Abstract
The thalamus is an essential structure in the mammalian forebrain conveying information topographically from the sensory periphery to primary neocortical areas. Beyond this initial processing stage, “higher-order” thalamocortical connections have been presumed to serve only a modulatory role, or are otherwise functionally disregarded. Here we demonstrate that these “higher-order” thalamic nuclei share similar synaptic properties with the “first-order” thalamic nuclei. Using whole cell recordings from layer 4 neurons in thalamocortical slice preparations in the mouse somatosensory and auditory systems, we found that electrical stimulation in all thalamic nuclei elicited large, glutamatergic excitatory postsynaptic potentials (EPSPs) that depress in response to repetitive stimulation and that fail to activate a metabotropic glutamate response. In contrast, the intracortical inputs from layer 6 to layer 4 exhibit facilitating EPSPs. These data suggest that higher-order thalamocortical projections may serve a functional role similar to the first-order nuclei, whereas both are physiologically distinct from the intracortical layer 6 inputs. These results suggest an alternate route for information transfer between cortical areas via a corticothalamocortical pathway.
INTRODUCTION
In each of the main sensory pathways, except olfaction, information is conveyed from sensory receptors eventually to the thalamus and then to primary cortical areas (Jones 2007). From there, the conventional view suggests that information is processed along a hierarchical series of corticocortical connections (Felleman and Van Essen 1991; Rouiller et al. 1991; Zeki and Shipp 1988), with “higher” areas integrating input from “lower” areas to produce increasingly complex receptive fields. In this framework, thalamic inputs are generally disregarded as information-bearing sources for “higher” cortical areas and, instead, are assigned a supporting role for regulating processes such as attention (Olshausen et al. 1993). However, the relevance of any functional assignment is uncertain because the physiological properties of the higher-order thalamocortical pathways have never been adequately elucidated.
Insight into the possible functional roles of these higher-order thalamocortical projections can be derived from related studies of inputs to the thalamus (Bartlett and Smith 2002; Li et al. 2003; Reichova and Sherman 2004), whose synaptic properties have been partitioned into two main types. The first type of input produces large excitatory postsynaptic potentials (EPSPs) by activating only ionotropic glutamate receptors and exhibiting synaptic depression (Li et al. 2003; Reichova and Sherman 2004). These synaptic inputs originate from information-bearing sources, such as the retinogeniculate pathway. The second type of input does not represent the main type of input to be relayed (e.g., the layer 6 corticogeniculate pathway) and, instead, produces small EPSPs that facilitate while activating both ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs) (Li et al. 2003; Reichova and Sherman 2004). These physiological criteria thus provide an ideal basis for comparing the possible roles of the first- and higher-order thalamocortical pathways. Such a characterization of synaptic properties is currently unavailable for the higher-order thalamocortical pathways and is only fragmentary for the first-order pathways (Gil et al. 1999; MacLean et al. 2006; Rose and Metherate 2001; Stratford et al. 1996).
Moreover, these criteria may extend to all cortical inputs, thus potentially establishing an empirical principle for identifying the major routes of information flow throughout the forebrain (Sherman and Guillery 1998, 2002). Thus we sought to compare the basic physiological properties of thalamocortical synapses with those originating from intrinsic cortical sources—that is, the input from layer 6 to layer 4. Anatomically, the intrinsic cortical sources provide an overwhelming majority (∼50%) of the input to a cortical column (Ahmed et al. 1994; Lee and Winer 2008b), whereas the thalamocortical input is an order of magnitude less (∼5%) (Ahmed et al. 1994; Latawiec et al. 2000; Lee and Winer 2008a). Whether such marked structural differences are further instantiated by distinct synaptic physiological properties remains an open question that we address directly here.
Therefore to investigate these issues, we used in vitro slice preparations in the mouse somatosensory (Agmon and Connors 1991) and auditory (Cruikshank et al. 2002) systems. In each system, the synaptic responses of layer 4 neurons to paired-pulse and high-frequency stimulation were measured and compared for both first- and higher-order pathways.
METHODS
Thalamocortical slice preparations were made from BALB/c mice (ages 10–16 days), as described in previous work (Reichova and Sherman 2004). Briefly, after isoflurane anesthesia and decapitation, whole brains were quickly placed into cool, oxygenated, artificial cerebrospinal fluid (ACSF) and blocked to preserve first- and higher-order thalamocortical pathways, as described for the somatosensory (Agmon and Connors 1991) and auditory (Cruikshank et al. 2002) systems. The blocking cuts were modified for the higher-order auditory slice as follows. A rostral coronal blocking cut was first made at 25° rostrocaudal from the dorsal surface. The brain was tipped forward on the blocked surface and a second horizontal blocking cut was made at 0° along the dorsal surface, which was then affixed to a stage such that slices were collected ventrodorsally. The somatosensory preparation was obtained with nearly equal efficacy for the first-order (∼50%; n = 16 of 30) and higher-order (∼50%; n = 16 of 36) pathways. The higher-order auditory slice had a somewhat lower success rate (∼25%; n = 12 of 52) than did the first-order slice (∼50%; n = 12 of 28). Thalamocortical slices (500 μm) were vibratomed (Campden Instruments, Lafayette, IN), and recovered in physiological ACSF (in mM: 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 glucose) for 1 h at 32°C. The slices were then placed in a submersion-type recording chamber on a modified microscope stage and maintained at 32°C with constant perfusion of ACSF.
