Abstract
The study presents analytical, simulation, and experimental analyses of amplitude cancellation of motor-unit action potentials (APs) in the interference electromyogram (EMG) and its relation to the size of the spike-triggered average (STA) EMG. The amount of cancellation of motor-unit APs decreases monotonically as a function of the ratio between the root mean square (RMS) of the motor-unit AP and the RMS of the interference EMG signal. The theoretical derivation of this association indicates a method to measure cancellation in individual motor units by STA of the interference and squared EMGs. The theoretical relation was examined in both simulated EMG signals generated by populations of 200 motor units and experimental recordings of 492 and 184 motor-unit APs in the vastus medialis and abductor digiti minimi muscles, respectively. Although the theoretical relation predicted (R2 = 0.95; P < 0.001) the amount of cancellation in the simulated EMGs, the presence of motor-unit synchronization decreased the strength of the association for small APs. The decrease in size of the STA obtained from the squared EMG relative to that extracted from the interference EMG was predicted by the experimental measure of cancellation (R2 = 0.65; P < 0.001, for vastus medialis; R2 = 0.26; P < 0.05, for abductor digiti minimi). The results indicate that cancellation of APs in the interference EMG can be analytically predicted and experimentally measured with STA from the discharge times of the motor units into the surface EMG.
INTRODUCTION
Spike-triggered averages (STAs) can be derived from the surface electromyogram (EMG) by triggering from the discharge times of a single motor neuron and averaging into the concurrently recorded surface EMG signals (Kakuda et al. 1991). STA into the interference and rectified EMGs has been used to extract single motor-unit potentials from the surface EMG to measure motor-unit synchronization (Milner-Brown et al. 1973, 1975), to estimate the number of motor units in a muscle (McComas et al. 1971; Slawnych et al. 1990; Stein and Yang 1990), to assess cross talk in the surface EMG (Farina et al. 2003), and to quantify motor-unit conduction velocity (Farina et al. 2002). It has often been assumed that STA into the rectified or squared EMG is not influenced by amplitude cancellation (Milner-Brown et al. 1975). However, cancellation of EMG amplitude, which is the result of the algebraic summation of positive and negative phases of motor-unit action potentials (Day and Hulliger 2001; Keenan et al. 2005), occurs before the interference signal is rectified (Keenan et al. 2006a).
Simulation analysis has shown that there is a link between the area of the STA obtained from the rectified (or squared) EMG and amplitude cancellation (Keenan et al. 2006a). This observation suggested that estimating the strength of motor-unit synchronization from the size of the STA derived from the rectified EMG (Milner-Brown et al. 1975) is limited by the unpredictable influence of amplitude cancellation. However, the association between amplitude cancellation and STA has not been demonstrated theoretically or confirmed experimentally. The purpose of this study was to derive an analytical expression for the amount of amplitude cancellation experienced by motor-unit potentials in the surface EMG and to examine its association with the size of the STA extracted from the surface EMG. The analytical derivations were evaluated with a computer model in which the amount of amplitude cancellation could be quantified and experimental recordings of motor-unit potentials were taken from the vastus medialis and abductor digiti minimi muscles.
METHODS
The approach begins with the derivation of an analytical expression for the degree of cancellation of motor-unit action potentials (APs) in the surface EMG and an explanation of how the amount of cancellation can be measured experimentally with STA. The analytical results are validated with a computational model and experimental recordings.
Cancellation of motor-unit action potentials
The RMS of the summation of two signals x1[n] and x2[n] is obtained as
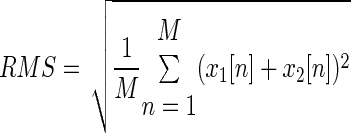 |
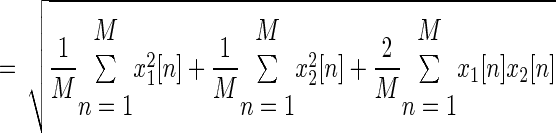 |
where M is the number of samples in the signals. The term (2/M) × ∑n=1M x1[n]x2[n] tends to zero for large M under the assumptions that x1[n] and x2[n] are uncorrelated and jointly ergodic for the cross-correlation. With these assumptions
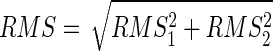 |
(1) |
where RMS1 and RMS2 are the RMS values of the two signals. The property in Eq. 1 is used in all of the following derivations, which limits them to low levels of motor-unit synchronization. The effect of synchronization on these derivations is addressed by the modeling approach and by experimental results on two muscles with different amounts of synchronization.
