Abstract
Computational studies are challenging the intuitive view that neurons with broad tuning curves are necessarily less discriminative than neurons with sharp tuning curves. In the context of somatosensory processing, broad tuning curves are equivalent to large receptive fields. To clarify the computational role of large receptive fields for cortical processing of somatosensory information, we recorded ensembles of single neurons from the infragranular forelimb/forepaw region of the rat primary somatosensory cortex while tactile stimuli were separately delivered to different locations on the forelimbs/forepaws under light anesthesia. We specifically adopted the perspective of individual columns/segregates receiving inputs from multiple body location. Using single-trial analyses of many single-neuron responses, we obtained two main results. 1) The responses of even small populations of neurons recorded from within the same estimated column/segregate can be used to discriminate between stimuli delivered to different surround locations in the excitatory receptive fields. 2) The temporal precision of surround responses is sufficiently high for spike timing to add information over spike count in the discrimination between surround locations. This surround spike-timing code (i) is particularly informative when spike count is ambiguous, e.g., in the discrimination between close locations or when receptive fields are large, (ii) becomes progressively more informative as the number of neurons increases, (iii) is a first-spike code, and (iv) is not limited by the assumption that the time of stimulus onset is known. These results suggest that even though large receptive fields result in a loss of spatial selectivity of single neurons, they can provide as a counterpart a sophisticated temporal code based on latency differences in large populations of neurons without necessarily sacrificing basic information about stimulus location.
INTRODUCTION
The body map in the primary somatosensory (S1) cortex (Chapin 1986; Chapin and Lin 1984; Mountcastle 1957; Penfield and Boldrey 1937) has been classically considered as a mosaic of columns/segregates (Favorov and Diamond 1990; Favorov and Kelly 1994a,b; Favorov and Whitsel 1988a,b; Favorov et al. 1987; Mountcastle 1957, 1997, 2003; Waters et al. 1995; Woolsey and Van der Loos 1970). A column/segregate is a discrete cortical module the neurons of which all have in common a small body area in their excitatory receptive fields. In the granular layer of S1 (layer IV) (Killackey and Ebner 1972), neurons typically display small excitatory receptive fields, mostly localized to the body area corresponding to a single column/segregate. Conversely, in the supragranular layers (layers II–III) and in the infragranular layers of S1 (layers V–VI), neurons are also activated by contacting remarkably large and irregularly shaped body areas, which represent the excitatory surround of the neuron's receptive fields and can vary greatly from neuron to neuron (Armstrong-James and Fox 1987; Chapin 1986; Chapin and Lin 1984; Ghazanfar and Nicolelis 1999; Haupt et al. 2004; Kleinfeld and Delaney 1996; Masino and Frosting 1996; Moore and Nelson 1998; Petersen and Diamond 2000; Peterson et al. 1998; Simons 1978; Tutunculer et al. 2006; Zhu and Connors 1999). These large and irregular receptive fields suggest that supra- and infragranular layers of S1 might have sacrificed the basic discrimination of stimulus location in favor of some other sensory or sensorimotor integration. Computational studies, however, are challenging this intuitive view of broad tuning curves (i.e., large receptive fields) being necessarily less discriminative than sharp tuning curves (i.e., small receptive fields) (Brown and Bäcker 2006; Montemurro and Panzeri 2006; Pouget et al. 1999; Seriès et al. 2004; Zhang and Sejnowski 1999). The computational potential of excitatory surround responses (i.e., broad tuning curves) in the primary somatosensory cortex remains largely unexplored.
In the present paper, we focus on the infragranular layers of S1 because their neurons have the largest receptive fields across layers (Chapin 1986). Large receptive fields of infragranular neurons are typically spatiotemporally organized in such a way that responses to stimuli delivered in the body area corresponding to the neuron's column/segregate display the greatest firing probability and the shortest latency, whereas excitatory surround responses display smaller firing probabilities and longer, more variable latencies (Ghazanfar and Nicolelis 1999; Petersen and Diamond 2000; Tutunculer et al. 2006). To investigate the computational implications of this spatiotemporal receptive field structure, we adopted the perspective of individual columns/segregates, which represent basic functional units of cortical integration and computation, receiving inputs from multiple body locations. We constructed two testable hypotheses: that surround responses of populations of infragranular neurons within the same column/segregate can be used to discriminate between individual surround locations and that the temporal precision of surround responses is sufficiently high for spike timing to provide additional information over spike count in the discrimination between surround locations.
To test these hypotheses, we bilaterally implanted microelectrode arrays into the forelimb/forepaw region of the rat infragranular primary somatosensory cortex to record the activity of large numbers of single neurons in response to tactile stimuli separately delivered to different locations on the forelimbs/forepaws under light anesthesia (Foffani et al. 2004; Tutunculer et al. 2006). We then used populations of neurons recorded from an estimated column/segregate to test the ability of their responses to discriminate between surround locations (1st hypothesis), using single-trial analysis techniques (Foffani and Moxon 2004) and information theoretic measures (Foffani et al. 2004). To assess the differential contribution of spike count and spike timing to the discrimination of surround locations (2nd hypothesis), we varied the bin size used to perform the classification (Foffani et al. 2004) and evaluated the effect of this manipulation on the ability of the population responses to discriminate between surround locations on a single-trial basis.
METHODS
Overview
Tactile stimuli were applied to 10 locations across the forelimb/forepaw while single neurons were recorded from arrays of microwire electrodes chronically implanted into the contralateral forelimb/forepaw S1 cortex. Stimulus locations were selected to allow no more than one location per column/segregate. We first evaluated the receptive field structure of these neurons. The neurons recorded from each animal were then subdivided into populations of cells recorded within an estimated column/segregate. Peristimulus time histograms (PSTHs) of each cell to each location stimulated (10 total) were generated and used as templates to discriminate stimulus location on a single-trial basis (PSTH-based classification) (Foffani and Moxon 2004). The ability of the population responses to discriminate between surround locations using spike count was evaluated by using a single 40-ms poststimulus bin in the PSTH templates and subsequent single trials. The ability of the same population responses to discriminate between surround locations using spike-timing was evaluated by using 10 4-ms poststimulus bins in the PSTH templates and subsequent single trials. We explicitly tested 1) whether surround responses of populations of infragranular neurons could be used to discriminate between individual surround locations and 2) whether spike timing provides additional information to spike count for the discrimination between surround locations. To further clarify the potential significance of spike timing in surround responses, we performed the following supplementary analyses: (i) we investigated whether spike timing was more informative when spike count is ambiguous, e.g., when discriminating close surround locations or when receptive fields are large; (ii) we studied how the additional information provided by spike timing over spike count depended on the number of neurons composing the population; (iii) we evaluated how much information was lost when using only the first spike per neuron in each single-trial response; (iv) and we investigated whether spike timing could be exploited to release the assumption that the time of stimulus onset is known. The receptive field structure of all neurons included in this study and their overall ability to discriminate stimulus location were reported in our previous works (Foffani et al. 2004; Tutunculer et al. 2006). Representative neurophysiological data are shown in Fig. 1. Details of the procedures follow.
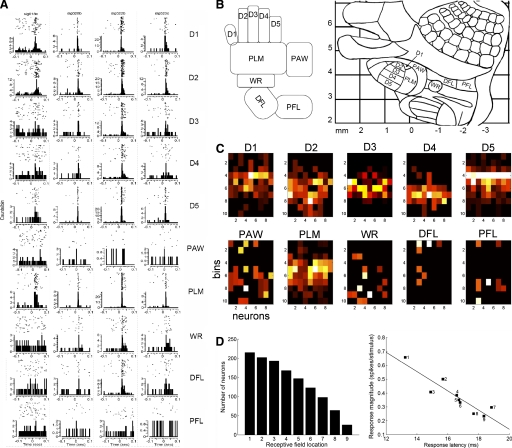
FIG. 1.
Response properties of infragranular neurons. A: raster plots and peri-stimulus time histograms (PSTHs) of a representative subset of 4 neurons (1 per column) simultaneously recorded from 1 rat responding to stimuli delivered to 10 locations on the contralateral forelimb (rows). Despite the sparseness of the locations stimulated, neurons responded with remarkable consistency. B: schematic representation of the locations stimulated (left, adapted from Tutunculer et al. 2006) and their corresponding distribution within the somatotopic arrangement of the primary somatosensory cortex (from Tutunculer et al. 2006, see also Chapin and Lin 1984). The locations stimulated included each of the digits (D1, D2, D3, D4, D5), the dorsal paw (PAW), the ventral palm (PLM), the wrist (WR), distal forelimb (DFL), and the proximal forelimb (PFL) (for further details, see Foffani et al. 2004; Tutunculer et al. 2006). C: spatiotemporal patterns of activity of 9 representative neurons with the same receptive field center (D5) selected from a population of neurons simultaneously recorded from 1 rat. For each image, the PSTHs of each cell in response to the labeled location (4-ms bin size; 100 stimuli) were color-coded and laid next to each other (x axis, neuron number; y axis, time poststimulus) such that each image is a population PSTH that represents the stimulus-specific response pattern for these cells. Colors are light-graded from maximal (1: white) to minimal (0: black) response. This population consistently responded to ≥6 of the 10 locations with stimulus-specific patterns of neural activity. D: excitatory receptive field structure for the entire dataset. Left: number of neurons (y axis) displaying significant responses to locations in their excitatory receptive fields (x axis). For each cell, receptive field locations were ranked by decreasing response magnitude, from the location that identified the neuron's column/segregate (1) to surround locations (2–9). Note that no cell responded to all 10 locations. Right: latency-magnitude scatter plot. When the stimulus was moved from the location that identified the neuron's column/segregate (1) to surround locations (2–9), response latencies (x axis) progressively increased as response magnitudes (y axis) decreased (Pearson r = −0.91, P = 0.0006; n = 9). Each latency-magnitude point represents the average over all neurons displaying significant responses to the receptive field location indicated by the corresponding number, as in the left plot. The SDs of the latencies within locations ranged between 4.3 and 5.9 ms (not shown).
