Abstract
Motoneuron activation is strongly influenced by persistent inward currents (PICs) flowing through voltage-sensitive channels. PIC characteristics and their contribution to the control of motoneuron firing rate have been extensively described in reduced animal preparations, but their contribution to rate modulation in human motoneurons is controversial. It has recently been proposed that the analysis of discharge records of a simultaneously recorded pair of motor units can be used to make quantitative estimates of the PIC contribution, based on the assumption that the firing rate of an early recruited (reporter) unit can be used as a measure of the synaptic drive to a later recruited (test) unit. If the test unit's discharge is augmented by PICs, less synaptic drive will be required to sustain discharge than required to initially recruit it, and the difference in reporter unit discharge (ΔF) at test recruitment and de-recruitment is a measure of the size of the PIC contribution. We applied this analysis to discharge records of pairs of motoneurons in the decerebrate cat preparation, in which motoneuron PICs have been well-characterized and are known to be prominent. Mean ΔF values were positive in 58/63 pairs, and were significantly greater than zero in 40/63 pairs, as would be expected based on PIC characteristics recorded in this preparation. However, several lines of evidence suggest that the ΔF value obtained in a particular motoneuron pair may depend on a number of factors other than the PIC contribution to firing rate.
INTRODUCTION
The excitatory drive that controls motoneuron firing rate is provided not only by ionotropic synaptic input but also by persistent inward currents (PICs) flowing through voltage-sensitive channels on the dendrites (Heckman et al. 2003). PICs have been shown to contribute substantially to the net excitatory drive to motoneurons in the cat, rat, mouse, and turtle (reviewed in Powers and Binder 2001) and are presumed to play an analogous role in the control of discharge in human motoneurons. Because PICs cannot be measured directly in human motoneurons, their contribution to motoneuron excitation can only be inferred from the characteristics of human motoneuron discharge behavior. It is for this reason that animal experiments are critical for interpreting human motoneuron discharge behavior, particularly when synaptically driven discharge and intracellular recordings can be obtained in the same preparation. The goal of the present study was to test the utility of a recently proposed technique for estimating PIC properties in human motoneurons by analyzing synaptically evoked motoneuron discharge in the decerebrate cat.
Gorassini et al. (2002) have proposed that PIC properties can be estimated in human motoneurons based on certain features of the discharge behavior of a pair of simultaneously active human motor units. The rationale is based on the idea that as the net excitatory synaptic drive to a motoneuron increases, the low-threshold dendritic channels meditating PIC are first activated around the threshold for repetitive discharge. They stay activated as synaptic drive is decreased, allowing the motoneuron to continue to fire at a lower level of synaptic drive than was originally required to initiate discharge. Because motoneurons innervating the same muscle often receive common synaptic input (Binder et al. 1996; Keen and Fuglevand 2004; Kirkwood and Sears 1978), Gorassini et al. (2002) argued that the firing rate of an early-recruited (reporter) unit can be used as a measure of the synaptic drive to a later-recruited (test) unit. If the test unit's discharge is augmented by PICs, less synaptic drive will be required to sustain discharge than required to initially recruit it, and the firing rate of the reporter unit will be higher when the test unit is recruited than when the test unit is de-recruited. The difference in firing rate (ΔF) is thought to reflect the relative magnitude of the PIC contribution to the total net excitatory drive to the test unit (Gorassini et al. 2002). Recordings from pairs of tail motoneurons in awake chronic and acute spinal rats supported the validity of this technique (Bennett et al. 2001). Positive ΔF were generally found for motor-unit pairs from chronic spinal but not from acute spinal animals, consistent with larger PICs present in motoneurons from chronic spinal animals (cf. Harvey et al. 2006).
The characteristics of PICs have also been extensively studied in spinal motoneurons in the unanesthetized, decerebrate cat based on somatic intracellular current- and voltage-clamp recordings (reviewed in Heckman et al. 2005). In this preparation, PICs are found in all motoneurons (Lee and Heckman 1998a,b, 1999), are more readily activated by synaptic inputs than by somatically injected current (Bennett et al. 1998), and provide more excitatory drive to motoneurons than any of the major synaptic input sources studied to date (Binder 2003). Therefore if positive ΔF values obtained from paired unit recordings reflect a prominent PIC contribution to excitation, positive ΔF values should be a consistent feature of paired discharge recordings of spinal motoneurons in the decerebrate cat.
Our results are consistent with this expectation; average ΔF values were positive in almost all cases. However, there was considerable variability in ΔF values that is likely to reflect the sensitivity of ΔF to several factors other than PIC amplitude. These multiple dependencies of ΔF limit the strength of inferences about the role of PIC in firing rate modulation that can be drawn from ΔF measurements.
METHODS
Surgical and experimental procedures
Data were collected from five adult cats (2.5–3.5 kg) with the approval of the Wright State University Institutional Animal Care and Use Committee. Anesthesia was induced in a closed chamber and maintained via a tracheal cannula throughout the initial dissection with a gaseous mixture of isofluorane (1.5–2.5%) in O2. Artificial respiration was adjusted to hold end-tidal CO2 between 3 and 4%. The right carotid artery and jugular vein were cannulated for monitoring blood pressure and administering fluids, respectively. In three experiments in which recordings were made from MG axons using micropipettes, the lumbosacral enlargement was exposed by a laminectomy from L4 to S1 to provide access to the L7 and S1 ventral roots. In these same experiments, the left hindlimb was dissected to expose the MG muscle nerve, and the triceps surae muscles were separated from their surrounding tissues. After separating the plantaris tendon, the remainder of the Achilles tendon was cut and attached to a servomotor that provided the muscle stretch stimulus. The TA-EDL tendon was also dissected free from surrounding structures to allow application of tonic stretch. In two experiments in which MG motor-unit activity was recorded using fine wire intramuscular electrodes, no laminectomy was performed, and only minimal dissection of the left hindlimb was performed to allow access to the MG muscle belly. In all experiments, animals were then mounted in a recording frame, and following ligation of the left carotid artery, an intercollicular decerebration was performed. Anesthesia was discontinued after the decerebration. At the end of the experiment, animals were killed using a lethal dose of KCl (oversaturated solution, intravenous).
