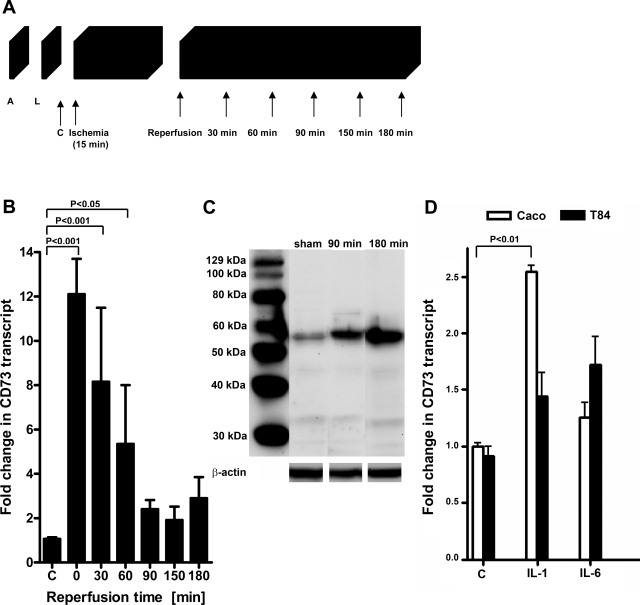
On the basis of previous studies showing tissue protection by extracellular adenosine generated via hypoxia-inducible CD73 (11, 12, 30), we hypothesized that CD73-dependent adenosine generation may play an important role during intestinal IR. Therefore, we investigated intestinal CD73 expression using our previously described model of intestinal IR (21,22,23). To define transcriptional effects of intestinal IR, intestinal epithelial scrapings were harvested at the indicated reperfusion time points following 15 min ischemia (Fig. 1A) and assessed via real-time RT-PCR. A strong induction of CD73 mRNA was observed immediately following ischemia (12.1±1.6-fold, P<0.001) with a steady decrease over the reperfusion time period (Fig. 1B). As the next step, we measured CD73 by Western blot analysis from intestinal epithelial scrapings and confirmed increases in CD73 protein after intestinal IR compared with sham controls (Fig. 1C). Taken together, these data support transcriptional and translational induction of CD73 during intestinal IR.
Figure 1.
CD73 is induced by intestinal IR. A) Transcriptional responses to intestinal IR were measured following isolation of RNA from intestinal epithelial scrapings from WT mice that were anesthetized (A) and after a midline laparotomy (L) were subjected to 15 min of ischemia, followed by the indicated reperfusion times. RNA was also isolated from control (C) mice that did not undergo IR. B) CD73 mRNA levels were determined by real-time RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and expressed as fold change ± se compared to sham (C) mice; 4 mice/group. C) Intestinal scrapings from WT sham mice or mice that were subjected to 15 min of ischemia followed by 90 or 180 min reperfusion were flash-frozen and lysed; proteins were resolved by SDS-PAGE and transferred to nitrocellulose, and membranes were probed with anti-CD73 antibody. A representative Western blot of two is shown. The same blot was reprobed for β-actin as a control for protein loading. D) Caco-2 and T84 human intestinal epithelial cells were stimulated with 10 ng/ml of human recombinant IL-1β or IL-6. After 24 h of treatment, CD73 transcript levels were measured via RT-PCR. Data were calculated relative to an internal housekeeping gene (β-actin) and expressed as fold change ± se compared to untreated control (C) cells; 3 samples/group.
In addition to hypoxia (11, 12, 30) and hypoxia-inducible factor-1 (HIF-1) (31), inflammatory cytokines may regulate CD73. For example, interferon-α was shown to up-regulate CD73 expression on endothelial cells in vitro and in human patients in vivo and correlate positively with increased enzymatic activity and adenosine production (32). To determine whether inflammatory cytokines regulate CD73 in intestinal epithelial cells, Caco or T84 cells were treated with either IL-1β or IL-6. After 24 h of treatment, CD73 transcript levels were measured via RT-PCR. Caco cells stimulated with IL-1β resulted in a significant increase in CD73 transcript levels, while T84 cells stimulated with IL-1β or IL-6 caused a mild increase in CD73 mRNA (Fig. 1D). Collectively, these results suggest that inflammatory cytokines contribute to the induction of CD73 during intestinal ischemia reperfusion injury.