Surgery and single-neuron recording
Recordings were made from Long-Evans rats (240–300 g), and all procedures were approved by the Institutional Animal Care and Use Committee at Drexel University and followed National Institutes of Health Guidelines. Experimental procedures for surgery, single neuron discrimination, somatosensory mapping and histology were described in our previous works (Foffani et al. 2004; Tutunculer et al. 2006).
In brief, to record from large numbers of neurons simultaneously and to minimize the effect of anesthesia on the somatosensory responses of the cells, arrays of 16 channel microwire electrodes (NB labs, Dennison, TX) were chronically implanted with stereotaxic techniques into the forepaw region of the infragranular primary somatosensory cortex of each hemisphere (coordinates 0.5 mm anterior to Bregma and 3.5 and 4.5 mm lateral to –1.5 posterior to bregma and 3.0 and 4.0 mm lateral, atlas of Paxinos and Watson) in five rats under pentobarbital sodium (Nembutal) anesthesia (50 mg/kg). Each electrode array consisted of two rows of eight 50-μm Teflon-coated stainless steel microwires. The spacing between microwires before implant was 200 μm. The arrays were oriented so that rows ran from rostral to caudal. Animals were allowed to recover 7–10 days, and then the responses of neurons to touch stimulation of the forepaw and forelimb were quantified under light Nembutal anesthesia (induction dose: 35 mg/kg, stage III-2) (Friedberg et al. 1999). Analogue signals were filtered (150 Hz to 1.5 kHz) and amplified using multi-neuron acquisition-processor (MNAP; Plexon, Dallas, TX), and single neurons were discriminated on each electrode using commercial software (Plexon). Single-neuron discrimination was based on thresholding, template matching, and principal-component analysis of waveforms. A representative example of discrimination of three neurons from a single electrode can be found in our previous work (Fig. 1, B and C, in Foffani et al. 2004). Most electrodes allowed us to discriminate one or two neurons.
Ten locations on each forelimb (5 digits, dorsum, palm, ankle, forearm, arm) were stimulated using a fine tipped metal probe (1 mm diam) that moved 0.5 mm (controlled through a piezoelectric element actuated by a Grass stimulator) and simultaneously sent TTL pulses to the MNAP to record the precise stimulus onset. Assumptions and implications of using this sparse stimulation protocol were fully discussed in our previous work (Tutunculer et al. 2006). For the purpose of this study, our protocol guarantees that different locations correspond to different columns/segregates (Favorov and Diamond 1990; Favorov and Kelly 1994a,b; Favorov and Whitsel 1988a,b; Favorov et al. 1987; Mountcastle 1997; Waters et al. 1995). Every location was stimulated 100 times with 100-ms-long step stimuli delivered at 0.5-Hz frequency. Using piezoelectric control, the deflection time of the metal probe was <0.5 ms, about one order of magnitude smaller than the jitter of neural responses (Foffani et al. 2004). This virtually immediate deflection time was aimed to approximate as much as possible the ideal engineering concept of a step stimulus and reproduces the situation of a very light, abrupt contact with an object. To control the magnitude of the stimuli at each location, the metal probe was first positioned on the skin, ensuring contact but no visual indentation under ×10 magnification. The metal probe was then moved 0.5 mm away from the skin, and the stimulation was started. The effect of the stimulus was viewed under ×10 magnification to ensure no movement of the digits or limb. These stimulus properties and the relatively large distance between the locations stimulated make the possibility of stimulus spread across locations extremely unlikely. All locations were stimulated within the same recording session to ensure that the same neurons were recorded in response to stimulation of all locations. All 100 stimuli were given to a location, and then the stimulator was moved to the next location. There was no randomization of stimuli. The frequency of stimulation of 0.5 Hz corresponds to twice the interstimulus interval previously shown not to influence subsequent responses (Chapin 1986). The spike times for all discriminated neurons were recorded during the experiment along with timestamps of the onset of the stimuli. Signals were stored in Nex (Plexon) and the single-trial bin counts (i.e., single-trial rate histograms, 1-ms bin size) of all the neurons were exported to Matlab (version 6.5, The Mathworks, Natick, MA) for further analysis.
The positions of the tips of the electrodes within the infragranular cortex were histologically verified at the end of the study (Foffani et al. 2004; Tutunculer et al. 2006). In addition, because using microwire arrays for chronic recordings neurons with large diameter can be easily discriminated, most cells in this study are likely to be layer V pyramidal neurons, similar to previous studies in the whisker cortex (Foffani and Moxon 2004; Ghazanfar and Nicolelis 1999).
Data analysis
STRUCTURE OF THE EXCITATORY RECEPTIVE FIELDS.
The structure of each neuron's receptive field was quantitatively studied using the PSTHs of the neural responses at 1-ms bin size as previously described in detail (Tutunculer et al. 2006). PSTH responses and receptive fields of individual neurons were previously described (Tutunculer et al. 2006, Figs. 2, 5, and 7) and examples are presented in Fig. 1. Briefly, a neural response was considered significant if it exceeded a threshold set as the average background activity of the neuron (evaluated from 100 to 5 ms before the stimulus) plus 3 SD, at least three bins were over the threshold, and the spiking activity between the first and the last significant bin was significantly greater than the background activity (nonpaired t-test, P < 0.001). We used a relatively conservative significance level to avoid false positives due to the high number of comparisons. We also empirically verified that this significance level was consistent with determination of presence or absence of response by visual inspection of the PSTH. The sum of count values in all bins between the first significant bin and the last significant bin evaluated for each individual PSTH was considered as response magnitude, expressed in spikes/stimulus. For each neuron, we estimated the identity of the column/segregate from the location that generated the greatest response magnitude. This estimation procedure directly derives from the classical definitions and neuropysiological observations about columns/segregates (Favorov and Diamond 1990; Favorov and Kelly 1994a,b; Favorov and Whitsel 1988a,b; Favorov et al. 1987; Mountcastle 1957, 1997; Waters et al. 1995; Woolsey and Van der Loos 1970). All the other locations where the neuron showed a significant excitatory response on the side of the body contralateral to the electrode were identified as surround locations. To reduce errors due to the finite number of locations stimulated, a neuron was included in the analyses only if the maximal response magnitude to stimulation of any location was strong enough (>0.2 spike/stimulus) to allow a reliable estimation of the neuron's column/segregate (Tutunculer et al. 2006). Detailed examples of cortical topographical distributions of neurons based on their estimated columns/segregates can be found in our previous work (Fig. 3 in Tutunculer et al. 2006). As expected, the responses with greatest magnitude—used to estimate the identity of a neuron's column/segregate—also displayed the shortest latency, whereas surround responses displayed progressively longer latencies as the response magnitude decreased (Fig. 1D).
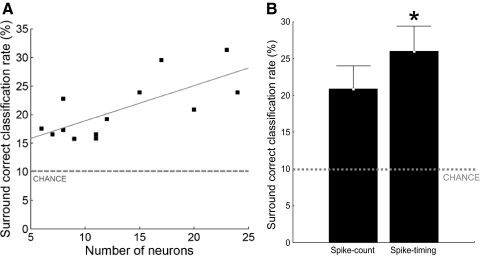
FIG. 2.
Single-trial discrimination between surround locations. A: scatter plot of the average classification performance of surround locations (expressed as correct classification rate, y axis) as a function of the number of neurons in the population (x axis). —, best linear fit of the data (Pearson r = 0.74, P = 0.0048); ···, chance classification performance (10%). Each square represents the average classification performance of 9 surround locations for 1 population of infragranular neurons recorded from within an estimated column/segregate. The classification performance was obtained with the PSTH-based classification method (Foffani and Moxon 2004) using spike count. B: spike count vs. spike timing. The average classification performance of surround locations (y axis) significantly increased (*) when spike timing was used for the discrimination as compared with spike count alone (x axis). Whiskers represent 95% confidence intervals. ···, chance classification performance (10%).
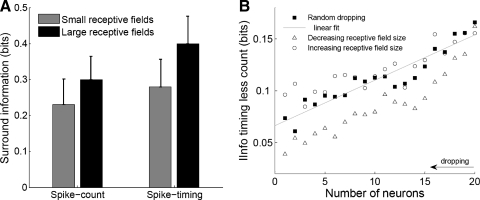
FIG. 3.