Recording motor axon/motor unit firing
MOTOR AXON PAIRS.
Records of MG motoneuron action potential firing were obtained by positioning glass micropipette electrodes (2 M K-acetate, 15–20 MΩ) into axons traversing ventral roots L7 or S1. MG axons were identified by the antidromic action potentials they produced on electrical stimulation (0.04-ms pulses, 1 Hz) through extracellular hook electrodes placed on the MG muscle nerve. These procedures were repeated to obtain intra-axonal records simultaneously from two individual MG motor axons. Intra-axonal voltages were amplified (×100) and filtered (0.1–20 kHz) on separate channels (Axoprobe). To eliminate the movement caused by strong segmental reflexes that can otherwise dislodge micropipettes, a paralytic agent (pancuronium bromide, 1 ml iv repeated as needed) was administered to maintain paralysis throughout data collection. This recording approach achieved absolute isolation of activity in two axons, thereby providing for discrimination of activity in one pair of motoneurons during strong reflexes that activated many motoneurons. However, the fact that a substantial fraction of motoneurons could not be activated reflexively reduced the data yield obtained by sampling only two axons at a time, and this was the basis for taking the additional approach of intramuscular recording described next.
MOTOR-UNIT PAIRS.
Extracellular records of MG motor-unit action potentials were obtained by inserting up to five pairs of microwires (twisted pairs of insulated stainless steel wires—50 μm, California Fine Wire) into the MG muscle. The wires (≤6 in long) were connected through separate unity-gain differential amplifiers to optically isolated amplifiers (gain: ×1,000; bandwidth: 30 Hz to 300 kHz). This approach yielded potentials that were reliably discriminated (see following text) from at least five individual motor units. The advantage of this approach over motor-axon recording was that motor-unit firing was recorded in every data sample. It was necessary, however, to constrain the level of reflex activity, which if too high, prevented confident discrimination of action potentials from individual motor units. These records were biased, therefore toward motoneurons with lower recruitment thresholds.
Voltage records from each electrode were recorded and digitized at ≥20 kHz (Spike2, Cambridge Electronic Design) on separate A/D channels and stored for off-line discrimination and analysis of motor axon or motor-unit firing. Discrimination of action potentials from individual axons or units was achieved using Spike 2 software to generate distinct voltage-profile templates (wave marks) for potentials in each recording channel. Unlike motor-axon records, multiple templates were typical for single channels of intramuscular records. Reliability of the motor-unit data included for analysis in this study was optimized by accepting only those cases for which templates were plainly distinct throughout each recruitment trial. In these cases, a smooth progression of firing rate was consistent with the absence of false negative discrimination and interspike histograms revealed no false positives. Motor units with dubious discrimination at any point during reflex contraction were discarded from further study.
Physiological activation of MG motoneurons
We recruited MG motoneurons and modulated their discharge rate primarily by pinching the skin around the ankle. The cutaneous and nocioceptive receptors in this area are innervated by the sural nerve, which provides a powerful excitatory input to MG motoneurons (e.g., Cope and Clark 1991; Prather et al. 2001, 2002). We also activated MG motoneurons by vibrating and/or stretching the triceps surae muscles. In some trials, excitatory stretch and cutaneous stimulation was combined with inhibition produced by tonic stretch of the TA-EDL muscles. The stimuli were applied alone and in combination to produce firing rate modulation of at least two MG motoneurons.
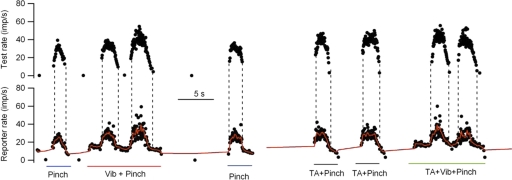
Figure 1 shows an example of discharge records obtained from a pair of MG motoneuron axons isolated in the L7 ventral root. Comodulation of the instantaneous discharge rates (black dots) of the two units was produced by pinching the skin around the ankle either alone or in combination with vibration of the triceps surae muscle (Vib) and/or tonic stretch of the TA-EDL muscles (TA). The dotted vertical lines mark the onset and offset of firing in the later recruited (test unit). As described in more detail in the following text, a number of characteristics of paired unit discharge were calculated based on the smoothed firing rate of the earlier recruited (reporter) unit (red lines in lower record).
FIG. 1.
Examples of motoneuron firing recorded from a pair of medial gastrocnemius (MG) motor axons. Black circles, instantaneous discharge rate of the earlier recruited (reporter) unit (bottom traces) and the later recruited (test) unit (top traces). The red trace represents the smoothed firing rate of the reporter unit. The vertical dotted lines show the times of onset and offset of discharge in the test unit. The stimuli used to elicit discharge are indicated below each epoch of repetitive discharge: pinch applied to sural dermatome, vibration applied to MG muscle, stretch applied to tibialis anterior (TA) muscle.
Data analysis
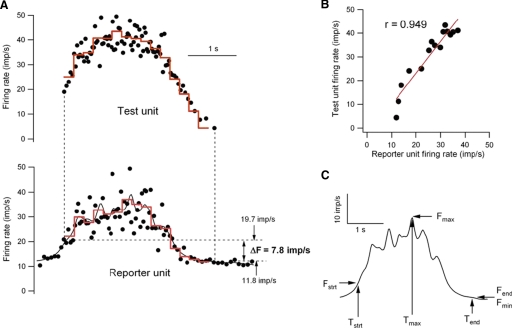
The times of spike occurrence of each discriminated unit were detected using Spike 2 spike recognition software and exported as text files for subsequent analysis in Igor (WaveMetrics). Instantaneous discharge rate was calculated as the reciprocal of each interspike interval, and the time associated with each frequency value was the midpoint of the interspike interval. An epoch of repetitive discharge was considered for further analysis if at least two motoneurons discharged for longer than one second at instantaneous rates >4 imp/s. Figure 2 illustrates the analysis of one such epoch. The filled circles in A are the instantaneous frequency of test unit (top trace) and reporter unit (bottom trace) versus time. The solid black line in lower trace is the smoothed firing rate of the reporter unit (Igor's Gaussian smoothing routine using 5 points before and after the point in question). ΔF is the difference in the value of the smoothed reporter rate at the time of the first and last test frequency value >4 imp/s (indicated by the vertical dashed lines). The red traces are firing rates averaged over 0.2-s time windows. The correlation of the relation between averaged test and reporter firing frequencies (referred to here as rate-rate relation) is shown in B. In many trials, the number of average rate points was low (<10), so even high linear correlation coefficients (r) were not statistically significant (i.e., P value for the t-ratio >0.05). For this reason, the rate-rate correlations were considered to be strong if the r2 value was >0.5 (i.e., r > 0.707). A number of other values are calculated from the smoothed reporter rate as shown in C. The amount of smoothed rate modulation is Fmax –Fmin. The normalized amount of rate modulation is (Fmax − Fmin)/(Tend –Tstrt). The average rates of smoothed reporter rate increase and decrease are given by (Fmax –Fstrt)/(Tmax − Tstrt) and (Fmax –Fend)/(TmaxTend).