CD73 inhibition accentuated injury following intestinal IR
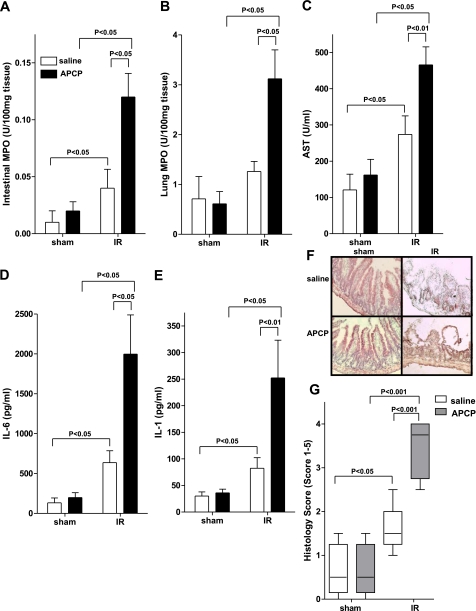
After having shown a robust induction of CD73 during intestinal IR injury, we next performed pharmacological studies to determine the functional role of CD73 in this model. WT mice were subjected to 15 min of superior mesenteric artery occlusion and 3 h of reperfusion following treatment with the specific CD73 inhibitor APCP (40 mg/kg/h, i.a.) or vehicle (saline). Consistent with previous studies (21,22,23), IR was associated with a significant increase in intestinal MPO activity (Fig. 2A; P<0.05). However, neutrophil tissue infiltration was dramatically increased following APCP-inhibition of CD73 (Fig. 2A; P<0.05). Similarly, increases in pulmonary MPO activity were significantly enhanced in mice exposed to IR injury following CD73 inhibition (Fig. 2B; P<0.05). As shown in Fig. 2C, APCP treatment prior to intestinal IR also resulted in a significant increase in secondary hepatic injury as measured by serum AST. Additionally, inhibition of CD73 caused a significant increase in serum IL-6 and IL-1β following IR (Fig. 2D, E, respectively). Histological analysis revealed that intestinal IR also significantly increased the intestinal injury score in APCP-treated mice compared to WT mice that were treated with vehicle (Fig. 2F, G). Taken together, these studies provide pharmacological evidence of CD73-mediated tissue protection during intestinal IR.
Figure 2.
Inhibition of CD73 by APCP increases injury. Injury was measured after WT mice were treated with APCP (40 mg/kg/h, i.a.) or sterile saline and subjected to 15 min of ischemia, followed by 3 h reperfusion. Injury was measured for the following parameters: Intestinal MPO (A), lung MPO (B), AST (C), IL-6 (D), IL-1 (E), H&E staining of jejunum sections (×200) (F), and quantification of ischemic injury (median±range; n=3–5 mice/group) (G). All other results are expressed as mean ± se; 8–12 mice/group.
Intestinal IR injury is increased in cd73−/− mice
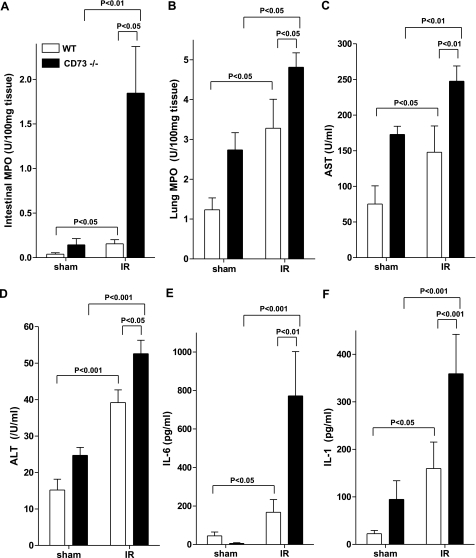
On the basis of these pharmacological findings demonstrating that CD73 inhibition enhances IR-induced injury, we next investigated the role of CD73 in protection against intestinal IR in cd73 gene-targeted mice (12). Following intestinal IR, cd73−/− mice demonstrated significantly higher intestinal MPO levels in comparison to littermate control (WT) mice (Fig. 3A; P<0.01). Similarly, pulmonary MPO levels were increased in cd73−/− mice (Fig. 3B; P<0.05). Biochemical analysis revealed that intestinal IR significantly increased serum AST (Fig. 3C) and ALT (Fig. 3D) concentrations in cd73−/− mice compared to littermate controls. Cd73−/− mice also demonstrated significantly higher IL-6 (Fig. 3E) and IL-1β (Fig. 3F) cytokine concentrations in the serum than WT control mice following intestinal IR. Taken together, these data reveal, for the first time, genetic evidence for CD73-dependent protection against intestinal IR.