Whole cell recordings were made under differential interference contrast (DIC) optics to identify thalamic and cortical sites for stimulation with pipettes containing intracellular solution (135 K-gluconate, 7 NaCl, 10 HEPES, 1–2 Na2ATP, 0.3 GTP, 2 MgCl2, and 0.5% biocytin at a pH of 7.3 obtained with KOH and osmolality of 290 mOsm obtained with distilled water). Current- or voltage-clamp recordings were made using the Axoclamp 2A amplifier and pCLAMP software (Axon Instruments, Union City, CA). Depolarizing current injections were used to determine the spiking characteristics of layer 4 neurons. Neurons were classified as regular spiking (RS) if they fired at slow adapting frequencies (<30 Hz) with small and slow afterhyperpolarizations (AHPs; 5–10 mV), in comparison to fast-spiking (FS) neurons that had higher maximal firing rates (>30 Hz), with large and fast AHPs (10–15 mV). The acquired data were digitized using a Digidata 1200 board and then stored in a computer for later analysis.
Thalamocortical axons were labeled in the slice by biocytin iontophoreses (40 μA, 16.7 mHz, 30 min) in the respective thalamic nuclei, then incubated overnight in ACSF at room temperature. Slices were placed in 4% paraformaldehyde for overnight fixation and staining was revealed using standard avidin-biotin-HRP histochemistry (Vector Laboratories, Burlingame, CA). For parvalbumin staining (Cruikshank et al. 2002), sections were first incubated in primary antibody (Sigma, St. Louis, MO) at a dilution of 1:1,000 at 4°C. Sections were then incubated at room temperature in biotinylated secondary antibody for 1 h and processed with standard avidin-biotin-HRP histochemistry (Vector Laboratories). For cytochrome oxidase staining, sections were processed using the method of Wong-Riley (1979).
Stock solutions of receptor antagonists were prepared in distilled water, diluted to their final concentration just before use, and were bath applied. The final bath concentration was generally estimated to be one fourth of the initial concentration based on the rate of injection. To block ionotropic glutamate receptors during high-frequency stimulation, 6,7-dinitroquinoxaline-2,3-dione (DNQX, 50 μM) for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801, 40 μM) for N-methyl-daspartate (NMDA) were used. To block γ-aminobutyric acid (GABA) receptors, gabazine (SR95531, 50 nM) for GABAA and 3-aminopropyl(cyclohexylmethyl)phosphinic acid (CGP46381, 50 nM) for GABAB were used.
Bipolar concentric electrodes were used for electrical stimulation in the thalamus (FHC, Bowdoinham, ME) and stimulation consisted of pulses of 0.1–0.2 ms delivered using an electrical stimulus protocol (Gil et al. 1999) to determine the intensity producing half successes/half failures (Fig. 5, C and D). This low-intensity stimulation was used to avoid the activation of corticothalamic fibers and fibers of passage; note that we do not suggest that our stimulation protocol activates single axons. The stimulation electrode was situated in the location that produced the most robust responses with the lowest stimulus intensity. A paired-pulse protocol delivered pulses (0.1–0.2 ms) separated by 25–200 ms and high-frequency stimulation consisted of pulses at 125 Hz for 800 ms. The paired-pulse protocol was also tested in the presence of GABA blockers.
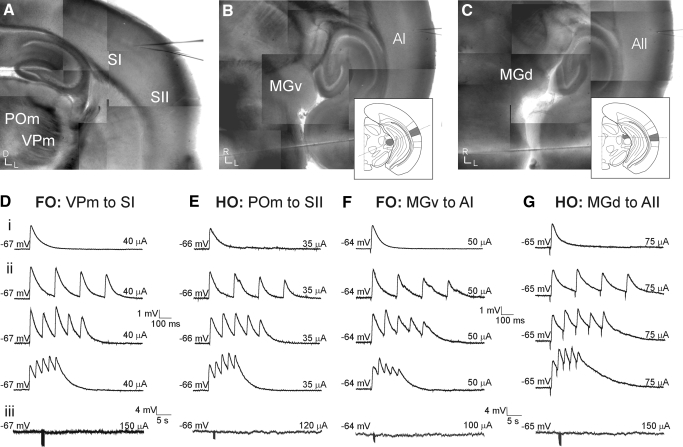
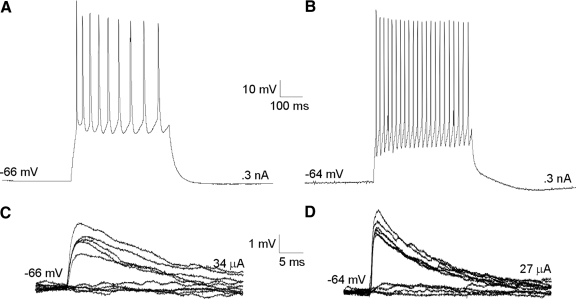
FIG. 5.
First-order and higher-order thalamocortical pathways exhibit similar physiological properties. A–C: photomontage of thalamocortical pathways in living slice preparations based on photomicrographs taken in the recording setup. The somatosensory slice (Agmon and Connors 1991) (A) contains both first-order (i.e., the ventral posterior medial nucleus [VPm] and SI) and higher-order (i.e., the posterior medial nucleus [POm] and SII) thalamocortical systems, whereas auditory slice (Cruikshank et al. 2002) preparations containing first-order (i.e., the ventral portion of the medial geniculate nucleus [MGv] and AI) (B) or higher-order (i.e., the dorsal portion of the medial geniculate nucleus [MGd] and AII). C: thalamocortical pathways are prepared from different blocking cuts (insets; see methods). D–G: whole cell, current-clamp recordings from single cells in cortical layer 4 in response to electrical thalamic stimulation in first-order (D, F) and higher-order (E, G) pathways of the somatosensory (D, E) and auditory (F, G) systems. Low-intensity electrical stimulation elicits large EPSPs (∼4 mV) (i) that depress in response to paired-pulse stimulation (ii; from top: 5, 10, 20 Hz). Pharmacologically blocking the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-daspartate receptors followed by high-frequency thalamic stimulation (125 Hz for 800 ms) does not evoke a metabotropic glutamate receptor response (iii).
