Abstract
From the beginning of the AIDS epidemic, stress has been a suspected contributor to the wide variation seen in disease progression, and some evidence supports this idea. Not all individuals respond to a stressor in the same way, however, and little is known about the biological mechanisms by which variations in individuals’ responses to their environment affect disease-relevant immunologic processes. Using the simian immunodeficiency virus/rhesus macaque model of AIDS, we explored how personality (sociability) and genotype (serotonin transporter promoter) independently interact with social context (stable or unstable social conditions) to influence behavioral expression, plasma cortisol concentrations, SIV-specific IgG, and expression of genes associated with Type I interferon early in infection. SIV viral RNA set-point was strongly and negatively correlated with survival as expected. Set-point was also associated with expression of interferon-stimulated genes, with CXCR3 expression, and with SIV-specific IgG titers. Poorer immune responses, in turn, were associated with display of sustained aggression and submission. Personality and genotype acted independently as well as in interaction with social condition to affect behavioral responses. Together, the data support an “interactionist” perspective (Eysenck, 1991) on disease. Given that an important goal of HIV treatment is to maintain viral set-point as low as possible, our data suggest that supplementing anti-retroviral therapy with behavioral or pharmacologic modulation of other aspects of an organism’s functioning might prolong survival, particularly among individuals living under conditions of threat or uncertainty.
Keywords: AIDS, simian immunodeficiency virus, stress, rhesus monkey, cortisol, antibody, coping, interferon, personality, serotonin transporter promoter polymorphism
1. Introduction
From early in the AIDS pandemic, psychosocial stress has been proposed as a contributor to the wide variation seen in the rate of HIV disease progression (Coates et al., 1984). Research has shown that the experience of stressors such as bereavement (Kemeny and Dean, 1995) and other negative life events (Leserman et al., 2002) are associated with indicators of more rapid disease progression in humans. Using the simian immunodeficiency virus (SIV)/rhesus macaque model of AIDS, we have demonstrated experimentally (Capitanio et al., 1998) and with a large archival cohort (Capitanio and Lerche, 1998) that social stress is associated with shorter survival in monkeys infected with SIV.
Not all individuals are affected equally by challenging circumstances, however; variation in individual traits has also been related to disease outcome. For example, Leserman et al. (2000) showed that HIV+ gay men who cope with stressful circumstances by using denial displayed an accelerated disease course, and Cole et al. (1997) found that individual differences in social sensitivity moderated the relationship between exposure to social rejection and disease progression in HIV+ gay men. An exploratory analysis of SIV-infected rhesus monkeys (Capitanio et al., 1999) suggested that the personality trait Sociability may be a relevant moderating factor when stressors are social in nature. Sociability is a commonly found personality dimension in nonhuman primates (Capitanio, 2004) and is akin to Extraversion in humans (Gosling and John, 1999). In rhesus monkeys, High-Sociable (HS) animals show higher frequencies of affiliation, greater sensitivity to social cues, and a greater tetanus-specific IgG response following social change compared to Low-Sociable (LS) animals (Capitanio, 1999, 2002; Maninger et al., 2003). Our earlier analysis (Capitanio et al., 1999) suggested that LS animals may be at greater risk for faster disease progression than are HS animals. Together, these and other studies suggest that, while stress may exert a main effect on disease progression, variation in psychosocial traits may moderate the host’s response to stress and affect disease course.
In stressful circumstances, an individual’s psychological functioning and the progress of disease may also be affected by factors that are not explicitly psychosocial. In the psychiatric literature, attention has focused on how candidate genes associated with brain monoaminergic activity, such as the serotonin (5-HT) transporter gene-linked polymorphic region (5-HTTLPR), are related to psychological characteristics such as aggressiveness, anxiety, neuroticism, etc. The serotonin transporter gene codes for a protein responsible for the presynaptic uptake of serotonin following neuronal release. The promoter is biallelic in rhesus monkeys and humans, with the short allele leading to reduced expression of the transporter protein. Both human and nonhuman primate evidence indicates that individuals possessing a short allele are more likely to display aggression and/or anxiety, particularly under adverse conditions (Lesch et al, 1997; Barr et al., 2003; Holmes et al., 2003; Caspi and Moffitt, 2006). While we are not aware of any studies evaluating the role of such genes in infectious disease, given the association between aggression/anxiety and disease processes (e.g., Moller et al., 1999; Zorilla et al., 1994), candidate genes such as these may have an important moderating influence.
Presumably, the influence of personality and genotype on disease processes will be mediated through their effects on physiological stress-response systems. In the context of HIV/SIV disease, there is some evidence that the sympathetic nervous system can affect disease course. In vitro work has shown that catecholamines associated with the sympathetic nervous system can accelerate HIV-1 replication (Cole et al., 1998) and Sloan et al. (2006) have demonstrated that SIV replicates at increased frequency in the vicinity of catecholaminergic varicosities in lymph nodes. Other research suggests that hypothalamic-pituitary-adrenal (HPA) function is associated with variation in the course of HIV/SIV disease (Leserman et al., 2000, 2002). Our animal model of social stress, for example, found reduced basal cortisol, and evidence of enhanced negative feedback, in monkeys that experienced stressful social conditions. These animals ultimately showed significantly shorter survival (Capitanio et al., 1998). In the current study, we focused on plasma cortisol concentration as a possible physiological mediator of host characteristics on immune and viral outcome measures.
The present research was undertaken to explore some mechanisms by which “person by situation” interactions affect SIV disease progression. Our principal measure of disease progression was viral RNA (vRNA) copy number measured at Week 10 post-inoculation (p.i.). By approximately 6 weeks p.i., vRNA levels typically stabilize (Staprans et al., 1999) around a “set-point” that reflects the ability of the immune system to regulate viral production. Viral set-point remains relatively stable over the course of disease until progression to AIDS. Remarkably, variation in set-point measured early in disease correlates very highly with survival. In our earlier study of SIV-infected rhesus monkeys (Capitanio et al., 1998), vRNA levels at Week 10 p.i. correlated −0.79 with survival. Comparably strong effects have been found by others for both SIV (e.g., Watson et al., 1997) and HIV (e.g., Mellors et al., 1996).
The strong correlation between early vRNA set-point and survival suggests that events early in infection are critically important to the progress of the disease. Recent work using the SIV/rhesus macaque model, for example, has shown the surprising impact of the virus on host defenses within the first few weeks of infection (e.g., Mattapallil et al., 2005; Li et al., 2005), and increased attention has focused on mechanisms associated with innate immunity (Levy et al., 2003; Ahmed et al., 2005). In general, the first line of defense against viral infection is the interferon system. Type I-interferons (IFNs) such as IFN-α are produced by virally-infected cells at high levels within hours of infection. IFNs upregulate transcription of effector proteins (such as Mx and OAS [2′-5′ oligoadenylate synthetase]), and together the interferon system can act in an autocrine and paracrine fashion to inhibit protein synthesis and induce an anti-viral state. IFNs also activate NK cells, which produce and secrete the Type II interferon, IFN-γ, and its effector molecules, such as CXCL9. Interferons can also affect adaptive immune responses (Biron, 1998). The role of interferon systems in HIV/SIV infection is not straightforward, however. On the one hand, a reduction in the number of circulating cells that produce Type 1 interferons is associated with progression (e.g., Soumelis et al., 2001). On the other hand, studies with SIV-infected monkeys have demonstrated that Type 1 interferon responses during acute SIV infection are ineffective in controlling viral replication (Abel et al., 2002), presumably because HIV can block the effector functions of interferon-induced proteins (e.g., Sen, 2001).
We had several goals for the present study. First, we wanted to confirm whether viral set-point is associated with survival as we and others have demonstrated. Second, because there is relatively little information on innate immune responses early in immunodeficiency virus infection, we wanted to describe how such measures change during early SIV infection, and then to identify which of these measures is associated with variation in vRNA. Our third and principal goal was to determine whether Sociability and serotonin transporter genotype interact with social condition to affect behavior, and how the behavioral responses were associated with the immune changes that were predictive of vRNA. We had several specific predictions: we expected that social stress would be associated with a poorer SIV-specific IgG response, which would confirm an earlier report from our laboratory (Capitanio et al., 1998); and we expected that animals in Unstable (but not in Stable) social conditions that were Low-Sociable, and animals in Unstable (but not in Stable) social conditions that possessed a short allele for the rhesus 5-HTTLPR (rh5-HTTLPR) would be most likely to 1) experience sustained agonistic behavior, resulting in 2) altered cortisol concentrations and changes in Type I interferon responses that would be associated with 3) higher vRNA. Our model for these relationships is illustrated in Figure 1, which also shows the specific conditions and variables that were examined in the present study.
