Abstract
Rats with impaired function in dorsomedial regions of the prefrontal cortex (dmPFC) are unable to maintain a behavioral response over a delay period. Here we report that neurons in this cortical region are prominently modulated after errors in a tone-cued, simple reaction time task and that inactivation of dmPFC attenuates a slowing of reaction times that is observed following errors. Using methods for chronic single-unit recording, we found that approximately one-third of dmPFC neurons were modulated after errors, and 28% of these neurons had increased posterror firing that persisted into the delay period of the following trial. In contrast to dmPFC, no such neurons were found in motor cortex. Our results implicate the dorsomedial prefrontal cortex in a form of retrospective working memory that improves task performance following errors.
INTRODUCTION
The rodent prefrontal cortex is comprised of the anterior cingulate, prelimbic, and infralimbic areas (Gabbott et al. 2005). During simple reaction time tasks, inactivation of the dorsal part of the prelimbic cortex and the adjacent pregenual anterior cingulate cortex, which we collectively call the dorsomedial prefrontal cortex (dmPFC), has been shown to increase the frequency of premature responding (Narayanan et al. 2006; Risterucci et al. 2003). Single neurons within dmPFC fire prominently on error trials (Narayanan and Laubach 2006). Furthermore the activity of delay-modulated neurons in dmPFC is predictive of premature responding (Narayanan and Laubach 2006). Rodent dmPFC is necessary for goal-directed behavior (Corbit and Balleine 2003; Killcross and Coutureau 2003; Ostlund and Balleine 2005) and is engaged in spatial working-memory tasks (Baeg et al. 2003; Batuev et al. 1990; Ragozzino et al. 1998). These studies suggest that error-sensitive neurons within dmPFC may maintain information about past outcomes (i.e., errors) to guide future actions.
In primates, neurons in medial prefrontal regions, including anterior cingulate, supplementary motor regions, and superior frontal gyrus, are prominently modulated after errors (Amiez et al. 2006; Niki and Watanabe 1976; Ridderinkhof et al. 2004; Rushworth et al. 2004, 2007; Schall et al. 2002; Walton et al. 2007). These studies implicate primate medial frontal areas in posterror processing. dmPFC in primates may not be directly homologous to rodent dmPFC (Preuss 1995; Uylings et al. 2003). Nevertheless, it has been argued that these areas mediate similar behavioral functions (Uylings et al. 2003). Across species, medial prefrontal cortex may monitor behavioral performance (van Veen et al. 2004) and integrate information about prior behavior to control future actions (Bush et al. 2002; Dalley et al. 2004; Ridderinkhof et al. 2004; Rushworth et al. 2004; Schall et al. 2002; Shima et al. 2007).
In the present study, we tested the hypothesis that rodent dmPFC is involved in posterror processing. Rats were trained to perform a simple reaction time task in which they held a lever down over a delay period of 1.0 s. Lever releases that occurred too early or too late were scored as errors and were unrewarded. After errors, animals showed a slowing of reaction times (RTs). That is, RTs were longer on trials that were preceded by an error than on trials that were preceded by a correct response. In one experiment, we inactivated dmPFC and found that rats showed attenuated posterror slowing of RTs. In a second experiment, we recorded neural activity in dmPFC and found that many dmPFC neurons increased their firing rates after errors and maintained such elevated firing into the delay period on the following trial. This pattern of neural activity was not observed in motor cortex. Together, our results suggest that dmPFC neurons are involved in posterror slowing and may mediate a form of retrospective working memory that improves task performance following errors.
METHODS
Twenty-two Long-Evans rats (aged 3–4 mo, male) were trained to perform a simple RT task using standard operant procedures and by motivation through water restriction (Narayanan et al. 2006). To perform this task correctly, animals had to press and hold a lever for a 1.0-s delay period and release the lever promptly (within 0.6 s) to receive a liquid reward (0.15 ml of water). The end of the delay period was signaled by a 100-ms, 72-dB, 8-kHz tone. Reaction time was defined as the latency between the end of the delay period and lever release. In some recording sessions, tones were omitted on 50% of trials (catch trials); no difference in behavior or neural activity has been found on such trials (Narayanan and Laubach 2006; Narayanan et al. 2006). If animals released the lever prior to the end of the 1.0-s delay or after the 0.6-s response window, then these trials were scored as errors (premature or late, respectively), and all behavioral devices (pump, lever, and houselight) were extinguished for 4–8 s (Fig. 1 A). Seven animals were tested in sessions without posterror timeouts and in sessions with distractors [lever vibration at 50 Hz using a vibration shaker (Brüel and Kjaer, Norcross, GA) and a 72-dB, 8-kHz tone presented for 1 s immediately after pump inactivation on 50% of pseudorandomly chosen correct trials].
