Abstract
Inadequate dietary n-3 polyunsaturated fatty acid (PUFA) content is associated with altered function of the CNS dopamine systems. In this study, the effects of dietary n-3 PUFA content was determined on dopamine cell number and morphology. Adult (postnatal day 70), male, Long-Evans rats were raised from conception on diets containing adequate (control) or negligible n-3 PUFAs. The number and morphology of tyrosine hydroxylase-positive cells in the substantia nigra pars compacta and ventral tegmental area were determined stereologically. The number of tyrosine hydroxylase-positive cells in rats fed the n-3 PUFA-deficient diet was 33.9% lower in the substantia nigra pars compacta and 33.7% lower in the ventral tegmental area than in those fed the control diet (P<0.05); however, the volume of tyrosine hydroxylase-positive cell bodies was not different between diet groups in either brain region. Rats fed the n-3 PUFA-deficient diet also exhibited dendritic depletion and isolation of tyrosine hydroxylase-positive cells compared to rats fed the control diet, which had clustering of tyrosine hydroxylase-positive cells and extensive dendritic arborization. These findings support a role for n-3 PUFAs in the survival of dopamine neurons and suggest that altered dopamine cell number, as well as function, contributes to the behavioral effects observed in rats raised on n-3 PUFA-deficient diets.
Keywords: omega-3 polyunsaturated fatty acid, docosahexaenoic acid, stereology, tyrosine hydroxylase, substantia nigra pars compacta, ventral tegmental area
Introduction
Dopamine is a major neurotransmitter in the central nervous system (CNS) with three major projections arising from the dopamine perikarya in the substantia nigra and ventral tegmental area [16]. The nigrostriatal pathway projects primarily from the substantia nigra pars compacta to the neostriatum, and is the component of the extrapyramidal motor system that degenerates in Parkinson’s disease. The mesocortical pathway projects primarily from the ventral tegmental area to the cortex, and is involved in cognitive function. Hypofunction of this system is associated with schizophrenia and attention deficit hyperactivity disorder [6, 7, 23, 24, 31, 35, 38]. The mesolimbic system projects primarily from the ventral tegmental area to limbic brain regions, such as the nucleus accumbens, and is involved in reinforcement and reward. The “dopamine hypothesis of schizophrenia” proposes that the positive symptoms of schizophrenia arise from hyperactivity of this pathway.
Long chain polyunsaturated fatty acids (LC-PUFAs) are a major component of the phospholipids that form the membranes of all cells. In the CNS, docosahexaenoic acid (DHA, 22:6n-3), which is derived from the essential fatty acid α-linolenic acid (18:3n-3), is the predominant species of LC-PUFA, representing about 15% of the total fatty acids [36]. Brain DHA accumulates primarily during late prenatal and early neonatal life, and is important for neurodevelopment. Inadequate accumulation of DHA, which can occur because of insufficient n-3 PUFA content in the maternal diet, neonatal diet, or the diet after weaning, is associated with suboptimal attentional and cognitive function. In addition, a growing body of epidemiological and clinical evidence suggests that low dietary and/or tissue levels of n-3 LC-PUFAs, particularly DHA, may contribute to the development or severity of neuropsychiatric disorders such as schizophrenia and attention deficit hyperactivity disorder, with early development as a period of particular vulnerability [26]. Concordant with these observations, studies in animals also indicated altered function of the mesolimbic and mesocortical dopamine systems in rats raised from conception with very low levels of brain DHA [5]. Alterations in behaviors associated with dopaminergic function are also noted in animals with inadequate accretion of DHA during development, though the effects vary depending on the magnitude of the decrease in DHA [10, 11, 18, 19, 33].
As a component of membrane phospholipids, the percentage of DHA influences the physicochemical properties of the membrane, and thus the function of a variety of membrane-bound proteins, including dopaminergic, GABAergic, and cholinergic receptors in vitro [12, 25, 39]. DHA and other LC-PUFAs also serve as precursors for inter- and intracellular signals, such as prostaglandins and neuroprotectin D1, and activate transcription factors [3, 13, 34]. In addition, DHA has anti-apoptotic effects in neuronal cells [34]. DHA may be of particular importance for dopamine neurons as it is a ligand for the retinoid X receptor (RXR) [8]. The RXR heterodimerizes with Nurr1, and promotes differentiation and survival of dopamine neurons, and modulates dopamine synthesis and function during both development and adult life [30, 40, 41]. Interactions of RXR and Nur77 also modulate dopaminergic function [20].