Surround spike-timing code A: large vs. small receptive fields. Surround responses conveyed more information about stimulus location and the additional information conveyed by spike timing over spike count was greater in subpopulations of neurons with large receptive fields compared with subpopulations of neurons with small receptive fields. Whiskers represent 95% confidence intervals. B: population coding. The additional information provided by spike timing over spike count, i.e., the information extracted with spike timing less the information extracted with spike count (y axis), linearly increased as the number of neurons increased (x axis). Random neuron dropping (▪) was compared with neuron dropping by increasing receptive field size (○) and by decreasing receptive field size (▵). The additional information extracted using spike timing over spike count significantly decreased when dropping neurons with the largest receptive field size compared with random neuron dropping.
SINGLE-TRIAL DISCRIMINATION BETWEEN SURROUND LOCATIONS.
To determine if excitatory surround responses could be used to discriminate between stimuli delivered to different surround locations, neurons recorded simultaneously from each individual animal were divided into populations of cells recorded from the same estimated column/segregate. Discrimination of stimulus location involved classifying single-trial responses using a PSTH-based classification method previously described and validated (Foffani and Moxon 2004; Foffani et al. 2004). Briefly, the PSTH-based classification method first creates a set of templates corresponding to each location stimulated (Fig. 1C). Each template corresponding to an individual location is constructed using the average responses of the neurons composing the population to all stimuli delivered to that location (i.e., the PSTHs). The PSTH-based classification method then classifies each single trial (i.e., population response to a single stimulus delivered to a single location) by assigning it to the location with the “closest” template in the Euclidean distance sense.
The properties of the PSTH-based classification method were thoroughly discussed in our previous works (Foffani and Moxon 2004; Foffani et al. 2004) but are restated here for clarity. On the one hand, because the PSTH-based method employs only first-order statistics (i.e., the means generated by the PSTHs) and the convergence of the estimate of the mean does not depend on the number of variables, the method is particularly appropriate for studying the differential contribution of spike count and spike timing to the somatosensory code, which requires changing the number of variables used for the classification (by changing the bin size). On the other hand, the PSTH-based method does not explicitly take into account correlations between neurons in the trial-to-trial variability, usually referred as noise correlations. However, stimulus-dependent noise-correlations seem to play a minor role in the somatosensory code (Petersen et al. 2001), allowing a decoder that neglects noise correlations to extract virtually 100% of the encoded information (Amari and Nakahara 2006; Panzeri et al. 2002; Pola et al. 2003). Noise correlations are therefore unlikely to affect the main results and conclusions of this work. Finally, the PSTH-based method is particularly effective in a complete “leave-one-out” cross-validation design, which refers to the situation in which for every single trial to be classified the training set is composed by all the other trials and the testing set is only the single trial itself. In fact, as the templates are constructed with average responses (the PSTHs), the training stage can be run just once on the entire dataset, and in the testing stage a single trial can be excluded from the training set by simply subtracting it from the template average with negligible increase of computational time.
In the computational experiments presented here, classifications were always performed between the 10 forelimb locations contralateral to the implanted array, including the location that identified the neurons' column/segregate, in complete leave-one-out cross-validation. A 40-ms duration response window (5–44 ms after contact) was employed for the classification. Classification performance was evaluated as correct classification rate (%). To verify the hypothesis that excitatory surround responses can be used to discriminate between individual surround locations, the average correct classification rate of the nine surround locations in each population of neurons within the same estimated column/segregate (n = 13) was submitted to a two-tailed one-sample Wilcoxon signed-rank test against chance classification rate. Chance classification rate is theoretically 10% because the location identifying the neurons' column/segregate was always conservatively included as a possible class in the classification.
To evaluate whether spike timing increases the ability of populations of neurons within the same column/segregate to discriminate between surround locations compared with spike count, we applied the PSTH-based classification described in the preceding text, dividing the 40-ms-long response window with different bin sizes: one 40-ms poststimulus bin for spike count, 10 4-ms bins for spike timing. Classification performance was evaluated as correct classification rate (%). To test the effect of spike timing, the average correct classification rate of surround locations in each population of neurons within the same estimated column/segregate (n = 13) was submitted to a two-tailed paired Wilcoxon signed-rank test (spike count vs. spike timing). The rationale for choosing 4-ms bins for spike timing is that we have previously shown that this bin size approximately corresponds to the trial-to-trial jitter of the neural responses and is therefore optimal for discriminating stimulus location in these experimental conditions (Foffani et al. 2004). As a control, we repeated the single-trial discrimination of stimulus location using a bin size of 1 ms.
The statistical analyses described in the preceding text relied on the theoretical chance classification performance (10%). We also empirically measured chance classification performance with a bootstrap analysis by running the single-trial discrimination of stimulus location after shuffling trials across locations. We found that chance correct classification rate for individual locations (n = 130) was 10.2 ± 13.7% (mean ± SD). This analysis allowed us to statistically test the ability of single populations to discriminate between surround locations, by applying, for each population and for both spike count and spike timing, an unpaired two-tailed Wilcoxon rank sum test between correct classification rate of surround locations (n = 9) and chance correct classification rate (n = 130). We also used the Spearman correlation coefficient to test whether the significance of surround discriminability on a population-by-population basis correlated with the number of neurons composing the population.
Previous studies in very similar experimental conditions have shown that response adaptation is not observed when stimuli are separated by ≥1 s (Chapin 1986). We carried out our experiments with an important margin of safety, using a stimulation frequency of 0.5 Hz. Nonetheless, we performed a control analysis to verify that our ability to discriminate stimulus location did not change due to the repetition of stimuli within locations. To this end, we repeated the PSTH-based classification on either the first 50 trials or the last 50 trials per location. We then used a paired two-tailed Wilcoxon signed-rank test to challenge the null hypothesis that correct classification rate was identical for the first 50 trials and for the last 50 trials per location. The methodological robustness of the PSTH-based classification method to the number of trials used in the analysis was studied in a previous work (Foffani and Moxon 2004).
INFORMATION THEORETIC MEASURES.
Correct classification rate is a good measure of classification performance to show that the activity of populations of neurons within the same column/segregate can be used to discriminate between individual surround locations, but this measure is not practical for quantifying the total amount of information conveyed by surround responses because percentages cannot be added. We therefore used a more rigorous measure of classification performance, mutual information, which is additive, i.e., the total information can be expressed as the sum of the information conveyed by responses to individual locations. Importantly here and throughout the paper we use the word “information” to refer to the mathematical concept of “mutual information” within the framework of Shannon's information theory. We are explicitly not attempting to quantify information at the level of perception. The mutual information I(s,r) between predicted stimulus locations s and real stimulus locations r was computed from the confusion matrix of the classification (Foffani et al. 2004; Franco et al. 2004; Gochin et al. 1994; Gruner 2002; Laubach et al. 2000; Panzeri et al. 1999; Rolls et al. 1997, 1998), according to the following formula
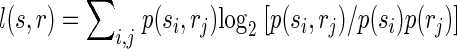 |
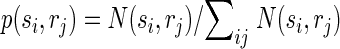 |
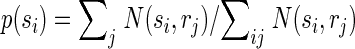 |
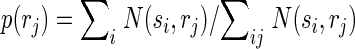 |
(1) |
where N(si,rj) is the number of times a response from location j is classified as having arisen from location i. Therefore Σi,j N(si,rj) represents the total number of trials (1,000 in our data), Σi N(si,rj) represents the total number of trials at location j (always 100 in our data), p(si) represents the probability that a response from any location is classified as having arisen from location i, and p(rj) is the probability that a response from location j is classified as having arisen from any location (prior probability of location j, in our data always = 1/10). Theoretical chance classification, when discriminating between 10 different locations, corresponds to 0 bits of mutual information. Perfect classification would provide 3.32 bits (i.e., log210) of mutual information, each individual location contributing to a maximum of 0.332 bits. The upward bias of the mutual information due to finite sampling was experimentally minimized by using 10 times as many trials per location (100) as the number of locations (10). We empirically measured chance classification performance with the same bootstrapping procedure used for chance correct classification rate. We found that chance classification corresponded to 0.007 ± 0.003 bits of mutual information for individual locations (n = 130). Note that the data processing inequality implies that the mutual information between predicted and actual stimuli is a lower bound for the mutual information between the neural responses and the actual stimuli (Schneidman et al. 2003).
The surround information, i.e., the information related to the discrimination between individual surround locations, was evaluated as the sum of the contributions provided by the responses from each of the nine surround locations, i.e., conservatively excluding the contribution provided by the responses from the location used to estimate the column/segregate
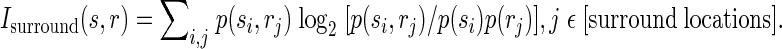 |
(2) |
To confirm that spike-timing increases the ability of population responses to discriminate between surround locations compared with spike count, the surround information estimated in each population of neurons within the same estimated column/segregate (n = 13) was submitted to a two-tailed paired Wilcoxon signed-rank test (spike count vs. spike timing).
DISCRIMINATING DISTANT LOCATIONS VERSUS CLOSE LOCATIONS.