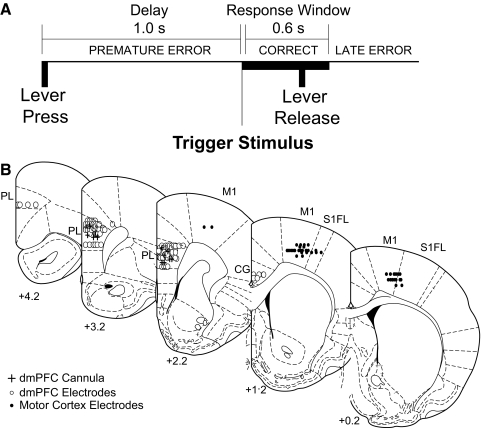
FIG. 1.
Behavioral task and electrode locations: Animals had to press and hold a lever for a 1.0-s delay period and release the lever promptly (i.e., within 0.6 s) to receive a liquid reward. The end of the delay period was signified by a 72-dB 8-kHz tone; in some recording sessions, the tone was omitted on 50% of trials. If animals released the lever prior to the end of the 1.0-s delay or after the 0.6-s response window, then these trials were scored as errors (premature or late, respectively), and all behavioral devices (pump, lever and houselight) were extinguished for 4–8 s. B: electrode locations: Recording sites in dorsomedial prefrontal cortex (dmPFC) are shown from 8 animals that were implanted with microwire electrodes arrays in dmPFC (white dots) and from 7 animals that were implanted with microwire electrode arrays in motor cortex (black dots) and cannula in dmPFC (black crosses). Reconstructions are shown in frontal planes based on atlas sections (Paxinos and Watson 1982).
Reversible inactivation of dmPFC was performed according to procedures described previously (Narayanan et al. 2005, 2006). Briefly, 33-gauge cannulae (Plastics One) were implanted into the dorsal prelimbic region (coordinates from bregma: AP +3.2, ML ±1.4, DV −3.6 @ 10° in the frontal plane; Fig. 1B) (Paxinos and Watson 1982) of seven fully trained animals via aseptic surgical procedures. One week after surgery, animals were lightly anesthetized with halothane via a nosecone for 7 min and tested in the simple RT task 45 min after recovery from anesthesia. On the first day of testing, 0.9% saline (Phoenix Scientific, St. Joseph, MO) was infused into dmPFC (control sessions). On the second day of testing, muscimol, a GABAA receptor agonist (Sigma-Aldrich, St Louis, MO) (Lomber 1999; Martin and Ghez 1999; Narayanan et al. 2006), was infused into dmPFC at 0.1 mg/ml (inactivation sessions). On the third day of testing, animals were run without manipulation. Infusion was conducted by inserting injectors into the guide cannula and 0.5 μl of infusion fluid was delivered per site at a rate of 15 μl/h (0.25 μl/min) (Martin and Ghez 1999) via a syringe infusion pump (KDS Scientific, Holliston, MA). After injection was complete, the injector was left in place for 2 min to allow for diffusion. Rats were tested in the simple RT task 45 min after the start of the infusions.
In these animals, microelectrodes configured in 4 × 4 arrays of 50-μm stainless steel wires (250 μm between wires; impedance measured in vitro at 100–300 kΩ; Neurolinc) were implanted into rat motor cortex (7 animals; coordinates from bregma: AP −0.5, ML ±2.5–3.5, DV −1.5 @ −25° in the frontal plane; 1 animal had poor recordings and was excluded from neural analyses) according to methods described in detail previously (Laubach et al. 2000; Narayanan and Laubach 2006; Narayanan et al. 2005). In eight additional animals, microelectrode arrays were implanted into the dorsal prelimbic region of rat frontal cortex (8 animals; coordinates from bregma: AP +3.2, ML ±1.4, DV −3.6 @ 10° in the frontal plane; ∼94% of electrodes were in prelimbic cortex) targeting coordinates of previous inactivation (Narayanan and Laubach 2006; Narayanan et al. 2006) (Fig. 1B).