When the AP of the generic motor unit m is summed with an interference EMG signal, the RMS of the combined signal is
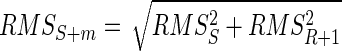 |
(2) |
where RMSS+m is the RMS of the EMG signal after the summation of the AP, RMSS is the RMS of the interference signal before the addition of the AP, and RMSR+1 is the RMS of the added AP.
The contribution of the AP to the RMS of the combined signal is smaller than the RMS of the AP due to cancellation (Eq. 2). The increase in RMS of the combined signal by the addition of the AP of motor unit m is the difference between RMSS+m and RMSS
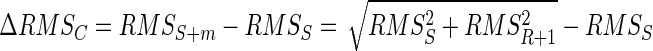 |
(3) |
The increase in RMS of the combined signal in the absence of cancellation (ΔRMSNC) is equal to RMSR+1. The contribution of the AP m to the RMS of the combined signal, as a percentage of its contribution without cancellation, is
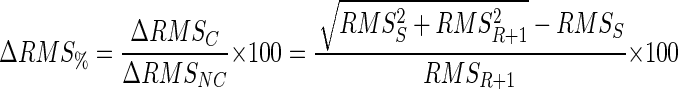 |
(4) |
Thus the amount of cancellation of the motor-unit AP is
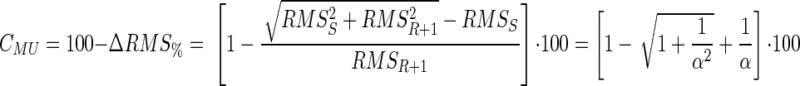 |
(5) |
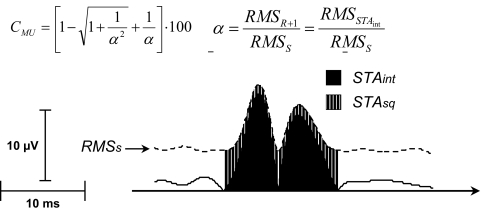
where α = (RMSR+1/RMSS) is the ratio between the RMS of the AP of motor unit m and the RMS of the initial interference signal (Fig. 1).
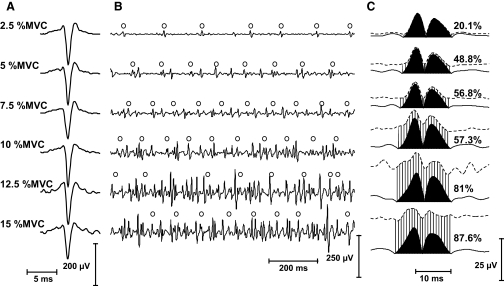
FIG. 1.
Estimation of amplitude cancellation for a motor-unit action potential by using spike-triggered averaging (STA). A motor unit was identified from the intramuscular electromyographic (EMG) recording in the vastus medialis muscle during a contraction at 7.5% maximal voluntary contraction (MVC). The action potentials (APs) of the motor unit were used to trigger averages from the interference (black area) and the squared EMGs. The average of the squared EMG was followed by computation of the square root (dashed area). With these measurements, the amount of amplitude cancellation can be estimated from Eq. 5.