To further our understanding of the role of spike timing in discriminating surround locations, we took advantage of the fact that as the cortical distance between a surround location and the neurons' column/segregate increases, the response magnitude decreases (Tutunculer et al. 2006). We tested whether spike timing was more informative to discriminate close surround locations, which have very similar response magnitudes compared with the location corresponding to the neurons' estimated column/segregate rather than distant locations, which have very different response magnitudes compared with the location corresponding to the neurons' estimated column/segregate. To this end, we reanalyzed the outcome of the classification by separating locations into two groups: the first group included the digits and the palm, the second group included the rest of the forelimb (dorsal paw, wrist, distal forelimb, proximal forelimb). The rationale for this grouping is that in our previous study the ventral palm always clustered with the digits (Tutunculer et al. 2006). Using only populations of neurons whose column/segregate was in the digits or palm, we considered the digits and the palm as close surround locations (excluding the location corresponding to the neurons' column/segregate) and the other locations as distant surround locations. Correct classification rate was used to quantify the ability of the population responses to discriminate close surround locations and distant surround locations using either spike count or spike timing.
LARGE RECEPTIVE FIELDS VERSUS SMALL RECEPTIVE FIELDS.
To test whether the impact of spike timing depended on the receptive field size of neurons, each population of neurons recorded within the same estimated column/segregate was divided into two subpopulations of neurons based on their receptive field size: one subpopulation was composed of neurons the receptive field size of which was smaller than the population median, the other subpopulation was composed by neurons the receptive field size of which was larger than the population median. Neurons the receptive field size of which was equal to the population median were excluded when necessary to guarantee that the two subpopulations had the same number of neurons. The information about the discrimination between surround locations extracted with spike count and the additional information extracted with spike timing were compared between the subpopulations of neurons with small receptive fields and the subpopulations of neurons with large receptive fields using the two-tailed paired Wilcoxon signed-rank test.
POPULATION CODING.
The dependence of the additional information provided by spike timing over spike count on the number of neurons composing the population was tested through a neuron dropping procedure (Foffani and Moxon 2004; Foffani et al. 2004). The additional information provided by spike timing over spike count (bits) was measured as the difference between the information extracted with 4-ms bin size and the information extracted with 40-ms bin size, and was averaged, for each number of neurons N, over all the available populations composed by at least N neurons. The Pearson coefficient was used for correlations.
To corroborate that the impact of spike timing depended on the receptive field size of neurons, we repeated the neuron dropping procedure after sorting neurons either by increasing or by decreasing receptive field size. We applied a paired t-test on the average information extracted after dropping one by one 10 neurons starting from 20, to challenge the null hypothesis that the additional information provided by spike timing over spike count did not differ when neurons were dropped randomly compared with increasing/decreasing receptive field size.
FIRST SPIKES.
To test whether the information provided by surround spike timing relied on a first-spike code, classification performance was obtained by applying the PSTH-based classification method considering only the first spike per neuron after each stimulus (Foffani et al. 2004; Panzeri et al. 2001).
DISCRIMINATION OF STIMULUS ONSET.
To test whether the knowledge of stimulus onset was a limiting assumption for the spike-timing code in surround responses, we performed a single-trial analysis to explicitly discriminate the time of stimulus onset. We used the same PSTH-based classification method in a manner similar to its use for stimulus location, but for each population of cells within the same estimated column/segregate, we classified bins (i.e., time poststimulus, at 4-ms bin size) rather than locations. For this analysis, we used only the responses to the location corresponding to the estimated column/segregate of each population of neurons. The average number of spikes per bin for each neuron represented the templates against which single-trial responses were compared. Because each bin is defined in relation to stimulus onset, classifying bins is equivalent to discriminating the time of stimulus onset. Classification performance was always expressed as bits of mutual information between predicted bins and real bins, using the same formula described for stimulus location (Eq. 1).
Both analyses for discrimination of stimulus location and discrimination of stimulus onset make an additional implicit assumption: that a stimulus occurred. To release this assumption, we extended the discrimination of stimulus onset to perform stimulus detection, i.e., to discriminate the presence or absence of a stimulus. To this end we discriminated, at 40-ms bin size, between the poststimulus window (5–44 ms, presence of stimulus) and a prestimulus window (−44 to −5 ms, absence of stimulus). Again for this analysis, we used only the responses to the location corresponding to the estimated column/segregate of each population of neurons. Classification performance was expressed in terms of correct classification rate (%). Because in this analysis the possible classes are two (presence or absence of stimulus), chance classification corresponds to 50%.
Throughout the text values are given as means ± SD. Results were considered significant at P < 0.05.
RESULTS
We studied the activity of 215 neurons recorded from the infragranular layers of the primary somatosensory cortex in five rats in response to tactile step stimuli applied to 10 discrete locations on the contralateral forelimb (5 digits, dorsum, palm, ankle, forearm, arm) (Foffani et al. 2004; Tutunculer et al. 2006). Neurons were grouped based on their estimated column/segregate [13 populations of 6–24 neurons (mean 13.1 neurons)], and we performed single-trial discrimination of stimulus location to test the ability of the population responses to discriminate between individual surround locations (i.e., between locations corresponding to other columns/segregates), using both spike count and spike timing. We performed the following supplementary analyses to clarify the computation role of the excitatory surround: (i) we investigated whether the impact of spike timing in discriminating between surround locations depended on the distances between locations and on the size of the neurons' receptive fields; (ii) we studied how the additional information provided by spike timing over spike count depended on the number of neurons composing the population; (iii) we evaluated how much information was lost when using only the first spike per neuron in each single-trial response; (iv) and we tested whether spike timing could be exploited to release the assumption that the time of stimulus onset is known.
Single-trial discrimination between surround locations
Our first hypothesis was that surround responses of populations of infragranular neurons within the same estimated column/segregate could be used to discriminate stimuli between individual surround locations. Because neurons displayed different response magnitudes to stimuli delivered to different locations, we tested whether the discrimination between surround locations could be achieved using only spike count. To assess the contribution from spike count, the neural responses were presented to the PSTH-based classification method in one single 40-ms bin. Of course each column/segregate could discriminate the location with largest response magnitude better than any surround location (Petersen and Diamond 2000). However, even using only spike count, the responses of populations of neurons within the same estimated column/segregate correctly classified the exact surround location where stimuli were delivered in 20.8 ± 5.2% of the trials; this is about twice what would be expected by chance (1-sample Wilcoxon: P = 0.0002; Fig. 2A). These results suggest that differences in response magnitude in infragranular neurons within the same column/segregate can be used to discriminate between stimulation of different surround locations on the body.
Next, we moved to our second hypothesis, that the temporal precision of surround responses is sufficiently high for spike timing to provide additional information over spike count in the discrimination between surround locations. To assess the contribution from spike timing, the neural responses were presented to the PSTH-based classification method in 10 4-ms bins. We found that the ability of populations of neurons within the same estimated column/segregate to discriminate between surround locations significantly increased using spike timing (26.0 ± 5.6%) compared with spike count (20.8 ± 5.2%; paired Wilcoxon: P = 0.0210; Fig. 2B). Note that the additional 5.2% of trials (i.e., 26.0-20.8) correctly classified using spike timing correspond to a 25% improvement of classification performance. This effect of spike timing improving the discrimination between surround locations could also be observed on a population-by-population basis. Surround locations were discriminated significantly better than chance (unpaired Wilcoxon at P < 0.05) in only 46% of the populations (6 of 13, unpaired Wilcoxon at P < 0.05) when using spike count, whereas that ratio rose to 92% (12 of 13) of the populations when using spike timing. As expected, the ability to discriminate between surround locations increased as the number of neurons composing the population increased (Spearman, spike count: R = 0.64, P = 0.0180; spike timing: R = 0.63, P = 0.0208; n = 13; Fig. 2A). As a control analysis, we found no significant differences in classification performance of individual locations between the first 50 trials and the last 50 trials, either using spike count (paired Wilcoxon: P = 0.56, n = 130) or spike timing (P = 0.41). Finally, the average 25% improvement of spike timing over spike count in the discrimination between surround locations was confirmed when classification performance was measured with mutual information (spike count: 0.36 ± 0.15 bits; spike timing: 0.45 ± 0.17 bits; Wilcoxon: P = 0.0002, n = 13). Further decreasing the bin size from 4 to 1 ms did not significantly change the overall information extracted in agreement with our previous work (Foffani et al. 2004).