To further understand the role of n-3 PUFAs on the CNS dopamine systems, the effects of dietary n-3 PUFA content were determined on dopaminergic cell number and morphology. We will show that young adult rats fed an n-3 PUFA-deficient diet throughout the lifespan have decreased numbers of dopamine neurons in both the substantia nigra and ventral tegmental area compared to those fed a diet containing adequate n-3 PUFAs.
Materials and Methods
Research was performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
The control diet was prepared from a purified powdered basal diet (TD00235, Teklad, Indianapolis, IN) and pure soybean oil (without hydrogenation) (70 g/kg). Accordingly, the control diet contained 4.20 g/kg α-linolenic acid (18:3n-3) and 33.81 g/kg linoleic acid (18:2n-6). The control diet is identical in composition to AIN-93G (Teklad, Indianapolis, IN), which meets all current nutrient standards for rat pregnancy and growth [32]. The deficient diet was the same as the control diet, except it was prepared with safflower oil (66.5 g/kg) and soybean oil (3.5 g/kg), and thus contained 0.38 g/kg α-linolenic acid (18:3n-3) and 45.96 g/kg linoleic acid (18:2n-6)
Male, Long-Evans rats (70 days old; n = 8, each from a different litter) were raised from conception on the experimental diets and were from the second litter produced by the respective dam. Rats used for breeding (>10 weeks old when initially mated; Harlan, Indianapolis, IN) were obtained at least 5 days prior to initiation of any treatments. Rats were housed in a temperature- and humidity-controlled animal facility with a 12-hour dark-light cycle (on at 0600h), food and water ad libitum, and were weighed and handled regularly. Male breeders were fed standard laboratory chow when not being mated. Dams were placed on the experimental diets at the time of initial mating, and were fed the respective diet for the duration of the study. Litters were culled to 8 pups on postnatal day 1 and weaned on postnatal day 20 Weaned offspring were group-housed and maintained on the maternal diet. Dams were re-mated 10 days after weaning the first litter. Using these procedures, second litter offspring raised on the deficient diet have brain phospholipid DHA contents that are roughly 45% lower than those raised on the control diet [28].
On postnatal day 70, rats were deeply anesthetized with Avertin (230 mg/kg, sc; Sigma Aldrich, St. Louis, MO), perfused with 0.1M phosphate buffered saline (PBS) (pH 7.2), followed by 4% paraformaldehyde in PBS, and decapitated. Skulls were post-fixed in the perfusion fixative at room temperature for at least 7 days, and then transferred into PBS for shipment to NeuroScience Associates.
Brains were extracted and sections (60 µm) prepared by NeuroScience Associates (Knoxville, TN) using MultiBrain™ Technology. Free-floating sections were stained for tyrosine hydroxylase (TH) with a 1:1500 dilution of TH primary antibody (Pelfreez, Milwaukee, WI), a goat anti-rabbit secondary antibody, and an avidin biotin complex (Vectastain ABC kit, Vector, Burlingame, CA). Adjacent sections were stained with cresyl violet. Samples were mounted on gelatinized (subbed) glass slides for viewing.
Tyrosine hydroxylase-positive and Nissl stained cells bodies in the substantia nigra pars compacta and ventral tegmental area were quantitatively and qualitatively evaluated for both neuronal number and cell body volume using stereological methods. Sections were initially screened for percentage areas of staining then reconstructed in 3 dimensions. Every sixth section containing the regions of interest (Bregma −4.70 to −6.30 mm) was selected from a random initial sort to ensure random overall sampling. The MultiBrain™ technology used for the histological procedure assures that brains embedded in gelatin prior to sectioning possess enough variance to provide a random and unbiased sampling of each of the 16 brain sections on each slide. The optical fractionator method and the Stereologer software package [27] were used to count stained cells using a Nikon Eclipse 80i microscope, linked to a Sony 3CCD Color Digital Video Camera, which operated an Advanced Scientific Instrumentation MS-2000 motorized Stage input into a Dell Precision 650 Server and a high resolution plasma monitor. Areas of interest were precisely outlined and checked against an atlas [29]. The inclusion grid was thenrandomly applied by the software. The stereology was performed at high magnification with 100x/1.4 aperture oil immersion lenses (yielding 3600x) which allows for clear visualization of the nucleolus and precise definition of the cell walls. An IUR volumetric assessment was performed on each counted neuron to assess cell body volume. The coefficient of error for all samples fell within the limits of 0.02–1.5 (n = 16).
