Abstract
How does the brain calculate the spatial orientation of the head relative to gravity? Psychophysical measurements are critical to investigate this question, but such measurements have been limited to humans. In non-human primates, behavioral measures have focused on vestibular-mediated eye movements, which do not reflect percepts of head orientation. We have therefore developed a method to measure tilt perception in monkeys, derived from the subjective visual vertical (SVV) task. Two rhesus monkeys were trained to align a light bar parallel to gravity and performed this task during roll tilts, centrifugation, and roll optokinetic stimulation. The monkeys accurately aligned the light bar with gravity during static roll tilts but also demonstrated small orientation-dependent misperceptions of the tilt angle analogous to those measured in humans. When the gravito-inertial force (GIF) rotated dynamically in the roll plane, SVV responses remained closely aligned with the GIF during roll tilt of the head (coplanar canal rotational cues present), lagged slightly behind the GIF during variable-radius centrifugation (no canal cues present), and shifted gradually during fixed-radius centrifugation (orthogonal yaw canal cues present). SVV responses also deviated away from the earth-vertical during roll optokinetic stimulation. These results demonstrate that rotational cues derived from the semicircular canals and visual system have prominent effects on psychophysical measurements of roll tilt in rhesus monkeys and therefore suggest that a central synthesis of graviceptive and rotational cues contributes to percepts of head orientation relative to gravity in non-human primates.
INTRODUCTION
When subjects move in a gravitational environment, the semicircular canals in the vestibular labyrinth sense angular head velocity and the otolith organs sense the vector sum of gravity and the inertial force produced by linear acceleration (gravito-inertial force, GIF). There is extensive evidence that the brain is able to use vestibular and other sensory cues to generate relatively accurate percepts of head orientation and motion (Merfeld and Zupan 2003) as well as appropriate oculomotor reflexes (Green and Angelaki 2003, 2004). To investigate the mechanisms used by the brain to accomplish these tasks, numerous studies have characterized vestibular-mediated eye movements in both human and non-human primates and tilt psychophysics in human subjects (see Guedry 1974 for a review). No psychophysical measurements of head tilt have been made in monkeys, although such studies could potentially utilize both standard motion protocols (e.g., tilt, translation, and centrifugation) and more invasive techniques (e.g., neural stimulation, ablation, and recording) that are not feasible in humans.
Psychophysical measurements are necessary to study the neural mechanisms used by non-human primates to estimate head orientation for several reasons. 1) percepts of head tilt cannot be deduced from eye movement responses (Zupan and Merfeld 2005). Torsional eye movements, for example, include an angular vestibuloocular reflex (VOR) component that is independent of gravity as well as utricular contributions that do not reflect roll tilt of the GIF (Angelaki 1998; Merfeld et al. 1996; Paige and Tomko 1991). 2) Recent work in human subjects suggests that qualitatively different neural mechanisms may be used by the brain to generate vestibular-mediated psychophysical (e.g., perceived tilt) and motor (e.g., eye movement) responses (Merfeld et al. 2005a,b). This divergence between perception and action has been observed in other sensory systems (e.g., vision, Goodale and Westwood 2004) and implies that direct psychophysical measures are required to study the neural substrates underlying perceptual processes. And 3) substantial differences in vestibular-mediated eye movements have been described in monkeys (Angelaki and Hess 1994; Wearne et al. 1999) and humans (Fetter et al. 1992; Landsberg et al. 1965; Merfeld et al. 2001), such as the more pronounced alignment of vestibular and optokinetic eye movements with the GIF in monkeys. These well-documented differences in oculomotor responses suggest that psychophysical responses in human and non-human primates may also differ substantially.
Two principal hypotheses have been proposed to explain how the brain estimates the gravitational (tilt) and inertial (translation) components of the ambiguous GIF transduced by the otolith organs. It has been suggested that the GIF is disambiguated based on its frequency content (Paige and Tomko 1991; Telford et al. 1997) with low-frequency shifts in the GIF interpreted as tilt and higher frequencies shifts interpreted as translation. Conversely, because head movements typically activate the canals and alter visual orientation cues, a second hypothesis is that the brain's estimates of head orientation and motion are based on a central synthesis of gravito-inertial and nonotolithic afferent signals (Merfeld and Zupan 2003; Young 1984). In rhesus monkeys, numerous eye movement studies are consistent with the sensory synthesis hypothesis as vestibular-mediated eye movements appear to result from an interaction between the rotational cues derived from the canals and the GIF information transduced by the otoliths (e.g., Angelaki et al. 1999). While similar mechanisms appear to contribute to percepts of head tilt in humans (Merfeld et al. 2001; Merfeld et al. 2005a,b), the neural processes that underlie tilt perception in monkeys remain unknown.
To investigate this problem, we have developed a method to measure tilt psychophysics in rhesus monkeys in the roll plane based on the subjective visual vertical (SVV) task widely used in human studies (Bohmer and Rickenmann 1995; Strupp et al. 2003; reviewed in Howard and Templeton 1966). Rhesus monkeys were trained to align a light bar parallel to the direction of gravity and were tested with this task during a variety of motion paradigms. We hypothesized that in rhesus monkeys, perception of head orientation in the roll plane depends at least in part on a synthesis of the GIF cues transduced by the otolith organs and rotational cues derived from semicircular canals and the visual system. We explicitly addressed this hypothesis by comparing SVV responses during three motion paradigms that shifted the GIF in the roll plane in a similar manner but provided substantially different canal rotational cues. Specifically, we compared SVV responses during roll tilt of the head (congruent roll canal cues), variable-radius centrifugation (no canal cues), and fixed-radius centrifugation (yaw canal cues orthogonal to the roll shift in GIF). We found that the rotational information provided by the canals had substantial effects on the SVV responses produced by roll tilt of the GIF. Furthermore, roll optokinetic stimulation also had a pronounced effect on SVV responses. Taken together, these results strongly suggest that a central synthesis of graviceptive and rotational sensory information contributes to neural estimates of head orientation with respect to gravity in rhesus monkeys. A preliminary description of a subset of these experiments has previously been published (Lewis et al. 2005).
METHODS
Experiments were performed in two 3.5-yr-old juvenile rhesus monkeys (weights: 3.6 and 3.9 kg) which had not participated in any prior experiments. All experimental methods were approved by the institutional animal care and use committee and were in accordance with USDA guidelines.
General methods
A head bolt was implanted in each monkey's skull under general (pentobarbital) anesthesia using aseptic techniques in the hospital's animal operating room. This appliance was necessary to immobilize the head during training and experiments. The head was held in a stereotactic frame, a triangular region of the scalp was removed, and the skull surface was cleaned carefully. Six to eight sterile titanium screws were then placed in the skull in an inverted orientation (head of screw beneath the skull), and the skull surface was covered with dental acrylic. A fiberglass head bolt was then laid into the acrylic, oriented such that the head was pitched forward ∼18° with respect to the stereotactic zero when the head was immobilized in the monkey chair. This head orientation placed the lateral canals close to alignment with the earth-horizontal plane and the vertical canals close to the earth-vertical plane (Blanks et al. 1985).
Subjective visual vertical training and testing
The animals sat in the primate chair and grasped a small steering wheel attached to the front of the chair. The wheel provided no orientation information and was connected to a potentiometer that measured the amplitude of the wheel rotation. This signal was used to rotate in roll the orientation of a light bar presented on a liquid crystal display (LCD) panel located 50 cm in front of the animal. To minimize visual orientation cues, the LCD panel and the entire visual surround were masked with black fabric, except for a circular aperture (27 cm diam) centered between the animal's eyes that contained the relevant visual display. This display was black except for the light bar and a visual reference bar that was used in the early stages of training. The visual displays were presented, and the animal's behavioral responses recorded, with programs using the Virtual Reality Utilities (VRUT) software package. During all SVV training and testing, the monkey's head was immobilized with the head bolt, but the rest of the body could move within the primate chair.
The monkeys were initially trained 4 day/wk for ∼6 mo to align the light bar parallel to the direction of gravity, using the training paradigm described in the following text. Throughout the subsequent periods of testing, the need for accurate performance on the SVV task was frequently reinforced. Once testing began, one full session each week was spent on the training task, and on testing days, each experiment was preceded by 5 min of training.
SVV TRAINING.
During each training trial, the bar appeared at a random orientation, the animal rotated the wheel until the bar was approximately upright, and a water reward was given. The light bar was then extinguished and reappeared at another random orientation for the next trial. An angular “window” about the true earth-vertical (which was not visible to the monkey) was used during training, and the animals received water only if they correctly rotated the line into the window and kept it there for 0.8 s. The size of the window was gradually reduced over time (to a minimum of ±4°) to increase the accuracy with which the animals oriented the bar. Initially the monkeys were trained with a visual reference line that indicated the true earth-vertical, and once this task was mastered, they were trained to align the light bar parallel to the earth-vertical without visual cues by gradually dimming and eventually eliminating the reference line.