Discriminating distant locations versus close locations
Response magnitudes progressively decrease as a stimulus is delivered at locations that are progressively more distant from the center of a neuron's receptive field (Tutunculer et al. 2006). Differences in response magnitudes are therefore smaller between close locations than between distant locations. It is therefore reasonable to expect that spike count would be more efficient for discriminating between distant locations than between close locations. Spike timing could then become particularly helpful for discriminating between close locations when differences in response magnitude are very small. We verified these predictions by considering the distance between locations in our analysis. Because our previous study suggested a functional separation between the forepaw and the rest of the forelimb (Tutunculer et al. 2006), we broke the analysis into digits + palm and the rest of the forelimb (dorsal paw, wrist, distal forelimb, proximal forelimb). Of 13 populations of neurons, 12 were recorded from column/segregates estimated to be within the digits or palm and were used for this analysis. The digits and the palm were considered close surround locations, whereas the other locations were considered distant surround locations. We found that distant surround locations were discriminated 2.6 times better than chance with spike count (25.9 ± 28.9%), and their discrimination showed little improvement with spike timing (28.5 ± 29.2%). Conversely close surround locations were poorly discriminated with spike count (16.4 ± 14.7%), but their discrimination considerably improved with spike timing (22.8 ± 16.4%). Note that the additional 6.4% of trials (i.e., 22.8-16.4) correctly classified using spike timing in close surround locations corresponds to a 39% improvement of classification performance for those locations. These results suggest that spike timing could be particularly critical to resolve the uncertainty between stimuli that are difficult to discriminate using spike count alone.
Large receptive fields versus small receptive fields
According to the results reported in the previous section, one would expect spike timing to be more informative about the discrimination between surround locations in neurons with large receptive fields than in neurons with small receptive fields. This prediction was tested by directly assessing the ability of neurons with small receptive fields to discriminate between surround locations compared with neurons with large receptive fields (Fig. 3A). This analysis was performed by separating each population of neurons into a subpopulation with receptive field size below the median and a subpopulation with receptive field size above the median. The average receptive field size was 4.9 ± 2.3 locations in the first group and 8.2 ± 2.7 locations in the second group. Classification performance using spike count was greater (paired Wilcoxon: P = 0.0081) in the subpopulations of neurons with large receptive fields (0.30 ± 0.12 bits) compared with the subpopulations of neurons with small receptive fields (0.23 ± 0.13 bits). Spike timing was more informative than spike count both in the subpopulations of neurons with large receptive fields (0.40 ± 0.14 bits) and in the subpopulations of neurons with small receptive fields (0.28 ± 0.14 bits). Importantly, the additional information conveyed by spike timing over spike count was greater in the subpopulations of neurons with large receptive fields (paired Wilcoxon: 0.0266), representing a 33% improvement of classification performance. These findings support the view of spike timing being particularly informative when receptive fields are large.
Population coding
Somatosensory information is distributed among neurons in the sense that the information carried by a population is greater than the information separately carried by any of the neurons that compose the population (Foffani et al. 2004; Ghazanfar et al. 2000; Nicolelis et al. 1998; Petersen et al. 2001). To investigate whether this population effect extends to the spike-timing code expressed by surround responses, we evaluated the dependence of the additional information provided by spike timing over spike count on the number of neurons in the population. This dependence is critical because if the additional information provided by spike timing over spike count about stimulus location decreased as the number of neurons increased, it would likely have little physiological significance in the brain. Our results show that the additional information provided by spike timing over spike count (i.e., the difference between the information extracted using spike timing and the information extracted using spike count) linearly increased with the number of neurons (Pearson: r = 0.94, P < 0.0001; Fig. 3B). Spike timing is therefore likely to be particularly informative in very large populations of neurons.
The results reported in the previous sections suggest that spike timing can overcome the ambiguity of spike count particularly when receptive fields are large. To verify this interpretation from the perspective of population coding, we performed the single-trial discrimination while dropping neurons in increasing or decreasing order of receptive field size (Fig. 3B). Compared with a random neuron dropping, we found that the additional information extracted using spike timing over spike count significantly decreased when dropping neurons with the largest receptive field size (paired t-test: P < 0.0001), whereas it did not change when dropping neurons with the smallest receptive field size (P = 0.29). These findings corroborate that spike-timing becomes particularly informative when receptive fields are large.
First spikes
To investigate whether most of the information about stimulus location is carried by the first spike in each neuron's response, we quantified how much information is lost by considering only first spikes while ignoring subsequent spikes in the classification. We found that using only first spikes it was possible to extract virtually all the information (98%) contained in the neural responses. First spikes constituted about 70% of the total spikes (Foffani et al. 2004), suggesting that ignoring ∼30% of the spikes led to negligible loss of information. This result is consistent with previous studies suggesting that under strong temporal constraints the somatosensory system could achieve rapid information processing with just one spike per neuron (Foffani et al. 2004; Johansson and Birznieks 2004; Petersen et al. 2001; Thorpe et al. 2001; VanRullen et al. 2005). This is important also because the first-spike code provides an intuitive neurophysiological interpretation of the information extracted with the PSTH-based classification method (Foffani et al. 2004), thereby clarifying the nature of the spike-timing code expressed by surround responses. In fact, the PSTH of a first-spike response represents the statistical distribution of the first-spike latency related to stimulus onset. Spike-timing precision therefore allows populations of neurons to discriminate latency differences between and within surround responses.
Discrimination of stimulus onset
An open question when studying how cortical neurons discriminate stimulus location is how do neurons know when the stimulus occurred (VanRullen et al. 2005). In fact, discriminating stimulus location from the activity of a population of neurons relies on the experimental assumption that stimulus onset is known. To investigate whether this was a limiting assumption for the surround spike-timing information, we performed an analysis to explicitly discriminate the time of stimulus onset. Essentially rather than asking whether the single-trial activity of a population of neurons in 10 bins (at 4-ms bin size) within a 40-ms-long poststimulus time window can be used to discriminate stimulus location, we used the PSTH-based classification method to test whether the single-trial activity of the neurons in a single bin could be used to discriminate the time of stimulus onset, i.e., to discriminate the identity of the bin between 10 possible bins, at 4-ms bin size, in the poststimulus time window. Using only the responses to the location corresponding to the estimated column/segregate, we confirmed the prediction that the activity of neurons in a single bin (bin size = 4 ms) in the poststimulus time window (window size = 40 ms) could be used to discriminate stimulus onset (0.43 ± 0.16 bits).
The preceding discrimination of stimulus onset still makes the implicit assumption that a stimulus occurred. To release this assumption, we extended the discrimination of stimulus onset to perform stimulus detection by discriminating between the poststimulus window (5–44 ms), which represented presence of stimulus, and a prestimulus window (−44 to −5 ms), which represented absence of stimulus. Again using only the responses to the location corresponding to the estimated column/segregate, we found that the presence or absence of a stimulus could be determined in 87.7 ± 9.8% of the trials. These results suggest that stimulus detection is a relatively easy task compared with discrimination of stimulus location and stimulus onset. Assuming that a stimulus occurred is therefore not a restrictive assumption.
Overall, these results suggest that the ability of populations of neurons within the same column/segregate to discriminate the time of stimulus onset could provide the internal temporal references necessary to physiologically exploit spike-timing precision for the complex discrimination between surround locations.
DISCUSSION
Using single-trial responses of populations of cortical neurons to punctuate tactile stimuli, we obtained two main results. First, the responses of even relatively small populations of infragranular neurons recorded from the same estimated cortical column/segregate in the rat primary somatosensory cortex (S1) can be used to discriminate between individual surround locations. Second, the temporal precision of surround responses is sufficiently high for spike timing to add information over spike count in the discrimination between surround locations. This surround spike-timing code (i) is particularly informative when latency differences can overcome the ambiguity of similar response magnitudes, e.g., when discriminating between close locations or when receptive fields are large, (ii) is relevant at the population level, (iii) is a first-spike code, (iv) and is not limited by the assumption that the time of stimulus onset is known. These results suggest that the spatiotemporal structure of the excitatory receptive fields of S1 infragranular neurons allows the activity of cortical columns/segregates to encode which other columns/segregates are being activated and that surround responses critically contribute to “the role of spike timing in the forelimb somatosensory cortex of the rat” (Foffani et al. 2004). In general, even though large receptive fields (i.e., broad tuning curves) result in a loss of spatial selectivity of single neurons, they can provide as a counterpart a sophisticated temporal code based on latency differences in large populations of neurons without necessarily sacrificing basic information about stimulus location.
Methodological considerations
The aim of the present work was to investigate the role of surround responses (i.e., large receptive fields) of cortical somatosensory neurons in the computational problem of discriminating stimulus location. It is first important to remark that we are exclusively talking about the receptive field excitatory surround of infragranular neurons and that different approaches should be adopted to investigate the classical inhibitory surround (see e.g., Derdikman et al. 2003; DiCarlo and Johnson 2002; DiCarlo et al. 1998; Sripati et al. 2006). Our sparse stimulation protocol was designed so that each location stimulated corresponded to a different cortical column/segregate. This protocol ensures that the possible error in the neurophysiological estimation of each neuron's column/segregate was smaller than the cortical distance between adjacent locations and that all surround responses were elicited by stimuli outside the body area corresponding to the neuron's column/segregate. We can therefore confidently conclude that spike timing provides additional information over spike count to discriminate between surround locations. In addition, because spike timing was particularly informative when discriminating between close surround locations, the importance of spike timing is likely to increase as the density of stimulation sites increases. As an important note, spike timing refers here to the temporal code based on latency differences within the neural responses to individual passive stimuli (as in Foffani et al. 2004; Panzeri et al. 2001; Petersen et al. 2001). This should not be confused with the temporal code based on latency differences between neural responses to subsequent sensorimotor events during active behaviors (as in Ahissar and Arieli 2001; Knutsen et al. 2006; Zacksenhouse and Ahissar 2006). Indeed these temporal codes are complementary and not mutually exclusive.