Neuronal ensemble recordings were made using a multi-electrode recording system (Plexon, Dallas, TX). Putative single neuronal units were identified on-line using an oscilloscope and audio monitor. The Plexon off-line sorter was used to analyze the signals off-line and to remove artifacts. Principal component analysis and waveform shape were used for spike sorting. Single units were identified as having consistent waveform shape, separable clusters in PCA space, average amplitude estimated at least three times larger than background activity, a consistent refractory period of ≥2 ms in interspike interval histograms, and consistent firing rates around behavioral events (as measured by a runs test of firing rates across trials around behavioral events; neurons with |z| scores >4 were considering “nonstationary” and were excluded). Analysis of neuronal activity and quantitative analysis of basic firing properties were carried out using Stranger (Biographics, Winston-Salem, NC), NeuroExplorer (Nex Technologies, Littleton, MA) and with custom routines for MATLAB. Peri-event rasters and average histograms were constructed around lever release, lever press, and tone offset.
Once experiments were complete, rats were anesthetized and killed by injections of 100 mg/kg sodium pentobarbital and then were transcardially perfused with either 10% formalin or 4% paraformaldehyde. Brains were sectioned on a freezing microtome, mounted on gelatin-subbed slides, and stained for Nissl with thionin.
The Animal Care and Use Committee at the John B. Pierce Laboratory approved all procedures.
RESULTS
In behavioral sessions from fifteen rats, 62 ± 3% (means ± SE) of trials were performed correctly. Errors occurred on 38 ± 3% of trials with 21 ± 2% of these being premature responses (lever released before the end of the delay period) and 17 ± 2% of these being late responses (lever released >0.6 s after the end of the delay period). Animals exhibited posterror slowing of RTs (defined as the time between stimulus onset at the end of the delay period and lever release); that is, RTs on correct trials that were preceded by errors were slower (0.283 ± 0.09 s) than RTs on correct trials that were preceded by correct responses [0.253 ± 0.008 s; paired T(1,24) = 3.08, P < 0.005; repeated-measures ANOVA F(1,1622) = 28.85, P ≪ 0.05; within-subjects analysis revealed that 9 of 15 animals exhibited posterror slowing; group analyses included 25 sessions from all 15 animals]. RTs were slowed following both premature errors [paired T(1,24) = 2.24, P < 0.03] as well as following late errors [paired T(1,20) = 3.61, P < 0.002]. There was no difference between RTs on trials preceded by premature errors (0.286 ± 0.01 s) and by late responses [0.292 ± 0.01 s; paired T(1,20) = 0.82, P < 0.42; 4 sessions with <5 late trials were excluded]. Posterror slowing was also observed in sessions without posterror timeouts [i.e., where houselights and devices were not extinguished after errors; paired T(1,6) = 3.84, P < 0.009]. However, if a distracting stimulus (tone and lever vibration lasting 1 s after pump activation after 50% of correct trials) was presented after correct trials, posterror slowing was eliminated [paired T(1,6) = 0.41, P < 0.70 on postdistractor trials]. This effect may have been due to increased variability of RTs following the distractor [postdistractor RT SEs = 0.02 s; postcorrect RT SEs = 0.014 s; paired T(1,6) = 2.19, P < 0.07; no difference between postdistractor and postcorrect RTs: paired T(1,6) = 1.05, P < 0.33].