FIG. 2.
Analysis of paired discharge records. A: instantaneous discharge rates (black dots) of the test unit (top) and reporter unit (bottom). The solid black line in the bottom trace is the smoothed firing rate of the reporter unit. The red lines represent the firing rates of the 2 units averaged over the same 0.2-s time bins. B: relation between the average rates of the test and reporter units (red lines in A). C: characterization of time course and amplitude of the modulation of smoothed firing rate in the reporter unit. See text for further details.
Values are presented as means ± SD. A one-sided t-test was used to determine if ΔF values were significantly greater than zero.
RESULTS
ΔF is positive for most unit pairs
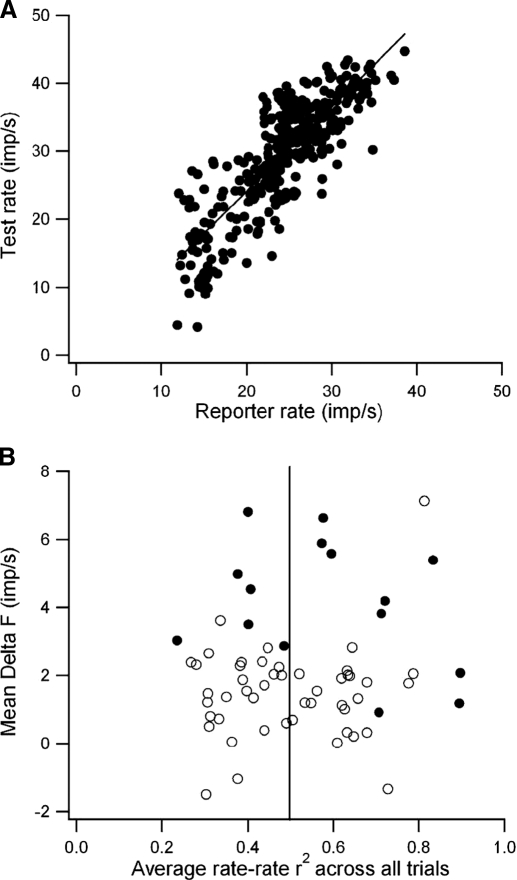
We report here on discharge characteristics from 63 motoneuron pairs in which we recorded at least five epochs in which the discharge of both motoneurons lasted >1 s. Fifteen pairs were obtained from dual intraxonal recordings, and 48 pairs were obtained with intramuscular recordings. Because the quantitative link between the magnitude of ΔF and the proportion of excitatory drive provided by PIC depends on the assumption that the firing rate of the reporter unit is “a sensitive and linear indicator of net excitatory synaptic drive at the time of the test unit recruitment and de-recruitment” (Gorassini et al. 2002), we initially restricted our analysis to trials in which the rate variations of the two units were highly correlated (r2 > 0.5), indicating that modulation of synaptic drive in the two units was similar. For these trials, mean ΔF values were positive in 58/63 pairs and were significantly greater than zero in 40/63 pairs (t-test, P > 0.05). In 43/63 pairs at least five epochs of firing had high rate-rate correlations. In this subset, mean ΔF values were significantly greater than zero in 37/43 comparisons.
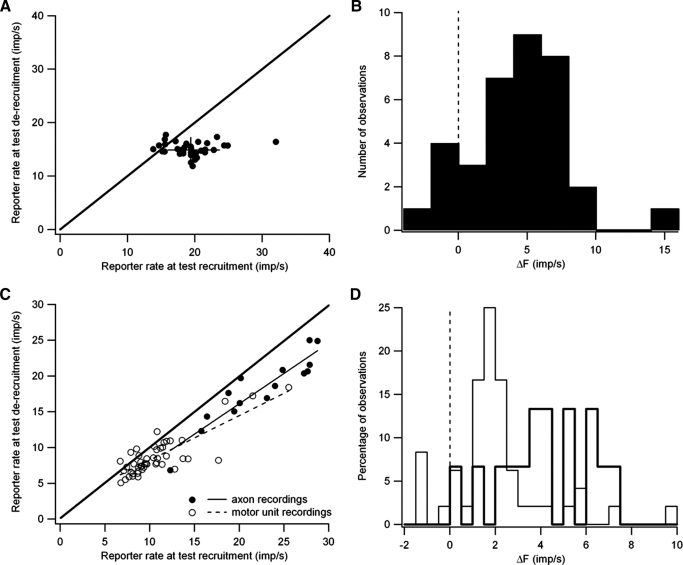
The intra-axonal recordings allowed more stable unit isolation (see methods), so that in all but one pair we were able to obtain more than five trials in which the rates of the two units were highly correlated, and in six pairs, we obtained ≥20 trials. Figure 3, A and B illustrate the data obtained from one particularly well-studied pair. For each trial in which the variations in mean firing rate (averaged over 0.2-s bins) of the reporter and test units were well-correlated (r2 > 0.5), ΔF was calculated as the difference in the value of the smoothed reporter rate at the time of test recruitment versus the time of test de-recruitment. Figure 3A shows the relation between the smoothed reporter rate at test de-recruitment versus test recruitment. Most points (30/35) fall below the line of identity, indicating that the test unit was de-recruited at a lower reporter rate than at recruitment, i.e., ΔF is positive, potentially indicating that more synaptic drive is needed to recruit the test unit than is needed to sustain its discharge. For this unit pair, there was a larger variation in the values of the smoothed reporter rate at test recruitment than at test derecruitment. However, this was not a consistent finding; across the entire sample the coefficients of variance for these two values were not significantly different.