Another issue to be considered is the possibility of experimental fluctuations in our recordings. Because our electrode implants were chronic, we could perform the experiments ≥1 wk after surgery; this allowed us to obtain very stable neuron recordings with light levels of anesthesia. Stable levels of light anesthesia were maintained at different times within the same session by giving small supplements when the rat consistently responded to tail pinch. On the other hand, stable levels of tactile stimulation across locations were maintained by using a piezoelectric actuator to control displacement of the stimulation probe and by using ×10 magnification to position the stimulation probe always at the same distance from the skin. However, the most convincing argument against a possible influence of experimental fluctuations in our results is probably the dependence of the surround spike-timing code on cortical distances. Furthermore, these hypothetical fluctuations would at most alter spike-count information but are unlikely to affect spike-timing information, rendering our results conservative.
Infragranular neurons can receive direct thalamic projections and paralemniscal inputs (Chmielowska et al. 1989; de Kock et al. 2007; Lu and Lin 1993; Manns et al. 2004) but are mostly considered as the main output of the primary somatosensory cortex (Ghazanfar and Nicolelis 1999; Killackey et al. 1989; Koralek et al. 1990). The receptive fields of these neurons are the largest across cortical layers (for brief review, see Tutunculer et al. 2006) and increase as the level of anesthesia decreases, reaching their maximum extent in awake conditions (Chapin and Lin 1984; Chapin et al. 1981; Erchova et al. 2002). Indeed, “the surprisingly large area of the cortex that can be excited by a single […] stimulus is not an artifact of anesthesia, but is an important integrative property of sensory processing also occurring in awake animals” (Ferezou et al. 2006). Our experimental anesthetized conditions represent a good model of passive unexpected stimuli during quiet behavior (Ferezou et al. 2006; Krupa et al. 2004). Even though different stimuli and attentional states produce different response patterns in thalamo-cortical networks (Aguilar and Castro-Alamancos 2005; Ferezou et al. 2006; Hirata et al. 2006; Krupa et al. 2004;), the computational role of the excitatory surround of infragranular neurons depicted in this study could be critical for clarifying the mechanisms of cortico-cortical (Nicolelis et al. 1998) and cortico-subcortical integration (Aguilar et al. 2003; Alitto and Usrey 2003; Canedo and Aguilar 2000; Castro-Alamancos 2004; Temereanca and Simons 2004) in normal behaviors. Nonetheless it needs to be stressed that inferences about perception based on anesthetized data should be cautious and that our experimental condition simply represents a model to rigorously quantify the computational potential of broad tuning curves (i.e., large receptive fields) in the context of somatosensory processing.
Stone in the pond
A punctuate stimulus applied to the skin causes a wave of neural activity to spread from the cortical column/segregate that corresponds to the location stimulated to cortical columns/segregates corresponding to surround locations (Ferezou et al. 2006; Petersen and Diamond 2000; Petersen et al. 2003a,b), similarly to a stone creating a series of expanding ripples when it is dropped into a pond. Within this analogy, the splash of the stone is the response corresponding to a specific column/segregate, whereas the expanding ripples are the surround responses of all other column/segregates. If the splash of the stone is directly observed, then it is straightforward to determine where the stone entered the pond (i.e., stimulus location). Even if the splash of the stone was not directly observed, it is still possible to infer where the stone entered the pond by analyzing the expanding ripples. Moreover, the expanding ripples also allow the inference of when the stone entered the pond. The power of this representation becomes apparent when multiple stones are dropped into the pond: even from a static picture (i.e., the equivalent of a 4-ms bin in the analogy), observing the patterns of the expanding ripples it is possible to determine where and when the stones entered the pond. It should be noted that the analogy with multiple stones does not include the nonlinearities observed in cortical responses when multiple stimuli are delivered. Nonetheless, the analogy illustrates how a topological network of neurons with broad tuning curves (i.e., large receptive fields) can represent a remarkably high variety of spatiotemporal patterns without sacrificing basic spatial information.
Comparison with the whisker cortex
One might wonder whether the present results are specific for the infragranular forelimb somatosensory cortex or could be extended to other layers, to the whisker cortex, or even to other sensory systems. Indeed the results described in the present study are based on a simple neurophysiological principle: that stimulation of a location corresponding to the column/segregate of a cortical neuron elicits the fastest and greatest response, whereas stimulation of surround locations elicits progressively delayed and smaller responses. This principle is certainly shared by the whisker cortex, in both the infragranular and supragranular layers (e.g., see Ferezou et al. 2006; Ghazanfar and Nicolelis 1999; Petersen and Diamond 2000; Petersen et al. 2003a,b; Wilent and Contreras 2004) and likely extends to the visual cortex (Bringuier et al. 1999; Hirsch and Martinez 2006; Martinez et al. 2005; Mountcastle 1997) and to the auditory cortex (Linden and Schreiner 2003).
In the whisker system, surround responses can contribute to the discriminability between whiskers (Petersen and Diamond 2000), and spike timing provides additional information to spike count about stimulus location (Foffani and Moxon 2004; Ghazanfar et al. 2000; Panzeri et al. 2001; Petersen et al. 2001). Given the functional similarities between the whisker cortex and the forelimb cortex (for further discussion, see Foffani et al. 2004; Tutunculer et al. 2006), we predict that also in whisker cortex—and likely in the cortical representations of other sensory systems as well—large populations of infra- or supragranular neurons will be able to discriminate between stimuli delivered at different surround locations, and spike timing will add information to spike count in discriminating between surround locations. The elegant computational convergence of somatosensory information from multiple body locations into columns/segregates, described here, probably uncovers a more general principle of the hierarchical laminar processing in the sensory neocortex.
Comparison with the monkey somatosensory cortex
Our study shows that spike-timing precision becomes particularly relevant when response magnitudes between stimuli are similar, which typically happens when receptive fields are large or when the locations stimulated are close. This finding can explain a counterintuitive result obtained by Nicolelis et al. (1998), who reported that in monkeys spike timing added information about stimulus location over spike count for populations of neurons recorded in the secondary somatosensory cortex but notably not for populations of neurons recorded in the primary somatosensory cortex. In view of our findings, this result is not unexpected because neurons in the secondary somatosensory cortex have larger receptive fields than neurons in the primary somatosensory cortex. It is therefore likely that the locations stimulated by Nicolelis et al. were not close enough to exploit spike timing in primary somatosensory cortex. Spike-timing precision could therefore provide the brain with an efficient mechanism to increase spatial discrimination between populations of neurons with overlapping receptive fields.