To ensure that they learned to align the bar with respect to the earth-vertical rather than a head or retinal reference, early in the course of training we introduced different static head orientations along the roll axis, including trials with the head upright (yaw axis aligned with gravity) and trials with the head tilted statically to the left or right. In all head positions, the animals were rewarded for rotating the light bar into the earth-vertical orientation. When this task was carried out accurately, they were trained while they were dynamically rotated about the roll axis.
SVV TESTING.
While the training paradigm required that the animal rotate the light bar into an angular window centered about the true earth-vertical, all data were acquired with a testing paradigm that did not impose any constraints on the placement of the light bar. We found by observing the animals' behavior during the task that they would rapidly rotate the light bar close to their desired orientation, make several quick corrections, and then pause. To capture this behavior accurately but not impose any constraints on the monkey's responses during data acquisition, we used a small tolerance window to determine when the pause associated with the desired response occurred. If the light bar remained within a small (typically 3°) window for a short interval (0.3–0.8 s), we concluded that the monkey had reached its desired response, and the bar orientation was written to the data file and the water reward was provided. If the monkey was actively rotating the light bar, conversely, the bar would exit the 3° window before the time criteria elapsed, the window would re-center about the bar at its new orientation, and this process would continue until the time criteria was met, signaling the end of the trial. The bar then disappeared and reappeared at a random orientation. The appropriate time criteria were determined empirically for each motion paradigm by observing the animals' behavioral responses while performing the SVV task. Longer time windows (e.g., 0.8 s) were used when the GIF orientation was stationary (e.g., static head tilts), while shorter time windows (e.g., 0.3 s) were used when the orientation of the GIF was changing (e.g., during centrifugation). Using this approach, we found that essentially all trials would terminate shortly after the monkey placed the bar in the desired orientation. During all data acquisition, therefore the animals were free to rotate the bar into any orientation and were not forced by the paradigm to respond in any preconceived fashion. Because there was no feedback regarding the accuracy of performance, the testing paradigm did not provide information that could shape or influence the animals' response to the SVV task.
While the monkeys were rewarded during the testing protocol each time they paused, they could not distinguish testing trials (which did not require a specific response) from training trials (which required that they align the light bar near the earth-vertical). If they were well motivated, they would pause only after they had rotated the light bar to their desired orientation. If the animal was not performing well on a given day, evidenced by erratic placement of the light bar during testing, the experiment would be terminated for the day to ensure that the learned behavior was not degraded by rewarding them for random responses.
Motion devices and paradigms
For all motion paradigms, the angular and linear positions of the monkey chair were recorded in Labview. Because the animal's head was fixed with respect to the chair, these measurements reflect the position of the head in three-dimensional space.
ROLL TILTS.
The monkeys were rotated about the nasal-occipital earth-horizontal axis centered between the eyes. These rotations were not computer controlled and were generated by a small 10 N-m DC motor that was controlled directly by a human operator. Rotational velocities were always within ±15% of the desired velocities indicated in the following text for each tilt paradigm.
STATIC ROLL TILTS.
The animals were maintained in different stationary head orientations for 30 s and were tilted slowly between head positions at a low (∼2°/s) angular velocity. The head was positioned in a random manner at 13 orientations, ranging from right-ear-down (RED), 30° to left-ear-down (LED), 30° in 5° increments. Typically 10–12 discrete SVV responses were obtained at each of the static tilt positions during each session. Tilt angles >30° were poorly tolerated by the monkeys and therefore were not used.
SLOW 30 DEG TILTS.
To approximate the shift in GIF orientation produced by the centrifugation paradigms (see following text), roll tilts from upright to 30° LED and RED orientations were performed over a period of 10 s at an approximate rate of 3°/s. The tilted positions were maintained for 10 s, and then the animal was returned to upright over a 10-s period.
RAPID ROLL TILTS.
The monkeys were rapidly rotated (angular velocity of ∼10°/s) from upright to 10, 20, and 30° LED and RED positions, held in each position for ∼10 s, and then returned to upright.
ROLL OPTOKINETIC STIMULATION.
The animals viewed a clockwise (CW) or counterclockwise (CCW) roll optokinetic (OK) stimulus on the LCD panel, which also displayed the light bar for the SVV task. The OK stimulus rotated at an angular velocity of 60°/s, spanned the central 30° of the visual field, and consisted of opaque circles (each spanning 1.8°) which were distributed randomly and filled 15% of the visual display. During OK stimulation, the monkeys were statically tilted in the roll plane from 20° LED to 20° RED in 10° increments. Data were acquired for intervals of 30 s while the head was statically tilted at each position.
CENTRIFUGATION.
Centrifugation experiments were performed on a computer-controlled centrifuge, consisting of a linear track that rotated about the earth-vertical axis and a sled that translated along the track. In these experiments, the head was upright and oriented such that the centripetal acceleration aligned with the inter-aural (IA) axis. This system operated with the following specifications: 95 cm track length, 1.0 g maximum linear acceleration, and 360°/s maximum angular velocity.
SINUSOIDAL VARIABLE-RADIUS CENTRIFUGATION.
To simulate the inter-aural acceleration produced by low-frequency sinusoidal IA translation, we employed the method of variable-radius centrifugation (Merfeld et al. 2001; Seidman et al. 1998). Specifically, the monkeys were slowly accelerated to an angular velocity of 230°/s about the earth-vertical axis, either CW or CCW when viewed from above. They were maintained at this constant velocity for 5 min with the chair centered at the rotational axis, allowing the yaw rotation cues derived from the semicircular canals to dissipate. Then the chair was translated sinusoidally at low frequencies (0.01–0.1 Hz) while the track continued to rotate. The amplitudes of translation (0.35 m for 0.01 Hz, 0.348 m for 0.05 Hz, and 0.342 m for 0.1 Hz translations) were chosen so that the net IA acceleration (centrifugal plus radial) modulated between right and left 0.58 g, with the centrifugal acceleration (rω2, where r is the displacement from the center of rotation and ω is the angular velocity) providing the majority of the IA acceleration. This sinusoidal variable-radius centrifugation paradigm tilted the GIF in the roll plane over a range of right/left 30° and mimicked the changes in inertial force that would be achieved with low-frequency, large-amplitude chair translation. To avoid abrupt linear accelerations, the translational motion for the two higher frequencies included a ramp-up period (1-half cycle for 0.05 Hz, 1 cycle for 0.1 Hz).
The Coriolis accelerations (2ω × v, where ω is the angular velocity and v the radial velocity) produced by these motion patterns (peak values: 0.02 g [0.01 Hz], 0.09 g [0.05 Hz], 0.17 g [0.1 Hz]) were always substantially smaller than the IA acceleration and were below the human perceptual threshold (0.03 g) for the lowest frequency (Melvill Jones and Young 1978; Travis and Dodge 1928). The Coriolis acceleration was also orthogonal to the IA acceleration that shifted the GIF in the roll plane, both spatially (as it was aligned with the naso-occipital axis) and temporally (as it was 90° out of phase with the IA acceleration). Furthermore, data were obtained with both clockwise (CW) and counterclockwise (CCW) angular velocities, which reversed the orientation of the Coriolis acceleration, but this did not influence the SVV responses. For these reasons, the Coriolis acceleration appeared to have a negligible influence on SVV responses in the roll plane during sinusoidal variable-radius centrifugation.
PARABOLIC VARIABLE-RADIUS AND FIXED-RADIUS CENTRIFUGATION.
These two centrifugation paradigms (Merfeld et al. 2001), in association with the roll tilt experiments, allowed us to evaluate the effects of canal rotational cues on perceived tilt when the GIF rotates in the roll plane. Specifically, canal cues were coplanar with the roll shift in GIF orientation during roll tilt, were absent during parabolic variable-radius centrifugation, and were orthogonal (yaw axis) to the roll GIF shift during fixed-radius centrifugation. In both of these centrifugation paradigms, trials were obtained with the chair rotating CW or CCW (viewed from above) with the monkey seated so that its face or back was oriented toward the direction of motion.
PARABOLIC VARIABLE-RADIUS CENTRIFUGATION.
The animals were centered over the rotational axis and the chair was accelerated to an angular velocity of 230°/s over a period of 60 s. After 5 min of rotation at a constant velocity (which allowed the yaw rotational cue sensed by the lateral canals to dissipate), the chair was translated along the IA axis from the center to an eccentricity of 0.35 m over a period of 10 s. The eccentric position (r) changed parabolically during the translation, introducing an IA centrifugal force (rω2) that increased parabolically from 0 to 0.58 g over 10 s (matching the IA force produced during fixed-radius centrifugation). The IA force shifted the orientation of the GIF in the roll plane from upright to 30° tilt toward the animal's right or left ear with the GIF tilt angle [equal to tan−1(rω2/g)] also shifting in an approximately parabolic manner. The chair remained at the eccentric position for 120 s and then returned to center over 10 s, removing the centrifugal force and returning the orientation of the GIF to upright. Recording of SVV responses began 15 s before the chair began to translate eccentrically and stopped 60 s after it returned to its centered position.
FIXED-RADIUS CENTRIFUGATION.