Experimental measurement of cancellation with STA
The ratio α = (RMSR+1/RMSS) in Eq. 5 can be computed experimentally by STA into the interference EMG (STAint) to provide the action potential s[n] of the triggering motor unit (Keenan et al. 2006a), and from which RMSR+1 can be computed (RMSR+1 = RMSSTAint). The number of triggers used experimentally should be sufficient to reduce the contribution of the other motor units to a negligible value with respect to s[n], and thus it depends on the amplitude of the interference EMG with respect to the amplitude of the motor-unit AP. The number of triggers for small motor-unit APs should be greater than that for larger motor-unit APs for a given amplitude of the interference EMG. The denominator of the ratio can be determined by taking the square root of the STA obtained from the squared EMG as follows
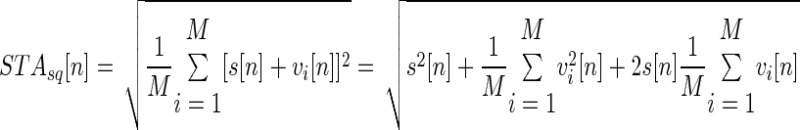 |
(6) |
where νi[n] is the interference signal without the motor-unit AP s[n]. The term (1/M) × ∑i=1M νi[n] in Eq. 6 tends to zero when M is large because it is the mean value of the EMG signal, whereas the term (1/M) × ∑i=1M νi2[n] is the RMS of the interference signal without the AP of the motor unit m (RMSS). With a sufficient number of triggers (Keenan et al. 2006a), Eq. 6 reduces to
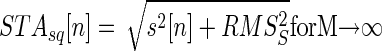 |
(7) |
where the baseline of STAsq[n] represents the RMS of the interference signal without the AP of motor unit m (Fig. 1). Thus STA into the interference EMG and into the squared EMG provide experimental estimates of RMSR+1 and RMSS that can be used in Eq. 5 to determine the amount of amplitude cancellation of the motor-unit AP.
The size of the STA obtained from the squared EMG (RMS of STAsq[n]) can be related to amplitude cancellation as follows
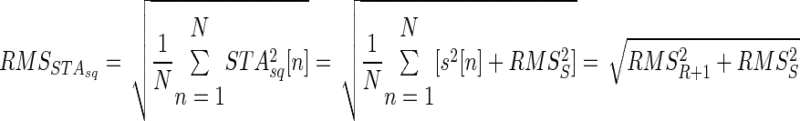 |
(8) |
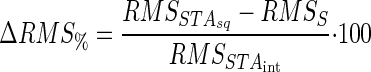 |
(9) |
and combining Eqs. 5 and 9 yields
 |
(10) |
The term (RMSSTAsq − RMSS)/RMSSTAint, which is equivalent to the ratio investigated by Keenan et al. (2006a) in simulation and used by Milner-Brown et al. (1975) to estimate motor-unit synchronization, is linearly related to cancellation (the complement to 1 indicates the amount of cancellation; Eq. 10). Because cancellation can be directly computed only from the RMS of the AP and of the interference signal (Eq. 5), the RMS of the STA into the squared EMG is a redundant measure: the size of the STA into the squared EMG can be obtained from other measures (in the absence of synchronization) and is the result of cancellation. This association was tested in experimental recordings. Equations 5 and 10 indicate that the size of the STA for the squared EMG can be predicted from the size of the AP and the amplitude of the interference EMG (Fig. 1)
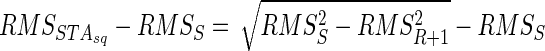 |
(11) |
Simulation procedure and analysis
The simulations were based on an anatomical model that consisted of a cylindrical volume conductor (Farina et al. 2004) with an anisotropic muscle layer, and isotropic bone, subcutaneous, and skin layers. The model is similar to one used in a previous study (Keenan et al. 2005) and the parameters are reported in Table 1.
TABLE 1.
Parameters of the cylindrical volume conductor model
| Model Parameter | Value |
|---|---|
| Tissue conductivities, S/m | |
| Bone | 0.02 |
| Muscle (radial and transverse) | 0.1 |
| Muscle (longitudinal) | 0.5 |
| Subcutaneous tissue | 0.05 |
| Skin | 0.1 |
| Muscle properties | |
| Number of motor units | 200 |
| Number of muscle fibers | 119,634 |
| Number of fibers in a motor unit (range) | 15–1,500 |
| Muscle cross-sectional area | 598 mm2 |
| Average fiber length | 120 mm |
| Skin thickness | 1 mm |
| Subcutaneous tissue thickness | 1 mm |
| Bone (radius) | 20 mm |
| Tendon ending spread | 5 mm |
| Innervation zone spread | 5 mm |
| CIS | 0–1 |
| Electrodes | |
| Rectangular, size | 5 × 1 mm |
| Interelectrode distance | 5 mm |
The muscle had an elliptical shape and was 30 mm in the transverse direction and 25.4 mm thick. The thickness of the subcutaneous layer was 1 mm. The intracellular APs of the muscle fibers were derived from the analytical description developed by Rosenfalk (1969). The innervation zones of the motor units (n = 200) were located at the center of the muscle. Average fiber length was 120 mm for all motor units and the locations of the end plates and tendon endings varied randomly (uniform distribution) over a range of 5 mm. The motor-unit territories were circular and distributed randomly throughout the muscle. The fibers of each motor unit were distributed with a density of 20 fibers/mm2 (Armstrong et al. 1988) and interdigitated with the fibers of many other units to yield a fiber density in the muscle of 200 fibers/mm2. When a portion of the motor-unit territory was constrained by the muscle boundary, the territory of the unit was modified to fit the muscle cross section (Keenan et al. 2005). Innervation numbers ranged from 15 to 1,500, based on an approximately 100-fold range of twitch forces (Elek et al. 1992), with an exponential distribution across the population (Fuglevand et al. 1993a).