Figure 3.
Injury is increased in cd73−/− mice. CD73-deficient (CD73−/−) mice or age, weight, and gender-matched littermate controls (WT) were subjected to 15 min of ischemia, followed by 3 h of reperfusion. Injury was measured for the following parameters: Intestinal MPO (A), lung MPO (B), AST (C), ALT (D), IL-6 (E), and IL-1 (F). All results are expressed as mean ± se; 8–12 mice/group.
Histological signs of intestinal injury by intestinal IR are increased in cd73−/− mice
To confirm enhanced injury observed in cd73−/− mice, we examined jejunum from WT littermate controls or cd73−/− mice. Intestinal IR resulted in loss and shortening of intestinal villi and marked intestinal epithelial cell denudation (Fig. 4A). However, the observed injury was significantly increased in cd73−/− mice compared to WT controls after intestinal IR, as semiquantitative histological analysis of these sections demonstrated an increase in the Chiu index (28), from 2 in WT mice to 3.5 in cd73−/− mice (Fig. 4A, B; P<0.01). Taken together, these data provide strong evidence for a role of CD73 in attenuating intestinal IR injury.
Figure 4.
Histological signs of intestinal IR injury are increased in cd73−/− mice. H&E staining of jejunum sections (×200) (A) and quantification of ischemic injury (B). Results are expressed as median ± range; n = 3–5 mice/group.
Intestinal adenosine concentrations are decreased in cd73−/− mice
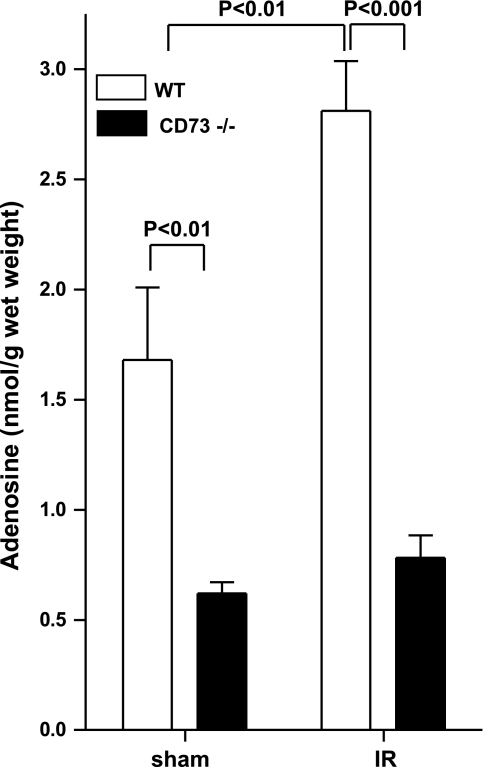
Next, we performed studies to confirm CD73-mediated phosphohydrolysis of AMP in intestinal IR injury by measuring adenosine tissue concentrations. To do this, we measured jejunal adenosine levels in tissues shock frozen immediately following 15 min of intestinal ischemia or in sham-operated controls. Intestinal adenosine concentrations were significantly increased following in WT IR mice compared to sham controls (Fig. 5; P<0.001). In contrast, baseline intestinal adenosine levels of cd73−/− were significantly lower than littermate controls (Fig. 5; 2.71±0.2-fold, P<0.01) and did not increase with intestinal ischemia (Fig. 5). Taken together, these data demonstrate that increases in intestinal adenosine during ischemia require CD73 activity.
Figure 5.
Increased intestinal adenosine concentrations with IR are attenuated in cd73−/− mice. WT or cd73−/− mice were subjected to 15 min of ischemia. The jejunum was snap frozen immediately prior to the end of ischemia (IR). In control mice, the jejunum was snap frozen without clamping of the superior mesenteric artery (sham). Results are expressed as the mean ± se of 6 mice per group.
Adenosine receptor stimulation decreased intestinal IR injury
To confirm that adenosine regulates intestinal IR injury, WT or cd73−/− mice were treated with NECA, a nonspecific adenosine receptor agonist. Stimulation of adenosine receptors resulted in a significant inhibition of neutrophil infiltration into the intestinal and lung tissues (Fig. 6A, B, respectively), as well as a significant decrease in serum ALT (Fig. 6C) and proinflammatory cytokine levels (Fig. 6D, E) in cd73−/− mice. In fact, treatment of cd73−/− mice with NECA resulted in a WT phenotype, suggesting that adenosine regulates intestinal IR injury via binding to adenosine receptors.