Figure 1.
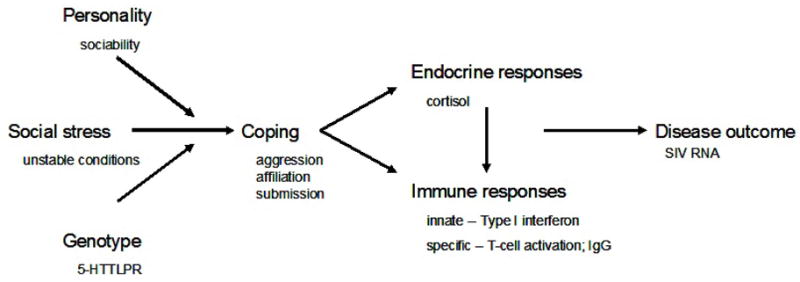
An interactionist model on disease, which shows expected relationships between host factors, social condition, coping responses, measures of endocrine and immune function, and disease outcome.
2. Materials and Methods
2.1. Subjects
Thirty-six adult male rhesus monkeys (Macaca mulatta), born and reared in half-acre outdoor corrals at the California National Primate Research Center, were selected from a larger cohort of 88 animals. All animals had been characterized on four personality dimensions, including Sociability, using established procedures that involved two independent observers conducting detailed observations of animals in their natal corrals, followed by ratings by the observers of each animal on a trait check-list (http://psychology.ucdavis.edu/capitanio/rhesus_personality.pdf), and factor analysis of ratings (Capitanio and Widaman, 2005). The 88 animals were rank ordered on the z-scored Sociability factor (which reflected a composite of the traits “affiliative”, “warm” and not “solitary”; Cronbach’s alpha=0.92), and animals were selected randomly from the top of the distribution (High Sociable: HS) and from the bottom of the distribution (Low Sociable: LS) with the provisos that a) HS and LS animals did not differ in age, b) animals did not have a history of health (especially chronic diarrhea and trauma) or behavioral (abnormal behaviors) problems, c) did not have extreme scores (beyond +/− 1.5–2.0 standard deviations from the mean) on any of the other three personality dimensions, and d) within each personality condition, were drawn from across the 15 corrals to insure that animals that would be placed together would initially be unfamiliar with each other. For LS animals, z-scores for Sociability ranged from −2.02 to −0.53, and for HS animals scores ranged from 0.52 to 2.02. At a mean age of 7.2 (range 5.4–9.4) years, the 36 subjects were relocated to individual housing indoors in standard-sized laboratory cages, and at a mean age of 9.0 years, animals were inoculated intravenously with 0.5 ml of either SIVmac251 (at 102.66 50% tissue culture infectious dose, n=24) or saline (n=12). Only two of the 24 inoculated animals were genetically related: one HS and one LS animal were first cousins.
2.2. Social conditions
Eighteen monkeys (12 inoculated, 6 control) were assigned to the Stable and 18 (12 inoculated, 6 control) were assigned to the Unstable social condition. Half of each assigned set were either HS or LS, and all group formations occurred among animals of the same personality type. Animals in the Stable condition met daily in groups of 3 (two inoculated, one control), and membership in each Stable group did not change across the experiment (except owing to euthanasia). Animals in the Unstable social condition met for an equivalent time each day, although group size and membership varied daily: two-, three-, and four-member groups were formed each day from among the pools of either 9 LS (6 inoculated, 3 control) or 9 HS (6 inoculated, 3 control) animals. Figure 2 shows the experimental design. Animals were formed into social groups for 100 min. per day, 3–5 days per week. We inoculated all animals after they had already experienced three formations of their respective groups, to insure that the experiences of animals in Stable and Unstable conditions were different at the point of inoculation. We note that the control animals were included primarily to insure that inoculated animals would have sufficient partners available to maintain the social conditions once inoculated animals were euthanized owing to advanced disease. Data from control animals are not included in any analyses.
Figure 2.
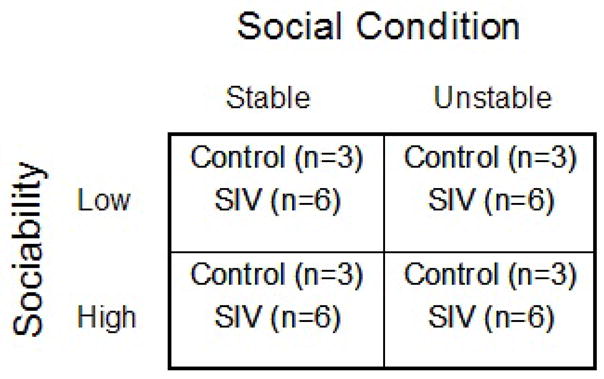
Schematic of experimental design.
2.3. Behavioral data
Behavioral data were collected using the Observer software package (Noldus, 1991) by trained observers who demonstrated greater than 85% inter-observer agreement. On each day, data were collected in several five minute observation sessions, and because blood sampling occurred at two- or four-week intervals (see below), behavioral data were summarized across two-week periods referred to as biweeks. Four categories of behavior were examined in the present study. “Affiliation” comprised proximity, contact, grooming, play, or sexual behavior and animals were considered “affiliative” if they spent, on average, at least 100 sec. of a five minute session engaging in any of those behaviors. The second category involved submissive behavior, specifically the display of the “present sex” posture, in which the initiator presented his hindquarters in the direction of another animal. The third category focused on aggression-received, and reflected an animal’s receiving either threats [facial expression including open mouth, hard stare, ear flaps, bark vocalization, lunges or aggressive movements], aggression [contact involving attacking, biting, slapping, pushing], or chases [fast, aggressive locomotion of one animal after another]. Finally, the fourth category focused on aggression-initiated, and reflected an animal’s initiations of threats, aggression, and chases.
Because our immune measures were taken at three time points (Weeks 2, 6, and 8 p.i.), we created separate behavioral indices, based on our four categories, to reflect these points. For the analysis of data at Week 2, the four indices reflected the number of days that the animals showed the requisite behaviors. During this biweek, animals experienced 7 days of social exposure; our measures reflected the number of days animals displayed affiliation (range 1–5), displayed submissive behavior (range 0–5), received aggression (range 0–3), or initiated aggression (range 0–3). For the Weeks 6 and 8 p.i. analyses, we focused on the number of biweekly periods (3 and 4, respectively) in which the behaviors were shown. Our interest was not in simple number of occurrences, but rather in the initiation or receipt of sustained behavior, and because the indices were generally not normally distributed, our indices were dichotomized. For the Week 6 analysis, sustained behavior (affiliation, submission, aggression-initiated, aggression-received) was defined as display at least once during all three biweekly periods versus display for less than three periods. For the Week 8 analysis, behavior was considered “sustained” if it was exhibited for 3 or 4 of the four biweekly periods. Table 1 shows the number of animals showing sustained behavior by these criteria.
Table 1.
Number of animals showing “sustained” behavior (see Section 2.3 for details).
| Affiliation | Submission | Aggression received | Aggression initiated | |
|---|---|---|---|---|
| Week 6 | 2 | 15 | 4 | 5 |
| Week 8 | 7 | 15 | 7 | 7 |
We note that during the period covered by this report (the first 10 weeks post-inoculation), two animals were euthanized between Weeks 8 and 10. Typically, we see very little change in social behavior in our groups until just before the animals meet criteria for euthanasia (see next section), and in many cases we see no changes in behavior. This is likely because our animals receive limited social experience each day, rather than being continuously socially housed. Consequently they are motivated to interact throughout their disease. Our data suggest that the illness of the two euthanized animals did not affect their behavior enough to influence our results.
2.4. Survival
Survival was defined as days to euthanasia, which was accomplished by overdose of pentobarbital, a method consistent with recommendations of the American Veterinary Medical Association. Euthanasia decisions were based on CNPRC’s standardized Criteria for Retrovirus Infected Macaques, and included weight loss > 15% in two weeks or 30% in 2 months, persistent anorexia with weight loss, or presence of neurological signs. Euthanasia decisions were made by CNPRC veterinarians who were unfamiliar with the hypotheses of the present study or group assignments of the animals.