For photostimulation, nitroindolinyl (NI)-caged glutamate (Sigma-RBI) was added at a concentration of 0.37 mM to the recirculating ACSF (Shepherd et al. 2003). Photolysis of the caged glutamate was done focally with an average beam intensity of 5 mW to give a 1-ms, 100-pulse light stimulus by Q-switching the pulsed UV laser (355-nm wavelength, frequency-tripled Nd:YVO4, 100-kHz pulse repetition rate; DPSS Lasers, Santa Clara, CA). Responses were analyzed using custom software written in Matlab (MathWorks, Natick, MA) and the traces were superimposed on a photomicrograph corresponding to the stimulation sites.
RESULTS
We examined the physiological properties of thalamocortical and intracortical synapses in vitro using slice preparations from the mouse somatosensory (Agmon and Connors 1991) and auditory (Cruikshank et al. 2002) systems (Figs. 1–3), which have been described previously. The somatosensory slice contained both the first-order (the ventral posterior medial nucleus [VPm] to cortical area SI) and higher-order (the posterior medial nucleus [POm] to cortical area SII) thalamocortical pathways (Spreafico et al. 1987) (Fig. 1 ). For the auditory slice, we used different slice preparations for the first- and higher-order pathways (see methods).
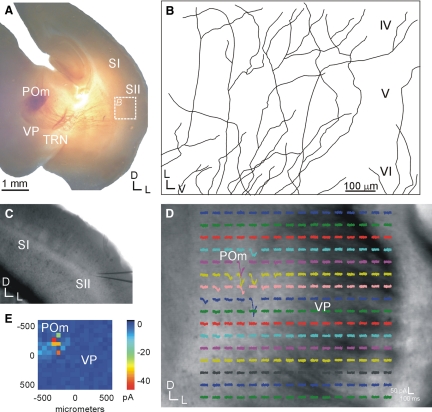
FIG. 1.
Higher-order thalamocortical pathways in the somatosensory slice are intact. A and B: focal biocytin injections in the posterior medial nucleus (POm) labels fiber tracts that traverse across the ventral posterior nucleus (VP), the thalamic reticular nucleus (TRN), and the internal capsule, before traversing into layer 4 of the secondary somatosensory cortex (SII) (B; tracings of labeled axons). C–E: photostimulation with caged glutamate in POm (D) results in excitatory postsynaptic currents (EPSCs) from a layer 4 neuron in SII (C). E: response map plot showing the mean EPSC at each stimulation site.
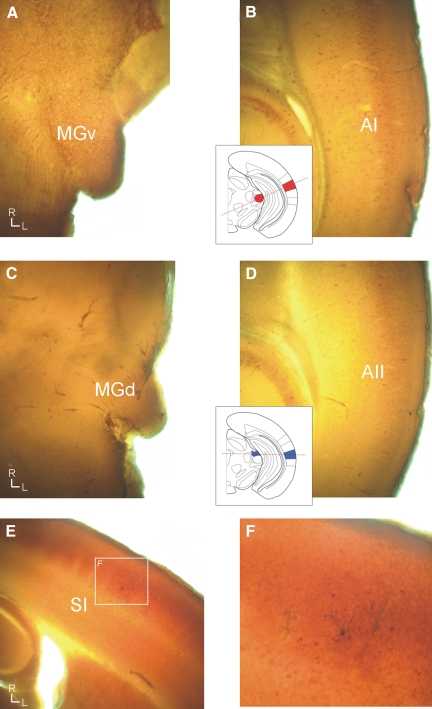
FIG. 3.
Parvalbumin distinguishes the first-order and higher-order auditory regions, whereas cytochrome oxidase distinguishes the first-order somatosensory area. A and B: the first-order auditory regions stain intensely for parvalbumin in the ventral portion of the medial geniculate nucleus (MGv) (A) and in the primary auditory area (AI) cell bodies (B). C and D: the higher-order auditory regions are weakly immunoreactive in both the dorsal portion of the medial geniculate nucleus (MGd) (C) and AII (D). E and F: cytochrome oxidase intensely labels the barrel region (E). F: a recovered biocytin-filled neuron in the primary somatosensory area (SI).
The higher-order thalamocortical projection has not previously been demonstrated in the somatosensory slice, but was verified here using biocytin injections in POm that labeled thalamocortical axons terminating in layer 4 of SII (Fig. 1, A and B) and also using photostimulation with caged glutamate in POm to elicit excitatory postsynaptic current (EPSC) responses in SII (Fig. 1, C–E). In all instances, biocytin injections placed in POm of electrophysiologically connected slices (n = 5) resulted in fibers terminating in layer 4 of SII (Fig. 1, A and B). In addition, photostimulation of POm resulted in EPSCs in SII (n = 4), which must originate from intact thalamocortical neurons in POm, since photostimulation activates only the soma or proximal dendrites of neurons and not fibers of passage or axons antidromically (Lam and Sherman 2007; Shepherd et al. 2003). POm is thus at least partly connected to SII in the slice. Both first-order and higher-order regions were readily distinguished in the living slice preparations under DIC optics. The ventral posterior medial nucleus had a characteristically dark crescent or half-moon appearance, whereas the posterior medial nucleus was lighter in appearance (Figs. 1D and 5A). SI was distinguished from SII by the presence of the barrel fields in SI, which appeared as a series of alternating dark and light regions in layer 4 (Figs. 1C and 5A) (Agmon and Connors 1991). These are also evident in slices postprocessed for cytochrome oxidase (Fig. 3, E and F) (Wong-Riley 1979).