Relevance for somatosensory processing and plasticity
Our results suggest that the discrete map of columns/segregates in the primary somatosensory cortex may offer local spatiotemporal references that allow the construction, by simple latency differences, of a precise spike-timing code in surround responses. Therefore the columnar and laminar organization of the somatosensory cortex (Mountcastle 1997) may be important not only to facilitate decoding stimulus location through the classical spike-count code (Panzeri et al. 2003; Petersen and Diamond 2000) but also to provide the spatial architecture for constructing spike-timing codes (Panzeri et al. 2001) distributed across multiple columns/segregates to facilitate decoding of more complex spatiotemporal somatosensory stimuli. The surround spike-timing code revealed here may represent a critical link between stimulus location and stimulus dynamics, transforming spatial information into temporal patterns and vice versa. It may also represent a simple way to both maximize the number of cortical columns/segregates involved in any given somatosensory task and to maximize the somatosensory information conveyed by individual columns/segregates, thereby optimizing the available computational resources in the somatosensory cortex. Within this view, the dramatic distortion of the body representation in cortical somatotopic maps, which optimizes the resolution of the local spike-count code by maximizing cortical distances between receptive field centers, will also optimize the resolution of the distributed spike-timing code by maximizing the latency differences between surround locations. This could explain, at least in part, the rich variety of spatiotemporal reorganization phenomena observed in cortical somatosensory maps under physiological and pathological conditions (Celikel et al. 2004; Diamond et al. 1994; Faggin et al. 1997; Finnerty et al. 1999; Pons et al. 1991; Wang et al. 1995; Tinazzi et al. 2000). In conclusion, the surround spike-timing code reflects an elegant convergence of somatosensory information from multiple body locations into individual columns/segregates, uncovering the computational potential of large receptive fields (i.e., broad tuning curves) in the primary somatosensory cortex.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant 2P50NS-24707. G. Foffani is partly supported by Fondo de Investigación Sanitaria del Instituto de Salud Carlos III Grant PI05 2322 and Consejería de Sanidad de la Junta de Comunidades de Castilla-La Mancha Grant 06056-00.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aguilar and Castro-Alamancos 2005.Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states J Neurosci 25: 10990–11002, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar et al. 2003.Aguilar J, Rivadulla C, Soto C, Canedo C. New corticocuneate cellular mechanisms underlying the modulation of cutaneous ascending transmission in anesthetized cats. J Neurophysiol 89: 3328–3339, 2003. [DOI] [PubMed] [Google Scholar]
- Ahissar and Arieli 2001.Ahissar E, Arieli A. Figuring space by time. Neuron 32: 185–201, 2001. [DOI] [PubMed] [Google Scholar]
- Alitto and Usrey 2003.Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol 13: 440–445, 2003. [DOI] [PubMed] [Google Scholar]
- Amari and Nakahara 2006.Amari S, Nakahara H. Correlation and independence in the neural code. Neural Comp 18: 1259–1267, 2006. [DOI] [PubMed] [Google Scholar]
- Arabzadeh et al. 2006.Arabzadeh E, Panzeri S, Diamond ME. Deciphering the spike train of a sensory neuron: counts and temporal patterns in the rat whisker pathway. J Neurosci 26: 9216–9226, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James and Fox 1987.Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 263: 265–281, 1987. [DOI] [PubMed] [Google Scholar]
- Brecht and Sakmann 2002.Brecht M, Sakmann B. Whisker maps of neuronal subclasses of the rat ventral posterior medial thalamus, identified by whole-cell voltage recording and morphological reconstruction J Physiol 538: 495–515, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringuier et al. 1999.Bringuier V, Chavane F, Glaeser L, Fregnac Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science 283: 695–699, 1999. [DOI] [PubMed] [Google Scholar]
- Brown and Bäcker 2006.Brown WM, Bäcker A. Optimal neuronal tuning for finite stimulus spaces. Neural Comput 18: 1511–1526, 2006. [DOI] [PubMed] [Google Scholar]
- Canedo and Aguilar 2000.Canedo A, Aguilar J. Spatial and cortical influences exerted on cuneothalamic and thalamocortical neurons of the cat. Eur J Neurosci 12: 2515–2533, 2000. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos 2004.Castro-Alamancos MA Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74: 213–247, 2004. [DOI] [PubMed] [Google Scholar]
- Celikel et al. 2004.Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci 7: 534–541, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin 1986.Chapin JK Laminar differences in sizes, shapes, and response profiles of cutaneous receptive fields in the rat SI cortex. Exp Brain Res 62: 549–559, 1986. [DOI] [PubMed] [Google Scholar]
- Chapin and Lin 1984.Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol 229: 199–213, 1984. [DOI] [PubMed] [Google Scholar]
- Chapin et al. 1981.Chapin JK, Waterhouse BD, Woodward DJ. Differences in cutaneous sensory response properties of single somatosensory cortical neurons in awake and halothane anesthetized rats. Brain Res Bull 6: 63–70, 1981. [DOI] [PubMed] [Google Scholar]
- Chmielowska et al. 1989.Chmielowska J, Carvell GE, Simons DJ. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol 285: 325–338, 1989. [DOI] [PubMed] [Google Scholar]
- de Kock et al. 2007.de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol 581: 139–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman et al. 2003.Derdikman D, Hildesheim R, Ahissar E, Arieli A, Grinvald A. Imaging spatiotemporal dynamics of surround inhibition in the barrels somatosensory cortex. J Neurosci 23: 3100–3105, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond et al. 1994.Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science 265: 1885–1888, 1994. [DOI] [PubMed] [Google Scholar]
- DiCarlo and Johnson 2002.DiCarlo JJ, Johnson KO. Receptive field structure in cortical area 3b of the alert monkey. Behav Brain Res 135: 167–178, 2002. [DOI] [PubMed] [Google Scholar]
- DiCarlo et al. 1998.DiCarlo JJ, Johnson KO, Hsiao SS. Structure of receptive fields in area 3b of primary somatosensory cortex in the alert monkey. J Neurosci 18: 2626–2645, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erchova et al. 2002.Erchova IA, Lebedev MA, Diamond ME. Somatosensory cortical neuronal population activity across states of anaesthesia. Eur J Neurosci 15: 744–752, 2002. [DOI] [PubMed] [Google Scholar]
- Faggin et al. 1997.Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci USA 94: 9428–9433, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorov and Diamond 1990.Favorov OV, Diamond ME. Demonstration of discrete place-defined columns–segregates–in the cat SI. J Comp Neurol 298: 97–112, 1990. [DOI] [PubMed] [Google Scholar]
- Favorov et al. 1987.Favorov OV, Diamond ME, Whitsel BL. Evidence for a mosaic representation of the body surface in area 3b of the somatic cortex of cat. Proc Natl Acad Sci USA 84: 6606–6610, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorov and Kelly 1994a.Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates. I. Development of afferent connections. Cereb Cortex 4: 408–427, 1994a. [DOI] [PubMed] [Google Scholar]
- Favorov and Kelly 1994b.Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates. II. Emergent functional properties. Cereb Cortex 4: 428–442, 1994b. [DOI] [PubMed] [Google Scholar]
- Favorov and Whitsel 1988a.Favorov O, Whitsel BL. Spatial organization of the peripheral input to area 1 cell columns. I. The detection of ‘segregates’. Brain Res 472: 25–42, 1988a. [DOI] [PubMed] [Google Scholar]
- Favorov and Whitsel 1988b.Favorov O, Whitsel BL. Spatial organization of the peripheral input to area 1 cell columns. II. The forelimb representation achieved by a mosaic of segregates. Brain Res 472: 43–56, 1988b. [DOI] [PubMed] [Google Scholar]
- Ferezou et al. 2006.Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 50: 617–629, 2006. [DOI] [PubMed] [Google Scholar]
- Finnerty et al. 1999.Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature 400: 367–371, 1999. [DOI] [PubMed] [Google Scholar]
- Foffani and Moxon 2004.Foffani G, Moxon KA. PSTH-based classification of sensory stimuli using ensembles of single neurons. J Neurosci Methods 135: 107–120, 2004. [DOI] [PubMed] [Google Scholar]
- Foffani et al. 2004.Foffani G, Tutunculer B, Moxon KA. Role of spike timing in the forelimb somatosensory cortex of the rat. J Neurosci 24: 7266–7271, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco et al. 2004.Franco L, Rolls ET, Aggelopoulos NC, Treves A. The use of decoding to analyze the contribution to the information of the correlations between the firing of simultaneously recorded neurons. Exp Brain Res 155: 370–384, 2004. [DOI] [PubMed] [Google Scholar]
- Friedberg et al. 1999.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol 81: 2243–2252, 1999. [DOI] [PubMed] [Google Scholar]
- Ghazanfar and Nicolelis 1999.Ghazanfar AA, Nicolelis MA. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex 9: 348–361, 1999. [DOI] [PubMed] [Google Scholar]
- Ghazanfar et al. 2000.Ghazanfar AA, Stambaugh CR, Nicolelis MA. Encoding of tactile stimulus location by somatosensory thalamocortical ensembles. J Neurosci 20: 3761–3775, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochin et al. 1994.Gochin PM, Colombo M, Dorfman GA, Gerstein GL, Gross CG. Neural ensemble coding in inferior temporal cortex. J Neurophysiol 71: 2325–2337, 1994. [DOI] [PubMed] [Google Scholar]
- Gruner 2002.Gruner CM Mutual information calculation using empirical classification. Neurocomputing 44–46: 1083–1088, 2002. [Google Scholar]
- Haupt et al. 2004.Haupt SS, Spengler F, Husemann R, Dinse HR. Receptive field scatter, topography and map variability in different layers of the hindpaw representation of rat somatosensory cortex. Exp Brain Res 155: 485–499, 2004. [DOI] [PubMed] [Google Scholar]
- Hirata et al. 2006.Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26: 4426–4436, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch and Martinez 2006.Hirsch JA, Martinez LM. Laminar processing in the visual cortical column. Curr Opin Neurobiol 16: 377–384, 2006. [DOI] [PubMed] [Google Scholar]
- Johansson and Birznieks 2004.Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci 7: 170–177, 2004. [DOI] [PubMed] [Google Scholar]
- Kaas and Collins 2003.Kaas JH, Collins CE. Anatomic and functional reorganization of somatosensory cortex in mature primates after peripheral nerve and spinal cord injury. Adv Neurol 93: 87–95, 2003. [PubMed] [Google Scholar]
- Kaas et al. 1983.Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci 6: 325–356, 1983. [DOI] [PubMed] [Google Scholar]
- Killackey and Ebner 1972.Killackey HP, Ebner FF. Two different types of thalamocortical projections to a single cortical area in mammals. Brain Behav Evol 6: 141–169, 1972. [DOI] [PubMed] [Google Scholar]
- Killackey et al. 1989.Killackey HP, Koralek KA, Chiaia NL, Rhodes RW. Laminar and areal differences in the origin of the subcortical projection neurons of the rat somatosensory cortex. J Comp Neurol 282: 428–445, 1989. [DOI] [PubMed] [Google Scholar]
- Kleinfeld and Delaney 1996.Kleinfeld D, Delaney KR. Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes. J Comp Neurol 375: 89–108, 1996. [DOI] [PubMed] [Google Scholar]
- Knutsen et al. 2006.Knutsen PM, Pietr M, Ahissar E. Haptic object localization in the vibrissal system: behavior and performance. J Neurosci 26: 8451–8464, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek et al. 1990.Koralek KA, Olavarria J, Killackey HP. Areal and laminar organization of corticocortical projections in the rat somatosensory cortex. J Comp Neurol 299: 133–150, 1990. [DOI] [PubMed] [Google Scholar]
- Krupa et al. 2004.Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MA. Layer-specific somatosensory cortical activation during active tactile discrimination. Science 304: 1989–1992, 2004. [DOI] [PubMed] [Google Scholar]
- Laubach et al. 2000.Laubach M, Wessberg J, Nicolelis MAL. Cortical ensemble activity increasingly predicts behavior outcomes during learning of a motor task. Nature 405: 567–471, 2000. [DOI] [PubMed] [Google Scholar]
- Linden and Schreiner 2003.Linden JF, Schreiner CE. Columnar transformations in auditory cortex? A comparison to visual and somatosensory cortices. Cereb Cortex 13: 83–89, 2003. [DOI] [PubMed] [Google Scholar]
- Lu and Lin 1993.Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res 10: 1–16, 1993. [DOI] [PubMed] [Google Scholar]
- Martinez et al. 2005.Martinez LM, Wang Q, Reid RC, Pillai C, Alonso JM, Sommer FT, Hirsch JA. Receptive field structure varies with layer in the primary visual cortex. Nat Neurosci 8: 372–379, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns et al. 2004.Manns ID, Sakmann B, Brecht M. Sub- and suprathreshold receptive field properties of pyramidal neurons in layers 5A and 5B of rat somatosensory barrel cortex. J Physiol 556: 601–622, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino and Frostig 1996.Masino SA, Frostig RD. Quantitative long-term imaging of the functional representation of a whisker in rat barrel cortex. Proc Natl Acad Sci USA 93: 4942–4947, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore and Nelson 1998.Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol 80: 2882–2892, 1998. [DOI] [PubMed] [Google Scholar]
- Montemurro and Panzeri 2006.Montemurro MA, Panzeri S. Optimal tuning widths in population coding of periodic variables. Neural Comput 18: 1555–1576, 2006. [DOI] [PubMed] [Google Scholar]
- Mountcastle 1957.Mountcastle VB Modality and topographic properties of single neurons in cat's somatic sensory cortex. J Neurophysiol 20: 408–434, 1957. [DOI] [PubMed] [Google Scholar]
- Mountcastle 2003.Mountcastle VB Introduction. Computation in cortical columns. Cereb Cortex 13: 2–4, 2003. [DOI] [PubMed] [Google Scholar]
- Mountcastle 1997.Mountcastle VM The columnar organization of the neocortex. Brain 120: 701–722, 1997. [DOI] [PubMed] [Google Scholar]
- Moxon 2003.Moxon KA, Foffani G. Neurorobotics as a means for assessing brain function after spinal injury. Proceedings of the 1st International IEEE EMBS Conference on Neural Engineering, 20–22 March 2003, pp: 95–98.