2.5. Blood sample collection and assays
Blood was collected from non-anesthetized animals at Week 4 p.i. at 1500–1530 h for cortisol assay, and under anesthesia (ketamine, 10mg/kg) at 0830–1100 for all other assays. Blood was drawn into non-heparinized syringes, was immediately transferred to tubes containing EDTA, and was spun at 3000 rpm at 4°C (cortisol) or 2000 rpm at room temperature (SIV measures) for 10 min. Plasma was removed and frozen at −80°C. The pellet was resuspended in RPMI (Irvine Scientific, Santa Ana, California) and peripheral blood mononuclear cells (PBMCs) were isolated using lymphocyte separation medium (MP Biomedicals, Solon, Ohio). Cells were washed then resuspended in freezing media (90% fetal bovine serum [Invitrogen, Carlsbad, California] and 10% dimethyl sulfoxide [Sigma, St. Louis, MO]) to a final concentration of 5×106 cells/ml. One ml aliquots were stored in liquid nitrogen until RNA isolation.
2.5.1. Cortisol
Plasma cortisol was assayed using commercially available kits (Diagnostic Products Corporation, Los Angeles, CA), and intra- and inter-assay coefficients of variation were 4.5% and 6.3%, respectively.
2.5.2. mRNA expression
Frozen PBMCs (drawn at one week prior to inoculation, and at Weeks 2 and 6 p.i.) were thawed and lysed immediately in Trizol. Gene expression was quantified relative to pre-inoculation levels for interferon-α (IFN-α), three genes stimulated by interferons (OAS, Mx, IP-10/CXCL10), interferon-γ (IFN-γ), one gene stimulated by IFN-γ and IFN-α (Mig/CXCL9), and CXCR3 (which is the chemokine receptor for CXCL9 and CXCL10 that is upregulated on activated T cells) using real-time RT-PCR according to published procedures (Abel et al., 2002, 2004). Because of the high intercorrelations among the three interferon-stimulated gene measures (correlation coefficients ranged from r= 0.50 to r= 0.67), a principal component referred to as ISG (interferon-stimulated genes) was created that accounted for 68.4% of the variance (Week 2) and 76.9% of the variance (Week 6). Gene expression data were missing for four animals, and analysis revealed that the pattern of missing data was unrelated to Sociability, Social condition, or serotonin transporter genotype (all p > .50).
2.5.3. SIV IgG
Anti-SIV IgG titers at Week 8 were determined using previously described methods (Lu et al., 1998).
2.5.4. SIV RNA
SIV RNA was quantified from a blood sample drawn at Week 10 (for the two animals euthanized between Weeks 8 and 10 p.i., the Week 8 sample was assayed) using real-time PCR as described (Leutenegger et al., 2001).
2.5.5. rh5-HTTLPR
Using the QIAamp DNA blood minikit (Qiagen, Valencia, CA), DNA extraction was performed on peripheral blood lymphocytes collected from EDTA-treated whole blood. PCR employed primers STPR5, 5′-GGCGTTGCCGCTCTGAATGC-3′ and STPR3, 5′-GAGGGACTGAGCTGGACAACCAC-3′ (Research Genetics, Huntsville, Ala) (Lesch et al., 1997) to amplify the short (−21bp) or long fragments of the rh5-HTTLPR using modifications of methods described by (Heils et al., 1996). PCR was performed in a Perkin Elmer 9700 thermal cycler with Roche Applied Science (Indianapolis, Indiana) reagents. PCR thermal cycler conditions for a 50ul reaction were as follows; 95deg/5min × 1, [56°C/30sec, 74°C/30sec, 94°C/30sec] × 40, 74°C/5min × 1. PCR reaction products underwent PST1 digest (New England Biolabs, Ipswitch, Massachusetts) and fragment analysis in ethidium bromide stained 5% Tris-Borate-EDTA buffered polyacrylamide mini-gels (Bio-Rad Laboratories, Hercules, California). Animal assignment to social condition was blind to genotype status; among the 24 SIV-inoculated monkeys, 14 displayed a homozygous long genotype (9 Stable and 5 Unstable) and 10 were heterozygotes (3 Stable and 7 Unstable). Fisher Exact tests revealed that genotype was unrelated to Social condition (p=.214) or to Sociability status (p=.680).
2.6. Statistical analysis
Analyses involved computation of Pearson product-moment correlation coefficients, use of multiple or logistic regression to compare the contributions of multiple independent variables to an outcome measure, and repeated measures analysis of variance to examine changes in measures over multiple time points. The anti-SIV IgG titer, and all measures of mRNA expression except CXCL9, were log-transformed owing to non-normality. Analyses were performed only on the 24 SIV-inoculated animals.
3. Results
3.1. Survival and vRNA
Survival of the 24 SIV-inoculated animals ranged from 56 to 713 days, with a mean of 302.5 days (SD=176.2 days). At Week 10 p.i., vRNA ranged from 4.67 to 7.57 (log10)copies/ml, with a mean of 5.96 (SD=0.78). Survival and vRNA were negatively correlated, with r = −0.73 (p<.001, n=24). Correlations between our independent variables (social condition, sociability, and serotonin transporter genotype) and our outcome measure, vRNA, were all non-significant (all p > .15).
3.2. Immune and viral measures
We first present descriptive information on our immune measures, examine changes in these measures over the first six weeks of infection, then identify measures that correlate with vRNA.
3.2.1. Immune changes following SIV inoculation
Substantial variation was found in all immune measures. Means and ranges are presented in Table 2. At Week 2 p.i., gene expression was significantly higher compared to pre-inoculation levels for Mx (t(20)= 10.27, p<.001), OAS (t(20)= 12.35, p<.001), CXCL10 (t(19)= 8.24, p<.001), and CXCL9 (t(20)= 5.32, p<.001), and expression of CXCR3 was significantly lower than pre-inoculation levels (t(20)= −2.57, p<.05). No change was found for IFN-α (p = .76) or IFN-γ (p = .35).
Table 2.
Mean and range for immune system measures.
| Measure1 | Week 2 p.i. | Week 6 p.i. | Week 8 p.i. |
|---|---|---|---|
| Interferon-alpha2 | 1.56 (0.22, 6.33) | 1.43 (0.09, 4.70) | -- |
| Mx2 | 11.17*** (0.92, 57.71) | 17.11***,† (1.25, 76.37) | -- |
| OAS2 | 10.11*** (1.27, 28.63) | 9.99*** (1.16, 26.68) | -- |
| CXCL102 | 12.51*** (1.11, 99.77) | 11.74*** (0.78, 51.81) | -- |
| ISG 3 | 0.00 (−2.20, 2.60) | 0.00 (−2.53, 1.39) | -- |
| Interferon-gamma2 | 1.36 (0.18, 4.48) | 3.32*,† (0.21, 21.89) | -- |
| CXCL9 | 3.85*** (0.003,9.10) | 3.05* (0.005,18.70) | -- |
| CXCR32 | 0.85* (0.30, 1.94) | 0.97 (0.24, 2.47) | -- |
| Anti-SIV IgG 4 | -- | -- | 3.55*** (0.00, 5.60) |
For all measures except ISG and anti-SIV IgG, values are fold-change (mean and range) compared to pre-inoculation values.
Measure was log-transformed for all subsequent analysis, owing to non-normality.
Interferon-Stimulated Genes. This measure is the principal component score for Mx, OAS, and CXCL10. Mean and range are presented.
Value is mean (and range) log10 titer.
p<.05,
p<.01,
p<.001; indicates significantly different from pre-inoculation levels (mRNA measures) or from zero (IgG).
significant increase from Week 2 to Week 6 p.i. for Mx (p<.05) and interferon-gamma (p<.001).
At Week 6 p.i., gene expression was significantly higher than pre-inoculation levels for Mx (t(20)=10.17, p<.001), OAS (t(20)=11.63, p<.001), CXCL10 (t(19)=9.17, p<.001), CXCL9 (t(20)=2.45, p<.05), and IFN-γ (t(20)=2.46, p<.05). No difference was evident for CXCR3 (p = .16) or for IFN-α (p = .87). At Week 8 p.i., SIV-specific IgG titers were significantly greater than zero (t(23)=11.52, p<.001).
Repeated measures analysis of variance suggested that mRNA expression increased between Weeks 2 and 6 p.i. only for Mx (F(1,20)=6.79, p<.05) and IFN-γ (F(1,20)=22.97, p<.001). Correlations between all Week 2 and Week 6 measures were significant: IFN-α (r= 0.62, p<.01, n=21), Mx (r= 0.85, p<.001, n=21), OAS (r= 0.65, p<.01, n=21), CXCL10 (r= 0.78, p<.001, n=20), IFN-γ (r= 0.74, p<.001, n=21), CXCL9 (r= 0.69, p<.01, n=21), and CXCR3 (r= 0.53, p<.05, n=21). The correlation coefficient for the composite interferon-stimulated gene (ISG) measure between Weeks 2 and 6 was r=0.77, p<.001, n=20).