Quantitative data are presented as the mean ± S.E.M. and were analyzed by Student’s t-test (two-tailed) (GraphPad InStat v.3). Differences were considered significant at P<0.05.
Results
Weights of rats fed the control and deficient diets on postnatal day 70 were 376 ± 16 g and 359 ± 13 g, respectively, and were not different between groups.
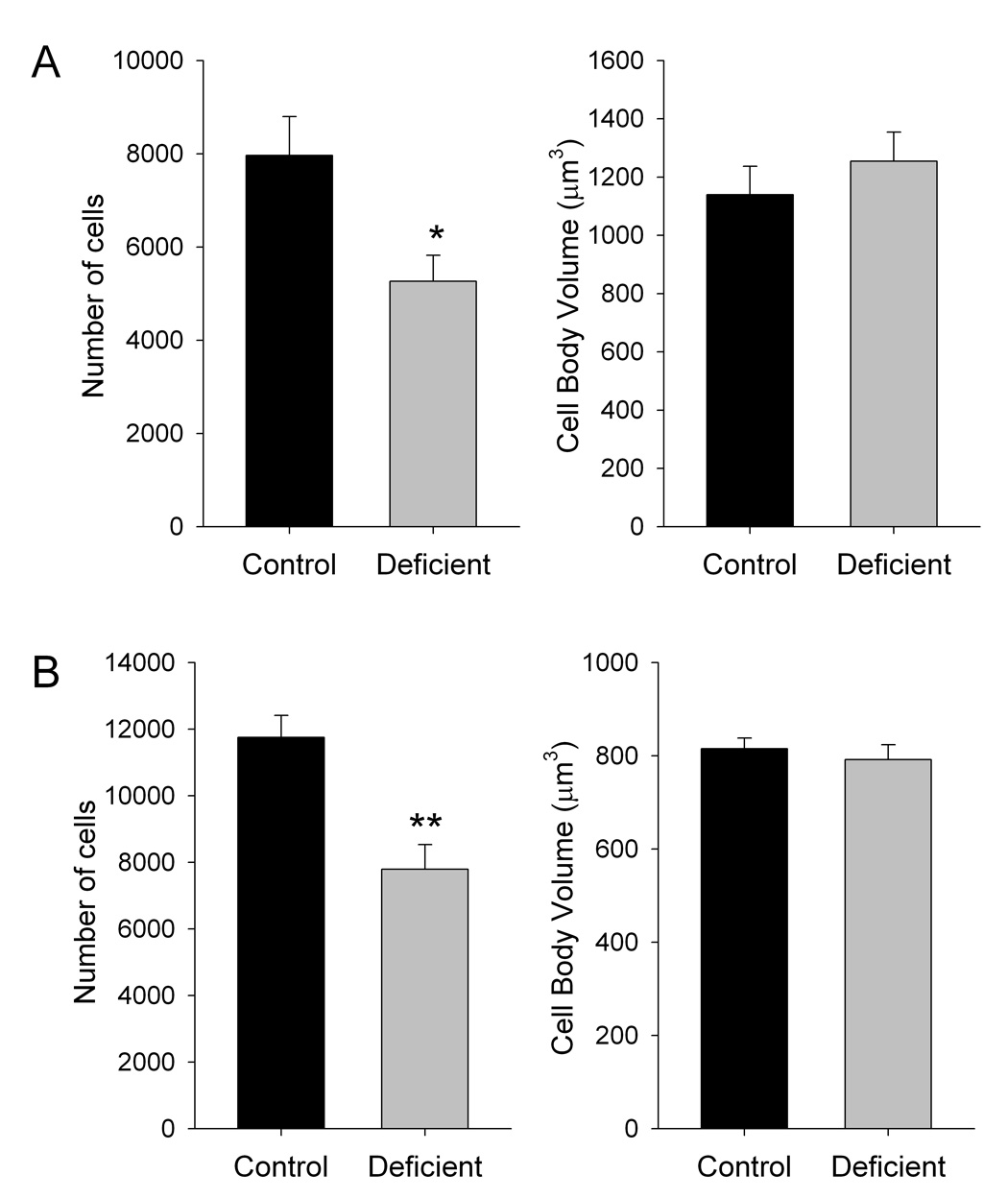
In the substantia nigra pars compacta, the number of tyrosine hydroxylase-positive cells in rats fed the deficient diet was 33.9% lower than in those fed the control diet (P<0.05) (fig. 1). In the ventral tegmental area, the number of tyrosine hydroxylase-positive cells in rats fed the deficient diet was 33.7% lower than in those fed the control diet (P<0.01). The volume of tyrosine hydroxylase-positive cell bodies was not difference between diet groups in either brain region.