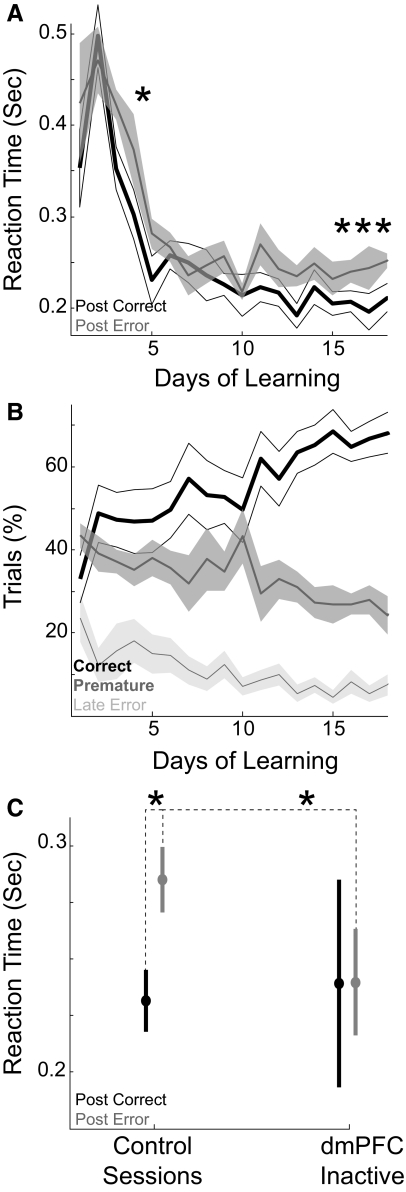
Posterror slowing developed consistently after 16 days of training [paired T(1,6) = 3.43, P < 0.01; learning experiments done with 7 separate animals; Fig. 2 A], several days after animals' performance reached criterion of 60% correct responses (reached on day 11 at 62 ± 7%; Fig. 2B). Early in training, animals rarely exhibited posterror slowing [only on day 5; paired T(1,6) = 3.28, P < 0.02], suggesting that posterror slowing emerged as a feature of skilled performance and not of learning the basic procedure associated with the simple RT task.
FIG. 2.
Posterror slowing of reaction times (RTs). A: RTs following correct trials (black line) decreased over learning but were consistently faster than RTs following error trials (gray line) only after the 16th day of training. Asterisk indicates significant posterror slowing (P < 0.05). B: learning of the simple RT task. Animals increased their correct responses (black line) over the course of several days, reaching 60% on the 11th day of training. Premature errors (dark gray line) decreased over training [paired T(1,6) = 10.2, P < 0.001 on day 1 vs. 11], and late errors (light gray line) decreased somewhat [paired T(1,6) = 2.00, P < 0.09 on day 1 vs. 11]. C: posterror slowing in control sessions was attenuated in sessions with dmPFC inactivated, as RTs became equivalent following errors and correct trials. Asterisk indicates significant posterror slowing (P < 0.05).
To assess the role of dmPFC in posterror slowing, muscimol was used to inactivate dmPFC in seven additional rats. With dmPFC inactivated, animals did not exhibit posterror slowing. That is, posterror RTs (0.252 ± 0.018 s, 95% CI = 0.217–0.288 s across animals) were equivalent to postcorrect RTs [0.250 ± 0.037 s, CI = 0.178–0.321 s; paired T(1,5) = 0.09, P < 0.94]. Importantly, these animals exhibited posterror slowing of RTs in control sessions [posterror RTs: 0.288 ± 0.011 s, CI = 0.267–0.3101 s; postcorrect RTs: 0.245 ± 0.011 s, CI = 0.224–0.265 s; paired T(1,6) = 2.95, P < 0.03] and in recovery sessions run 24 h after the inactivation sessions [posterror RTs: 0.265 ± 0.015 s, CI = 0.235–0.294 s; postcorrect RTs: 0.241 ± 0.021 s, CI = 0.200–0.283 s; paired T(1,6) = 2.49, P < 0.05]. The loss of posterror slowing of RTs in dmPFC inactivation sessions was due to speeding of posterror RTs in dmPFC inactivation sessions [paired T(1,6) = 2.61, P < 0.04; Fig. 2C]. Note that with dmPFC inactivated, animals' correct responding significantly decreased compared with control sessions [dmPFC inactivation sessions: 40 ± 4% of responses, CI = 33–48%; control sessions: 58 ± 3%, CI = 52–64%; paired T(1,6) = 4.01, P < 0.007], primarily because of increased premature responding [dmPFC inactivation sessions: 41 ± 5% of responses, CI = 30–52%; control sessions: 25 ± 3%, CI = 22–30%; paired T(1,6) = 2.80, P < 0.03] (Narayanan and Laubach 2006; Narayanan et al. 2005; Risterucci et al. 2003).
These results implicate dmPFC in posterror slowing of RTs. To test this idea, we recorded from 194 single rodent dmPFC neurons (15 sessions, 8 animals) during simple RT task performance. Of these, 30% (58 of 194) of dmPFC neurons had significant posterror differences in delay-related firing (0.25–1 s after lever press; Wilcoxon rank-sum P < 0.05), more than were influenced by the outcome (correct vs. error) of the second trial back (14 of 194, or 7%; χ2 = 33.02, P ≪ 0.001). That is, neural activity of these neurons during the delay period while animals were waiting to respond was significantly different depending on whether the previous trial was correct or resulted in an error.