3.2.2. Immune measures and vRNA
Bivariate analysis showed that higher Week 10 p.i. vRNA levels were associated with reduced CXCR3 expression at Week 6 p.i. (r= −0.62, p<.01, n=21), greater ISG expression (using the principal component measure) at Week 6 (r= 0.54, p<.05, n=20), and a lower SIV-IgG titer at Week 8 (r= −0.69, p<.001, n=24). Correlations between the three measures ranged from −0.33 to 0.32, but statistically were not significant (all p>.15). In a multiple regression analysis, all three measures remained significant predictors of vRNA; the adjusted R2 was 0.664 (n=20). Because gene expression data for our other immune measures (IFN-α, IFN-γ, and CXCL9) were unrelated to vRNA, they are not considered further in this analysis.
3.3. Effects of Social Condition, Sociability, and Serotonin Genotype on behavioral and immune measures
In this section, we present three sets of analyses for each post-inoculation time-point (Weeks 2, 6, and 8 p.i.). First, we examine how the three independent variables (social condition, Sociability, and serotonin transporter genotype) affected coping responses as indexed by our four behavioral measures (affiliation, submissive behavior, aggression-initiated, and aggression-received). Next, for those behavioral measures that were significantly influenced by the independent variables, we compute correlation coefficients with the immune system measures that were predictive of vRNA (i.e., ISG, CXCR3, anti-SIV IgG titer). Finally, we explore whether the independent variables are themselves directly related to the immune system measures. Because we expect genotype and Sociability to moderate the effect of social condition, we conduct parallel analyses for animals in Stable and for animals in Unstable social conditions. Significant results at each time point are summarized in Table 3.
Table 3.
Summary of correlational analyses. See Section 3.3 for sample size information for each correlation.
| Full Sample | Unstable Condition | Stable Condition | |
|---|---|---|---|
| Week 2 p.i. | |||
| Independent variables and behavior | |||
| Unstable, sust. affiliation | r = −0.52** | X | X |
| Sociability, sust. submission | NS | r = −0.76** | NS |
| Behaviors and immune measures | |||
| Sust. submission, ISG | r = 0.51* | r = 0.91*** | NS |
| Independent variables and immune measures | |||
| Sociability, ISG | NS | r = −0.75* | NS |
| Week 6 p.i. | |||
| Independent variables and behavior | |||
| Unstable, sust. aggression initiated | r = 0.51* | X | X |
| Unstable, sust. aggression received | r = 0.45* | X | X |
| rh5-HTTLPR2, aggression initiated1 | NS | r = 0.65* | NS |
| Behaviors and immune measures | |||
| Aggression initiated1, CXCR3 | r = −0.44* | NS | NS |
| Aggression initiated1, ISG | NS | NS | r = 0.76* |
| Independent variables and immune measures | |||
| rh5-HTTLPR2, ISG | r = −0.53* | NS | r = −0.61 (p=.06) |
| Week 8 p.i. | |||
| Independent variables and behavior | |||
| rh5-HTTLPR2, sust. aggression initiated | r = 0.57** | r = 0.66* | ND |
| Unstable, sust. aggression received | r = 0.64** | NS | ND |
| Behaviors and immune measures | |||
| Sust. aggression received, anti-SIV IgG | r = −0.45* | NS | ND |
| Independent variables and immune measures | |||
| All analyses NS. | |||
This measure of aggression-initiated is number of biweekly periods.
rh5-HTTLPR is coded as l/l homozygotes = 0, s/l heterozygotes = 1.
p<.05,
p<.01,
p<.001.
X = no analysis possible; NS = not statistically significant; ND = no analysis possible owing to no data for this group.
3.3.1. Week 2
Independent variables and behavior
Independent variables were significantly associated only with our measures of affiliation and sustained submission. Across the full sample, reduced affiliation was associated with experiencing Unstable social conditions (r= −0.52, p<.01, n=24) and with being heterozygous for the serotonin transporter (r= −0.45, p<.05, n=24). When both independent variables were entered into a multiple regression, however, only social condition was significantly associated with affiliation (t(21)= −2.23, p<.05).
Display of sustained submissive behavior was associated with being low in Sociability, but only for animals in the Unstable social condition. This was indicated by a significant correlation for the Unstable condition (r= −0.76, p<.01, n=12), and a non-significant correlation for the Stable condition (p = .73) (Figure 3a).
Figure 3.
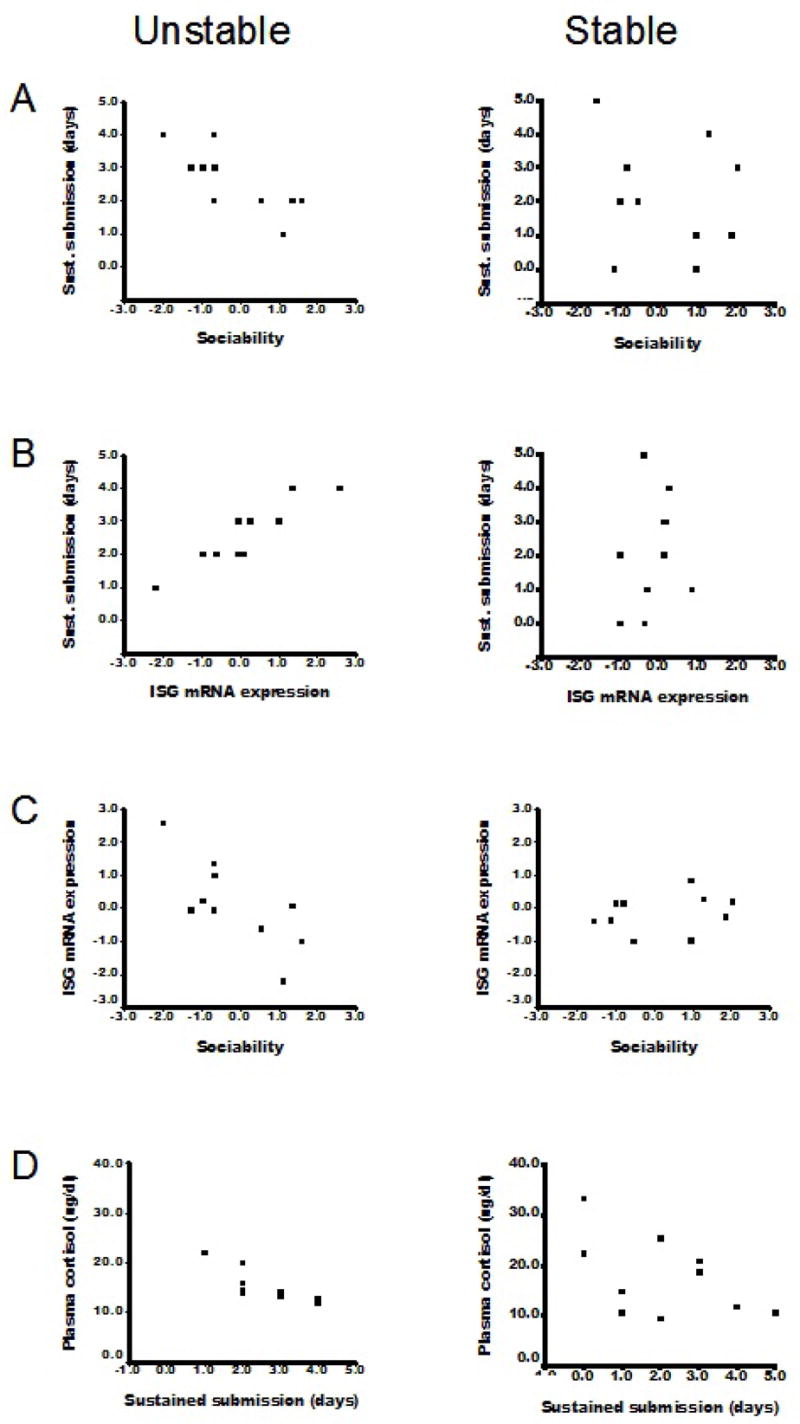
Significant relationships among variables for animals in Unstable social conditions (left panels), and nonsignificant relationships for animals in Stable social conditions (right panels). In the Unstable condition, animals that displayed more submissive behavior during the first two weeks p.i. a) were lower in Sociability, b) had greater expression of interferon stimulated genes (ISG), and d) had lower basal plasma cortisol concentrations at Week 4 p.i. Sociability was also directly related to ISG mRNA expression (c).