The motor units had a mean muscle fiber conduction velocity of 4.0 ± 0.35 m/s (Farina et al. 2000; Troni et al. 1983), with the slowest conduction velocity assigned to the smallest motor unit (Andreassen and Arendt-Nielsen 1987). The surface-recorded, motor-unit potential consisted of the sum of the APs of the muscle fibers belonging to the motor unit. The simulated EMG signals were detected with bar electrodes (5 × 1 mm), arranged in bipolar configurations with 5-mm distance between electrodes. The center of the detection system was between the innervation zone and tendon. EMG signals were computed at 4,096 samples/s.
Activation of the motor-unit pool was modeled as a constant force contraction for 20 s. The distribution of recruitment thresholds for the motor neurons was determined from an exponential function with many low-threshold neurons and progressively fewer high-threshold neurons (De Luca et al. 1982; Moritz et al. 2005). Each motor unit began discharging at 8 pulses per second (pps), once excitation exceeded the assigned recruitment threshold of the unit, and discharge rates increased linearly with increased excitation; the rate was 3 pps per 10% increase in excitation. Excitation corresponded to the simulated net synaptic input onto the motor neuron pool (Heckman and Binder 1991) and it was assumed that all neurons received the same level of excitation. As a consequence, the simulated input–output functions of the neurons matched the observed relations between discharge rate and injected current (Kernell 1965).
Maximal excitation was denoted as the level of input necessary to bring the last recruited motor neuron to its assigned peak discharge rate, and values of excitation were expressed as a percentage of this maximum. As described by Fuglevand et al. (1993a), the first recruited unit had a maximal discharge rate of 35 pps, whereas the peak discharge rate decreased linearly with increasing recruitment threshold so that the last recruited unit had a peak discharge rate of 25 pps. The last unit was recruited at 50% of maximal excitation. The discharge rate was modeled as a random process with a Gaussian distribution, with the coefficient of variation for discharge rate set at 20%. Activation levels from 2.5 to 25% (2.5% increments) of the maximum excitation were simulated. Ten motor-unit populations were simulated at each excitation level with a random location of the motor units within the muscle tissue.
Motor-unit synchronization was examined by adjusting the timing of selected APs to impose a temporal association with APs discharged by different motor neurons, as described by Yao et al. (2000). Briefly, motor-unit synchronization was imposed by applying a function that selected a percentage of the APs discharged by each motor unit to be synchronized with the discharges of six other motor units. Discharges of the reference motor unit were randomly selected and, when a discharge of another randomly selected motor unit occurred within ±30 ms of the discharge by the reference motor unit, this discharge time was aligned with the reference unit with a Gaussian distribution that had an SD of 1.67 ms centered on the reference unit. This process was repeated until the discharges of six other motor units were aligned with the discharge of the reference motor unit for each discharge of the reference motor unit selected for this adjustment, and ad seriatim for all motor units in the pool. The percentage of discharges of the reference motor unit to be synchronized with other units was determined to obtain specified levels of common input strength (CIS), which is the frequency of extra synchronous discharges (Nordstrom et al. 1992). To determine the level of motor-unit synchronization across the motor-unit pool, CIS values were calculated for every motor unit with the 15 preceding and the 15 succeeding motor units (Keenan et al. 2006a). In this way, CIS values were fixed in a range 0–1, irrespective of excitation level.