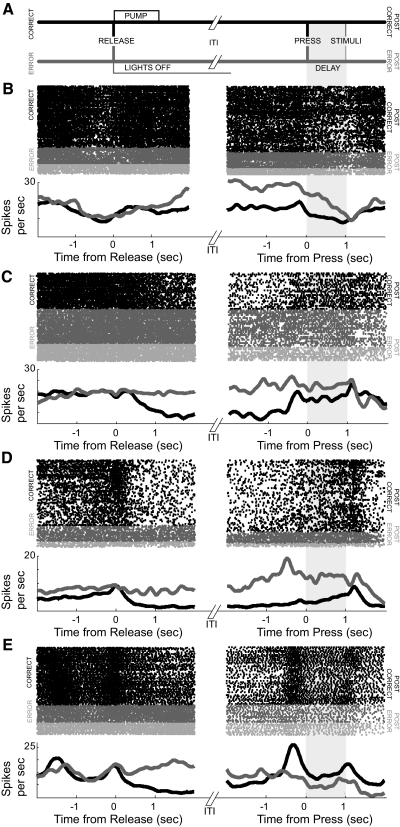
Two-thirds of neurons (39 of 58, or 64%) with posterror differences in delay-related firing rate also had significant posterror differences in firing rate during the intertrial interval (ITI; 1–2 s after lever release; Wilcoxon rank-sum P < 0.05; mean ITI = 7.21 ± 0.45 s). Most of these neurons (26 of 39, 67%) had increased firing rates after errors. Of these, 16 neurons (of 58, or 28%; 8% of all dmPFC neurons) had significantly increased posterror activity that persisted into the delay period of the following trial. Examples of such neurons are shown in Fig. 3. Sometime after the lever release, the firing rates of such neurons diverged by ∼25% (left panels) depending on trial outcome (correct or error). On the following trial, when rasters were sorted by the previous trial outcome (i.e., if the previous trial was correct or an error; right panels), differences in firing rate could persist into the delay period of the following trial when animals were holding down the lever. Few of these neurons fired differently on premature errors (dark gray) versus late errors (light gray; 3 of 39; 7%; no different from chance at P < 0.05: χ2 = 0.21, P < 0.64).
FIG. 3.
Errors influence persistent activity of dmPFC neurons. A: sequence of events following correct and error trials. Following correct trials (top line, black), a pump is activated at a latency of 100 ms after lever release and is kept on for 1 s. Following error trials (bottom line, gray) the house lights and all behavioral devices are extinguished for 4–8 s. Houselights then come on 1.5 ± 0.2 s prior to posterror lever presses. B–E: examples of neurons that fired differently depending on trial outcome are shown. B and C: neurons fired more if trials ended in error (gray colors, left panel) than if trials were correct and rewarded (black, left panel). Increased posterror firing (gray colors, right panel) persisted into the delay period of the following trial, and for these neurons, was more than postcorrect firing (black, right panel). Shaded region after lever press on right panel was used to identify delay-related posterror differences in firing rate. No difference was observed between premature (dark gray) and late (light gray) errors; see text.
Some dmPFC neurons showed increased delay-related firing after errors as well as after correct trials. To quantify this effect, we calculated a modulation index for posterror differences in delay-related firing rate according to the following formula: (FRpostCorrect − FRpostError)/(FRpostCorrect + FRpostError).
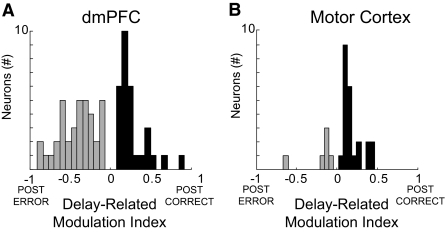
This index is close to 1 if delay-related firing is stronger after correct trials. Conversely, this index is close to −1 if delay-related firing is stronger after error trials. For the subpopulation of neurons that showed significant differences in delay-related firing rate as a function of preceding trial outcome, 45% of 58 neurons had stronger delay-related firing following correct trials and 55% of 58 neurons had stronger delay-related firing following error trials (Fig. 4 A).