Behavior and immune measures
Sustained submission was associated with greater ISG mRNA expression for the full sample (r= 0.51, p<.05, n=20), but separate analyses suggested that this relationship held only for animals in the Unstable social conditions (Unstable: r= 0.91, p<.001, n=10; Stable: p= .52) (Figure 3b). Affiliation was unrelated to ISG mRNA expression, and neither affiliation nor submission correlated significantly with other immune measures at this time point.
Independent variables and immune measures
Finally, a significant correlation was found between Sociability and ISG mRNA expression, but only among animals in the Unstable social conditions (r= −0.75, p<.05, n=10; Stable: p = .51 [Figure 3c]). Serotonin transporter genotype was unrelated to any measure in the Week 2 p.i. analyses.
Summary
These results indicate that, within the first two weeks post-inoculation, Low Sociable animals in the Unstable, but not in the Stable, social condition displayed more submissive behavior and had higher ISG expression at Week 2 p.i. Intercorrelations for Sociability, submissive behavior, and ISG expression were high and significant for animals in Unstable conditions, but were non-significant for animals in Stable social conditions (Figure 3a–c). In a formal test of whether the effect of Sociability on ISG mRNA expression among animals in the Unstable condition was mediated by display of sustained submission, both submission and Sociability were entered into a multiple regression equation to predict the ISG activity. Results indicated full mediation: sustained submission remained a significant predictor of ISG expression (t(7)=3.4, p<.05) while Sociability did not (p = .74), indicating that the effect of Sociability on ISG mRNA expression is mediated through Sociability’s effects on display of sustained submissive behavior.
3.3.2. Week 6
Independent variables and behavior
As expected, animals in the Unstable social condition initiated more sustained aggression (r= 0.51, p = .01, n=24), and received more sustained aggression (r= 0.45, p<.05, n=24). Serotonin genotype was marginally related to sustained aggression initiated (r= 0.40, p = .054, n=24). Sociability was not associated with behavior, either for the full sample or in separate analyses for animals in Stable and in Unstable social conditions.
Since we hypothesized that animals in the Unstable condition that were heterozygous for the serotonin transporter genotype would display increased aggression, we examined these data more closely, inasmuch as our measure of “sustained aggression” was a conservative measure, requiring animals to display aggression during all 3 biweekly periods. We conducted an analysis of variance using as our outcome measure the number of biweekly periods during which animals displayed aggression, and using serotonin genotype and social condition as independent variables. Animals in Unstable conditions displayed significantly more aggression than did animals in Stable social conditions, as indicated by a main effect for social condition (F(1,20)= 12.58, p<.01), but this effect was moderated by serotonin genotype, as indicated by a significant social condition by genotype interaction (F(1,20)= 10.53, p<.01). Figure 4 indicates that s/l heterozygotes in Unstable conditions displayed aggression during a mean of 2.6 (out of a total of 3.0) biweekly periods, whereas heterozygotes in Stable conditions displayed no aggression. For purposes of further analyses below, we calculated correlation coefficients separately for animals in Unstable and for Stable conditions, between genotype and the number of biweekly periods of aggression initiated. For the Unstable condition, the correlation was significant (r= 0.65, p<.05, n=12, and for the Stable condition, the correlation was not (p = .09). Thus, Unstable social conditions were associated with more sustained aggression initiated and received, and, as expected, the majority of the aggression-initiated was displayed by animals in this condition that were heterozygous for the serotonin transporter genotype.
Figure 4.
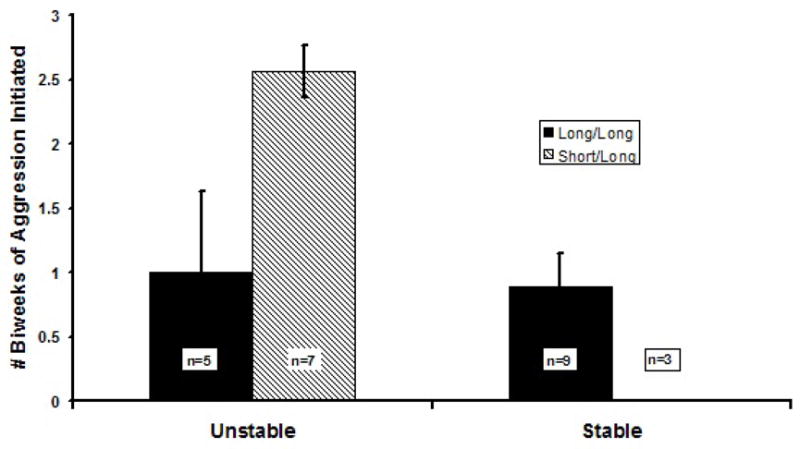
At six weeks post-inoculation, genotype for the rhesus serotonin transporter promoter polymorphism is associated with number of biweekly periods that individuals displayed aggression.
Behavior and immune measures
When we examined the relationships between measures of aggression initiated and received with measures of immune activity, we found that, for the full sample, animals that displayed aggression in more biweekly periods had lower CXCR3 expression (r= −0.44, p<.05, n=21). Separate analyses of the social conditions revealed that, among animals in the Stable (but not the Unstable) social condition, animals that initiated aggression in more biweekly periods had greater ISG activity (r= 0.76, p<.05, n=10).
Independent variables and immune measures
Analyses of the relationships between independent variables and immune system measures showed that, for the full sample, animals that were l/l homozygous for the serotonin transporter promoter showed greater ISG mRNA expression (r= −0.53, p<.05, n=20). In separate analyses, this relationship was not significant for animals in the Unstable condition (p = .19), but among animals in the Stable social condition, the relationship was marginally significant (r= −0.61, p = .06, n=10), indicating that l/l homozygotes had more ISG activity.
Summary
At Week 6 p.i., more sustained aggression was found among animals in the Unstable social conditions, and particularly by animals in this condition that were heterozygous for the rh5-HTTLPR. Overall, animals that displayed more aggression had lower CXCR3 expression, which is associated with higher vRNA. Among animals in Stable social conditions, however, some aggression was displayed (Figure 4), but it was performed exclusively by l/l homozygotes. These animals had higher ISG expression, which is also associated with higher vRNA. Together, these data indicate that aggression was displayed by animals with different genotypes in the two social conditions (s/l heterozygotes in the Unstable condition, and l/l homozygotes in the Stable condition), but generally appeared to have negative consequences: reduced CXCR3 expression (across all animals), and increased ISG expression (animals in Stable conditions only).
3.3.3. Week 8
Independent variables and behavior
Results for this analysis were very similar to those reported above for Week 6. Across the full sample, sustained aggression initiated (defined as displaying aggression in 3 or 4 of the possible 4 biweekly periods) was displayed by animals in the Unstable social condition (r= 0.64, p = .001, n=24) and by animals heterozygous for serotonin genotype (r= 0.57, p<.01, n=24). In a logistic regression with sustained aggression initiated as the outcome, only serotonin genotype remained a significant predictor (p<.05). In fact, sustained aggression initiated was displayed only by animals in Unstable conditions, and among these animals, heterozygotes displayed significantly more sustained aggression (r= 0.66, p<.05, n=12). In addition, receipt of sustained aggression was also associated with Unstable social conditions (r= 0.64, p = .001, n=24). Further correlational analyses involving Sociability with the full sample or with the samples from the Stable/Unstable social conditions separately were all non-significant.
Behavior and immune measures
Based on earlier work (Capitanio et al., 1998), we expected that receipt of sustained aggression would be inversely associated with antibody titer. In the present study we confirmed this result: monkeys that received sustained aggression showed significantly lower IgG titers (r= −0.45, p<.05, n=24). Seven animals received sustained aggression, all of which were in the Unstable condition (Chisq(1)=9.9, p = .002). Mean log antibody titer for these seven animals was 63% of the titer for the remaining 17 animals. IgG titer was not related to sustained aggression initiated.
Independent variables and immune measures
Neither social condition, Sociability, nor serotonin genotype were statistically related to anti-SIV IgG titers at Week 8 p.i., nor did we find relationships between IgG and Sociability or genotype for animals in Stable or Unstable social conditions separately.