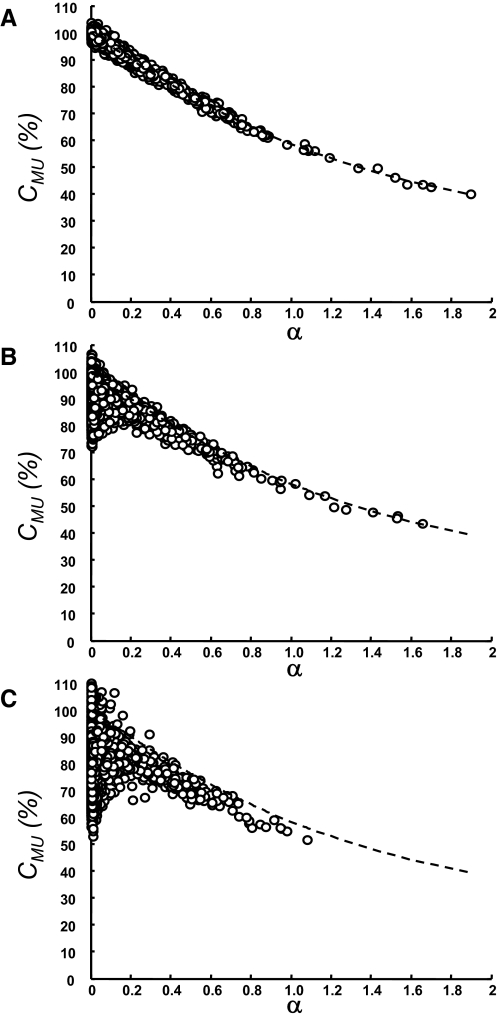
An amplitude cancellation index was calculated for all simulated motor units at each excitation level (Keenan et al. 2006a): 1) the RMS was calculated from the motor-unit AP; 2) the difference between the RMS of the interference EMG before and after removing all the APs of the reference motor unit divided by the number of APs of the reference unit was subtracted from the value derived from step 1; and 3) the value from step 2 was divided by the value from step 1 and multiplied by 100. These steps correspond to a direct calculation of cancellation, which is not possible under experimental conditions. Amplitude cancellation was also computed from the simulated signals using Eq. 5, which corresponds to a measure that can be made under experimental conditions, and compared with the direct measure of cancellation from steps 1–3. The direct measure of cancellation and that derived from Eq. 5 were compared by linear regression analysis. The simulations served to prove the validity of Eq. 5 in realistic synthetic signals. This relation cannot be examined in experimental recordings because the direct measure of cancellation is not available. However, the association between Eq. 5 and the reduction in size of the STA into the squared EMG was experimentally tested in two muscles. The association between Eq. 10 (reduction in size of the STA into the rectified EMG) and amplitude cancellation has been established with simulations (Keenan et al. 2006a; their Fig. 3); the results from that study were confirmed with the present simulation analysis and are not reported here.
FIG. 3.
Measurement of amplitude cancellation (CMU) in the simulated EMG signals. Interference EMG signals were simulated at excitation levels that ranged from 2.5 to 25%. A CMU value of 100% denotes complete cancellation of the motor-unit potential in the interference signal. A: the motor-unit discharge patterns were uncorrelated [common input strength (CIS) = 0]. B: a moderate level of synchronization was included (CIS = 0.2). C: a high level of synchronization was simulated (CIS = 1). Cancellation of the motor-unit AP (circles) at each level of excitation was computed directly by comparing the increase in RMS in the interference signal when the motor unit was added to the sum of the RMS values for the interference signal and the motor-unit AP. This comparison cannot be performed under experimental conditions. Cancellation was also computed from Eq. 5 (dashed line). The direct measurement of cancellation (circles) corresponded to the analytical prediction (dashed line) with some variability for high levels of cancellation due to the variance in the estimation of the signal RMS from 20-s simulated contractions. Note that this variability may cause the estimated cancellation to be >100% in A, due to estimation variance.
Experimental procedures and signal analysis
Two groups of 10 (5 men; age, mean ± SD, 29.1 ± 5.8 yr) and 8 (6 men; age, mean ± SD, 30.3 ± 4.2 yr) healthy subjects participated in the study. One muscle (vastus lateralis or abductor digiti minimi) was investigated in each group. The study was conducted in accordance with the Declaration of Helsinki, approved by the local Ethics Committee, and written informed consent was obtained from all subjects prior to participating in the study.