Figure 1. Effects of dietary n-3 PUFA content on dopaminergic cell numbers and cell body volume in the substantia nigra pars compacta (A) and ventral tegmental area (B).
Data are presented at the mean ± S.E.M. (n = 8 per group). *P<0.05, **P<0.01 by Students t-test (two-tailed).
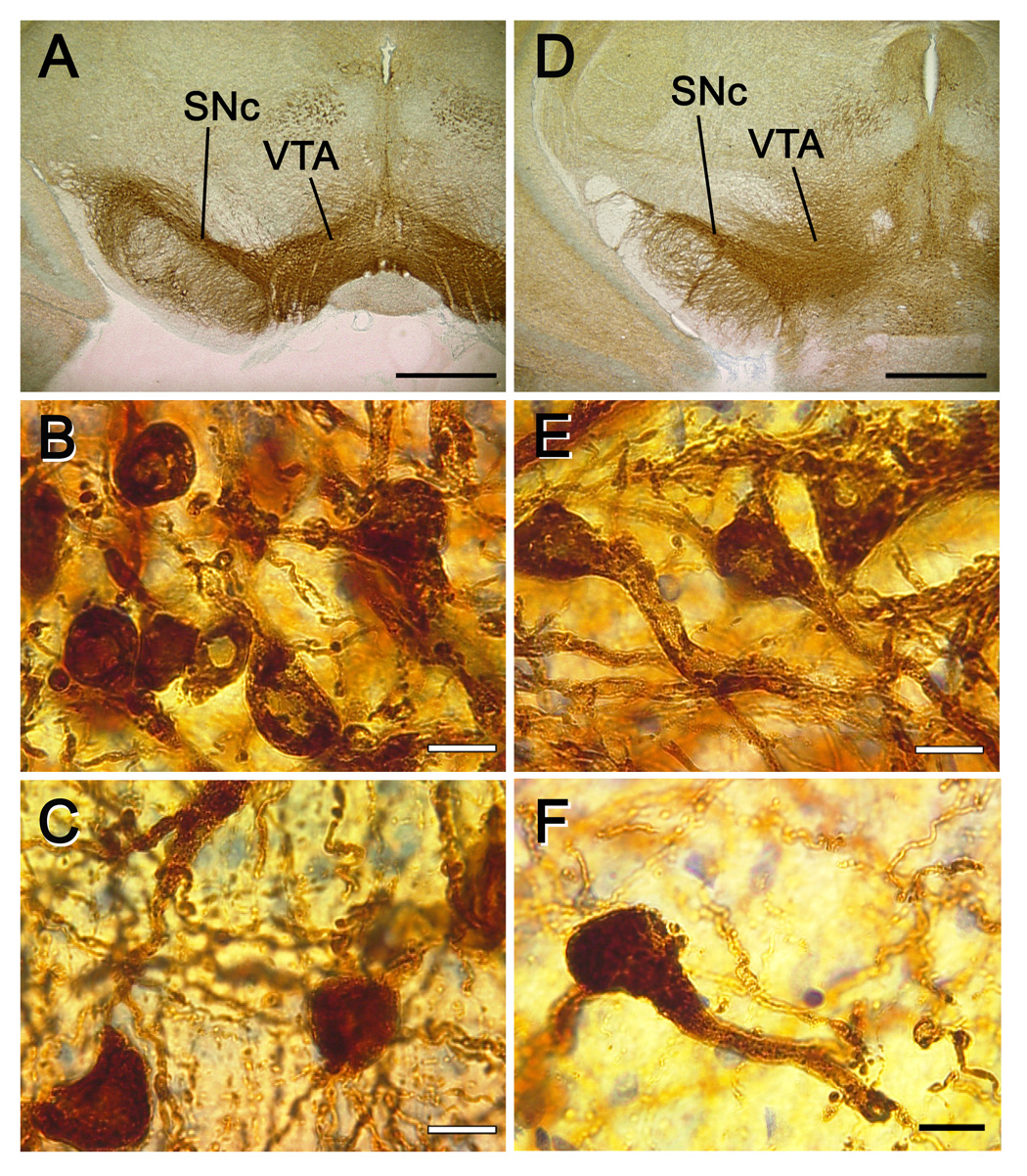
High power examination of morphology revealed that the area of the substantia nigra pars compacta was smaller in rats fed the deficient diet and had fewer tyrosine hydroxylase-positive projections to the pars reticulata compared to rats fed the control diet (fig. 2). Rats fed the deficient diet also exhibited dendritic depletion and isolation of tyrosine hydroxylase-positive cells compared to rats fed the control diet, which had clustering of tyrosine hydroxylase-positive cells and extensive dendritic arborization in both the substantia nigra pars compacta and the ventral tegmental area.