Figure 6.
Treatment with NECA, a nonspecific adenosine receptor agonist, decreases IR injury. Injury was measured after WT or cd73−/− mice were treated with NECA (0.1 mg/kg, i.p., plus 0.1 mg/kg, s.c.) or sterile saline and subjected to 15 min of ischemia, followed by 3 h of reperfusion. Injury was measured for the following parameters: intestinal MPO (A), lung MPO (B), ALT (C), IL-6 (D), and IL-1 (E). All results are expressed as mean ± se; 4–8 mice/group.
Reconstitution of cd73−/− mice with soluble 5′-nucleotidase resulted in a WT phenotype
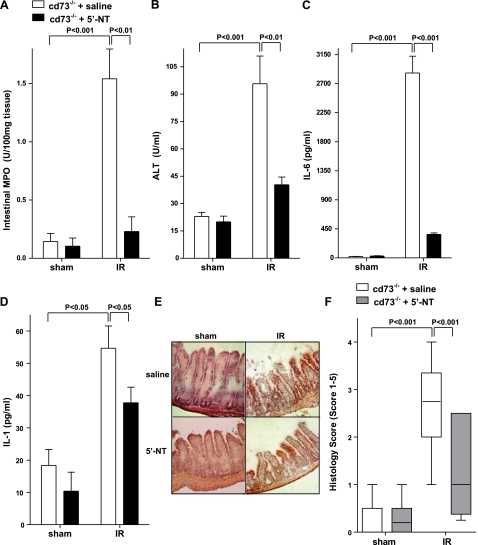
As proof of principle and to demonstrate that an increase in functional injury parameters in cd73−/− mice reflects lack of ecto-5′-nucleotidase enzyme activity, we next reconstituted cd73−/− mice with soluble 5′-nucleotidase (5′-NT) from Crotalus atrox venom (12) and subjected them to intestinal IR. As shown in Fig. 7, reconstitution of cd73−/− mice with soluble 5′-nucleotidase attenuated injury, as demonstrated by a significant decrease in intestinal MPO (Fig. 7A), ALT (Fig. 7B), and proinflammatory cytokines IL-6 (Fig. 7C) and IL-1β (Fig. 7D). Histological analysis confirmed that 5′-NT treatment was associated with reconstitution of a WT phenotype (Fig. 7E, F). Taken together, these results confirm our genetic studies, demonstrating that CD73 plays a crucial role in protection against intestinal IR-induced injury.
Figure 7.
Reconstitution of cd73−/− mice with soluble 5′-nucleotidase decreases injury in cd73−/− mice. Injury was measured after cd73−/− mice were reconstituted with 5′-nucleotidase (2 U bolus prior to ischemia, followed by 40 U/kg/h, i.a.) or sterile saline and subjected to 15 min of ischemia, followed by 3 h reperfusion. Injury was measured for the following parameters: Intestinal MPO (A), ALT (B), IL-6 (C), IL-1 (D), H&E staining of jejunum sections (×200) (E), and quantification of ischemic injury (F) (median±range; n=3–5 mice/group). All other results are expressed as mean ± se; 8–12 mice/group.
Treatment of intestinal ischemia with soluble 5′-nucleotidase in WT mice decreased IR injury
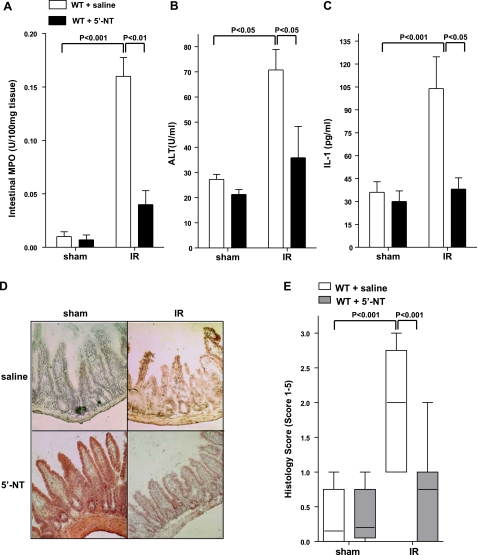
We next pursued the usefulness of 5′-NT treatment of intestinal ischemia in WT mice. For this purpose, we treated WT mice with 5′-nucleotidase prior to intestinal IR (12). As shown in Fig. 8A–C, injury following intestinal IR was inhibited by treatment of WT mice with soluble 5′-nucleotidase. Intestinal histological analysis confirmed this protection (Fig. 8D, E). Taken together, these data demonstrate a therapeutic effect of treatment with soluble 5′-nucleotidase in intestinal IR.