The monkeys were first translated 0.35 m to the right or left of the rotational axis and were then rotated in yaw about an earth-vertical axis at an acceleration of 23°·s−1·s−1 for 10 s to a constant velocity of 230°/s while their eccentric position remained constant. Like the parabolic variable-radius paradigm, this motion protocol provided a parabolic increase in IA centrifugal force which increased from 0 to 0.58 g over 10 s, shifting the orientation of the GIF in the roll plane from upright to 30° tilted toward the right or left ear. After 120 s of constant-velocity rotation, the animals were decelerated in a symmetric manner to an angular velocity of zero. Recording of SVV responses began 15 s before the chair began to rotate and stopped 60 s after its angular velocity returned to zero.
Data analysis
SIGN CONVENTION.
Head tilts in the roll plane were defined as positive when the top of the head rotated toward the right and negative when it rotated toward the left (Fig. 1 inset) . GIF tilts in the roll plane were defined as positive when the GIF rotated toward the right ear (e.g., consistent with a rightward head tilt) and negative when it rotated toward the left ear (consistent with a leftward head tilt). The SVV error was defined as the angle between the light bar and the true earth-vertical and was defined as positive when the top of the bar deviated to the left of the earth-vertical and negative when it deviated to the right. Similar to prior studies (e.g., Bortolami et al. 2006), we refer to the sum of the head tilt (HT) angle and the SVV error as the perceived head tilt (PT), although the exact relationship between the PT and the monkeys' perception of head tilt is complex and considered extensively in the discussion.
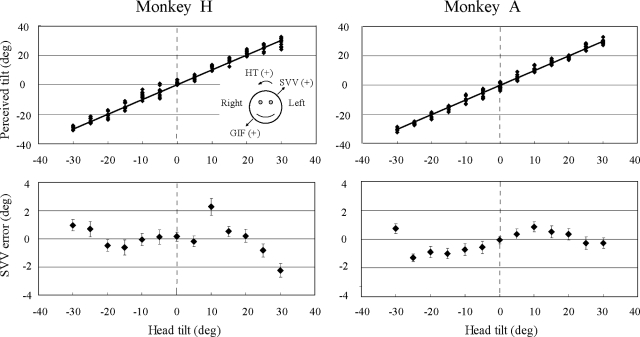
FIG. 1.
Subjective visual vertical (SVV) responses during static roll tilt for the 2 monkeys. Top: perceived tilt (PT) plotted against the actual head tilt (HT) for 1 experimental session, where PT = HT + SVV error. Positive values indicate tilts toward the right-ear-down orientation. The solid lines represent perfectly veridical responses (PT = HT). Bottom: SVV error plotted against head tilt for all 8 static roll tilt experimental sessions in each animal. The mean ±1 SE are illustrated, and the bias for each animal (+0.73° for monkey H, +0.1° for monkey A) has been subtracted from the SVV error values. In these and subsequent plots of SVV error vs. head tilt angle, positive SVV errors associated with positive (rightward) head tilts indicate overestimation of tilt angle as do negative SVV errors associated with negative (leftward) head tilts. Inset: sign orientation for HT, SVV, and gravito-inertial force (GIF) orientation. Head tilts toward the right-ear-down orientation are positive, GIF shifts toward the right ear (as would occur with a rightward head tilt) are positive, and SVV deviations toward the left are positive.
For roll head tilts, for example, if a 20° rightward (positive) head tilt resulted in a 3° leftward (positive) deviation of the SVV response, the PT was overestimated (20 + 3 = 23°). For the centrifugation paradigms, where the head remained upright (e.g., HT was 0), the PT was equivalent to the SVV error. For example, during centrifugation trials that rotated the GIF toward the right ear (positive deviation), the monkey would be predicted to rotate the light bar toward the left (positive SVV error), aligning it approximately parallel to the GIF.
ROLL TILTS.
Because these motions were not computer-controlled, each rotation had slightly different dynamic characteristics. We therefore could not superimpose the PT responses as a function of time for multiple trials and hence could only evaluate the dynamic characteristics of the PT responses in a qualitative manner. The quantitative analysis for the roll tilt experiments focused on the relationship between the actual and perceived head tilt while the head was stationary and in motion.
SINUSOIDAL VARIABLE-RADIUS CENTRIFUGATION.
Each trial consisted of multiple sinusoidal translations (3 cycles for 0.01 Hz, 10 cycles for 0.05 Hz, and 20 cycles for 0.1 Hz). For each trial, the cycles were superimposed and a sine wave was fit to both the motion stimuli (GIF tilt) and the PT responses, using a least-mean-square linear regression to the equation x(t) = B + Accos(ωt) + Assin(ωt), where B is the DC bias, Ac is the amplitude of the cosine component, and As is the amplitude of the sine component. B, Ac, and As all have units of degrees. The values for these parameters were determined for each trial and then were averaged across multiple trials for each animal and specific motion parameters (frequency of translation, direction of angular rotation). The gain and phase of the response was calculated from the averaged values, with the gain defined as (1/30)(As2 + Ac2)1/2 and the phase defined as tan−1(As/Ac). The covariance matrix (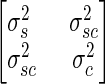 ) was calculated using standard methods that yield the variance of the sine (σs2) and cosine (σc2) component as the diagonal elements and the covariance (σsc2) of the sine and cosine components on the off-diagonal elements. The covariance ellipse, which is the two-dimensional extension of the one-dimensional confidence interval, was constructed using multivariate methods detailed elsewhere (e.g., Johnson and Wichern 1982). The SD of the amplitude is half the width of the ellipse in the radial direction and the SD of the phase is half the ellipse width in the perpendicular direction.
) was calculated using standard methods that yield the variance of the sine (σs2) and cosine (σc2) component as the diagonal elements and the covariance (σsc2) of the sine and cosine components on the off-diagonal elements. The covariance ellipse, which is the two-dimensional extension of the one-dimensional confidence interval, was constructed using multivariate methods detailed elsewhere (e.g., Johnson and Wichern 1982). The SD of the amplitude is half the width of the ellipse in the radial direction and the SD of the phase is half the ellipse width in the perpendicular direction.
PARABOLIC VARIABLE-RADIUS AND FIXED-RADIUS CENTRIFUGATION.
For each trial, the tilt gain was defined as the peak perceived tilt (PT) while the IA force was present, normalized by dividing by the amplitude of the GIF tilt (30°); PT decay was the magnitude of the decline in PT while the IA force was maintained, expressed as a percentage of the peak PT; and the aftereffect was the magnitude of the reversal in PT that occurred after the IA force was removed, expressed as a percentage of the peak PT. To characterize the temporal nature of the shift in PT during the trial, we determined the time required for the PT to shift to 0.63 of its maximal deviation. We chose this time to define the dynamics of the PT shift because there was considerable variability in the period needed for the PT shift to maximize; less variability was evident if we examined a point while the PT was changing fairly rapidly rather than the time required for the response to plateau. While the PT shifts were not consistently exponential and hence were not fit by a specific function, the time to 0.63 of peak corresponds to the time constant of an exponential function. To combine multiple centrifugation trials graphically, a linear interpolation was made between consecutive PT responses during each trial, allowing us to generate a continuous response variable, from which the mean and standard error of the PT over time could be calculated and plotted.
RESULTS
Roll tilts
STATIC ROLL TILTS.
When the monkeys were statically tilted in roll, their SVV responses aligned closely with the earth-vertical, yielding PT responses that closely approximated the actual HT as shown in Fig. 1, top, for a single test session. When the results of the eight static tilt sessions were combined for each monkey (Fig. 1, bottom), it is evident that the SVV errors were quite small over the ±30° tilt range we tested. Both monkeys, however, overestimated the head tilt angle when the head was tilted ≤20° and tended to slightly underestimate the head tilt angle for larger tilts, consistent with the Aubert and Muller effects observed in humans (Guedry 1974). Although this position-dependent modulation of the SVV error was subtle, it was significant for each animal (monkey H: 1-way ANOVA, P < 0.001, F = 6.2, dof = 12; monkey A: 1-way ANOVA on ranks, P < 0.001, H = 54.4, dof = 12).
SLOW TILTS.