Figure 2. Representative photomicrographs of tyrosine hydroxylase-positive dopamine neurons from rats fed control or n-3 PUFA-deficient diets.
A. Control diet, 2x magnification (bar = 100 µm). B. Control diet, SNc, 100x magnification, (bar = 1 µm). C. Control diet, VTA, 100x magnification, (bar = 1 µm). D. Deficient diet, 2x magnification (bar = 100 µm). E. Deficient diet, SNc, 100x magnification, (bar = 1 µm). F. Deficient diet, VTA, 100x magnification, (bar = 1 µm). A–C and D–F are from the same animal, respectively, under the same light and staining conditions. Abbreviations: SNc – substantia nigra pars compacta. VTA – ventral tegmental area.
Discussion
The present findings clearly demonstrate lower numbers of dopamine neurons in rats fed a diet deficient in n-3 PUFAs, a treatment that has been previously shown to reduce brain phospholipid DHA content by roughly 45% [28]. Furthermore, dopaminergic neurons in the substantia nigra pars compacta and ventral tegmental area were reduced by the same proportion, suggesting that the effects of the n-3 PUFA-deficient diet were not selective for either population of cells.
In contrast to the present findings, a previous study reported that decreased brain DHA content resulted in decreased neuron size in the hippocampus, hypothalamus, piriform cortex, and entorhinal cortex [1, 2]. The studies of Ahmad et al. [1, 2], however, did not examine the dopaminergic perikarya, and differed from the present study in that rats were fed a diet lacking .α-linolenic acid-deficient for three generations, resulting in a greater decrease in brain DHA content (−87%) than in the rats used in this study, and cell body volumes were determined by different methods. These methodological issues may have contributed to the differences between the results of these studies. It is also likely that inadequate dietary n-3 PUFA content produces different effects on specific types of neurons in various brain regions.
The mechanism by which the n-3 PUFA-deficient diet resulted in lower numbers of dopaminergic cells in the substantia nigra pars compacta and ventral tegmental area must be determined in future studies. The anti-apoptotic actions of DHA [34] and its role as an RXR ligand [8, 30] may contribute to this effect. Whether the apparent neuroprotective effects of a diet containing adequate n-3 PUFAs occur during early development and/or later in the lifespan also remains to be determined.
Regardless of the mechanism by which the n-3 PUFA-deficient diet resulted in decreased numbers of dopaminergic neurons, decreases in cell number could contribute to the behavioral alterations observed in animals raised on similar diets, such as changes in motor activity and exploratory behavior in rats and increased stereotyped behavior in rhesus monkeys [10, 11, 18, 19, 33]. These alterations in cell number may also contribute to the alterations in dopaminergic neurochemistry observed in previous studies. For example, using a multigenerational model of n-3 PUFA-deficiency in which rats have a larger decrease in brain DHA content (−75%) than those in this study, Chalon and colleagues found a number of alterations in terminal fields of the mesolimbic dopamine system. Of note, amphetamine-stimulated dopamine release and the densities of dopamine-immunoreactive vesicles, D2 receptor mRNA, and the vesicular monoamine transporter (VMAT2) were decreased in the frontal cortex [9, 43, 44]. In the nucleus accumbens, amphetamine-stimulated dopamine release and the density of VMAT2 were decreased, while basal dopamine release and D2 receptor density were increased [9, 42–44]. However, in contrast to the present finding of differences in dopamine cell number in both the ventral tegmental area and the substantia nigra pars compacta , neurochemical parameters in the striatum were relatively unaffected in the multigenerational DHA deficiency model [9, 17]. This apparent discrepancy may be due to the magnitude of the decrease in dopaminergic cells produced by our manipulation of brain DHA content. Clinical Parkinsonism is not typically apparent until patients have lost roughly 80% of their nigrostriatal dopamine neurons. Accordingly, if a decrease in the number of nigrostriatal neurons of the magnitude observed in this study (−34%) also occurs in the multigenerationally DHA-deficient rat [9, 17], the decrease in cell number might be insufficient to produce significant alterations in the parameters measured.