Figure 8.
Treatment with soluble 5′-nucleotidase decreases injury in WT mice. Injury was measured after WT mice were treated with 5′-nucleotidase (2 U bolus prior to ischemia, followed by 20 U/kg/h, i.a.) or sterile saline and subjected to 15 min of ischemia, followed by 3 h of reperfusion. Injury was measured for the following parameters: Intestinal MPO (A), ALT (B), IL-1 (C), H&E staining of jejunum sections (×200) (D), and quantification of ischemic injury (median±range; n=3–5 mice/group) (E). All other results are expressed as mean ± se; 8–12 mice/group.
Mechanism of CD73 regulation
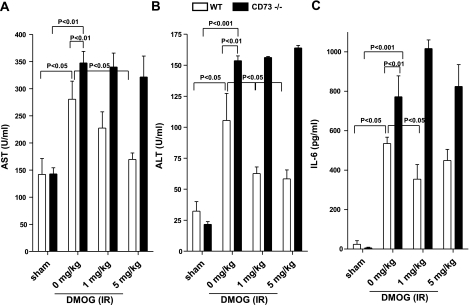
One way that oxygen homeostasis is coordinated is through the transcriptional regulator HIF-1 (33). Recently, much attention has focused on HIF-1 as a key regulator of a wide variety of oxygen-regulated genes, including CD73 (31). To determine whether the observed protective effects of CD73-dependent adenosine production during intestinal IR are regulated by HIF-1, WT or cd73−/− mice were treated i.p. with 40 or 200 mg/kg of dimethyloxaloylglycine (DMOG), a nonspecific prolyl hydroxylase inhibitor, which activates HIF-1 activity by inhibiting proteasomal degradation of the HIF-1α subunit. As shown in Fig. 9, WT mice treated with DMOG showed a significant decrease in AST (Fig. 9A), ALT (Fig. 9B) and IL-6 (Fig. 9C) following intestinal IR. In contrast, DMOG had no effect on cd73−/− mice (Fig. 9A–C). These data suggest that HIF-1 regulates CD73 during intestinal IR and thus activation of HIF-1 inhibits intestinal IR injury.
Figure 9.
HIF-1 activation decreases IR injury in WT but not cd73−/− mice. Injury was measured after WT or cd73−/− mice were treated with DMOG (40 or 200 mg/kg, i.p., 4 h prior to ischemia) or sterile saline and subjected to 15 min of ischemia, followed by 3 h of reperfusion. Injury was measured for the following parameters: AST (A), ALT (B), and IL-6 (C). All results are expressed as mean ± se of 4–8 mice/group.
DISCUSSION
In the present study, we pursued the contribution of extracellular adenosine production in protection against intestinal IR-induced injury. Transcriptional and translational profiling of intestinal tissue revealed a prominent induction of epithelial cell CD73. Pharmacological inhibition or targeted gene deletion of CD73 significantly increased injury following intestinal IR demonstrating the importance of this enzyme in acute ischemia. Moreover, soluble 5′-nucleotidase treatment was associated with lower levels of injury. Taken together, these studies suggest that manipulation of CD73 enzymatic activity to increase extracellular adenosine production represents a therapeutic strategy for the treatment of acute ischemic injury of the intestine and that deficiency of CD73 may result in enhanced injury during intestinal ischemic events.
Our results showing transcriptional and translational induction of intestinal epithelial cell CD73 are consistent with previous studies that found that exposure of intestinal epithelial cells to ambient hypoxia (2% oxygen) resulted in a robust induction of CD73 transcript, protein, and function (11, 31). Similarly, studies in cd73−/− mice demonstrated increased vascular leakage syndrome and pulmonary edema on hypoxia exposure (8% oxygen over 4 h) (12), confirming a role of CD73-dependent adenosine production in multiple-organ injury during hypoxic events. Studies examining the CD73 gene promoter found a binding site for hypoxia-inducible factor (HIF)-1α and inhibition of HIF-1α expression resulted in attenuation of hypoxia-inducible CD73 expression (31), demonstrating a HIF-1α-dependent pathway for CD73 induction by hypoxia. In addition to hypoxia, HIF-1α protein levels were induced in the intestinal mucosa and persisted after 1 and 3 h of reperfusion in a rat model of intestinal IR injury (34). Furthermore, in the present study, we show that cd73−/− mice were resistant to the effects of the HIF-1 activator, DMOG, while WT mice treated with DMOG demonstrated a dose-dependent decrease in intestinal IR injury markers. Therefore, it is likely that the observed protective effects of CD73-dependent adenosine production during intestinal IR are transcriptionally coordinated by HIF and that activation of HIF-1 may provide an additional potential therapeutic approach to decrease intestinal IR injury.