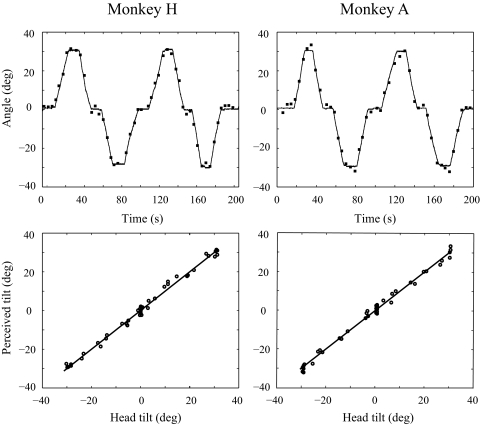
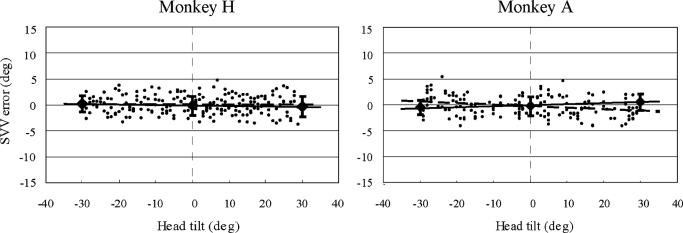
When the monkeys were tilted 30° over a period of 10 s (average velocity of 3°/s), the PT responses closely aligned with the actual HT angle (Fig. 2). The monkeys' responses were highly accurate while the HT angle was stationary at the upright or left/right 30° down positions and were also accurate while the head was in motion between these static orientations. When all 15 slow tilt cycles were combined for each monkey (Fig. 3), the SVV errors while the head was head stationary at the three static positions were essentially indistinguishable from the SVV errors measured while the head was slowly tilting. For monkey H, linear regression of the static and dynamic SVV errors as a function of head position showed nearly identical offsets and slopes (static offset = −0.08, dynamic offset = 0.08; static slope = −0.01, dynamic slope = −0.002), and the static and dynamic responses were statistically indistinguishable (t-test on the means, P = 0.34; F-test on the variances P > 0.5). Similarly for monkey A, linear regression yielded static and dynamic slopes that were nearly identical (static slope = 0.02, dynamic slope = −0.03) and offsets that were similar (static = −0.07, dynamic = −0.28). Again the static and dynamic SVV errors did not differ statistically (Mann-Whitney test on means, P = 0.38; F-test on variances, P > 0.1). These results demonstrate that during roll tilts with an angular head velocity of ∼3°/s, the monkeys were able to accurately estimate the tilt angle while in motion with no measurable lag between changes in head orientation and perceived head tilt.
FIG. 2.
Examples of SVV responses during slow roll tilts (30° over 10 s) for each monkey. Top: PT responses (▪) and HT angle (—) vs. time. Bottom: PT responses vs. HT angle. The PT closely approximated the HT angle, both while the head was stationary and moving. The solid lines represent perfectly veridical responses (PT = HT).
FIG. 3.
SVV error plotted against head tilt during the slow 30° roll tilts. Each plot shows the data obtained from 15 tilt cycles. ⧫, the mean errors while head was statically positioned at right and left 30° and upright orientations ±1 SE. •, the individual responses while the head was in motion between these static positions. Linear regressions for the static (—) and dynamic (- - -) responses are also illustrated.
RAPID ROLL TILTS.
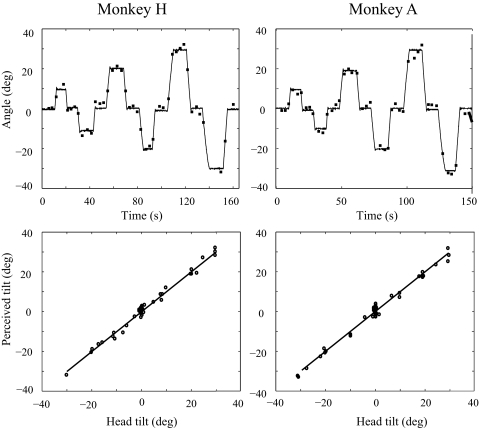
Even during relatively fast roll tilts the PT tilt responses closely aligned with the actual HT (Fig. 4). For monkey H, eight full trials were analyzed (each consisting of tilts from upright to right or left 10, 20, and 30° and back to upright), resulting in 281 SVV error measurements with a root mean squared (RMS) value of 1.96°. Seven trials were analyzed for monkey A, yielding 304 SVV error measurements with a RMS value of 1.86°.
FIG. 4.
Examples of SVV responses during rapid roll tilts between the upright position and left or right 10, 20, and 30°. Top: PT and HT angle vs. time. Bottom: PT vs. HT (solid lines, PT = HT).
Roll optokinetic stimulation
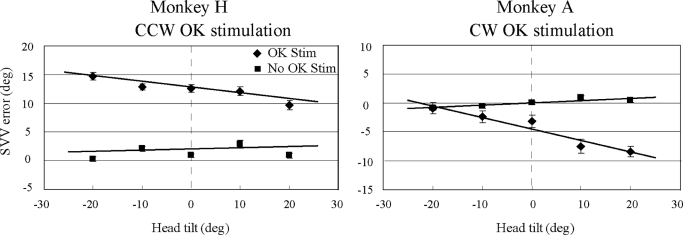
When the monkeys viewed a roll OK stimulus in the absence of visual orientation cues, their SVV errors deviated in the direction of the OK motion. Monkey H viewed a CCW stimulus and the SVV errors deviated to the left, consistent with an illusion of roll tilt to the right. Monkey A viewed a CW stimulus and the SVV errors deviated to the right, consistent with an illusion of roll tilt to the left (Fig. 5: ⧫, OK stimulus present; ▪, OK stimulus absent). The influence of the OK stimulus on the SVV error was significant for each monkey [P < 0.001 (Mann-Whitney rank sum test)] when pooled data with and without OK stimulation for each animal were compared. For both monkeys, the magnitude of the illusionary tilt produced by the OK stimulus was larger when the head was statically tilted in the direction opposite the illusionary tilt and was smaller when the head was tilted in the same direction as the illusionary tilt (Fig. 5). For monkey H, the illusionary tilt toward the right was largest with the head tilted leftward. While a linear regression of the SVV error without OK stimulation in this monkey had a small rightward bias (1.4°) and a slope close to zero (0.02), a regression of the responses with the OK stimulation present yielded a much larger rightward bias (12.4°) but also a nonzero negative slope (−0.11). Similarly for monkey A, the illusionary tilt was toward the left and was largest in magnitude with the head tilted rightward. For this monkey, linear regression of the SVV errors without OK stimulation yielded no bias (0°) and small positive slope (0.04), whereas regression of responses during OK stimulation showed a large leftward bias (−4.5°) and a substantial negative slope (−0.2, Fig. 5). Two-way ANOVA confirmed that the effect of the OK stimulus on the SVV errors was dependent on head orientation (P < 0.001 for the interaction between OK state and head tilt angle for each monkey).
FIG. 5.
Mean SVV error (⧫) ± SE for all optokinetic trials (7 counterclockwise trials for monkey H, 6 clockwise trials for monkey A) compared with the mean SVV error in the same head orientations without the optokinetic stimulus (▪). Linear regressions of the 2 data sets [optokinetic (OK) present, OK absent] are also illustrated for each animal. For monkey H, the OK stimulus rotated counterclockwise and induced an illusion of tilt toward the right (leftward = positive SVV errors); for monkey A, the OK stimulus rotated clockwise and induced a leftward illusion of tilt (rightward = negative SVV errors).
Centrifugation
SINUSOIDAL VARIABLE-RADIUS CENTRIFUGATION.
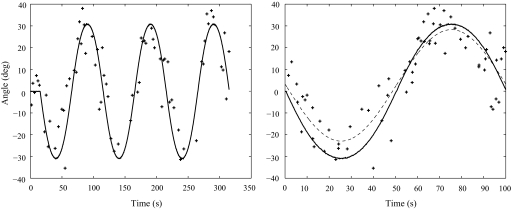
Perceived tilt aligned relatively closely with the GIF tilt for 0.01 Hz translations (gains near 0.8, phase shifts close to 0; Fig. 6, Table 1). As the frequency of the sinusoidal translations increased, the amplitude of the PT declined somewhat (gains near 0.7 for 0.05 Hz, 0.6 for 0.1 Hz; Table 1). Because the timing criteria used for SVV data acquisition (see methods) introduced a delay of 0.3 s for each SVV response, an obligate phase lag [1.1° (0.01 Hz), 5.4° (0.05 Hz), and 10.8° (0.1 Hz)] was introduced. Correcting for the phase lag associated with the SVV task (Table 1) yields fairly small phase shifts for the three translational frequencies we tested (0–5.0° for 0.01 Hz, −13.0 to −3.7° for 0.05 Hz, and −9.1 to 1.8° for 0.1 Hz with negative values indicating a lag). The bias for all trials was positive (toward the left), consistent with a small misperception of roll tilt toward the right for both animals during all stimulus conditions.
FIG. 6.
Example of SVV responses during sinusoidal variable-radius centrifugation for monkey A at 0.01-Hz, counterclockwise chair rotation. Left: —, the sinusoidal modulation in GIF angle; +, individual PT responses. Right: the cycles in the trial superimposed. —, the GIF angle; - - -, a sinusoidal fit to the PT responses.
TABLE 1.