FIG. 3.
Positive ΔF values for a single motor-axon pair (A and B) and for the entire sample of units (C and D). A: smoothed reporter rate at the time of test unit de-recruitment versus the smoothed reporter rate at the time of test recruitment for all trials (35) in which the rate modulation of the test and reporter units were well correlated. Diagonal line is the line of identity. Vertical and horizontal lines indicate mean ±1 SD for reporter rate at test de-recruitment and recruitment, respectively. B: distribution of ΔF values calculated as the difference if smoothed reporter rate at test recruitment and de-recruitment for the same motor-axon pair. C: averaged smoothed reporter rate at the time of test unit de-recruitment versus the smoothed reporter rate at the time of test recruitment for motor-axon pairs (filled circles) and motor-unit recordings (open circles). Diagonal line is the line of identity. D: Distribution of average ΔF values for motor-axon pairs (bold lines) and motor-unit pairs (thin lines).
Figure 3B shows the distribution of ΔF values for the motor-axon pair examined in A. The mean ΔF value (4.41 ± 3.62 imp/s) was significantly greater than zero, and the majority of ΔF values fell between 2 and 8 imp/s. All of the negative ΔF values and two of the three ΔF values between 0 and 2 imp/s were obtained in trials in which the test unit was recruited shortly after the reporter unit (<0.25 s). Figure 3C summarizes data obtained from all motor-axon pairs exhibiting high rate-rate correlations. As was the case for the example pair shown in Fig. 3A, the average smoothed reporter rate at the time of test de-recruitment was almost always lower (58/63 pairs) than the rate at the time of test recruitment. Reporter firing rates at both test recruitment and de-recruitment were significantly lower for motor-unit pairs (open circles) than for motor-axon pairs (filled circles). The mean reporter rate at test recruitment was 10.71 ± 3.69 imp/s for motor-unit recordings versus 22.29 ± 5.17 imp/s for motor-axon recordings (t = −9.60, P < 0.001), and the reporter rate at test de-recruitment was comparably lower as well (8.67 ± 2.77 imp/s for motor-unit recording vs. 18.06 ± 4.76 imp/s for motor-axon recording; t = −9.52, P < 0.001). These differences are consistent with the idea that our motor-unit recordings preferentially sampled motoneurons with recruitment thresholds lower than those sampled in our motor-axon recordings (see methods). For both types of recordings, there was a significant positive correlation between the values of the reporter unit at test recruitment and de-recruitment (motor-unit recordings: dashed line, r = 0.830, P < 0.001; motor-axon recordings: r = 0.921, P < 0.001).
Figure 3D shows the distribution of ΔF values for motor-axon (thick lines) and motor-unit pairs (thin lines). The mean ΔF values for motor-unit pairs were significantly lower than those obtained from the motor-axon pairs (2.03 ± 2.07 vs. 4.22 ± 2.02 imp/s; unpaired t = 3.60, P < 0.001). Nonetheless, there was overlap in the distributions of ΔF values for the two samples, and Fig. 3C shows that for the four motor-unit pairs in which the reporter rate at test recruitment was >15 imp/s, mean ΔF values (reflected in the horizontal distance from the line of identity) were similar to those obtained from the motor-axon pairs (5.73 vs. 4.22). Indeed, for motor-unit pairs, there was a positive correlation between ΔF and the reporter rate at test recruitment (r = 0.679, P < 0.001).
The lower ΔF values in the more slowly discharging, lower threshold motoneurons could reflect a lower PIC contribution to rate modulation of lower threshold motoneurons. The available experimental evidence from intracellular recordings in this preparation indicates that the peak PIC amplitude is similar across the MG motoneuron pool and that PICs are more persistent in lower threshold units (Lee and Heckman 1998a), predicting a larger PIC contribution to recruiting and sustaining discharge in low-threshold motoneurons. However, it is likely that a number of factors other than PIC amplitude may affect measured ΔF values, including the amount of rate modulation in the reporter unit and the relative recruitment thresholds of the reporter and test units.
Relation of ΔF values to the amount of rate modulation
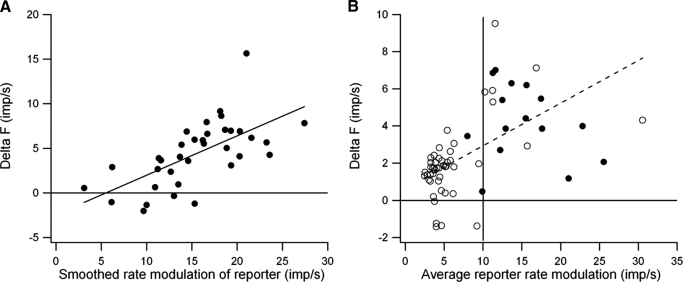
Figure 4A illustrates the relation between ΔF values and the amount of rate modulation in the reporter unit during the time that the test unit was active for the motor-axon pair shown in Fig. 3, A and B. For this unit pair, there was a significant positive correlation between ΔF values and the amount of rate modulation in the reporter unit. Overall significant positive correlations between these two measures were obtained in about a third of the motoneuron pairs (5/15 motor-axon pairs and 14/48 motor-unit pairs). Because the amount of rate modulation in the reporter unit should be related to the variation in synaptic drive to the two units, and ΔF values are thought to reflect the PIC contribution to firing rate modulation (Gorassini et al. 2002), this result is consistent with graded activation of PIC by synaptic input, although other factors may contribute (see discussion). For example, this relation might simply reflect the fact that the maximum ΔF value is constrained by the amount of reporter rate modulation; if the reporter unit shows little or no modulation, ΔF values must also be small.
FIG. 4.
Dependence of ΔF on the amount of reporter rate modulation. A: relation between ΔF values and reporter rate modulation for the motor-axon pair shown in Fig. 3, A and B. B: relation between average ΔF and the averaged smoothed rate modulation of the reporter unit at test recruitment for motor-axon (•) and motor-unit pairs (○), --- least-squares linear regression for data from motor-unit pairs (r = 0.54, P < 0.01).