- Nicolelis et al. 1998.Nicolelis MA, Ghazanfar AA, Stambaugh CR, Oliveira LM, Laubach M, Chapin JK, Nelson RJ, Kaas JH. Simultaneous encoding of tactile information by three primate cortical areas. Nat Neurosci 1: 621–630, 1998. [DOI] [PubMed] [Google Scholar]
- Panzeri et al. 2001.Panzeri S, Petersen RS, Schultz SR, Lebedev M, Diamond ME. The role of spike timing in the coding of stimulus location in rat somatosensory cortex. Neuron 29: 769–777, 2001. [DOI] [PubMed] [Google Scholar]
- Panzeri et al. 2003.Panzeri S, Petroni F, Petersen RS, Diamond ME. Decoding neuronal population activity in rat somatosensory cortex: role of columnar organization. Cereb Cortex 13: 45–52, 2003. [DOI] [PubMed] [Google Scholar]
- Panzeri et al. 2002.Panzeri S, Pola G, Petroni F, Young MP, Petersen RS. A critical assessment of different measures of the information carried by correlated neuronal firing. BioSystems 67: 177–185, 2002. [DOI] [PubMed] [Google Scholar]
- Panzeri et al. 1999.Panzeri S, Treves A, Schultz S, Rolls ET. On decoding the responses of a population of neurons from short time windows. Neural Comput 11: 1533–1577, 1999. [DOI] [PubMed] [Google Scholar]
- Penfield and Boldrey 1937.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443, 1937. [Google Scholar]
- Petersen et al. 2003a.Petersen CC, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23: 1298–1309, 2003a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen et al. 2003b.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen and Diamond 2000.Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci 20: 6135–6143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen et al. 2001.Petersen RS, Panzeri S, Diamond ME. Population coding of stimulus location in rat somatosensory cortex. Neuron 32: 503–514, 2001. [DOI] [PubMed] [Google Scholar]
- Petersen et al. 2002.Petersen RS, Panzeri S, Diamond ME. Population coding in somatosensory cortex. Curr Opin Neurobiol 12: 441–447, 2002. [DOI] [PubMed] [Google Scholar]
- Peterson et al. 1998.Peterson BE, Goldreich D, Merzenich MM. Optical imaging and electrophysiology of rat barrel cortex. I. Responses to small single-vibrissa deflections. Cereb Cortex 8: 173–183, 1998. [DOI] [PubMed] [Google Scholar]
- Pola et al. 2003.Pola G, Thiele A, Hoffmann KP, Panzeri S. An exact method to quantify the information transmitted by different mechanisms of correlational coding. Network 14: 35–60, 2003. [DOI] [PubMed] [Google Scholar]
- Pons et al. 1991.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252: 1857–1860, 1991. [DOI] [PubMed] [Google Scholar]
- Pouget et al. 1999.Pouget A, Deneve S, Ducom JC, Latham PE. Narrow versus wide tuning curves: what's best for a population code? Neural Comput 11: 85–90, 1999. [DOI] [PubMed] [Google Scholar]
- Rolls et al. 1998.Rolls ET, Treves A, Robertson RG, Georges-François P, Panzeri S. Information about spatial view in an ensemble of primate hippocampal cells. J Neurophysiol 79: 1797–1813, 1998. [DOI] [PubMed] [Google Scholar]
- Rolls et al. 1997.Rolls ET, Treves A, Tovee MJ. The representational capacity of the distributed encoding of information provided by populations of neurons in primate temporal visual cortex. Exp Brain Res 114: 149–162, 1997. [DOI] [PubMed] [Google Scholar]
- Schneidman et al. 2003.Schneidman E, Bialek W, Berry MJ. Synergy, redundancy, and independence in population codes. J Neurosci 23: 11539–11553, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series and Latham 2004.Series P, Latham PE. Pouget A. Tuning curve sharpening for orientation selectivity: coding efficiency and the impact of correlations. Nat Neurosci 7: 1129–1135, 2004. [DOI] [PubMed] [Google Scholar]
- Simons 1978.Simons DJ Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820, 1978. [DOI] [PubMed] [Google Scholar]
- Sripati et al. 2006.Sripati AP, Yoshioka T, Denchev P, Hsiao SS, Johnson KO. Spatiotemporal receptive fields of peripheral afferents and cortical area 3b and 1 neurons in the primate somatosensory system. J Neurosci 26: 2101–2114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temereanca and Simons 2004.Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41: 639–651, 2004. [DOI] [PubMed] [Google Scholar]
- Thorpe et al. 2001.Thorpe S, Delorme A, Van Rullen R. Spike-based strategies for rapid processing. Neural Netw 14: 715–725, 2001. [DOI] [PubMed] [Google Scholar]
- Tinazzi et al. 2000.Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguiere F, Fiaschi A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain 123: 42–50, 2000. [DOI] [PubMed] [Google Scholar]
- Tutunculer et al. 2006.Tutunculer B, Foffani G, Himes BT, Moxon KA. Structure of the excitatory receptive fields of infragranular forelimb neurons in the rat primary somatosensory cortex responding to touch. Cereb Cortex 16: 791–810, 2006. [DOI] [PubMed] [Google Scholar]
- VanRullen et al. 2005.VanRullen R, Guyonneau R, Thorpe SJ. Spike times make sense. Trends Neurosci 28: 1–4, 2005. [DOI] [PubMed] [Google Scholar]
- Wang et al. 1995.Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378: 71–75, 1995. [DOI] [PubMed] [Google Scholar]
- Waters et al. 1995.Waters RS, Li CX, McCandlish CA. Relationship between the organization of the forepaw barrel subfield and the representation of the forepaw in layer IV of rat somatosensory cortex. Exp Brain Res 103: 183–197, 1995. [DOI] [PubMed] [Google Scholar]
- Wilent and Contreras 2004.Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci 24: 3985–3998, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey and Van der Loos 1970.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17: 205–242, 1970. [DOI] [PubMed] [Google Scholar]
- Zacksenhouse and Ahissar 2006.Zacksenhouse M, Ahissar E. Temporal decoding by phase-locked loops: unique features of circuit-level implementations and their significance for vibrissal information processing. Neural Comput 18: 1611–1636, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang and Sejnowski 1999.Zhang K, Sejnowski TJ. Neuronal tuning: to sharpen or broaden? Neural Comput 11: 75–84, 1999. [DOI] [PubMed] [Google Scholar]
- Zhu and Connors 1999.Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol 81: 1171–1183, 1999. [DOI] [PubMed] [Google Scholar]