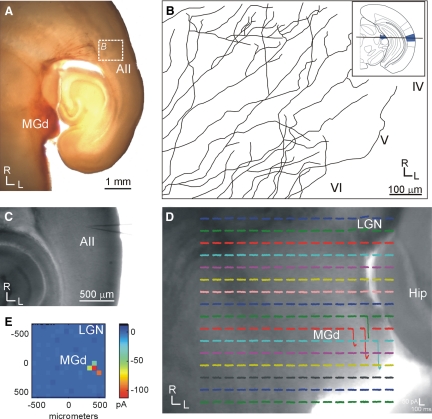
For the auditory system, first-order (the ventral portion of the medial geniculate body [MGv] to cortical area AI; Figs. 3, A and B and 5B) and higher-order (the dorsal portion of the medial geniculate body [MGd] to cortical area AII; Figs. 2, 3, C and D, and 5C) thalamocortical slices were prepared using different blocking cuts (see methods). The higher-order projection from MGd to AII has not previously been demonstrated in a slice, but was verified here using biocytin injections in MGd that labeled thalamocortical axons terminating in layer 4 of AII (Fig. 2, A and B) and using photostimulation in MGd to elicit EPSCs in AII (Fig. 2, C–E). In all instances, biocytin injections of MGd (n = 4) of electrophysiologically connected slices resulted in fibers extending into layer 4 (Fig. 2, A and B). Photostimulation in MGd (n = 3) also elicited responses from AII (Fig. 2, C–E). Thus MGd is at least partly connected to AII in the slice. The cytoarchitecture of first- and higher-order regions was subtle under DIC optics, although the laminar arrangement of MGv neurons could occasionally be discerned from the more anisotropic character of MGd (Winer 1992). Areal and nuclear determinations were validated post hoc in each case with parvalbumin immunocytochemistry (Cruikshank et al. 2002), which differentiated the first-order thalamic nucleus (MGv) (Fig. 3A) and cortical area (AI) (Fig. 3B) from their higher-order equivalents (Fig. 3, C and D). In the thalamus, parvalbumin intensely stained the neuropil in MGv (Fig. 3A), whereas MGd is weakly stained (Fig. 3C), which is apparent from a comparison with surrounding structures. In the cortex, parvalbumin-stained neuronal cell bodies are highly clustered in AI (Fig. 3B), but stained more diffusely in AII (Fig. 3D).
FIG. 2.
Higher-order thalamocortical pathways are intact in the modified auditory slice; conventions as in Fig. 1. A and B: focal biocytin injections in the dorsal division of the medial geniculate body (MGd) labels intact fibers that traverse around the lateral geniculate nucleus, thalamic reticular nucleus, and internal capsule, before penetrating into layer 4 of the second auditory area (AII) (B; tracings of labeled axons). Inset in B indicates the horizontal blocking angle for preparing the higher-order auditory slice (see methods). C–E: photostimulation with caged glutamate in MGd (D) results in EPSCs in a layer 4 neuron in AII (C). E: mean EPSC at each stimulation site.
First- and higher-order thalamocortical synapses
For each of these preparations, we obtained whole cell, current-clamp recordings from layer 4 neurons in response to low-intensity electrical stimulation in the thalamus (Fig. 4, C and D). We studied the evoked EPSPs in a total of 56 neurons (46 regular spiking; 10 fast spiking). Regular-spiking (RS) neurons fired at slow adapting frequencies (23.7 ± 4.2 Hz) with small afterhyperpolarizations (AHPs; 4.8 ± 0.9 mV), whereas fast-spiking (FS) neurons had higher maximal firing rates (56.2 ± 5.8 Hz), with large AHPs (11.3 ± 1.8 mV) (Fig. 4, A and B). RS neurons had an average resting potential of −65.3 ± 4.8 mV and input resistance of 291.4 ± 120.3 MΩ, whereas FS neurons had an average resting potential of −62.8 ± 6.9 mV and input resistance of 167.6 ± 79.9 MΩ. These cortical neurons (Fig. 4, A and B) were distributed as follows: somatosensory first-order (n = 16, activation of the ventral posterior medial nucleus to area SI); somatosensory higher-order (n = 16, activation of the posterior medial nucleus to area SII); auditory first-order (n = 12, activation of the ventral portion of the medial geniculate body to area AI); and auditory higher-order (n = 12; activation of the dorsal portion of the medial geniculate body to area AII). In all thalamocortical pathways, thalamic stimulation (20–70 μA) elicited large-amplitude (∼4 mV), monosynaptic EPSPs (Fig. 5, D–G, row i), as determined by their short latency (5–7 ms), low onset jitter (<0.5 ms), monophasic time course, and small-amplitude variance (Table 1).
FIG. 4.
Intrinsic properties and low-intensity stimulation. The majority of recorded layer 4 neurons were regular spiking neurons (A, C) (n = 46), whereas fast-spiking (FS) neurons constituted a relative minority (B, D) (n = 10). C and D: low-intensity electrical stimulation, determined as the intensity that resulted in half successes/half failures (Gil et al. 1999), in the thalamocortical pathways resulted in large excitatory postsynaptic potentials (EPSPs) that were slightly larger in FS neurons.
TABLE 1.
Summary statistics for first and second pulse amplitudes (EPSP1 and EPSP2), latencies (Lat), jitter, rise times (RT), and mean paired-pulse ratio at 10 Hz
| SI | SII | AI | AII | |
|---|---|---|---|---|
| EPSP1, mV | 4.8 ± 2.2 | 3.5 ± 1.5 | 3.5 ± 1.7 | 2.7 ± 1.0 |
| Lat1, ms | 5.1 ± 2.3 | 6.1 ± 2.7 | 7.4 ± 1.5 | 7.0 ± 2.0 |
| Jitter1, ms | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.2 |
| RT1, ms | 9.0 ± 3.9 | 8.1 ± 3.8 | 7.9 ± 1.6 | 11.4 ± 2.9 |
| EPSP2, mV | 2.9 ± 1.3 | 2.1 ± 0.9 | 2.3 ± 1.5 | 1.9 ± 0.7 |
| Lat2, ms | 5.2 ± 2.3 | 5.9 ± 2.7 | 7.4 ± 1.6 | 6.6 ± 1.9 |
| RT2, ms | 8.5 ± 3.2 | 7.5 ± 2.5 | 8.0 ± 2.0 | 10.2 ± 2.1 |
| Paired-pulse ratio, EPSP2/EPSP1 | 0.61 ± 0.13 | 0.60 ± 0.13 | 0.64 ± 0.15 | 0.65 ± 0.14 |
Values are means ± SD.