Sinusoidal variable-radius centrifugation
| Gain | Phase | Bias | n | |
|---|---|---|---|---|
| Monkey H | ||||
| 0.01 Hz | ||||
| CW | 0.82 ± 0.01 | 5.00 ± 0.95 | 2.90 ± 0.47 | 5 |
| CCW | 0.80 ± 0.06 | 1.46 ± 1.85 | 2.53 ± 0.61 | 5 |
| 0.05 Hz | ||||
| CW | 0.72 ± 0.08 | −7.46 ± 2.49 | 3.99 ± 0.73 | 6 |
| CCW | 0.68 ± 0.08 | −3.67 ± 2.47 | 4.03 ± 0.68 | 7 |
| 0.1 Hz | ||||
| CW | 0.64 ± 0.06 | 0.23 ± 3.58 | 2.36 ± 0.85 | 5 |
| CCW | 0.61 ± 0.04 | 1.83 ± 3.13 | 5.22 ± 0.57 | 5 |
| Monkey A | ||||
| 0.01 Hz | ||||
| CW | 0.81 ± 0.07 | 0.80 ± 2.59 | 0.91 ± 1.25 | 5 |
| CCW | 0.77 ± 0.05 | 0.00 ± 1.32 | 0.43 ± 1.58 | 5 |
| 0.05 Hz | ||||
| CW | 0.70 ± 0.05 | −13.00 ± 1.89 | 3.20 ± 0.92 | 6 |
| CCW | 0.70 ± 0.06 | −9.43 ± 3.32 | 1.21 ± 0.79 | 5 |
| 0.1 Hz | ||||
| CW | 0.63 ± 0.05 | −9.05 ± 4.36 | 2.61 ± 0.83 | 4 |
| CCW | 0.60 ± 0.08 | −4.50 ± 8.06 | 0.42 ± 0.45 | 4 |
Values are means ± SE. n is the number of trials that were averaged. Gain, phase, and bias of the subjective visual vertical (SVV) responses were calculated as described in methods; negative phase indicates a lag between the stimulation (tilt of the gravito-inertial force) and the SVV response. Phases were corrected for the obligate 0.3-s delay associated with the timing criteria of the SVV task (see results). Phase and bias are in degrees. CW, clockwise; CCW, counter clock wise.
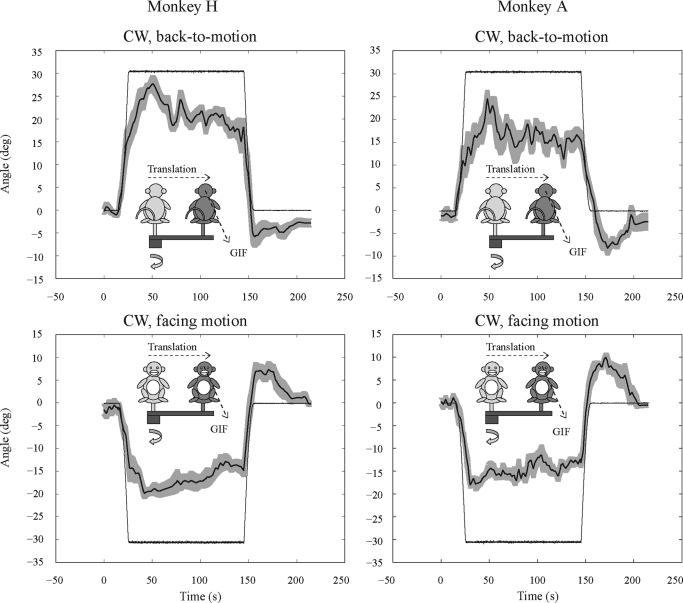
PARABOLIC VARIABLE-RADIUS CENTRIFUGATION.
When IA force was added during parabolic variable-radius centrifugation, perceived head tilt shifted rapidly toward alignment with the GIF but clearly lagged behind the change in GIF orientation (Fig. 7). While the GIF tilt reached 63% of its maximum after 6.3 s, PT did not reach 63% of its peak shift until 9–15 s (Table 2). PT approached but did not reach the 30° tilt of the GIF for any direction of rotation (CW or CCW) or head orientation (facing motion, back-to-motion). The tilt gains (peak PT/30°) ranged from 0.90 to 0.94 when the PT was toward the right-ear-down orientation (positive values) and were somewhat smaller (range: 0.65–0.74) when the PT was toward the left-ear-down orientation (negative values; Fig. 7, Table 2).
FIG. 7.
Averaged SVV responses during parabolic variable-radius centrifugation for clockwise chair rotations, facing and back-to-motion. Plotted are the mean PT (—) ±1 SE ( ) for 4–8 combined centrifugation trials and the GIF angle. Motion paradigms are illustrated by the insets.
) for 4–8 combined centrifugation trials and the GIF angle. Motion paradigms are illustrated by the insets.
TABLE 2.
Parabolic variable- and fixed-radius centrifugation
| Time to 0.63 × peak, s | Gain | PT Decay, % | Aftereffect, % | n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. Variable-radius | ||||||||||
| Monkey H | ||||||||||
| CW, back (+) | 12.9 ± 0.9 | 0.94 ± 0.02 | 41.5 ± 4.1 | 27.7 ± 1.5 | 8 | |||||
| CCW, face (+) | 11.3 ± 1.1 | 0.90 ± 0.02 | 52.2 ± 4.5 | 23.3 ± 3.6 | 6 | |||||
| CW, face (−) | 12.0 ± 1.5 | 0.68 ± 0.03 | 35.3 ± 6.03 | 42.6 ± 6.6 | 6 | |||||
| CCW, back (−) | 14.4 ± 2.1 | 0.74 ± 0.03 | 41.9 ± 8.1 | 36.5 ± 4.3 | 5 | |||||
| Monkey A | ||||||||||
| CW, back (+) | 14.4 ± 2.5 | 0.93 ± 0.03 | 51.6 ± 5.3 | 34.4 ± 4.8 | 5 | |||||
| CCW, face (+) | 9.2 ± 0.6 | 0.90 ± 0.07 | 67.8 ± 4.4 | 37.8 ± 1.6 | 4 | |||||
| CW, face (−) | 11.7 ± 1.1 | 0.65 ± 0.01 | 32.3 ± 2.3 | 55.4 ± 3.1 | 4 | |||||
| CCW, back (−) | 12.1 ± 1.1 | 0.69 ± 0.05 | 60.0 ± 2.9 | 52.2 ± 10.8 | 4 | |||||
| B. Fixed-radius | ||||||||||
| Monkey H | ||||||||||
| CW, back (+) | 46 ± 2.3 | 0.87 ± 0.03 | 0 | 1.1 ± 3.4 | 6 | |||||
| CCW, face (+) | 33 ± 2.9 | 0.73 ± 0.03 | 0 | 0 | 10 | |||||
| CW, face (−) | 36 ± 4.3 | 0.71 ± 0.05 | 0 | 7.0 ± 5.1 | 5 | |||||
| CCW, back (−) | 34 ± 3.9 | 0.76 ± 0.04 | 9.2 ± 6.5 | 3.9 ± 2.4 | 5 | |||||
| Monkey A | ||||||||||
| CW, back (+) | 37.4 ± 2.5 | 0.79 ± 0.04 | 7.6 ± 2.8 | 2.5 ± 1.7 | 5 | |||||
| CCW, face (+) | 42.7 ± 2.1 | 0.81 ± 0.04 | 11.1 ± 1.2 | 7.4 ± 2.4 | 4 | |||||
| CW, face (−) | 38.8 ± 2.7 | 0.77 ± 0.15 | 0 | 0 | 4 | |||||
| CCW, back (−) | 34.5 ± 3.0 | 0.76 ± 0.04 | 6.6 ± 5.9 | 5.3 ± 3.0 | 5 | |||||
Values are means ± SE. Time to 0.63 × peak is the time in seconds required for the SVV response to reach 0.63 of its maximum value, measured from the onset of the centrifugal force application (t = 15 s). Gain is the peak SVV deviation divided by the peak tilt of the GIF (30°). Perceived head tilt (PT) decay is defined as the magnitude of the decline in perceived tilt (maximum PT − minimum PT) during sustained centrifugal force expressed as a percentage of the maximum PT. Aftereffect is the magnitude of the reversal in PT that occurred after the centrifugal force was removed expressed as a percentage of the maximum PT. Centrifugation trials that rotate the GIF toward the right ear are indicated by plus (+) signs and trials that rotate it toward the left ear by minus (−) signs (see Fig. 1, inset).
The PT decayed substantially during sustained tilt of the GIF (Fig. 7). The amount of PT decay ranged from 35 to 68% of the peak PT (Table 2). A substantial reversal in the PT (aftereffect) occurred when the IA force was removed (Fig. 7), ranging from 23 to 55% of the peak PT (Table 2).
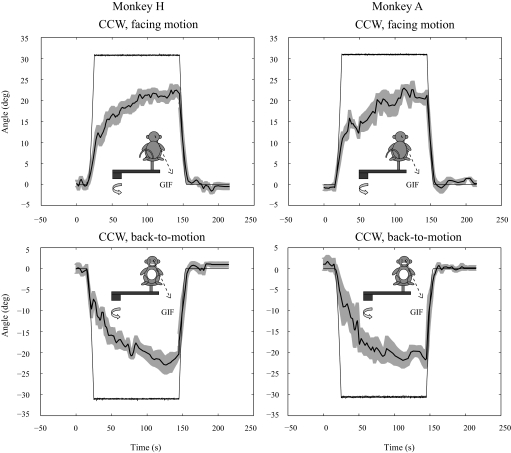
FIXED-RADIUS CENTRIFUGATION.