Figure 4B shows the relation between average ΔF values and the average amount of reporter rate modulation for both motor-axon (•) and motor-unit pairs (○). On average the reporter units in the motor-unit sample showed significantly less rate modulation than those in the sample of motor-axon pairs (6.09 ± 4.84 vs. 15.17 ± 4.95 imp/s, t = 6.31, P < 0.001). Nonetheless, the motor-unit pair sample covered a wider range of reporter rate modulation and exhibited a significant positive correlation (r = 0.538, P < 0.01) with ΔF values. For cases in which the average reporter rate modulation is >10 imp/s, the average ΔF values for the motor-unit and motor-axon pairs were not significantly different (5.61 ± 2.22 vs. 4.56 ± 1.85 imp/s, t = 1.08, NS).
Relation of ΔF values to choice of reporter unit
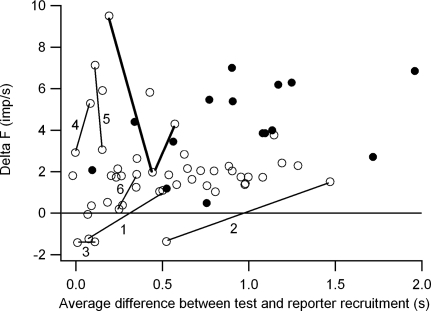
The reporter units used for calculating ΔF values were recruited over a range of time intervals relative to the recruitment of the test unit, which we take as an indication of the range of relative recruitment thresholds of the two units. Figure 5 shows the relation between average ΔF values and the average time between the recruitment of the reporter and test units. Overall, there was no relation between ΔF and relative recruitment threshold. However, 3/4 negative ΔF values were obtained for motoneuron pairs that were recruited within 0.25 s of one another (i.e., pairs that had similar recruitment thresholds). The paired motor-unit data included several recordings from more than two motor units, so that the same test unit could be paired with more than one reporter unit. Of the 48 motor-unit pairs, 20 pairs were derived from 10 test units paired with 2 different reporter units, and 15 pairs were derived from 5 test units paired with 3 different reporter units. In some cases (4 of the test units paired with 2 different reporters and 2 of the test units paired with 3 different reporters), the choice of reporter units made little difference and the ΔF values derived from different reporters differed by <1 imp/s. However, in the remaining cases, ΔF values could vary widely depending on which reporter unit was chosen. In six cases in which test units were paired with two different reporter units, one or both pairs had reporter-test combinations with similar recruitment thresholds. These four cases are represented in Fig. 5 by open circles connected by thin lines. In two cases (1 and 2), ΔF values were negative when the reporter and test units were recruited within 0.6 s of one another and positive for the reporter-test combination with the larger recruitment difference. In one case (3), both ΔF values were negative and both reporter-test combinations exhibited similar recruitment thresholds. In the remaining cases (4–6), both reporter-test combinations showed similar recruitment thresholds, and the ΔF values, although both positive, differed by >1 imp/s. A similar wide variation in ΔF values was seen in one case in which the test unit was paired with three different reporter units (open circles connected by bold lines). Overall these results suggest that ΔF values can depend on the choice of reporter unit and can also vary widely for reporter-test combinations with similar recruitment thresholds.
FIG. 5.
Relation between ΔF values and the average difference in the recruitment time of the reporter and test units. ○, from motor-unit pairs; •, from motor-axon pairs. —, ΔF values obtained for the same test motor unit using different reporter units. See text for further details.
Relation of ΔF values to co-variation of firing rates of reporter and test units
As described in the preceding text (see methods), the degree of co-variation of the firing rates of the reporter and test units was assessed by calculating the average firing rate of both units over the same 0.2-s time bins (Fig. 2A, red traces) and calculated the linear correlation between average firing rate values throughout the trial (Fig. 2B). Ideally, if the reporter firing rate is to be considered a “a sensitive and linear indicator of net excitatory synaptic drive” to the test unit (Gorassini et al. 2002), then a tight linear correlation between the rates of the two units should be obtained not only for individual trials but also when the firing rates of the two units are compared across all trials. Figure 6A shows the relation between the averaged firing rate values of the test unit versus those of the reporter unit compiled across all trials that exhibited high rate-rate correlations (r2 > 0.5) for one motor-axon pair. The variations in test and reporter firing rates were highly correlated across all trials (r = 0.831), and the correlation coefficient calculated across all trials was similar to the mean correlation coefficient for individual trials (0.864), indicating that not only were variations in test and reporter firing rates highly correlated in individual trials but that the slopes and intercepts of these linear relations showed relatively little trial-to-trial variation. However, in many other cases the correlation coefficients calculated for rate-rate variations compiled across all trials were significantly lower than the average correlation coefficients for individual trials, indicating that the linear relation between test rate and reporter rate varied from trial to trial.
FIG. 6.
A: correlation between test rate and reporter rate (averaged over 0.2-s time bins) compiled from all 35 trials with high rate-rate correlations for the motor-axon pair of Fig. 3, A and B. B: relation between average ΔF values and average r2 value for rate-rate correlations for all units and trials. Note that ΔF values are predominantly positive even in cases when the average rate-rate correlations are low.
Figure 6B illustrates the relation between the average ΔF values and the average r2 value for rate-rate correlations across all trials for all pairs. Positive ΔF values clearly predominate even for motor-axon and motor-unit pairs in which the firing rates do not tightly co-vary for most of the trials. In a large proportion (27/63) of the pairs, the rate variations of the two units were not correlated in the majority of the trials. Nonetheless, positive ΔF values were often obtained in trials in which the rate-rate correlations were low, and the mean ΔF values for these 27 pairs were only slightly lower when all trials were included compared with those based only on trials with high rate-rate correlations (2.01 ± 1.77 vs. 2.79 ± 2.60 imp/s), although this difference was significant (t = 2.17, P < 0.05).