All of the thalamocortical EPSPs depressed in response to paired-pulse stimulation (Fig. 5, D–G, row ii) and were most depressed at higher frequencies (Fig. 5, D–G, row ii, top vs. bottom trace). Since these synapses also depress in the presence of pharmacological blockers of GABA receptors, the effect cannot be accounted for by feedforward inhibition. We quantified this effect by measuring the ratio of response amplitudes to the first and second pulses (EPSP2/EPSP1) and examined their subsequent distributions (Fig. 6). For all thalamocortical pathways, paired-pulse ratios were distributed unimodally at values <1.0, with an average value of 0.64 ± 0.14 at 10 Hz (Fig. 6A). This ratio decreased with increasing frequency, with the lowest values (0.54 ± 0.15) found at 40 Hz (Fig. 6B; Table 2). This finding of large, depressing EPSPs is indicative of synapses with high-release probabilities (Gil et al. 1997, 1999) and is common to both first- and higher-order thalamocortical pathways.
FIG. 6.
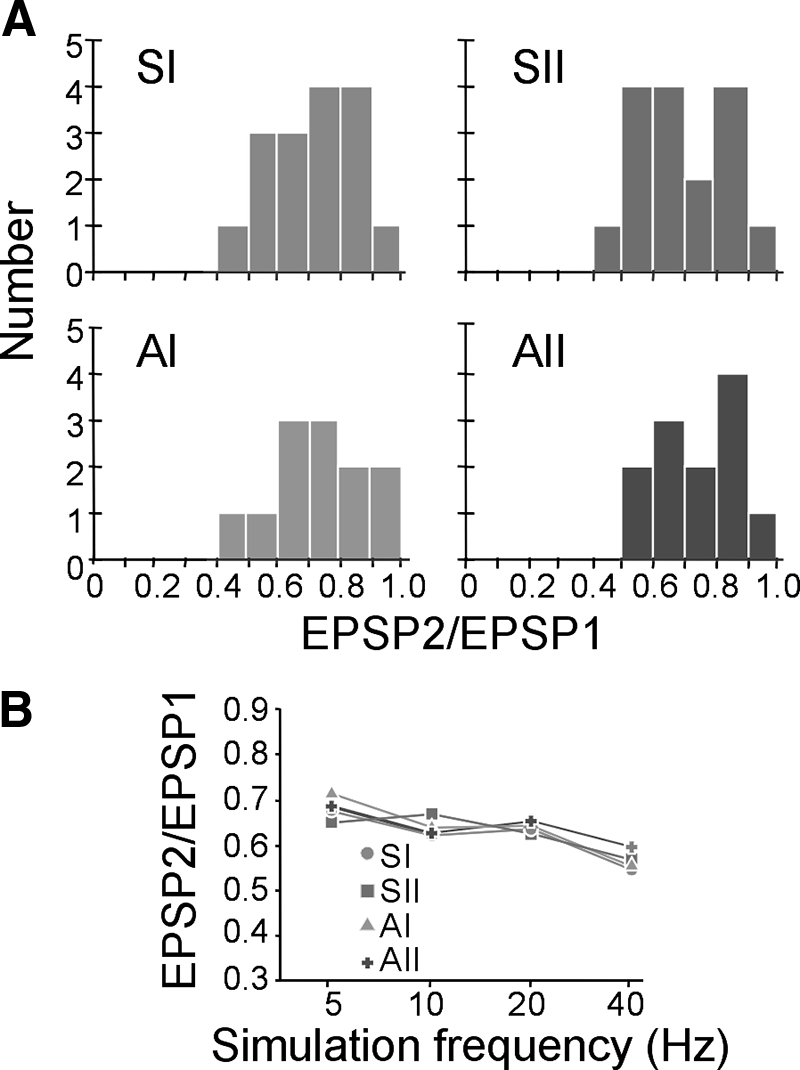
First-order and higher-order thalamocortical synapses exhibit paired-pulse depression. A: paired-pulse ratios (second:first pulse amplitude, or EPSP2/EPSP1) in somatosensory (SI, SII) and auditory (AI, AII) pathways at 10 Hz are distributed <1.0 for both first-order (SI, AI) and higher-order (SII, AII) pathways. B: at increasing stimulation frequencies (5, 10, 20, 40 Hz), paired-pulse ratios (mean ± SD) generally decrease (P < 0.05, ANOVA).
TABLE 2.
Paired-pulse ratios (EPSP2/EPSP1) in the thalamocortical and intracortical (layer 6 to layer 4) pathways at different stimulation frequencies
| 5 Hz | 10 Hz | 20 Hz | 40 Hz | |
|---|---|---|---|---|
| Thalamocortical | 0.69 ± 0.10 | 0.64 ± 0.14 | 0.62 ± 0.11 | 0.54 ± 0.15 |
| Intracortical | 1.09 ± 0.11 | 1.07 ± 0.15 | 1.10 ± 0.09 | 1.14 ± 0.09 |
Values are means ± SD.
Another physiological property that could potentially distinguish the first- and higher-order pathways is a differential response of postsynaptic mGluRs (Reichova and Sherman 2004). Thus to test whether thalamocortical pathways activate an mGluR response, iGluRs were pharmacologically blocked with DNQX (for AMPA receptors) and MK-801 (for NMDA receptors) in the same neurons that were used to test paired-pulse responses. High-frequency stimulation (HFS) was then applied in the thalamus (125 Hz for 800 ms), which is often required to elicit mGluR responses (McCormick and Von Krosigk 1992). In all of the tested thalamocortical pathways, thalamic HFS failed to elicit an mGluR response, even at very high intensities (>100 μA) (Fig. 5, D–G, row iii), underscoring the physiological similarity of both first-order and higher-order thalamocortical synapses. Thus first-order and higher-order thalamocortical projections are indistinguishable based on all of these tested parameters, suggesting that their functional roles may also be identical.