Unlike the parabolic variable-radius protocol, when IA force was added during fixed-radius centrifugation, PT shifted very slowly, lagging well behind the shift in GIF orientation (Fig. 8). PT did not reach 63% of its peak value until 33–46 s (Table 2), substantially longer than the time-frame associated with parabolic variable-radius centrifugation (9–15 s). The PT had similar gains in the fixed and parabolic variable-radius protocols (Table 2) although less difference was evident between positive (right-ear down) and negative (left-ear-down) directions of illusionary tilt in the fixed-radius paradigm (Fig. 8, Table 2). Finally, no meaningful decay or reversal of the PT occurred with the fixed-radius paradigm.
FIG. 8.
Averaged SVV responses during fixed-radius centrifugation for counterclockwise chair rotations, facing and back-to-motion. Plotted are the mean PT ± SE for 4–10 combined centrifugation trials and the GIF angle. Motion paradigms are illustrated by the inset.
DISCUSSION
We have developed and implemented a method to measure percepts of head tilt in rhesus monkeys using a task derived from the subjective visual vertical and have measured SVV responses during a number of motion paradigms that rotated the head or the GIF in the roll plane. As discussed in detail in the following text, our principal finding is that psychophysical measurements of roll tilt in rhesus monkeys, like humans, appear to depend on a central interaction between the gravito-inertial cues sensed by the otolith organs and the rotational cues sensed by the canals and the visual system. We will first discuss a number of issues that relate to the psychophysical task we employed in the monkeys and then will discuss the significance of our results.
Measuring tilt psychophysics with the subjective visual vertical
A principal tenet of this study is that the placement of the light bar during the SVV task provided an indication of the monkey's perceived head orientation in the roll plane. A number of issues must be considered regarding this contention, including factors that are specific to the training and testing methods we employed in non-human primates and factors that pertain to the subjective visual vertical test more generally.
SVV TASK IN MONKEYS.
When the SVV is used to test human subjects, they are instructed to align the light bar so that they perceive it to be parallel to the earth-vertical. The principal constraint with non-human primates is that we cannot provide similar verbal instructions and hence must rely on a training paradigm to teach them how to perform the SVV task. Because we cannot know the monkeys' actual perception of the earth-vertical, we utilized a training paradigm that rewarded the monkeys when they aligned the light bar close to the true earth-vertical during static and dynamic roll tilts of the head. It is quite clear that the monkeys were trained to orient the light bar with respect to the earth-vertical rather than some other reference frame. While their SVV responses closely approximated the true earth-vertical with the head upright or tilted in roll (Fig. 1), other potential reference frames, such as the longitudinal axis of the body or the retinal vertical (Suzuki et al. 1997) rotated away from the earth-vertical during roll tilts.
A discrepancy existed between the training task (e.g., rotate the light bar parallel to gravity) and the desired task (e.g., rotate the light bar parallel to the perceived direction of gravity), and this may have affected our results. The training approach made the implicit assumption that during roll tilts the perceived earth-vertical was relatively close to the true earth-vertical. Two observations support this contention: 1) because human subjects orient the light bar close to the earth-vertical when they are tilted in roll over the relatively small range we employed in our study (±30°), we hypothesized that monkeys would also have a close correspondence between the perceived and actual direction of gravity during roll tilts; and 2) during training, the gravitational vector appeared to be a stable, reproducible reference for the monkeys, and there was never evidence that they were learning to suppress or overcome any bias that would reflect a significant disparity between the perceived and actual earth-vertical.
These observations suggest a relatively close correspondence between the perceived and actual direction of gravity during roll tilts of the head, but they do not indicate that these two orientations are identical. For example, during the testing paradigm (which placed no constraints on the animals' responses), the monkeys showed small orientation-dependent misperceptions of the earth-vertical during roll tilt (see Fig. 1). It is possible that these errors may have been larger in the naïve monkeys and were reduced by training. Although the monkeys' placement of the light bar during the SVV task appears to be similar but not identical to their perception of the earth-vertical, we can still draw meaningful conclusions because we were able to compare responses on multiple testing paradigms. Several of the paradigms, for example, varied canal rotational cues while maintaining similar gravito-inertial cues. Direct comparison of the SVV responses during these paradigms allows us to examine the dependence of tilt percepts on the interaction between canal and otolith inputs even if the monkeys' SVV responses do not correspond exactly with their perceived earth-vertical.
The monkeys were trained during roll tilts (where the orientation of the GIF is always aligned with the earth-vertical), but we postulated that during testing they would align the light bar close to their perceived direction of gravity even when it was dissociated from the earth-vertical (12 o'clock position) by inertial cues. Our results demonstrate clearly that when the head remained upright and the GIF rotated in the roll plane, SVV responses shifted toward alignment with the GIF away from the earth-vertical orientation (Figs. 6–8).
In summary, the monkeys were trained to align the light bar parallel to the earth-vertical, the training rule most likely introduced some (presumably small) discrepancies between the SVV responses and the perceived earth-vertical, and the animals were able to generalize the training rule to align the light bar close to the perceived direction of gravity even when it tilted away from the earth-vertical orientation. Our results, which are discussed in detail in the following text, demonstrate a large number of features that have been described previously in human subjects. The large number of similarities observed between species would be highly improbable if the monkeys' SVV responses did not capture percepts of head orientation that are reasonable close to those obtained in human subjects who are given explicit verbal directions to align a light bar with the perceived vertical.
SVV AS A MEASURE OF PERCEIVED HEAD ORIENTATION.
It is evident from human studies that different psychophysical tests such as the SVV, subjective postural vertical, and somatosensory bar, result in different estimates of roll tilt in normal subjects and patients with vestibular disorders (Jarchow and Mast 1999; Mast 2000; Merfeld et al. 2001; Wade and Curthoys 1997). We employed the SVV task because it was the method that could be taught most readily to rhesus monkeys and because the preponderance of the human tilt psychophysical literature utilizes this approach. While SVV responses in humans (and presumably monkeys) diverge from the true earth-vertical for large head tilts, these perceptual errors are relatively small for the limited range of tilt angles we employed (Van Beuzekom and Van Gisbergen 2000).
The possible effect of torsional eye position on the perceived spatial orientation of a visual line remains uncertain (reviewed in Mast 2000). Although most studies indicate that ocular torsion only minimally influences SVV measurements (see for example Haustein 1992; Haustein and Mittelstaedt 1990), there is evidence that the SVV could be offset by a magnitude close the amount of torsion (Wade and Curthoys 1997). We cannot directly address this possibility in our study, as we did not measure torsional eye position or make other psychophysical measures that were independent of vision. Given the small amplitude of ocular counter-rolling produced by roll tilt, IA translation, and centrifugation (Angelaki 1998; Suzuki et al. 1997; Wearne et al. 1999); however, it is unlikely that the SVV responses we recorded in rhesus monkeys were substantially altered by the influence of ocular torsion.
SVV responses during static tilt of the head or the GIF
ROLL TILT OF THE HEAD.
When the head was statically tilted in the roll plane, the monkeys aligned the light-bar close to the earth-vertical (Fig. 1, top), indicating a nearly veridical perception of the head tilt angle. Small orientation-dependent inaccuracies were observed in both animals (Fig. 1, bottom), however, with a slight overestimation of smaller tilt angles (≤20°) and possibly an underestimation of larger angles. These findings are qualitatively similar to the orientation dependence of SVV responses described in humans (A and E effects) (Guedry 1974; Van Beuzekom and Van Gisbergen 2000). The relatively small perceptual errors observed in the two monkeys may relate to their extensive training, as they were taught to rotate the light bar within ±4° of the true earth-vertical.
ROLL TILT OF THE GIF.
Sustained 30° tilts of the GIF during parabolic variable- and fixed-radius centrifugation resulted in a shift in PT toward alignment with the GIF, but the perceived tilt indicated by the SVV responses systematically underestimated the extent of GIF tilt (by an average of 21%, Figs. 7 and 8). In contrast, the PT responses during static 30° roll tilts of the head were larger, underestimating the head tilt angle by only 3.5% on average (Fig. 1). The reasons for this discrepancy are uncertain. The centrifugation paradigms were associated with either a yaw rotational cue that gradually decayed (fixed-radius) or an absence of canal rotational cues (variable-radius). It is possible that the presence of orthogonal canal cues or the absence of coplanar cues affected the maximum PT that developed during the 120 s of GIF tilt, but this seems unlikely because the effect of the canal cues would presumably dissipate during the sustained period of centrifugation. Similarly, the small difference in the magnitude of the GIF during roll tilt (1 g) and centrifugation (1.16 g) appears unlikely to influence the perceived tilt in a significant manner. It is possible that residual visual information may have contributed to the differences in PT observed in the tilt and centrifugation paradigms. While we attempted to minimize visual orientation cues (see methods), a small amount of light was produced by the LCD panel that displayed the SVV light bar. Because the visual display and surrounding draping remained upright during all experiments, any residual visual information indicating the direction of gravity could have reduced the amplitude of the SVV shift away from the earth-vertical during centrifugation. Other nonvestibular, nonvisual sensory cues, such as tactile information from the chair, could also have affected the monkeys' responses during centrifugation.