Summary
Unstable social conditions led to some animals receiving sustained aggression. These animals were not identifiable as having a specific genotype or Sociability status, but they did have a lower SIV-specific antibody titer, which was associated with elevated vRNA.
3.4. The role of plasma cortisol
To identify neuroendocrine pathways that might mediate effects of social stress on immune measures, we examined basal concentrations of plasma cortisol at Week 4 p.i. Our previous research indicated that Unstable social conditions were associated with low basal concentrations of cortisol, which result from enhanced negative feedback regulation of the HPA axis (Capitanio et al., 1998). Our expectation was that animals experiencing greater stress would show lower concentrations, and this expectation was confirmed: when all animals were considered together, lower basal cortisol concentrations were found among animals showing more sustained submission during the first two weeks p.i. (r= −0.46, p<.05, n=24) and during the first 6 weeks p.i. (r= −0.38, p<.07, n=24). Correlations with other behaviors were nonsignificant. When separate analyses were conducted for animals in Stable and Unstable social conditions, however, correlation coefficients between sustained submission and cortisol concentrations were significant only for the Unstable condition during the first two weeks p.i. (r= − 0.76, p<.01, n=12) (Figure 3d), and during the first 6 weeks p.i. (r= −0.57, p = .05, n=12). Correlations between cortisol concentrations and other behaviors among animals in the Unstable condition, and all correlations between cortisol and behavior in the Stable condition, were nonsignificant (all p>.32).
Because sustained submissive behavior at Week 2 was associated with Sociability and with greater ISG mRNA expression among animals in the Unstable condition, we correlated plasma cortisol concentrations with these two variables. Lower basal cortisol concentrations were significantly associated with low Sociability (r= 0.66, p<.05, n=12) and with greater ISG expression (r= −0.65, p<.05, n=10). The correlations among animals in the Stable condition were non-significant (p>.20).
Summary
Within the first few weeks following SIV inoculation, low Sociability was associated with increased submission, lower basal plasma cortisol concentrations indicative of stress, and greater ISG mRNA expression. These relationships were significant only for animals in Unstable social conditions and not for animals in Stable social conditions.
4. Discussion
In immunodeficiency virus disease, viral set-point, which reflects a relatively stable balance between viral replication and immune control of the virus, and which is established within the first several weeks after infection, is a strong predictor of disease course. The present study supported our model (Figure 1) of the multiple influences that can act independently and in combination over a time course of a few weeks to affect a disease outcome: viral set-point, which is strongly associated with survival, was predicted by three measures of immune function, the SIV-specific IgG response, and expression of interferon-stimulated genes and CXCR3. These immunologic measures were related to specific patterns of behavior displayed in the social conditions, resulting from the interaction of host factors (personality and genotype) with social condition. Below, we discuss these results in relation to the three original goals of the study.
4.1. vRNA and survival
We (Capitanio et al., 1998) and others (e.g., Mellors et al., 1996; Watson et al., 1997) have found that viral set-point is a strong predictor of survival. Our current data are consistent with these results: vRNA copy number at Week 10 p.i. correlated r= −0.73 with survival, indicating that 53% of the variance in survival was explained by this single measure. Knowledge of this relationship has informed therapeutic practice in that a major goal of anti-retroviral therapy is to reduce viral load to as low as possible, in order to prolong life. Still, it is remarkable that survival for a disease with such a long time course can be predicted from such an early measure. Increasing evidence, however, mostly from the SIV model of AIDS, has revealed the intense activity of the virus within the first days and weeks following infection. For example, data presented by Mattapallil et al. (2005) and Li et al. (2005) have indicated massive infection of CD4+ memory T-cells in gut-associated lymphoid tissue and elsewhere in the body in the first days following acute infection, and death of most of these cells within the first few weeks p.i. These data have resulted in a new view of immunodeficiency virus infection that suggests that the magnitude of the early destruction “cripples the immune system at the outset of infection and sets the stage for its eventual failure” (Picker and Watkins, 2005, p. 431). Presumably, any factors that can reduce the magnitude of this early destruction, and hence reduce set-point, will have beneficial effects.
4.2. Immune responses in early SIV infection
As with other viral infections, a variety of innate responses cope with the initial events following SIV infection. We found no increase in IFN-α mRNA expression at Week 2 or Week 6 p.i. in PBMC. This result is consistent with evidence that IFN-α expression in PBMCs is seen earlier than Week 2 (and more transiently) in SIV infection (Abel et al., 2002). In contrast, expression of genes coding IFN-α’s effector molecules OAS, Mx, and CXCL10, was substantial and sustained, with 10–12-fold increases at Week 2 p.i. compared to pre-inoculation levels, with continued (or increased, in the case of Mx) expression at Week 6 p.i. Unfortunately, as others have found (Abel et al., 2002), activation of the IFN-α system is inadequate to control SIV replication. In vitro work (Shirazi et al., 1994) with HIV suggests that HIV-encoded tat can interfere with the inhibitory effects of IFNs, and our data show that greater expression of these interferon-stimulated genes is strongly associated with increased vRNA set-point. Thus, while mechanisms associated with IFN-α’s ineffectiveness in HIV and SIV infection remain to be determined, at a minimum it appears that the greater expression of interferon-stimulated genes in PBMCs reflects poorer control of viral replication.
Interferon-γ (IFN-γ) is produced and secreted by a variety of cell types in immunodeficiency virus infection. In the present study, we found no increase in mRNA expression of IFN-γ at Week 2 p.i., although there was increased expression of one of IFN-γ’s effector molecules, CXCL9, a pattern of response similar to that seen for IFN-α and its effectors. In contrast, by Week 6 p.i., expression of IFN-γ had increased significantly. It is possible that the Week 2 p.i. responses for IFN-γ and CXCL9 reflect upregulation among innate immune cells, such as NK cells and macrophages, while the increased expression at Week 6 reflected upregulation in CD8+ T-cells. Because neither IFN-γ nor CXCL9 expression was associated with vRNA set-point, no further analyses were performed. Future work should distinguish better between the two broad roles that IFN-γ can play in SIV infection, however, one of which reflects its secretion as an effector molecule of CD8+ cytotoxic T-cells (and hence an anti-viral role), and the other as a mediator of inflammation (which can promote viral replication). Whether psychological and/or social factors differentially affect these two processes should also be considered.
We found that mRNA expression of CXCR3 was significantly lower than pre-inoculation levels at Week 2 p.i., but had increased by Week 6 p.i. to be no longer different from pre-inoculation levels Nevertheless, at Week 6 p.i. substantial variation was seen in this measure (Table 2 shows considerably more variation at Week 6 compared to Week 2), and this variation was significantly negatively correlated with vRNA. CXCR3 is the chemokine receptor for CXCL9 and CXCL10, and is found on activated Th1-cells. Other studies (Abel et al., 2004, Sarkar et al., 2003) have reported positive relationships between CXCR3 expression and vRNA, but have focused on terminal endpoints and tissue analysis. Lower expression of CXCR3 in PBMC at the early time points studied here could reflect recruitment of Th1 cells by CXCL10 to lymphoid tissue, and hence their relative absence in peripheral blood. Consistent with this idea, greater expression of both CXCL10 and CXCR3 in lymph nodes has been found in SHIV-infected monkeys that were unprotected, compared to those that were protected, in a vaccine trial (Abel et al., 2004, Sarkar et al., 2003).
Finally, we found that the quantity of SIV-specific IgG produced at Week 8 p.i. was a strong predictor of vRNA. The role of antibodies in immunodeficiency virus disease remains unclear. On the one hand, the strength of the antibody response has been long-known to correlate with survival in SIV-infected monkeys (Daniel et al., 1987). On the other hand, actual neutralizing activity of these antibodies is generally weak (Desrosiers et al., 1989), leading to little success in developing a vaccine based on humoral immunity (but see McCann et al., 2005). Interestingly, however, anti-SIV IgG levels were uncorrelated with our other predictors of vRNA, namely ISG and CXCR3 expression, suggesting multiple routes whereby psychosocial (or other) factors might impact viral set-point.
4.3. Host factors, social condition, and coping
As we have demonstrated earlier (Capitanio et al., 1998), Unstable social conditions were associated with reduced affiliation and sustained aggressive behavior, and individuals that were recipients of sustained aggression had lower anti-SIV IgG. Clearly, however, not all animals responded to the Unstable social conditions in the same way behaviorally, and the differences in response were associated with different host characteristics (Sociability, rh5-HTTLPR genotype), and with different immune responses. We first discuss the behavioral responses seen in the social conditions, then consider mechanisms whereby coping patterns could be related to immune measures.