Nonlinear rate-rate relations
In many of the motor unit pairs, the similar ΔF values simply reflected the fact that there was little rate modulation in either the test or reporter unit so that ΔF values were low for all trials. However, in other cases for both motor-axon and motor-unit pairs, the test unit showed large variations in rate that were associated with much smaller variations in the rate of the reporter unit. In many of these cases, the rate-rate plots for individual trials exhibited a counter-clockwise hysteresis, in which the firing rates of the test and reporter units increased and decreased in parallel at the beginning and end of the trial, but following the initial increase in rate, the reporter rate declined while the test unit rate was still increasing. Figure 7A illustrates six rate-rate plots that exhibit this behavior, all taken from the same motor-axon pair. Positive ΔF values were obtained in all six of these trials in spite of the fact that none of them exhibited a strong rate-rate correlation. Figure 7B shows the average firing rate versus time for the reporter (filled circles) and test units (open circles) for two of these trials. The reporter unit shows much less rate modulation than the test unit and the reporter rate begins to decline prior to the test rate. One potential explanation for the fact that all of these trials have positive ΔF values is that the discharge rate of the reporter unit could provide a reasonable estimate of the synaptic drive to the test unit at the times of test recruitment and de-recruitment, without necessarily reflecting the level of synaptic drive to the test unit throughout the entire trial (see discussion). In contrast, if the firing rate of the reporter unit exhibits saturation prior to the recruitment of the test unit, this would lead to a smaller ΔF value and an underestimate of the PIC contribution to rate modulation.
FIG. 7.
A: rate-rate relations from 6 trials from the same motor-axon pair. B: rate vs. time for the reporter unit (filled circles) and test unit (○) for 2 of these trials.
Dependence of rate-rate correlation on source of synaptic input
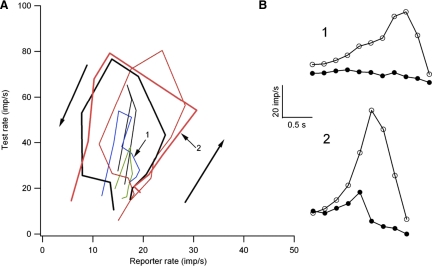
Co-modulation of firing rate was measured for nine motor-axon pairs driven to fire by different individual and combined sources of synaptic input (see Fig. 1). In seven of these nine cases, the rate-rate relations obtained with different synaptic inputs did not differ significantly. However, in the other two cases, rate-rate relations for at least one of the synaptic inputs differed significantly from the others as shown in Fig. 8. In both of these cases, there was a strong positive correlation between the firing rates of the test and reporter units when they were driven by skin pinch in combination with MG muscle vibration (red symbols). For the pair shown in Fig. 8B, a similar correlation was obtained when TA stretch was superimposed (green symbols). However, for the other synaptic input combinations, there was either no correlation in the firing rates of the two units or a correlation with a significantly different slope. These results suggest that although motoneurons innervating the same muscle generally receive similar synaptic inputs regardless of the input source, this is not always the case. Together with the variability in ΔF values when different reporter units are used with the same test unit, these results suggest that the firing rate of a given reporter unit is likely to bear a variable relation to the net excitatory input to the test unit.
FIG. 8.
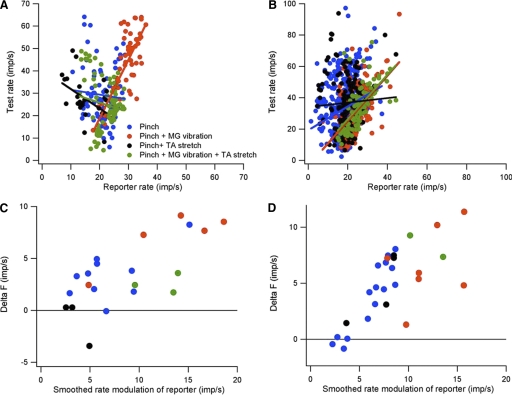
Effects of source of synaptic input on rate-rate relations, rate modulation and ΔF values. A and B: relations between reporter and test firing rates for 2 different motor-axon pairs in response to 4 different combinations of synaptic input. C and D: relation between ΔF values and amount of reporter rate modulation for the motor-axon pairs shown in A and B.
Figure 8, C and D, shows the relation between ΔF values and smoothed reporter rate modulation for the four different sources of synaptic input for the same motor-axon pairs shown in A and B. In both cases, both ΔF values and the amount of reporter rate modulation were highest when skin pinch was combined with muscle vibration and lowest when skin pinch was combined with TA stretch; the other two inputs produced intermediate values. These results suggest that estimates of the contribution of intrinsic currents to motoneuron excitation can also depend on the source of synaptic input.
DISCUSSION
This study was designed to examine critically a recently proposed technique for estimating the contribution of intrinsic PICs to firing rate modulation in motoneurons Because this technique represents the only available method to derive quantitative estimates of the PIC contribution to the excitation of human motoneurons, it is important to test its validity in a preparation in which PICs are well documented and known to make a prominent contribution to the excitatory drive to motoneurons (cf. Heckman et al. 2003). The technique is based on the idea that the difference in the firing rate (ΔF) of a lower threshold (reporter) unit and the time of recruitment and de-recruitment of a higher threshold (test) unit is proportional to the PIC contribution to the net excitatory drive to the test unit (Gorassini et al. 2002). We found that ΔF values were positive in nearly all unit pairs studied, consistent with the notion that PIC is reflected as a positive ΔF value. Our average ΔF values (4.2 imp/s for motor-axon pairs and 2.0 imp/s for the motor-unit pairs) are comparable to an average ΔF value of 3.6 imp/s measured from motor-unit pairs in human subjects (Gorassini et al. 2002). The use of intra-axonal recordings allowed us to record from higher threshold motoneuron than would generally be possible with motor unit recordings. The larger ΔF values in these high-threshold motoneurons could reflect a relatively greater PIC contribution to their excitation but could also result from the fact that rate modulation of the reporter units was greater in motor-axon pairs (see Fig. 4B). Comparison of ΔF values for motor-unit pairs that were biased toward lower threshold units in the samples from both human and decerebrate cat suggests that the PIC contribution to motoneuron excitation is relatively greater in normal human subjects. We also found large variability in ΔF values, indicating that PIC is highly variable across trials and across units and/or that ΔF is dependent on factors other than PIC amplitude.