Both animals showed a substantial decay in PT (mean: 48%) during the sustained GIF tilt produced by parabolic variable-radius centrifugation, followed by reversal in PT (mean: 39%) when the centrifugal force was removed. Qualitatively similar observations have been made in human subjects tested with variable-radius protocols (Seidman et al. 1998). Although the decline in PT could reflect habituation or adaptation, the reversal (aftereffect) indicates that a central or peripheral adaptive process must have contributed to the response decay observed during centrifugation.
SVV responses during dynamic tilt of the head or the GIF
EVIDENCE FOR CANAL-OTOLITH INTERACTIONS.
The canal-otolith interaction model of GIF resolution (Merfeld and Zupan 2003; Merfeld et al. 1993) proposes that a central synthesis of canal and otolith cues contributes to the brain's estimate of head orientation. This model predicts therefore that percepts of head orientation should be affected by changes in canal-mediated rotational cues when gravito-inertial cues remain invariant. To investigate this hypothesis, we utilized three motion paradigms that shifted the GIF in the roll plane in a similar manner but provided different canal cues. Specifically the slow roll tilt paradigm rotated the GIF by 30° over 10 s at a constant velocity and was associated with coplanar roll canal cues; the parabolic variable- and fixed-radius centrifugation paradigms rotated the GIF by 30° over 10 s in an approximately parabolic manner with either no canal cues (variable radius) or with orthogonal yaw canal cues (fixed radius).
Similar to prior reports in human subjects (Merfeld et al. 2001; Seidman et al. 1998; Stockwell and Guedry 1970), we found that the canal cues had a substantial effect on the dynamics of tilt perception. During roll tilt, the SVV responses aligned with gravity without any appreciable delay, and the accuracy of the PT was the same if the head was moving or stationary. The congruent canal and otolith information during roll tilt appeared to facilitate the central processing that allowed the brain to rapidly and accurately estimate the underlying motion pattern (Stockwell and Guedry 1970). In contrast, parabolic variable-radius centrifugation shifted PT responses toward alignment with the GIF more slowly, and it therefore appears that the absence of a congruent canal cue slowed the neural processing that identified the motion pattern as a roll tilt of the head. Finally, fixed-radius centrifugation shifted SVV responses toward the GIF most slowly, and it is likely that the large orthogonal yaw rotational cue inhibited the development of the roll tilt percept, possibly because a sensory conflict developed when the GIF was tilted in the presence of a yaw rotational cue while the head was upright (Graybiel and Brown 1951; Merfeld et al. 2001). Taken together, these results provide convincing evidence that a central interaction between canal and otolith cues (Curthoys 1996) contributes to neural estimates of head orientation in rhesus monkeys.
EVIDENCE FOR FREQUENCY SEGREGATION.
While these results are consistent with predictions made by the canal-otolith interaction model of GIF resolution (e.g., Merfeld and Zupan 2002; Merfeld et al. 1993), they do not exclude contributions from the frequency-segregation model, which proposes that lower frequency shifts in GIF orientation are interpreted by the brain as gravitational in origin and higher frequency shifts as inertial (Paige and Tomko 1991). Percepts of roll tilt during sinusoidal variable-radius centrifugation, in particular, appear to support contributions from frequency-segregation. Similar to prior reports in human subjects (Glasauer 1995; Merfeld et al. 2005a; Zupan and Merfeld 2005), illusions of roll tilt were greatest at the lowest frequency and became smaller as the frequency of translation increased. While these findings suggest that high and low-pass filters may disambiguate the shifts in GIF produced by head translation (Merfeld et al. 2005; Paige and Tomko 1991), the frequency independence of the phase is not consistent with a simple filtering mechanism (Glausauer 1995).
SVV responses during roll optokinetic stimulation
Although the optokinetic stimulus we used was relatively small, filling only the central 30° of the visual field, both monkeys developed substantial shifts in SVV responses when exposed to the OK stimulation (mean magnitude of the shift was 12.4° for monkey H and 4.5° for monkey A). While we did not measure torsional eye position, prior studies have shown only small torsional deviations of the eyes during roll optokinetic stimulation (Ibbotson et al. 2005; Zupan and Merfeld 2003). Therefore it is unlikely that the shift in SVV responses resulted solely from changes in torsional eye position. Furthermore, prior work in human subjects has demonstrated that the SVV shifts produced by OK stimulation are not a visual illusion because subjective postural and visual vertical responses were affected similarly (Dichgans et al. 1972). Although we could not perform these postural controls in our animals, it is highly likely that roll OK stimulation induced an illusion of roll tilt in the rhesus monkeys. The tilt illusion was consistent with the OK cues produced during true roll tilt with perceived head orientation shifting toward the right during CCW OK stimulation and toward the left during CW stimulation. Because an illusion of roll tilt was produced by visually mediated rotational cues without shifts in GIF orientation, percepts of head orientation in monkeys, like humans (Dichgans et al. 1972, 1974; Zupan and Merfeld 2003) appear to depend at least in part on a central fusion of (visual) rotational cues and the otolith cues that sense the GIF.
The size of the SVV deviation depended on an interaction between the OK stimulation and static head orientation as the deviations were larger when the head was tilted in the direction of the optokinetic stimulation. For example, monkey A viewed a CW OK stimulus and the shift in SVV responses toward the right (negative in Fig. 5) was larger when the head was statically tilted toward the right. The basis of this interaction in rhesus monkeys, which is the reverse of that described in human subjects (Dichgans et al. 1974), is uncertain. Because the ocular counter-rolling associated with static head tilt in the direction of the OK stimulation should shift the torsional position of the eyes in the opposite direction (Suzuki et al. 1998), counter-rolling would be expected to reduce not augment any component of the SVV shift that results from torsional deviation of the eyes. A more likely explanation for these results is that the roll OK stimulation rendered the monkey's estimate of the earth-vertical less reliable and they responded by orientating the light bar closer to their own longitudinal axis.
Comparing psychophysical and oculomotor responses in monkeys
Vestibular-mediated perceptual and oculomotor responses appear to utilize qualitatively similar mechanisms in rhesus monkeys. Roll tilts, for example, produce veridical shifts in perceived tilt as well as eye movements that are compensatory for roll (torsion) without a significant horizontal (translational VOR) response (Angelaki et al. 1999). Sinusoidal variable-radius centrifugation elicits percepts of roll tilt that decline as the frequency increases, similar to the frequency-dependence of the torsional eye movement response during IA translation (Telford et al. 1997), and both tilt perception and torsional eye movements have compensatory phases during low-frequency translations (Angelaki 1998). Finally, fixed-radius centrifugation produces a gradual shift in both PT and in the rotational axis of the eye (Wearne et al. 1999). These results suggest that both the perceptual and oculomotor correlates of roll tilt in rhesus monkeys depend, at least in part, on a central synthesis of rotational and gravito-inertial cues.
Conclusions and future directions
Our results suggest that tilt psychophysics in rhesus monkeys and humans are qualitatively similar. In both species, the perception of roll tilt, measured with a subjective visual vertical task, appears to depend on a synthesis of the otolith cues that sense gravity and linear acceleration and the canal and visual cues that sense head rotation. We cannot make quantitative comparisons between rhesus and human psychophysical results, however, given the small number of monkeys studied, the inherent variability in SVV responses, and the differences in the motion paradigms employed in various studies. Future experiments that include a larger number of monkeys and motion paradigms that are identical to those used with human subjects would facilitate direct quantitative comparisons between human and non-human primates. The psychophysical approach we have developed also allows the use invasive experimental techniques such as vestibular stimulation (Lewis et al. 2006) and ablation (Lewis et al. 2007), and neural recordings to facilitate the study of spatial orientation in non-human primates.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grants DC-6909 to R. F. Lewis and DC-4158 to D. M. Merfeld.