Layer 6 to layer 4 synapse
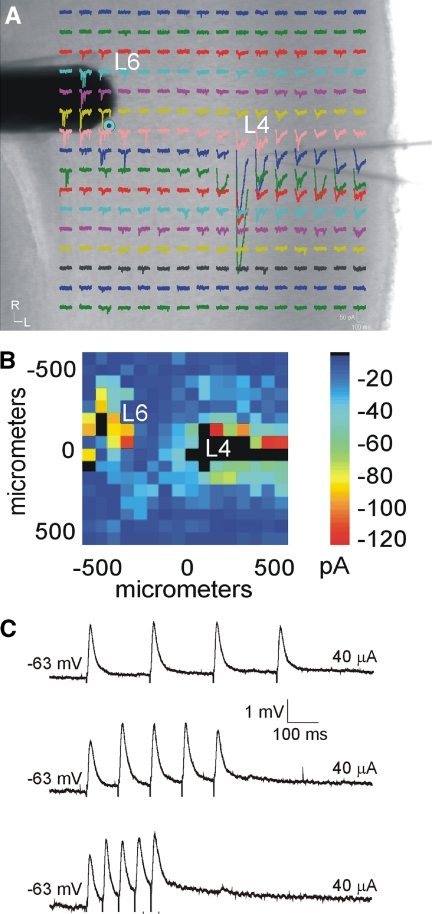
To test other cortical synapses, we then investigated the intracortical pathway from layer 6 to layer 4. A reason for doing this is that earlier reports (Stratford et al. 1996; Tarczy Hornoch et al. 1999) indicated that such input evokes a very different EPSP pattern from the thalamocortical pattern reported here—i.e., a facilitating response—and we sought to demonstrate with our techniques that such a pattern could be seen for other inputs to layer 4 cells. We thus activated inputs from layer 6 to layer 4 in a total of 12 neurons from first-order (SI: n = 3; AI: n = 3) and higher-order (SII: n = 3; AII: n = 3) areas. As an aid for identifying the precise location of layer 6 inputs, photostimulation with caged glutamate (Lam and Sherman 2007; Papageorgiou et al. 1999; Shepherd et al. 2003) was used in some experiments (n = 8) to map the intracortical inputs to layer 4 (Fig. 7, A and B). A stimulating electrode was then targeted to the layer 6 region that elicited the largest photostimulation response (Fig. 7A).
FIG. 7.
Intracortical projections from layer 6 to layer 4 differ physiologically from the thalamocortical projection. A and B: photostimulation with caged glutamate in the cortex (AII) identifies the location of layer 6 inputs to layer 4. A: whole cell, voltage-clamp recordings from layer 4 (L4) in response to photostimulation at 256 separate cortical loci (colored traces). A concentric bipolar electrode was targeted to the region in layer 6 (L6) that elicited the strongest photostimulation response (blue dot). B: response map plot illustrating the mean EPSC amplitude within 100 ms of the stimulus onset. C: whole cell, current-clamp recordings in layer 4 following paired-pulse electrical stimulation in layer 6 elicits facilitating EPSPs (from top: 5, 10, 20 Hz).
Electrical stimulation in layer 6 produced EPSPs in layer 4 neurons that facilitated in response to paired pulses, which became more pronounced at higher frequencies (Fig. 7C, top vs. bottom trace), in agreement with previous studies (Stratford et al. 1996; Tarczy Hornoch et al. 1999). Indicative of this, the average paired-pulse ratios were lowest at 5 Hz (1.09 ± 0.11) and highest at 40 Hz (1.14 ± 0.09) (Table 2). At each frequency, these ratios were significantly different compared with the thalamocortical ratios (P < 0.05, t-test) (Table 2).
DISCUSSION
Methodological considerations
In this study, we investigated the synaptic properties of the thalamocortical and intracortical inputs to layer 4 in the first- and higher-order pathways of the auditory and somatosensory systems. Whereas in vitro slice preparations for the first-order thalamocortical pathways have previously been developed for each system (Agmon and Connors 1991; Cruikshank et al. 2002), higher-order thalamocortical slice preparations have not been described. In our experiments, we found that the somatosensory slice preparation described by Agmon and Connors (1991) also preserved intact fibers from POm to SII (Fig. 1). However, for the higher-order auditory slice, we developed a different slice preparation from that previously described by Cruikshank et al. (2002), to preserve the higher-order pathway from MGd to AII (see methods). We found that although this slice could be reliably obtained, because of the more convoluted path of the higher-order thalamocortical pathways, it had a somewhat lower success rate.
We verified that the projections in these higher-order pathways were intact both anatomically and physiologically. Biocytin injections in the higher-order nuclei of both somatosensory and auditory slice preparations labeled fibers that penetrated into layer 4 of the corresponding higher-order cortical areas. Furthermore, photostimulation of these higher-order nuclei in the slice resulted in excitatory responses in the connected higher cortical regions. Since photostimulation can activate only neuronal cell bodies or proximal dendrites and not fibers of passage or axons antidromically (Lam and Sherman 2007; Shepherd et al. 2003), this strongly indicates that at least partial projections are intact in each higher-order preparation. Although photostimulation could conceivably activate SII polysynaptically, this appears unlikely, since the response latency is similar to photostimulation studies of the FO TC synapse in the somatosensory slice (Bureau et al. 2006). In the auditory slice, polysynaptic activation could not account for responses in AII since AI and AII are not present in the same slice plane, thus indicating that MGd and AII are directly connected in the slice. Thus the combination of anatomical tract tracing and photostimulation validate that these slice preparations contain intact connections from higher-order thalamic nuclei to higher-order cortical areas in both the somatosensory and auditory pathways.