Perhaps most importantly, decreased numbers of dopamine neurons in the substantia nigra pars compacta could confer increased vulnerability to insults and pathological processes that contribute to the development of Parkinson’s disease. For example, exposure to certain toxins in utero, such as bacterial endotoxin, decreases the number of nigrostriatal dopamine neurons at birth, and leads to increased sensitivity to subsequent Parkinsonism-inducing treatments in rats [21, 22]. Accordingly, the present findings suggest that increased dietary n-3 PUFA content or supplements might be useful attenuating the underlying pathology of Parkinson’s disease, which appears to involve apoptotic processes [37]. Brain fatty acid composition, however, was not different between postmortem Parkinson’s patients and controls [15]. While this may appear discrepant with the present findings and a potential role for decreased DHA in Parkinson’s disease, the decreased numbers of dopamine neurons observed in this study may be the result of decreased neurogenesis and/or increased apoptosis during pre- and early post-natal development. Brain DHA content at that developmental stage is a function primarily of maternal diet and milk composition [14], which in humans might not be reflected in adult brain DHA levels. Low DHA consumption and tissue levels were associated with higher risk of Alzheimer’s disease, another neurodegenerative disease, and DHA had beneficial effects in animal models of the disease, though clinical efficacy remains to be demonstrated [4].
In conclusion, the present data indicate that decreased dietary n-3 PUFAs result in decreased numbers of dopaminergic neurons in both the substantia nigra pars compacta and ventral tegmental area. This supports a role for n-3 PUFAs in the survival and maintenance of dopamine neurons. This decrease in numbers of dopaminergic neurons in animals raised on an n-3 PUFA-deficient diet could confer increased vulnerability to other insults that affect these neurons.
Acknowledgements
The authors thank Paul F. Davis, Marlies K. Ozias, and Guillermo Lona for technical assistance. Supported by NIH R01 MH067398 (BL), P30 HD02528 (BL, JDR), and P20 RR016475 from the INBRE Program of the National Center for Research Resources (BL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr. Neurol. 2002;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad A, Murthy M, Greiner RS, Moriguchi T, Salem N., Jr A decrease in cell size accompanies a loss of docosahexaenoate in the rat hippocampus. Nutr. Neurosci. 2002;5:103–113. doi: 10.1080/10284150290018973. [DOI] [PubMed] [Google Scholar]
- 3.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent. Fatty Acids. 2007;77:287–293. doi: 10.1016/j.plefa.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Cummings JL. Frontal-subcortical circuits and human behavior. Arch. Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 7.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 8.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 9.Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J. Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 10.Enslen M, Milon H, Malnoe A. Effect of low intake of n-3 fatty acids during development on brain phospholipid fatty acid composition and exploratory behavior in rats. Lipids. 1991;26:203–208. doi: 10.1007/BF02543972. [DOI] [PubMed] [Google Scholar]
- 11.Fedorova I, Salem N., Jr Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochem. J. 1986;25:830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 13.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv. Exp. Med. Biol. 2004;554:27–43. doi: 10.1007/978-1-4757-4242-8_5. [DOI] [PubMed] [Google Scholar]
- 15.Julien C, Berthiaume L, Hadj-Tahar A, Rajput AH, Bedard PJ, Di Paolo T, Julien P, Calon F. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem. Int. 2006;48:404–414. doi: 10.1016/j.neuint.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill, New York: 2000. [Google Scholar]
- 17.Kodas E, Page G, Zimmer L, Vancassel S, Guilloteau D, Durand G, Chalon S. Neither the density nor function of striatal dopamine transporters were influenced by chronic n-3 polyunsaturated fatty acid deficiency in rodents. Neurosci. Lett. 2002;321:95–99. doi: 10.1016/s0304-3940(01)02481-8. [DOI] [PubMed] [Google Scholar]