Sources of variability in ΔF
In many unit pairs, there was a significant positive correlation between the amount of rate modulation in the reporter unit and ΔF values. Because both firing rate and PIC activation are potentially modulated by the net excitatory synaptic input, this correlation might reflect graded synaptic activation of PIC. In other words, when a motoneuron is first recruited at a given level of excitatory synaptic input, the channels contributing to the total PIC are not maximally activated—further increases in net excitatory input are needed to fully activate the channels. Thus as excitatory synaptic input increases, both the total amount of rate modulation and the total PIC contribution to rate modulation increase as well. A greater PIC contribution should be associated with more stable or widespread dendritic plateau potentials, leading to a larger difference between the synaptic input required to recruit and de-recruit a motoneuron, which will be reflected as a larger ΔF. Alternatively, the correlation could simply reflect that fact that ΔF is limited by the amount of rate modulation in the reporter unit; for example, if the reporter unit only changes its rate by 5 imp/s over the time in which the test unit is active, the maximum ΔF is 5. This could potentially affect maximum ΔF values in human motoneurons, many of which do not exhibit marked rate modulation during slow, moderate isometric contractions (e.g., Monster and Chan 1977). Limited rate modulation is also seen in pathological conditions (Gemperline et al. 1995).
We found that ΔF showed the greatest variability and could even be negative when the recruitment thresholds of the reporter and test units were similar. Further, ΔF values for a given test unit could be either positive or negative depending on which reporter unit was chosen. One possible explanation for these results is that the degree of PIC activation in the reporter unit varies over the time that the test unit is active. Because PIC contributes to rate modulation, this implies that even if the reporter and test units receive identical synaptic inputs, the firing rate of the reporter unit will bear a variable relation to the amount of synaptic input to the test unit.
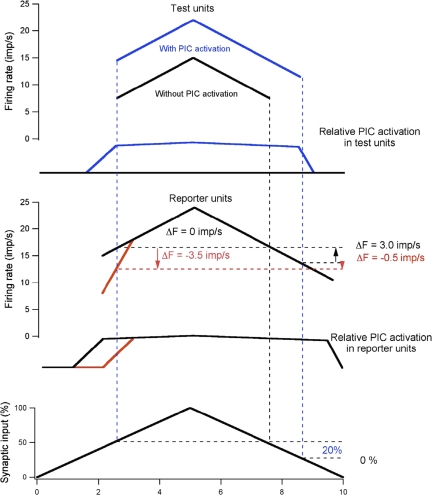
Figure 9 is a schematic illustration of the potential effects of PIC activation on ΔF values for hypothetical reporter and test units (top 2 panels) firing in relation to identical, slowly increasing then decreasing excitatory synaptic drive (bottom panel). For reporter units, PIC is activated and graded by synaptic drive (middle panel), but spike threshold and PIC onset times vary; PIC is activated either before (black lines) or at the same time (red lines) as spike threshold. For test units (top panel), PIC is either activated (blue lines) or not (black lines). Pairings of reporter and test units under these conditions yield ΔF values that vary from positive to negative values (see color-coded vertical dashed lines projected onto firing rate plots). First, we consider cases in which PIC is activated in the test unit (blue traces in top panel). Increasing synaptic drive activates PIC in this test unit prior to the onset of repetitive firing, so that its firing rate changes relatively little over most of the time course of reporter unit activation. It can be seen from the bottom panel that the synaptic input to the test unit is greater at recruitment than at de-recruitment of the reporter unit, suggesting that PIC contributes 20% of the total excitatory drive. Using this test unit, ΔF was positive when paired with the black reporter unit, but negative when paired with the red reporter unit. For the other test unit in which synaptic excitation fails to activate PIC (black traces in top panel), ΔF values are nonpositive (0 or −3.5 imp/s) regardless of when PIC is activated in a reporter unit. While these highly schematized conditions represent only a subset of possible relations between synaptic input, PIC activation and motoneuron rate modulation, they illustrate potential causes of variation in ΔF.
FIG. 9.
Schematic diagram of potential relations between persistent inward current (PIC) activation in reporter and test units and ΔF values. Firing rates of 2 different test units (top) and 2 different reporter units (middle) in response to an identical excitatory synaptic command (bottom). The recruitment times of the 2 test units are the same as they are for the 2 reporter units, but the contribution of PIC activation to the firing rate modulation differs. The blue traces in the top panel show the time course of PIC activation and firing rate in a test unit in which PIC is nearly fully activated prior to recruitment, whereas the black traces show PIC activation and firing rate modulation in a test unit in which synaptic input fails to activate PIC. The red and black traces in the middle panel show PIC activation and firing rate modulation in 2 different reporter units, 1 in which PIC is nearly fully activated prior to recruitment (black) and one in which PIC is activated after recruitment (red). ΔF calculated for the blue test unit against the black reporter unit is 3.0 imp/s, whereas that calculated against the red reporter unit is −0.5 imp/s. For the black test unit in which PIC is not activated, ΔF calculated against the black reporter unit is 0 imp/s, whereas that calculated against the red reporter unit is −3.5 imp/s. The bottom panel shows that PIC activation contributes 20% of the excitatory drive to the blue test unit and 0% of the drive to the black test unit. See text for further details.
Assumption of correlated unit firing
The ability to use reporter firing rate as an accurate estimate of the relative synaptic drive to the test unit also depends on the assumption of a linear relation between the relative net synaptic excitatory drives to two units. Strong linear correlations between the firing rates of the two units support this assumption. A surprising finding in the present study was that ΔF values were predominantly positive even when the firing rates of the two units were not well correlated. However, the paired unit technique of estimating PIC only requires that the reporter unit reflect the relative synaptic drive to the test unit at the time of test recruitment and de-recruitment; over the remainder of the trial, the rates of the two units could vary independently. Independent variation could arise due to rate saturation of the reporter unit (Johns and Fuglevand 2004) in which case the test unit could show large changes in firing rate that are not associated with an appreciable change in firing rate of the reporter unit, leading to a low rate-rate correlation. This behavior could arise if strong PIC activation in the reporter unit acted as a shunt to minimize the effects of further increases in synaptic activation (cf. Oakley et al. 2001) or if the relative balance of excitation and inhibition changed so that increased excitatory drive in the test unit was associated with a greater contribution of inhibitory input to the reporter unit (Heckman and Binder 1993). Alternatively, the rates of the two units could co-vary in a monotonic fashion but with different rate-rate relations for increasing versus decreasing synaptic input (see Fig. 7). The counter-clockwise hysteresis in the rate-rate relations shown in Fig. 7A could reflect strong PIC activation in the test unit after recruitment, leading to a rapid increase in test rate that is not associated with a comparable increase in the firing rate of the reporter unit, as suggested by Bennett et al. (2001) to explain similar behavior in tail motor units in rats with chronic spinal cord injury.