4.3.1. The importance of coping
Our data highlight the importance of coping responses when considering immunologic and disease-related outcomes. This is evident from the analysis (Section 3.1) showing that neither Sociability, social condition, nor serotonin genotype was directly associated with vRNA, yet the analyses reported in the remainder of the paper demonstrate a series of complex (and strong, especially for animals in Unstable conditions) relationships among our variables: sustained display of submissive behavior within the first two weeks (associated with low Sociability) was correlated with increased ISG activity; sustained display of aggression within the first six weeks (most of which was performed by animals in Unstable groups with s/l rh5-HTTLPR genotype) was associated with decreased CXCR3 expression in PBMCs; and aggression in Stable groups (associated with the l/l rh5-HTTLPR genotype) was associated with increased ISG activity. The gene expression data that were associated with each pattern of behavior were related to an increased viral set-point, suggesting that these patterns were maladaptive. What do these behaviors suggest about coping styles that might be maladaptive in the context of the social conditions presented to the animals?
When previously unfamiliar adult male rhesus monkeys meet, a series of agonistic interactions take place that define the dominance hierarchy within the group. Such interactions would have been seen on the first day of either the Stable or Unstable condition. By the fourth day of the group formations (which corresponds to the first day post-inoculation in the present study), however, tensions would be substantially lower in Stable groups, where animals could show more affiliation with only occasional reinforcements of the dominance hierarchy, often through use of non-contact behaviors such as threats or displacements. Animals that continued to display aggression in Stable groups, however, were likely to be the animals that were not “satisfied” with their position in the hierarchy, and may have been using aggression to better their social position. The fact that this aggression (over the first six weeks of the study) was performed in Stable groups only by animals possessing the l/l rh5-HTTLPR genotype, which is usually considered the more “benign” genotype, is consistent with the idea that their use of aggression may have been more instrumental and deliberate. Nevertheless, our data suggest such a response had a cost – increased ISG activity, through an as-yet unidentified mechanism.
In contrast, in the Unstable groups, periodic eruptions of aggression would be more frequent and the tension more sustained. This would be especially true in the first two weeks of Unstable group formations, as animals are establishing and re-establishing relationships every day. Insuring that one isn’t harmed during periodic eruptions would be very important, and there are different ways one can accomplish this. One way of coping with this tension is to keep a “low profile”, by avoiding interaction, and when necessary, sending clear non-threatening signals, such as moving out of the way and by exhibiting fear grimaces from a distance. Animals higher in Sociability appear to use this strategy when encountering unfamiliar animals (Capitanio, 1999, 2002). Some LS animals, however, handled the tension in Unstable groups by displaying more “present-sex” postures, and our mediational analysis revealed that this submissive behavior mediated the relationship between personality and higher ISG activity. “Present-sex” is a behavior that is usually not given at a distance, but rather occurs after a higher-ranked animal has already approached. Other data suggest that LS animals have poorer social skills; for example, when presented with videotaped displays of an unfamiliar monkey displaying aggressive behaviors, LS animals sit and stare – they show significantly less activity, tend to have higher durations of looking at the videotaped animal, and take nearly twice as long to gaze avert as do HS animals (Capitanio, 2002). Sustained displays of submission during the first two weeks, then, probably reflects a more passive style of coping in response to the aggression and tension seen in the early Unstable group formations.
It’s clear from Figure 4, however, that aggression continued in the Unstable groups over the first six weeks of the study, and this aggression was displayed primarily by animals possessing an s/l genotype for the serotonin transporter promoter. When combined with experience of adverse circumstances, such as nursery-rearing, possession of a short allele has been linked to sustained aggression (including aggression with little provocation) and anxiety (Barr et al., 2003), and with lower cerebrospinal fluid levels of 5-HIAA, a metabolite of serotonin (Bennett et al., 2002) in rhesus monkeys. Insofar as Unstable social conditions can be considered an “adverse” circumstance, our behavioral data are consistent with those of Barr et al. (2003). It appears then, that the sustained aggression in the Unstable groups may have been displayed by animals that tend to react impulsively to relatively innocuous events – an animal walking too close, or an animal that is sitting in a preferred location in the cage. Such an impulsive response style was associated with reduced CXCR3 expression on PBMCs. And of course, the targets of this aggression themselves paid a cost – decreased SIV-specific IgG by Week 8 p.i. Both of these immune outcomes were associated with elevated vRNA.
4.3.2 Physiological mechanisms associated with adverse immune outcomes
How might these patterns of coping – sustained submission and sustained aggression – be associated with a poorer disease outcome? As our model (Figure 1) indicates, we focused on a measure from one physiological stress-response system early in disease, basal concentrations of plasma cortisol. Previously (Capitanio et al., 1998), we found that Unstable social conditions resulted in lower basal cortisol concentrations, which was associated with enhanced negative feedback characteristic of chronic stress (e.g., Yehuda et al., 1995). In the current study, LS monkeys were more likely than HS monkeys to respond to Unstable conditions by down-regulation of their HPA axis and up-regulation of ISG transcription. What we do not know at this point is whether these relationships are causal or merely associations arising due to the influence of other unmeasured variables. Further, it’s unclear whether the possible mediating effects of glucocorticoids are operating at the level of IFN-α (e.g., evidence suggests that glucocorticoids can regulate IFN-α production: e.g., Norbiato et al., 1996), or are directly affecting transcription at the level of IFN-α’s effector molecules Mx, OAS, and CXCL-10.
While we were able to link submissive behavior, ISG expression, Sociability and cortisol among animals in Unstable (but not Stable) social conditions, we did not find cortisol concentrations to be related to our other two principal immune measures, CXCR3 expression and SIV-specific IgG. Of course, other mechanisms exist by which the central nervous system, the source of coping strategies, can interact with the immune system. Elsewhere, we have shown (Sloan et al., 2006) that SIV replicates preferentially in the vicinity of catecholaminergic varicosities in lymph nodes. It’s likely that continued activation of the sympathetic-adrenal-medullary system, associated with initiating impulsive aggression or being a frequent target of such aggression, could have an impact on immune responses in lymphoid tissue.
An additional mechanism is suggested by our finding that serotonin transporter genotype is an important contributor to behavioral and immune processes associated with a disease outcome, a result that we believe is completely novel. Serotonin is produced outside of the central nervous system, primarily in the gut, and is taken up by various cell types, including immune cells, which also contain serotonin receptors and transporters (Mossner and Lesch, 1998). Furthermore, there is increasing evidence for interaction between serotonin and cytokines in PBMCs (e.g., Tsao, et al., 2006). Together, these data present the intriguing possibility that pharmacologic manipulation of serotonin function may not only have an indirect effect on disease by reducing anxiety or facilitating more adaptive coping with stressors, but may also have a direct effect through modulation of immune function. Such a mechanism remain to be explored.
4.3.3 Limitations of the present study
We acknowledge several limitations of our study. First, we reiterate that our emphasis has been on the first 10 weeks after infection, and that our outcome measure, vRNA, while a strong predictor of survival, is not the sole predictor, inasmuch as it accounted for about half the variation in survival. While our data suggest that early psychosocial events are important in establishing a disease course, events occurring beyond the establishment of viral set-point are also likely to explain part of the remaining variation in survival. For example, the early death of an unusually aggressive animal (perhaps with an s/l genotype) may make the Unstable social conditions somewhat less stressful, permitting the expression of more affiliation. This example underscores the important role of context in social behavior, and as contexts change, the influences of particular host factors may also change. This was demonstrated in the present report by the result that Sociability was more influential in the first two weeks p.i., and serotonin genotype was more influential at later time points. A future report will examine the effects of psychosocial factors on disease course beyond the 10 week p.i. time point. A second limitation is related to the idea of social context. We specifically created our groups to be homogeneous in terms of Sociability – only LS animals or only HS animals ever interacted – and it’s unknown whether the negative effects of Low Sociability would be seen in groups that had a more mixed personality composition. Finally, although our results regarding the influence of 5-HTTLPR are intriguing, we consider them provisional at this time. Animals were not assigned to social condition based on genotype, and although there were no statistically significant relationships between genotype and either Sociability status or social condition, the cells in our experimental design did not contain equal numbers of l/l homozygotes and s/l heterozygotes (see Figure 4). We believe our results do suggest, however, that neural genotype, a host factor that is important for psychosocial functioning, can have biobehavioral consequences that may impact a disease process.