In the present study, we show that pharmacological inhibition or genetic deletion of CD73 due to decreased adenosine generation significantly increased infiltration of neutrophils into the intestinal tissue following intestinal IR. Human intestinal epithelial cells express surface CD73 abundantly on their apical domain and to a lesser extent on the basolateral domain (35). In vitro results show that neutrophils migrate across intestinal mucosa (36, 37) and release 5′-ATP, which is rapidly converted to 5′-AMP (14, 38). 5′-AMP is subsequently degraded into adenosine at the apical pole by CD73 anchored within the intestinal epithelial luminal membrane (38, 39), where it functions as a paracrine and/or autocrine mediator of chloride secretion in intestinal epithelial cells (38). The ability of intestinal epithelial cells to respond to 5′-AMP with chloride secretion and hence, water secretion, is an important component of the epithelial barrier that protects the intestine by preventing translocation of bacteria, bacterial products, and antigens to lamina propria (40, 41). A previous study showed that CD73 inhibition using APCP resulted in a significant increase in hypoxia-induced intestinal permeability (31), suggesting that hypoxia-induced CD73 in vivo may provide a protective mechanism for the intestinal barrier during episodes of decreased oxygen delivery. Furthermore, it has been shown that adenosine binds to surface-expressed adenosine receptors on neutrophils to limit excessive accumulation of neutrophils within tissues, and as such, functions as a feedback loop to attenuate potential tissue injury (30). CD39 and CD73 were identified as critical control points to attenuate excessive neutrophil accumulation during hypoxia (30). Thus, one mechanism for adenosine protection in the intestine may be neutrophil-intestinal epithelial crosstalk.
Our results also demonstrate that a nonselective adenosine receptor agonist inhibited intestinal IR injury, suggesting that CD73-dependent adenosine production modulates intestinal IR injury via adenosine receptor stimulation. In terms of specific adenosine receptors, previous studies on the contribution of adenosine receptor signaling in the intestine have revealed conflicting results. Treatment with an A1 receptor agonist was shown to protect against intestinal IR injury in the rat (42). However, other studies demonstrate that the A2BAR mediates enhancement of chloride secretion induced by adenosine (43, 44). Additionally, we previously demonstrated that A2BAR is selectively up-regulated by hypoxia and that A2BAR antagonists effectively neutralize ATP-mediated changes in posthypoxic endothelial permeability (11). A2BAR is also involved in resealing of endothelia during transendothelial migration of neutrophils (45), particularly during conditions of limited oxygen availability (11). Furthermore, the A2BAR is expressed in intact human intestinal epithelia (43) and appears to be the only adenosine receptor present in T84 cells in both apical and basolateral membranes (43, 46). However, the importance of the A2AAR was shown to play a critical role in regulation of colitis (47). Further studies using a combined pharmacological and genetic approach using adenosine receptor-deficient mice will be required to identify adenosine signaling mechanisms involved in modulation of IR injury to the small intestine.
In the present study, we demonstrate that CD73 plays an antiinflammatory role during gastrointestinal ischemia/reperfusion injury. Reconstitution studies with adenosine analogs or with soluble 5′-nucleotidase suggest that the tissue-protective effects of CD73 in this model are related to its enzymatic function and role in elevating extracellular adenosine levels. In contrast, some previous studies suggest that CD73 may function in mediating lymphocyte adhesion to the endothelium—independent of its enzymatic function. As such, it has been suggested that engagement of CD73 on the lymphocyte surface increases integrin-mediated binding of lymphocytes to endothelium, providing a possible mechanism for regulating lymphocyte–endothelial cell contacts in high endothelial venules (48,49,50). In contrast to these findings, more recent studies of gene-targeted mice for cd73 show an anti-inflammatory and tissue-protective role of CD73, which is related to its functional role in elevating extracellular adenosine levels (12, 24, 51, 52).