Comparison with previous studies
We found that the synaptic properties of first- and higher-order thalamocortical inputs were similar physiologically. In all cases, the thalamocortical pathways exhibited depressing responses to paired-pulse stimulation, but did not exhibit a metabotropic glutamate response. In contrast, we found that the intracortical layer 6 inputs to layer 4 exhibited paired-pulse facilitation.
Previous studies of the first-order somatosensory (Gil et al. 1997), auditory (Rose and Metherate 2001), and visual (MacLean et al. 2006) thalamocortical pathways have demonstrated that paired-pulse depression is a general feature of these synapses in first-order pathways. In addition, prior studies of intrinsic cortical connectivity in the first-order somatosensory (Gil et al. 1999) and visual (Stratford et al. 1996; Tarczy Hornoch et al. 1999) pathways have demonstrated facilitating synapses in the layer 6 to layer 4 projection. The present results extend these findings by applying these synaptic properties to the higher-order pathways in the somatosensory and auditory system. In addition, this study further extends these earlier findings by correlating paired-pulse effects and the absence of a metabotropic glutamate response in the thalamocortical pathways, thus identifying a specific synaptic response type, whose functional significance is discussed in the following text. Although the higher-order visual pathways were not investigated in this study, we speculate that an extension of these physiological properties to those synapses seems likely, but a definitive answer remains for future investigations.
Drivers and modulators
The physiological similarity of all thalamocortical projections in the somatosensory and auditory systems (Gil et al. 1999; Rose and Metherate 2001) suggests that these higher-order thalamocortical synapses, like those in the first-order system, are highly reliable and efficient transmitters of information and do not serve a mere modulatory role as previously suggested (Olshausen et al. 1993). Moreover, our data indicate that there may be no major differences in input properties between first- and higher-order thalamocortical pathways and, just as inputs from first-order nuclei (e.g., the lateral geniculate, ventral portion of the medial geniculate, and ventral posterior nuclei) are the major influence in creating the first step of processing in primary cortical areas (VI, AI, SI), higher-order thalamic nuclei (e.g., the pulvinar, dorsal portion of the medial geniculate, and posterior medial nuclei) may exert an equivalent influence on higher cortical areas (VII, AII, SII, etc.) (Sherman and Guillery 2002, 2005).
In contrast, the physiological difference observed between the thalamocortical and intrinsic layer 6 inputs resembles the two glutamatergic response types found in the thalamus (Li et al. 2003; Reichova and Sherman 2004), which have been termed “drivers” and “modulators” of information (Sherman and Guillery 1998). In this model, drivers are identified as the main pathways for information flow, whereas modulators modify the processing of that information, but not merely by simple gain changes (Bolz and Gilbert 1986). Our finding of analogous properties in the thalamocortical and intrinsic layer 6 projections suggests the possibility that the driver/modulator distinction that has proven so useful for inputs to thalamic relay cells may be fruitfully extended to cortex.
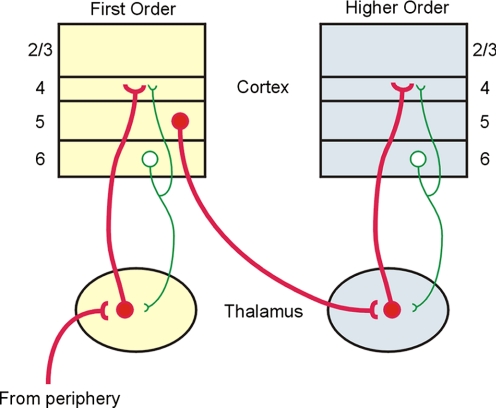
Although both first- and higher-order thalamocortical pathways may be considered “drivers” of cortical activity, most of the information being transmitted by higher-order thalamocortical projections does not arise from the sensory periphery. Rather, both physiological and anatomical data suggest that it originates from layer 5 of the first-order area (Reichova and Sherman 2004; Van Horn and Sherman 2004) (Fig. 8). Therefore we conclude that the higher-order thalamic nuclei are uniquely positioned to act as key intermediaries in the transfer of information from one cortical area to the next via a cortico-thalamocortical route (Sherman and Guillery 2002) (Fig. 8). This view of cortical information processing contrasts with the prevailing notion that such communication is mediated solely by the network of direct corticocortical connections (Felleman and Van Essen 1991; Rouiller et al. 1991; Zeki and Shipp 1988). Although not mutually exclusive, the transthalamic route proposed here may expand the functional repertoire of cortical communication by using the unique functional modes of the thalamus, such as gating (Cox et al. 1998) and burst versus tonic (Sherman 2001) response modes of relay cells. Moreover, the hierarchical routes of cortical information flow that have been previously suggested are largely based on neuroanatomical criteria (Felleman and Van Essen 1991; Rouiller et al. 1991; Zeki and Shipp 1988). Our limited validation of the driver/modulator criteria in cortical synapses suggests that these functional assignments may also be applied to the corticocortical pathways and, consequently, may reveal unexpected functional relationships among cortical areas.
FIG. 8.
Model of interareal communication via a corticothalamocortical pathway. In this model, driver projections (red, thick lines) represent the major information route and, in the example shown, the route from the first-order to the higher-order cortical area involves a corticothalamocortical pathway emanating from layer 5 of the first-order area. Also shown are modulatory pathways (green lines) from layer 6 both to layer 4 and to thalamus.
GRANTS
This work was supported by National Institutes of Health Grants R01 EY-003038, R01 DC-008794 to S. M. Sherman, and F32 NS-054478 to C. C. Lee.