4.3.4. Conclusion
We have identified innate and specific immune mechanisms through which personality and genotype interact with social context to affect viral set-point in SIV infected monkeys. Our data extend to the psychosocial realm the idea that events occurring early in immunodeficiency virus infection are critically important to the progress of the disease. Specifically, our data demonstrate that the social environment can interact with individual neurogenetic and personality characteristics to create complex “joint risk factors” that modulate gene expression dynamics that shape the trajectory of chronic disease. Understanding the mechanisms that underlie this “interactionist” perspective on disease (Eysenck, 1991) may have implications for treatment. In addition to helping individuals improve their “fit” with their environments, which would presumably reduce stress, our data suggest that intervening to affect the specific mechanisms associated with other, relevant aspects of organismal functioning could also be beneficial. In the present study, for example, we found that when individuals are stressed, personality characteristics are associated with HPA activity that is related to innate immune function. Cole et al. (1998, 2001, 2003) has also demonstrated that individuals with high autonomic activity show elevated viral set-point and impaired response to HAART (Cole et al., 1998, 2001, 2003). Perhaps pharmacologic modulation of HPA, autonomic, or serotonergic activity could supplement anti-retroviral therapy and prolong survival, particularly among those individuals living under conditions of threat or uncertainty, or those with particular neural genotypes.
Acknowledgments
We thank E. Tarara, C. Brennan, C. Stanko, and K. Cooman for behavioral data collection, M. Marthas for viral stock, C. Miller for availability of CNPRC Immunology Core facilities, D. Wolf for genotyping of 5-HTTLPR, and the veterinary and animal care staffs of CNPRC for expert technical assistance and care of the animals. K. Kopnisky, as well as two anonymous reviewers, provided helpful comments on earlier drafts of this paper. Supported by grants from the National Institutes of Health (MH049033, RR000169). Housing and care of the animals was in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care and the Public Health Service, and procedures were approved by the UC Davis Institutional Animal Care and Use Committee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-α/β) and IFN-α/β-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel K, La Franco-Scheuch L, Rourke T, Ma Z-M, de Silva V, Fallert B, Beckett L, Reinhart TA, Miller CJ. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J Virol. 2004;78:841–854. doi: 10.1128/JVI.78.2.841-854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed RK, Biberfeld G, Thorstensson R. Innate immunity in experimental SIV infection and vaccination. Mol Immunol. 2005;42:251–258. doi: 10.1016/j.molimm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes, Brain and Behavior. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Sem Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. Am J Primatol. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Sociability and responses to video playbacks in adult male rhesus monkeys (Macaca mulatta) Primates. 2002;43:169–177. doi: 10.1007/BF02629645. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality factors between and within species. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organizations. Cambridge University Press; Cambridge: 2004. pp. 13–33. [Google Scholar]
- Capitanio JP, Lerche NW. Social separation, housing relocation, and survival in simian AIDS: A retrospective analysis. Psychosom Med. 1998;60:235–244. doi: 10.1097/00006842-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Baroncelli S. The relationship of personality dimensions in adult male rhesus macaques to progression of simian immunodeficiency virus disease. Brain Behav Immun. 1999;13:138–54. doi: 10.1006/brbi.1998.0540. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Nat Acad Sci. 1998;95:4714–4719. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Widaman KF. Confirmatory factor analysis of personality structure in adult male rhesus monkeys (Macaca mulatta) Am J Primatol. 2005;65:289–294. doi: 10.1002/ajp.20116. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Coates TJ, Temoshok L, Mandel J. Psychosocial research is essential to understanding and treating AIDS. Amer Psychol. 1984;39:1309–1314. doi: 10.1037//0003-066x.39.11.1309. [DOI] [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: Mediation by the autonomic nervous system. Biol Psychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Taylor SE. Social identity and physical health: Accelerated HIV progression in rejection-sensitive gay men. J Pers Soc Psychol. 1997;72:320–335. doi: 10.1037//0022-3514.72.2.320. [DOI] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein Kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- Cole SW, Nailiboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc Nat Acad Sci. 2001;98:12695–12700. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, Sehgal PK, Hunsmann G, Schmidt DK, King NW, Desrosiers RC. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68:3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC, Wyand MS, Kodama T, Ringler DJ, Arthur LO, Sehgal PK, Letvin NL, King NW, Daniel MD. Vaccine protection against simian immunodeficiency virus infection. Proc Nat Acad Sci. 1989;86:6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ. Personality, stress and disease: An interactionist perspective. Psychol Inquiry. 1991;2:221–232. [Google Scholar]
- Gosling SD, John OP. Personality dimensions in nonhuman animals: A cross-species review. Curr Dir Psychol Sci. 1999;8:69–75. [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;15:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Dean L. Effects of AIDS-related bereavement on HIV progression amon New York city gay me. AIDS Educ Prev. 1995;7:36–47. [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poutska F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: Alternative biallelic variation in rhesus monkeys. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gaynes BN, Barroso J, Golden RN, Perkins DO, Folds JD, Evans DL. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu H, Perkins DO, Silva SG, Folds JD, Evans DL. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. Amer J Psychiatry. 2000;157:1221–1228. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Higgins J, Matthews TB, Tarantal AF, Luciw PA, Pedersen NC, North TW. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res Hum Retrovir. 2001;17:243–251. doi: 10.1089/088922201750063160. [DOI] [PubMed] [Google Scholar]
- Levy JA, Scott I, Mackewicz C. Protection from HIV/AIDS: the importance of innate immunity. Clin Immunol. 2003;108:167–174. doi: 10.1016/s1521-6616(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma Z-M, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Lu X, Kiyono H, Lu D, Kawabata S, Torten J, Srinivasan S, Dailey PJ, McGhee JR, Lehner T, Miller CJ. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Capitanio JP, Mendoza SP, Mason WA. Personality influences tetanus-specific antibody response in adult male rhesus macaques after removal from natal group and housing relocation. Am J Primatol. 2003;61:73–83. doi: 10.1002/ajp.10111. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- McCann CM, Song RJ, Ruprecht RM. Antibodies: Can they protect against HIV infection? Curr Drug Targets – Infect Dis. 2005;5:95–111. doi: 10.2174/1568005054201580. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Moller J, Hallqvist J, Diderichsen F, Theorell T, Reuterwall C, Ahlbom A. Do episodes of anger trigger myocardial infarction? A case-crossover analysis in the Stockholm Heart Epidemiology Program (SHEEP) Psychosom Med. 1999;61:842–849. doi: 10.1097/00006842-199911000-00019. [DOI] [PubMed] [Google Scholar]
- Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Br Behav Immun. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- Noldus LPJJ. The observer: A software system for collection and analysis of observational data. Behav Res Meth, Instr Comp. 1991;23:415–429. [Google Scholar]
- Norbiato G, Bevilacqua M, Tarcisio V, Clerici M. Glucocorticoids and interferon-alpha in the acquired immunodeficiency syndrome. J Clin Endocr Metab. 1996;81:2601–2606. doi: 10.1210/jcem.81.7.8675584. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Murphey-Corb M, Montelaro RC, Reinhart TA. Expression of IFN-Y induced CXCR3 agonist chemokines and compartmentalization of CXCR3+ cells in the periphery and lymph nodes of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrone. J Med Primatol. 2003;32:247–264. doi: 10.1034/j.1600-0684.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- Shirazi Y, Popik W, Pitha PM. Modulation of interferon-mediated inhibition of human immunodeficiency virus type 1 by Tat. J Interferon Res. 1994;14:259–263. doi: 10.1089/jir.1994.14.259. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced SIV replication adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- Staprans SI, Dailey PJ, Rosenthal A, Horton C, Grant RM, Lerche N, Feinberg MB. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J Virol. 1999;73:4829–4839. doi: 10.1128/jvi.73.6.4829-4839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT, Change WW, Chen CL, Wu SR, Fan CW, Lo HY. Serotonin transporter mRNA expression is decreased by lamivudine and ribavirin and increased by interferon in immune cells. Scand J Immunol. 2006;63:106–115. doi: 10.1111/j.1365-3083.2005.01715.x. [DOI] [PubMed] [Google Scholar]
- Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson PR, Hu S-L, Haigwood NL. Plasma viremia in macaques infected with simian immunodeficiency virus: Plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Zorilla EP, Redei E, DeRubeis RJ. Reduced cytokine levels and T-cell function in healthy males: relation to individual differences in subclinical anxiety. Br Behav Immun. 1994;8:293–312. doi: 10.1006/brbi.1994.1028. [DOI] [PubMed] [Google Scholar]