In summary, the present study demonstrates that CD73-mediated phosphohydrolysis of AMP is a critical control point for endogenous adenosine generation and modulation of injury during intestinal IR. Genetic deficiency of CD73 may result in excessive injury during acute gastrointestinal ischemia. The significant attenuation of intestinal injury following ischemia by soluble 5′-nucleotidase treatment also suggests possible new strategies to ameliorate the consequences of intestinal ischemia.
Acknowledgments
The authors thank Marion Faigle, Stephanie Zug, Matthias Nagel, Renate Riehle, Daniel Schuler, and Ann-Kathrin Stenz for technical assistance. In addition, the authors thank Linda F. Thompson (Oklahoma Medical Research Foundation, Oklahoma City, OK, USA) for providing gene-targeted mice. This study was supported in part by U.S. National Institutes of Health grants DK-067782 (M.L.H.), HL-52886 (G.L.S.), HL-56086 (G.L.S.), DE-17821 (G.L.S.) and DE-16191 (G.L.S.); Fortune grant F1211269 (M.L.H. and H.K.E.); European Society for Anaesthesiology Research grant D3008762 (M.L.H. and H.K.E.); and German Research Foundation (DFG) grant EL274/2–2 (H.K.E.).
References
- Carden D L, Granger D N. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tendler D A. Acute intestinal ischemia and infarction. Semin Gastrointest Dis. 2003;14:66–76. [PubMed] [Google Scholar]
- Mallick I H, Yang W, Winslet M C, Seifalian A M. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- Eltzschig H K, Collard C D. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- Turnage R H, Guice K S, Oldham K T. The effects of hypovolemia on multiple organ injury following intestinal reperfusion. Shock. 1994;1:408–412. doi: 10.1097/00024382-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M V. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- McCallion K, Harkin D W, Gardiner K R. Role of adenosine in immunomodulation: review of the literature. Crit Care Med. 2004;32:273–277. doi: 10.1097/01.CCM.0000098026.12020.45. [DOI] [PubMed] [Google Scholar]
- Cronstein B N. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Eltzschig H K, Ibla J C, Furuta G T, Leonard M O, Jacobson K A, Enjyoji K, Robson S C, Colgan S P. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L F, Eltzschig H K, Ibla J C, Van De Wiele C J, Resta R, Morote-Garcia J C, Colgan S P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H K, Abdulla P, Hoffman E, Hamilton K E, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman K A, Coe I R, Colgan S P. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H K, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson S C, Colgan S P. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- Eltzschig H K, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- Kaminski P M, Proctor K G. Extracellular and intracellular actions of adenosine and related compounds in the reperfused rat intestine. Circ Res. 1992;71:720–731. doi: 10.1161/01.res.71.3.720. [DOI] [PubMed] [Google Scholar]
- Kaminski P M, Proctor K G. Actions of adenosine on nitro blue tetrazolium deposition and surface pH during intestinal reperfusion injury. Circ Res. 1990;66:1713–1719. doi: 10.1161/01.res.66.6.1713. [DOI] [PubMed] [Google Scholar]
- Kaminski P M, Proctor K G. Attenuation of no-reflow phenomenon, neutrophil activation, and reperfusion injury in intestinal microcirculation by topical adenosine. Circ Res. 1989;65:426–435. doi: 10.1161/01.res.65.2.426. [DOI] [PubMed] [Google Scholar]
- Grisham M B, Hernandez L A, Granger D N. Adenosine inhibits ischemia-reperfusion-induced leukocyte adherence and extravasation. Am J Physiol. 1989;257:H1334–H1339. doi: 10.1152/ajpheart.1989.257.5.H1334. [DOI] [PubMed] [Google Scholar]
- Guckelberger O, Sun X F, Sevigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal J B, Robson S C. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- Stahl G L, Xu Y, Hao L, Miller M, Buras J A, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Montalto M C, Pfeiffer K J, Hao L, Stahl G L. Murine model of gastrointestinal ischemia associated with complement-dependent injury. J Appl Physiol. 2002;93:338–345. doi: 10.1152/japplphysiol.00159.2002. [DOI] [PubMed] [Google Scholar]
- Hart M L, Ceonzo K A, Shaffer L A, Takahashi K, Rother R P, Reenstra W R, Buras J A, Stahl G L. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson M A, Osswald H, Thompson L F, Unertl K, Eltzschig H K. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- Vakeva A P, Agah A, Rollins S A, Matis L A, Li L, Stahl G L. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Reilly T P, Elkahloun A G, George J W, Pohl L R. Macrophage migration inhibitory factor in drug-induced liver injury: a role in susceptibility and stress responsiveness. Biochem Biophys Res Commun. 2002;294:225–230. doi: 10.1016/S0006-291X(02)00466-7. [DOI] [PubMed] [Google Scholar]