Utility of the ΔF technique for assessing PIC contribution to the excitation of human motor units
Much of the variation in ΔF values that we observed is likely to have arisen from variations in the time course and magnitude of synaptic inputs to the motoneurons. Our epochs of firing exhibited a wide range of rate modulation, duration, rate of rise, and decline, and the epochs were typically much shorter in duration than those reported by Gorassini et al. (2002). Tighter control over the time course of synaptic input would likely result in more consistent and reliable estimates of ΔF. The original application of the paired unit technique was based on the behavior of human motor units during slow, smooth, and symmetric increases and decreases in isometric torque (Gorassini et al. 2002). For these rates and magnitudes of contraction, the firing rate of the motor units exhibited a linear relation to isometric torque and presumably to the net excitatory synaptic drive as well. Thus it may be possible by limiting the speed and size of contraction to avoid situations where the reporter unit input-output gain changes markedly. Nonlinear regions of reporter gain can also be avoided by choosing test units that are recruited after any initial steep increase in reporter firing rate (cf. Bennett et al. 2001; Gorassini et al. 2004). These conditions are likely to be optimal for estimating PIC based on ΔF values. However, the need for precise control over synaptic input may limit the utility of this technique, particularly for estimating changes in PIC properties following CNS injury, when voluntary control is impaired or where epochs of firing are elicited by reflex inputs. Similar problems may arise in studying normal movements that are not made under biofeedback and that have short durations typical of a variety of normal movements.
Even when conditions are optimal for estimating PIC from ΔF values, negative results may be difficult to interpret. For example, although PICs in rat tail motoneurons are small after acute spinal injury (Harvey et al. 2006), they are still present. Nonetheless, significant positive ΔF values were not obtained for motor-unit pairs in this condition (Bennett et al. 2001), suggesting that the lack of positive ΔF values does not necessarily indicate the absence of PICs. Preliminary simulations using a compartmental motoneuron model suggest that positive values of ΔF require that PIC in the dendrites is of sufficient magnitude to support a stable depolarization of the dendrite, which in turn requires that the local dendritic current-voltage relation has a region of negative slope (Cope et al. 2006). PICs that are smaller than this critical magnitude may still make a prominent contribution to rate modulation without yielding positive ΔF values. Improved computer models of motoneuron behavior that incorporate what is known about PIC properties may help researchers use multiple aspects of motoneuron behavior to assess PIC contributions to recruitment and rate modulation in normal and pathological situations.
GRANTS
This work was supported by Ohio Board of Regents Research Incentive Program and National Institute of Neurological Disorders and Stroke Grant NS-049324.
Acknowledgments
We thank Dr. Marc Binder for comments on the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bennett 1998.Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998. [DOI] [PubMed] [Google Scholar]
- Bennett 2001.Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001. [DOI] [PubMed] [Google Scholar]
- Binder 2003.Binder MD Intrinsic dendritic currents make a major contribution to the control of motoneuron discharge. J Physiol 552: 665, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder 1996.Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Handbook of Physiolog. Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. New York: Oxford, 1996, sect. 12, p. 3–53.
- Cope 1991.Cope TC, Clark BD. Motor-unit recruitment in the decerebrate cat: several unit properties are equally good predictors of order. J Neurophysiol 66: 1127–1138, 1991. [DOI] [PubMed] [Google Scholar]
- Cope 2006.Cope TC, Powers RK, Nardelli P. Graded amplification of synaptic inputs: evidence from paired motor axon recordings. Soc Neurosci Abstr 637: 622, 2006. [Google Scholar]
- Gemperline 1995.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve 18: 1101–1114, 1995. [DOI] [PubMed] [Google Scholar]
- Gorassini 2004.Gorassini M, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004. [DOI] [PubMed] [Google Scholar]
- Gorassini 2002.Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002. [DOI] [PubMed] [Google Scholar]
- Harvey 2006.Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman 1993.Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol 69: 1005–1008, 1993. [DOI] [PubMed] [Google Scholar]
- Heckman 2005.Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. [DOI] [PubMed] [Google Scholar]
- Heckman 2003.Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26: 688–695, 2003. [DOI] [PubMed] [Google Scholar]
- Johns 2004.Johns RK, Fuglevand AJ. Evidence for intrinsic mechanisms underlying firing rate saturation in human motor neurons. Soc Neurosci Abstr, 188, 2004.
- Keen 2004.Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol 91: 57–62, 2004. [DOI] [PubMed] [Google Scholar]
- Kirkwood 1978.Kirkwood PA, Sears TA. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol 275: 103–134, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee 1998a.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998a. [DOI] [PubMed] [Google Scholar]
- Lee 1998b.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998b. [DOI] [PubMed] [Google Scholar]
- Lee 1999.Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999. [DOI] [PubMed] [Google Scholar]
- Monster 1977.Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40: 1432–1443, 1977. [DOI] [PubMed] [Google Scholar]
- Oakley 2001.Oakley JC, Schwindt PC, Crill WE. Dendritic calcium spikes in layer 5 pyramidal neurons amplify and limit transmission of ligand-gated dendritic current to soma. J Neurophysiol 86: 514–527, 2001. [DOI] [PubMed] [Google Scholar]
- Powers 2001.Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001. [DOI] [PubMed] [Google Scholar]
- Prather 2002.Prather JF, Clark BD, Cope TC. Firing rate modulation of motoneurons activated by cutaneous and muscle receptor afferents in the decerebrate cat. J Neurophysiol 88: 1867–1879, 2002. [DOI] [PubMed] [Google Scholar]
- Prather 2001.Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysioy 85: 43–53, 2001. [DOI] [PubMed] [Google Scholar]