- 18.Levant B, Ozias MK, Carlson SE. Sex-specific effects of brain LC-PUFA composition on locomotor activity in rats. Physiol. Behav. 2006;89:196–204. doi: 10.1016/j.physbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav. Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Levesque D, Rouillard C. Nur77 and retinoid X receptors: crucial factors in dopamine-related neuroadaptation. Trends Neurosci. 2007;30:22–30. doi: 10.1016/j.tins.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp. Neurol. 2004;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Lou H, Henricksen L, Bruhn P. Focal cerebral dysfunction in developmental learning disabilities. Lancet. 1991;335:8–11. doi: 10.1016/0140-6736(90)90136-s. [DOI] [PubMed] [Google Scholar]
- 24.Lou H, Henricksen L, Bruhn P, Borner H, Nielson J. Striatal dysfunction in attention deficit hyperkinetic disorder. Arch. Neurol. 1989;46:48–52. doi: 10.1001/archneur.1989.00520370050018. [DOI] [PubMed] [Google Scholar]
- 25.Malnoe A, Milon H, Reme C. Effect of in vivo modulation of membrane docosahexaenoic acid levels on the dopamine-dependent adenylate cyclase activity in rat retina. J. Neurochem. 1990;55:1480–1485. doi: 10.1111/j.1471-4159.1990.tb04929.x. [DOI] [PubMed] [Google Scholar]
- 26.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Mouton P. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Baltimore, MD: The Johns Hopkins University Press; 2002. [Google Scholar]
- 28.Ozias MK, Carlson SE, Levant B. Maternal parity and diet (n-3) polyunsaturated fatty acid concentration influence accretion of brain phospholipid docosahexaenoic acid in developing rats. J. Nutr. 2007;137:125–129. doi: 10.1093/jn/137.1.125. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- 30.Perlmann T, Wallen-Mackenzie A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004;318:45–52. doi: 10.1007/s00441-004-0974-7. [DOI] [PubMed] [Google Scholar]
- 31.Pickar D, Breier A, Hsiao JK, Doran AR, Wolkowitz OM, Pato CN, Konicki PE, Potter WZ. Cerebrospinal fluid and plasma monoamine metabolites and their relation to psychosis: implications for regional brain dysfunction in schizophrenia. Arch. Gen. Psychiatry. 1990;47:641–648. doi: 10.1001/archpsyc.1990.01810190041006. [DOI] [PubMed] [Google Scholar]
- 32.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 33.Reisbick S, Neuringer M, Hasnain R, Connor WE. Home cage behavior of rhesus monkeys with long-term deficiency of omega-3 fatty acids. Physiol. Behav. 1994;55:231–239. doi: 10.1016/0031-9384(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 34.Salem N, Jr, Litman B, Kim H-Y, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–960. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 35.Satterfield JH, Dawson ME. Electrodermal correlates of hyperactivity in children. Psychophysiology. 1971;8:191–197. doi: 10.1111/j.1469-8986.1971.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 36.Sinclair AJ. Long chain polyunsaturated fatty acids in the mammalian brain. Proc. Nutr. Soc. 1975;34:287–291. doi: 10.1079/pns19750051. [DOI] [PubMed] [Google Scholar]
- 37.Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann. Neurol. 2003;53 Suppl 3:S61–S70. doi: 10.1002/ana.10489. discussion S70-62. [DOI] [PubMed] [Google Scholar]
- 38.Weinberger DR. Schizophrenia and the frontal lobe. Trends Neurosci. 1988;11:367–370. doi: 10.1016/0166-2236(88)90060-4. [DOI] [PubMed] [Google Scholar]
- 39.Witt M-R, Nielsen M. Characterization of the influence of unsaturated free fatty acids on brain GABA/benzodiazepine receptor binding in vitro. J. Neurochem. 1994;62:1432–1439. doi: 10.1046/j.1471-4159.1994.62041432.x. [DOI] [PubMed] [Google Scholar]
- 40.Zetterstrom RH, Lindqvist E, Mata de Urquiza A, Tomac A, Eriksson U, Perlmann T, Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur. J. Neurosci. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 41.Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 42.Zimmer L, Delion Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, Chalon S. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J. Lipid Res. 2000;41:32–40. [PubMed] [Google Scholar]
- 43.Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci. Lett. 2000;284:25–28. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 44.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am. J. Clin. Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]