Acknowledgments
We thank C. Varela, Y. Lam, and D. Llano for experimental advice and J. S. Roseman for histology assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Agmon and Connors 1991.Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991. [DOI] [PubMed] [Google Scholar]
- Ahmed et al. 1994.Ahmed B, Anderson JC, Douglas RJ, Martin KAC, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol 341: 39–49, 1994. [DOI] [PubMed] [Google Scholar]
- Bartlett and Smith 2002.Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience 113: 957–974, 2002. [DOI] [PubMed] [Google Scholar]
- Bolz and Gilbert 1986.Bolz J, Gilbert CD. Generation of end inhibition in the visual cortex via interlaminar connections. Nature 320: 362–365, 1986. [DOI] [PubMed] [Google Scholar]
- Bureau et al. 2006.Bureau I, von Saint Paul F, Svoboda K. Interdigitated lemniscal and paralemniscal pathways in the mouse barrel cortex. PLoS Biol 4: 2361–2371, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox et al. 1998.Cox CL, Zhou Q, Sherman SM. Glutamate locally activates dendritic outputs of thalamic interneurons. Nature 394: 478–482, 1998. [DOI] [PubMed] [Google Scholar]
- Cruikshank et al. 2002.Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol 87: 361–384, 2002. [DOI] [PubMed] [Google Scholar]
- Felleman and Van Essen 1991.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991. [DOI] [PubMed] [Google Scholar]
- Gil et al. 1997.Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19: 679–686, 1997. [DOI] [PubMed] [Google Scholar]
- Gil et al. 1999.Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortcal synaptic connections: quanta, innervation, and reliability. Neuron 23: 385–397, 1999. [DOI] [PubMed] [Google Scholar]
- Jones 2007.Jones EG The Thalamus. Cambridge, UK: Cambridge Univ. Press, 2007, p. 1708.
- Lam and Sherman 2007.Lam YW, Sherman SM. Different topography of the reticulothalamic inputs to first- and higher-order somatosensory thalamic relays revealed using photostimulation. J Neurophysiol 98: 2903–2909, 2007. [DOI] [PubMed] [Google Scholar]
- Latawiec et al. 2000.Latawiec D, Martin KA, Meskenaite V. Termination of the geniculocortical projection in the striate cortex of the monkey: a quantitative immunoelectron microscope study. J Comp Neurol 419: 306–319, 2000. [DOI] [PubMed] [Google Scholar]
- Lee and Winer 2008a.Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol 507: 1879–1900, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee and Winer 2008b.Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol 507: 1920–1943, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Bickford 2003.Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contributions to short-term synaptic plasticity. J Neurophysiol 90: 3429–3440, 2003. [DOI] [PubMed] [Google Scholar]
- MacLean et al. 2006.MacLean JN, Fenstermaker V, Watson BO, Yuste R. A visual thalamocortical slice. Nat Methods 3: 129–134, 2006. [DOI] [PubMed] [Google Scholar]
- McCormick and Von Krosigk 1992.McCormick DA, Von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA 89: 2774–2778, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen et al. 1993.Olshausen BA, Anderson CH, Van Essen DC. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J Neurosci 16: 1180–1192, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou et al. 1999.Papageorgiou G, Ogden DC, Barth A, Corrie JET. Photorelease of carboxylic acids from 1-acyl-7-nitroindolines in aqueous solution: rapid and efficient photorelease of l-glutamate. J Am Chem Soc 121: 6503–6504, 1999. [Google Scholar]
- Reichova and Sherman 2004.Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol 92: 2185–2197, 2004. [DOI] [PubMed] [Google Scholar]
- Rose and Metherate 2001.Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol 106: 331–340, 2001. [DOI] [PubMed] [Google Scholar]
- Rouiller et al. 1991.Rouiller EM, Simm GM, Villa AEP, de Ribaupierre Y, de Ribaupierre F. Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas. Exp Brain Res 86: 483–505, 1991. [DOI] [PubMed] [Google Scholar]
- Shepherd et al. 2003.Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38: 277–289, 2003. [DOI] [PubMed] [Google Scholar]
- Sherman 2001.Sherman SM Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24: 122–126, 2001. [DOI] [PubMed] [Google Scholar]
- Sherman and Guillery 1998.Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators.” Proc Natl Acad Sci USA 95: 7121–7126, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman and Guillery 2002.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357: 1695–1708, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman and Guillery 2005.Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. Cambridge, MA: MIT Press, 2005.
- Spreafico et al. 1987.Spreafico R, Barbaresi P, Weinberg RJ, Rustioni A. SII-projecting neurons in the rat thalamus: a single- and double-retrograde-tracing study. Somatosens Res 4: 359–375, 1987. [DOI] [PubMed] [Google Scholar]
- Stratford et al. 1996.Stratford KJ, Tarczy-Hornoch K, Martin KAC, Bannister NJ, Jack JJB. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382: 258–261, 1996. [DOI] [PubMed] [Google Scholar]
- Tarczy Hornoch et al. 1999.Tarczy Hornoch K, Martin KAC, Stratford KJ, Jack JJB. Intracortical excitation of spiny neurons in layer 4 of cat striate cortex in vitro. Cereb Cortex 9: 833–843, 1999. [DOI] [PubMed] [Google Scholar]
- Van and Sherman Horn 2004.Van Horn SC, Sherman SM. Differences in projection patterns between large and small corticothalamic terminals. J Comp Neurol 475: 406–415, 2004. [DOI] [PubMed] [Google Scholar]
- Winer 1992.Winer JA The functional architecture of the medial geniculate body and the primary auditory cortex. In: Springer Handbook of Auditory Research. The Mammalian Auditory Pathway: Neuroanatomy, edited by Webster DB, Popper AN, Fay RR. New York: Springer-Verlag, 1992, vol. 1, p. 222–409. [Google Scholar]
- Wong-Riley 1979.Wong-Riley M Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171: 11–28, 1979. [DOI] [PubMed] [Google Scholar]
- Zeki and Shipp 1988.Zeki S, Shipp S. The functional logic of cortical connections. Nature 335: 311–317, 1988. [DOI] [PubMed] [Google Scholar]