- Pfaffl M W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C J. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- Delabar U, Kloor D, Luippold G, Muhlbauer B. Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;724:231–238. doi: 10.1016/s0378-4347(98)00580-5. [DOI] [PubMed] [Google Scholar]
- Eltzschig H K, Thompson L F, Karhausen J, Cotta R J, Ibla J C, Robson S C, Colgan S P. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta G T, Comerford K M, Louis N, Karhausen J, Eltzschig H K, Hansen K R, Thompson L F, Colgan S P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela J, Henttinen T, Yegutkin G G, Airas L, Kujari A M, Rajala P, Jalkanen S. IFN-alpha induced adenosine production on the endothelium: a mechanism mediated by CD73 (ecto-5′-nucleotidase) up-regulation. J Immunol. 2004;172:1646–1653. doi: 10.4049/jimmunol.172.3.1646. [DOI] [PubMed] [Google Scholar]
- Maxwell P H. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90:791–797. doi: 10.1113/expphysiol.2005.030924. [DOI] [PubMed] [Google Scholar]
- Koury J,D E, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, Feinman R. Persistent HIF-1alpha activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock. 2004;22:270–277. doi: 10.1097/01.shk.0000135256.67441.3f. [DOI] [PubMed] [Google Scholar]
- Strohmeier G R, Thomas W I L, Patapoff W, Thompson L F, Carlson S L, Moe S J, Carnes D K, Mrsny R J, Madara J L. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C A, Colgan S P, Madara J L. Interactions of neutrophils with epithelial cells: lessons from the intestine. J Am Soc Nephrol. 1994;5:138–152. doi: 10.1681/ASN.V52138. [DOI] [PubMed] [Google Scholar]
- Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J L, Patapoff T W, Gillece-Castro B, Colgan S P, Parkos C A, Delp C, Mrsny R J. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeier G R, Lencer W I, Patapoff T W, Thompson L F, Carlson S L, Moe S J, Carnes D K, Mrsny R J, Madara J L. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S, MacNaughton W K, Appleyard C B, Chadee K, Wallace J L. Persistent epithelial dysfunction and bacterial translocation after resolution of intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G635–G644. doi: 10.1152/ajpgi.2001.281.3.G635. [DOI] [PubMed] [Google Scholar]
- Freeman S L, MacNaughton W K. Nitric oxide inhibitable isoforms of adenylate cyclase mediate epithelial secretory dysfunction following exposure to ionising radiation. Gut. 2004;53:214–221. doi: 10.1136/gut.2003.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozacmaka V H, Sayan H, Igdemb A A, Cetinb A, Ozacmakc I D. Attenuation of contractile dysfunction by atorvastatin after intestinal ischemia reperfusion injury in rats. Eur J Pharmacol. 2007;562:138–147. doi: 10.1016/j.ejphar.2007.01.061. [DOI] [PubMed] [Google Scholar]
- Strohmeier G R, Reppert S M, Lencer W I, Madara J L. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- Kolachala V, Asamoah V, Wang L, Srinivasan S, Merlin D, Sitaraman S V. Interferon-gamma down-regulates adenosine 2b receptor-mediated signaling and short circuit current in the intestinal epithelia by inhibiting the expression of adenylate cyclase. J Biol Chem. 2005;280:4048–4057. doi: 10.1074/jbc.M409577200. [DOI] [PubMed] [Google Scholar]
- Lennon P F, Taylor C T, Stahl G L, Colgan S P. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman S V, Wang L, Wong M, Bruewer M, Hobert M, Yun C H, Merlin D, Madara J L. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem. 2002;277:33188–33195. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- Naganuma M, Wiznerowicz E B, Lappas C M, Linden J, Worthington M T, Ernst P B. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- Airas L, Hellman J, Salmi M, Bono P, Puurunen T, Smith D J, Jalkanen S. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. J Exp Med. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airas L, Niemela J, Salmi M, Puurunen T, Smith D J, Jalkanen S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airas L, Niemela J, Jalkanen S. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function-associated antigen-1-dependent mechanism. J Immunol. 2000;165:5411–5417. doi: 10.4049/jimmunol.165.10.5411. [DOI] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson L F, Osswald H, Eltzschig H K. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig H K. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]