Acknowledgments
We thank F. Persson, S. Park, D. Channer, F. Mast, L. Zupan, and R. Terry.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Angelaki 1998.Angelaki DE Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. III. Responses to translation. J Neurophysiol 80: 680–695, 1998. [DOI] [PubMed] [Google Scholar]
- Angelaki and Hess 1994.Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. I. Vestibuloocular reflex. J Neurophysiol 71: 1222–1249, 1994. [DOI] [PubMed] [Google Scholar]
- Angelaki 1999.Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJM. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci 19: 316–327, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks 1985.Blanks RHI, Curthoys IS, Bennett ML, Markham CH. Planar relationships of the semicircular canals in rhesus and squirrel monkeys. Brain Res 340: 315–324, 1985. [DOI] [PubMed] [Google Scholar]
- Bohmer and Rickenmann 1995.Bohmer A, Rickenmann J. The subjective visual vertical as a clinical parameter of vestibular function in peripheral vestibular diseases. J Vest Res 5: 35–45, 1995. [PubMed] [Google Scholar]
- Bortolami 2006.Bortolami SB, Pierobon A, DiZio P, Lackner JR. Localization of the subjective vertical during roll, pitch, and recumbent yaw body tilt. Exp Brain Res 173: 364–373, 2006. [DOI] [PubMed] [Google Scholar]
- Curthoys 1996.Curthoys I The delay of the oculographic illusion. Brain Res Bull 40: 407–412, 1996. [DOI] [PubMed] [Google Scholar]
- Dichgans 1974.Dichgans J, Diener HC, Brandt T. Optokinetic-graviceptive interaction in different head positions. Acta Otolaryngol 78: 391–398, 1974. [DOI] [PubMed] [Google Scholar]
- Dichgans 1972.Dichgans J, Held R, Young LR, Brandt T. Moving visual scenes influence the apparent direction of gravity. Science 178: 1217–1219, 1972. [DOI] [PubMed] [Google Scholar]
- Fetter 1992.Fetter M, Tweed D, Hermann W, Wohland-Braun B, Koenig E. The influence of head position and head reorientation on the axis of eye rotation and the vestibular time constant during postrotatory nystagmus. Exp Brain Res 91: 121–128, 1992. [DOI] [PubMed] [Google Scholar]
- Glasauer 1995.Glasauer S Linear acceleration perception: frequency dependence of the hilltop illusion. Acta Otolaryngol Suppl 520: 37–40, 1995. [DOI] [PubMed] [Google Scholar]
- Goodale 2004.Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol 14: 203–211, 2004. [DOI] [PubMed] [Google Scholar]
- Graybiel 1951.Graybiel A, Brown R. The delay in visual reorientation following exposure to a change in direction of resultant force on a human centrifuge. J Gen Psychol 45: 143–150, 1951. [Google Scholar]
- Green 2003.Green AM, Angelaki DE. Resolution of sensory ambiguities for gaze stabilization requires a second neural integrator. J Neurosci 23: 9265–9275, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green 2004.Green AM, Angelaki DE. An integrative neural network for detecting inertial motion and head orientation. J Neurophysiol 92: 905–925, 2004. [DOI] [PubMed] [Google Scholar]
- Guedry 1974.Guedry F. Psychophysics of vestibular sensation. In: Handbook of Sensory Physiology, edited by Kornhumber HH. New York:Springer-Verlag, 1974, p. 1–154.
- Haustein 1992.Haustein W Head-centric visual localization with lateral body tilt. Vision Res 32: 669–673, 1992. [DOI] [PubMed] [Google Scholar]
- Haustein 1990.Haustein W, Mittelstaedt H. Evaluation of retinal orientation and gaze direction in the perception of the vertical. Vision Res 30: 255–262, 1990. [DOI] [PubMed] [Google Scholar]
- Howard 1966.Howard I, Templeton W. Human Spatial Orientation. London: Wiley, 1966.
- Ibbotson 2005.Ibbotson MR, Price NS, Das VE, Hietanen MA, Mustari MJ. Torsional eye movements during psychophysical testing with rotating patterns. Exp Brain Res 160: 264–267, 2005. [DOI] [PubMed] [Google Scholar]
- Jarchow 1999.Jarchow T, Mast FW. The effect of water immersion on postural and visual orientation. Aviat Space Environ Med 70: 879–886, 1999. [PubMed] [Google Scholar]
- Johnson 1982.Johnson RA, Wichern DW. Applied Multivariate Statistical Analsysis. Engelwood Cliffs, NJ: Prentice-Hall, 1982.
- Landsberg 1965.Landsberg M, Guedry F, Gaybriel A. Effect of changing resultant linear acceleration relative to the subject on nystagmus generated by angular acceleration. Aerospace Medicine 36: 456–460, 1965. [Google Scholar]
- Lewis 2006.Lewis RF, Haburcakova C, Gong W, Merfeld DM. Effects of semicircular canal activation on perceived head orientation. ARO Abstr 1107, 2006.
- Lewis 2007.Lewis RF, Haburcakova C, Gong W, Lee D, Wall C, Merfeld DM. Vestibular influences on tilt perception and postural control in rhesus monkey. Soc Neurosci Abstr 399: 1, 2007. [Google Scholar]
- Lewis 2005.Lewis RF, Haburcakova C, Merfeld DM. Tilt psychophysics measured in non-human primates. Ann NY Acad Sci 1039: 294–305, 2005. [DOI] [PubMed] [Google Scholar]
- Mast 2000.Mast F Does the world rock when the eyes roll? Swiss J Psycho 59: 89–101, 2000. [Google Scholar]
- Meluill 1978.Melvill Jones G, Young LR. Subjective detection of vertical acceleration: a velocity-dependent response? Acta Otolaryngol 85: 45–53, 1978. [DOI] [PubMed] [Google Scholar]
- Merfeld 2005a.Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol 94: 186–198, 2005a. [DOI] [PubMed] [Google Scholar]
- Merfeld 2005b.Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined TiltandTranslation. J Neurophysiol 94: 199–2005, 2005b. [DOI] [PubMed] [Google Scholar]
- Merfeld 1996.Merfeld DM, Teiwes W, Clarke AH, Scherer H, Young LR. The dynamic contributions of the otolith organs to human ocular torsion. Exp Brain Res 110: 315–321, 1996. [DOI] [PubMed] [Google Scholar]
- Merfeld 1993.Merfeld DM, Young LR, Oman CM, Shelhamer MJ. A multidimensional model of the effect of gravity on the spatical orientation of the monkey. J Vestib Res 3: 141–161, 1993. [PubMed] [Google Scholar]
- Merfeld 2002.Merfeld DM, Zupan LH. Neural processing of gravito-inertial cues in humans. III. Modeling tilt and translation responses. J Neurophysiol 87: 819–833, 2002. [DOI] [PubMed] [Google Scholar]
- Merfeld 2003.Merfeld DM, Zupan LH. Influence of rotational cues on the neural processing of gravito-inertial forces. In: Levels of Perception, edited by Harris L, Jenkins L. New York: Springer-Verlag, 2003, p. 341–373.
- Merfeld 2001.Merfeld DM, Zupan LH, Gifford CA. Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol 85: 1648–1660, 2001. [DOI] [PubMed] [Google Scholar]
- Paige 1991.Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol 65: 1170–1182, 1991. [DOI] [PubMed] [Google Scholar]
- Seidman 1998.Seidman SH, Telford L, Paige GD. Tilt perception during dynamic linear acceleration. Exp Brain Res 119: 307–314, 1998. [DOI] [PubMed] [Google Scholar]
- Stockwell 1970.Stockwell CW, Guedry FE. The effect of semicircular canal stimulation during tilting on the subsequent perception of the visual vertical. Acta Otolaryng 70: 170–175, 1970. [DOI] [PubMed] [Google Scholar]
- Strupp 2003.Strupp M, Glasauer S, Schneider E, Eggert T, Glaser M, Jahn K, Brandt T. Anterior canal failure: ocular torsion without perceptual tilt due to preserved otolith function. J Neurol Neurosurg Psychiatry 74: 1336–1338, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki 1997.Suzuki Y, Kase M, Kato H, Fukushima K. Stability of ocular counterrolling and Listing's plane during static roll-tilts. Invest Ophthalmol Vis Sci 38: 2103–2111, 1997. [PubMed] [Google Scholar]
- Telford 1997.Telford L, Seidman SH, Paige GD. Dynamics of squirrel monkey linear vestibuloocular reflex and interactions with fixation distance. J Neurophysiol 78: 1775–1790, 1997. [DOI] [PubMed] [Google Scholar]
- Travis 1928.Travis RC, Dodge R. Experimental analysis of the sensorimotor consequences of passive oscillation—rotatory and rectilinear. Psych Mon 38: 1–96, 1928. [Google Scholar]
- Van Beuzekom 2000.Van Beuzekom AD, Van Gisbergen JAM. Properties of the internal representation of gravity inferred from spatial-direction and body-tilt estimates. J Neurophysiol 84: 11–27, 2000. [DOI] [PubMed] [Google Scholar]
- Wade 1997.Wade SW, Curthoys IS. The effect of ocular torsional position on perception of the roll-tilt of visual stimuli. Vision Res 37: 1071–1078, 1997. [DOI] [PubMed] [Google Scholar]
- Wearne 1999.Wearne S, Raphan T, Cohen B. Effects of tilt of the gravito-inertial acceleration vector on the angular vestibuloocular reflex during centrifugation. J Neurophysiol 81: 2175–2190, 1999. [DOI] [PubMed] [Google Scholar]
- Young 1984.Young LR. Perception of the body in space: mechanisms. In: Handbook of Physiology. The Nervous System, edited by Darian-Smith I. Bethesda, MD: Am. Physiol. Soc., 1984, sect. 1.
- Zupan 2003.Zupan LH, Merfeld DM. Neural processing of gravito-inertial cues in humans. IV. Influence of visual rotational cues during roll optokinetic stimulation. J Neurophysiol 89: 390–400, 2003. [DOI] [PubMed] [Google Scholar]
- Zupan 2005.Zupan LH, Merfeld DM. Human ocular torsion and perceived roll responses to linear acceleration. J Vestib Res 15: 173–183, 2005. [PMC free article] [PubMed] [Google Scholar]