FIG. 4.
Posterror modulation indices. Posterror (gray) or postcorrect (black) delay-related modulation indices plotted for dmPFC (A) and for motor cortex (B). Although dmPFC neurons could have greater firing rate following errors and correct trials, dmPFC had significantly more neurons with posterror delay activity than motor cortex, while motor cortex had significantly more neurons with postcorrect delay activity than dmPFC.
To compare these patterns of neural activity to that of the motor cortex, we recorded from 80 motor cortical neurons (6 animals, 8 sessions) during the simple RT task. Of these, 18 neurons (23%) had both delay- and ITI-related differences in firing rates following correct and error responses, a fraction that was similar to our data from dmPFC (χ2 = 0.20, P < 0.67). In contrast to dmPFC, there were no neurons in motor cortex that showed significantly increased posterror activity that persisted into delay period of the following trial. Furthermore, most motor cortex neurons with delay-related posterror differences in firing rate had stronger delay-related firing following correct trials (23 of 29, or 79%; χ2 = 6.41, P < 0.01) and less delay-related firing following error trials (6 of 29, or 20%; χ2 = 5.73, P < 0.02) when compared with dmPFC (Fig. 4B).
DISCUSSION
We tested the hypothesis that rodent dmPFC is involved in posterror processing. We inactivated and recorded from rodent dmPFC during a simple RT task in which animals exhibited posterror slowing of RTs. We report two main findings: inactivation of dmPFC attenuated posterror slowing by speeding posterror RTs and many dmPFC neurons increased their firing after errors. These data establish that, in rats, dmPFC neuronal activity is sensitive to posterror slowing in simple RT tasks. As neuronal signals in dmPFC persisted from one trial to the next, the subpopulation of dmPFC neurons that are sensitive to posterror slowing may mediate a form of retrospective memory that is used to monitor prior task performance.
Neurons in motor cortex also had posterror differences in firing rate. However, these neurons tended to fire more after correct trials (Fig. 4B), and we could find no examples of neurons with persistent posterror differences in firing rate, suggesting that motor cortex patterns of posterror activity were distinct from that observed in rodent dmPFC.
Previous work from our lab has demonstrated that dmPFC inactivation dramatically increases premature responding and speeds RTs (Narayanan et al. 2006), leading to the hypothesis that dmPFC exerts an inhibitory influence over responding (Narayanan et al. 2005, 2006; Risterucci et al. 2003). The present data suggest that rats may benefit from dmPFC inhibitory control to slow RTs after errors. We note that RTs in dmPFC inactivation sessions are more variable; suggesting that without dmPFC-mediated inhibitory control, RTs may become faster and more erratic.
In addition to inhibitory control, rodent dmPFC has been implicated in maintaining task-relevant information (Baeg et al. 2003; Batuev et al. 1990; Ragozzino et al. 1998). We find further evidence for mnemonic processing in our simple RT task based on the posterror activity of dmPFC neurons. This activity may function in a type of retrospective memory that could be used to improve task performance following an error.
Behavioral experiments were used to assess the effects of distractors on posterror slowing. This manipulation attenuated posterror slowing, suggesting that animals may be attending to recent trial outcomes. By contrast, removing the normal contextual cues associated with the ITI (such as the houselights) had no effect of posterror slowing. We note that dmPFC neural activity did not simply transiently increase and then decrease after errors. Instead, activity increased and persisted to the next trial following an error. Taken together, these data suggest that such neural processing might represent a form of retrospective working memory for trial outcome, i.e., a form of distractor-sensitive working memory (Baddeley 1987). In the present study, it is difficult to determine which aspect of past task performance animals are maintaining—i.e., whether it is trial outcome (correct vs. error) or reward history (rewarded vs. unrewarded). Future experiments that manipulate reward contingencies will be needed to dissociate these possibilities.
Our findings converge with reports that primate medial frontal regions are prominently involved in posterror processing (Emeric et al. 2007; Schall et al. 2002; van Veen et al. 2004; Walton et al. 2007) as well as in action selection (Bush et al. 2002; Rushworth et al. 2004, 2007; Shima et al. 2007). During simple RT performance, inactivation of dmPFC decreased delay-related activity in motor cortex (Narayanan and Laubach 2006), suggesting that rodent dmPFC may exert top-down control over neurons in rodent motor cortex that are responsible for executing movements (Donoghue et al. 1992; Laubach et al. 2000; Neafsey et al. 1986). Taken together, these data suggest that in both primates and rodents, medial prefrontal regions may be involved in integrating information about task performance to achieve supervisory control of sensorimotor processes (Dalley et al. 2004; Rushworth et al. 2004; Schall et al. 2002).
GRANTS
This work was supported by funds from the National Science Foundation, Kavli Institute at Yale, and the John B. Pierce Laboratory for ML and from an National Institutes of Health training grant to the Yale Medical Scientist Training Program and an Army Research Office grant for N. S. Narayanan.
Acknowledgments
We thank N. Horst for carefully reading this manuscript and M. Leslie and J. Schall for thoughtful feedback on a previous version of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Amiez et al. 2006.Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex 16: 1040–1055, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley 1987.Baddeley A Working Memory. Oxford, UK: Oxford Univ. Press, 1987.
- Baeg et al. 2003.Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177–188, 2003. [DOI] [PubMed] [Google Scholar]
- Batuev et al. 1990.Batuev AS, Kursina NP, Shutov AP. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res 41: 95–102, 1990. [DOI] [PubMed] [Google Scholar]
- Bush et al. 2002.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA 99: 523–528, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit and Balleine 2003.Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res 146: 145–157, 2003. [DOI] [PubMed] [Google Scholar]
- Dalley et al. 2004.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784, 2004. [DOI] [PubMed] [Google Scholar]
- Donoghue et al. 1992.Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res 89: 1–19, 1992. [DOI] [PubMed] [Google Scholar]
- Emeric et al. 2007.Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Pare M, Pouget P, Stuphorn V, Taylor TL, Schall JD. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res 47: 35–49, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott et al. 2005.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177, 2005. [DOI] [PubMed] [Google Scholar]
- Killcross and Coutureau 2003.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex 13: 400–408, 2003. [DOI] [PubMed] [Google Scholar]
- Laubach et al. 2000.Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behavior outcomes during learning of a motor task. Nature 405: 567–571, 2000. [DOI] [PubMed] [Google Scholar]
- Lomber 1999.Lomber SG The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods 86: 109–117, 1999. [DOI] [PubMed] [Google Scholar]
- Martin and Ghez 1999.Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86: 145–159, 1999. [DOI] [PubMed] [Google Scholar]
- Narayanan et al. 2006.Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 2006. [DOI] [PubMed]
- Narayanan et al. 2005.Narayanan NS, Kimchi EY, Laubach M. Redundancy and synergy of neuronal ensembles in motor cortex. J Neurosci 25: 4207–4216, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan and Laubach 2006.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52: 921–931, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey et al. 1986.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res 396: 77–96, 1986. [DOI] [PubMed] [Google Scholar]
- Niki and Watanabe 1976.Niki H, Watanabe M. Cingulate unit activity and delayed response. Brain Res 110: 381–386, 1976. [DOI] [PubMed] [Google Scholar]
- Ostlund and Balleine 2005.Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci 25: 7763–7770, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos and Watson 1982.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1982. [DOI] [PubMed]
- Preuss 1995.Preuss T Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7: 1–24, 1995. [DOI] [PubMed] [Google Scholar]
- Ragozzino et al. 1998.Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci 112: 293–303, 1998. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof et al. 2004.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 306: 443–447, 2004. [DOI] [PubMed] [Google Scholar]
- Risterucci et al. 2003.Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci 17: 1498–1508, 2003. [DOI] [PubMed] [Google Scholar]
- Rushworth et al. 2007.Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol 17: 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- Rushworth et al. 2004.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8: 410–417, 2004. [DOI] [PubMed] [Google Scholar]
- Schall et al. 2002.Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron 36: 309–322, 2002. [DOI] [PubMed] [Google Scholar]
- Shima et al. 2007.Shima K, Isoda M, Mushiake H, Tanji J. Categorization of behavioral sequences in the prefrontal cortex. Nature 445: 315–318, 2007. [DOI] [PubMed] [Google Scholar]
- Uylings et al. 2003.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res 146: 3–17, 2003. [DOI] [PubMed] [Google Scholar]
- van Veen et al. 2004.van Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS. Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn 56: 267–276, 2004. [DOI] [PubMed] [Google Scholar]
- Walton et al. 2007.Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage 36, Suppl 2: T142–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]