Because the theoretical derivations are limited to low levels of motor-unit synchronization, two muscles with different amounts of synchronization were selected: the vastus medialis obliquus muscle (n = 10 subjects) and the abductor digiti minimi muscle (n = 8 subjects). Motor-unit synchronization is generally greater in hand muscles compared with large-limb muscles (Datta et al. 1991; De Luca et al. 1993; Kim et al. 2001).
The measurements on the vastus medialis were obtained with the subject seated comfortably on a chair with a belt fixing the position of the hip and the right thigh. The knee joint was flexed to 90°. A strap connected by a chain to a load cell was attached to the ankle to measure the extension force exerted by quadriceps femoris. The measurements on the abductor digiti minimi were obtained by fixing the fifth finger in a custom-made brace to record the force exerted during an isometric contraction of the muscle (Politecnico di Turin, Torino, Italy).
The subjects performed three maximal voluntary contractions (MVCs) with the knee extensors or abductor digiti minimi, with each trial separated by 2 min of rest. The greatest force was used as the reference MVC for the submaximal contractions. Surface and intramuscular EMG electrodes were then mounted.
A linear array of equally spaced surface electrodes (vastus medialis: 8 bar electrodes 5 mm long, 1-mm diameter, 5-mm interelectrode distance; abductor digiti minimi: 7 circular electrodes, 1-mm diameter, 2.5-mm interelectrode distance) was located between the most distal innervation zone and the distal tendon region of each muscle (Masuda et al. 1985). The array electrodes were used so that the maximal bipolar EMG amplitude could be detected (Merletti et al. 2003). The orientation of the array was based on visual inspection of the surface EMG signals detected in test contractions with the criterion of minimal change in the shape of the APs across channels. The skin was lightly abraded at the location selected for array placement. Surface EMG signals were amplified and a bipolar signal was derived from the center of the array (EMG16, LISiN, OT Bioelettronica, Turin, Italy, bandwidth 10–500 Hz), sampled at 2,048 Hz, and stored after 12-bit A/D conversion.
Two pairs of wire electrodes made of Teflon-coated stainless steel (A-M Systems, Carlsborg, WA; diameter 50 μm) were inserted into the muscle with 25-gauge needles at locations 10–20 mm proximal to the surface array for both muscles. One needle was inserted in line with the direction of the array, the other at about 10 mm in a transverse distance. The insulated wires were cut to expose only the cross section at the tip. The needles were inserted to a depth of a few millimeters below the muscle fascia and removed to leave the wire electrodes inside the muscle. The intramuscular EMG signals were amplified and provided a bipolar recording (Counterpoint EMG, Dantec Medical, Skovlunde, Denmark) that was band-pass filtered (500 Hz to 5 kHz). A reference electrode for both the surface and intramuscular recordings was placed around the ankle (for vastus medialis) or wrist (for abductor digiti minimi).
The subject performed a series of 30-s contractions at forces that ranged from 2.5 to 25% MVC (2.5% increments) for the vastus medialis and from 2.5 to 12.5% MVC (2.5% increments) for the abductor digiti minimi, in a randomized order. The rest periods varied with the target force and were the same for both muscles: 3 min for forces of 2.5–10% MVC, 15 min for 12.5–20% MVC, and 20 min for 22.5–25% MVC. The different ranges of force analyzed for the two muscles were due to a greater complexity of the signals from the abductor digiti minimi muscle that impeded accurate decomposition of the intramuscular signals at higher force levels.
In a separate experimental session, the level of motor-unit synchronization in the two muscles was measured from the two groups of subjects. The subjects were provided with feedback of the intramuscular signal recorded from one of the two locations and were asked to activate a motor unit at about 8 pps for 2 min (Semmler et al. 1999). The task was repeated for both wire locations and for as many motor units as could be controlled with the feedback.
The motor-unit APs were identified in the intramuscular recordings with a decomposition algorithm (McGill et al. 2005). The discharge times of the identified motor units from the wire in line with the array were used as triggers to average into the interference surface EMGs obtained with the array electrode during the constant-force contractions. The RMS of the STA into the interference and squared (with subsequent square root) EMG signals was computed for each motor unit. The degree of cancellation was measured for each detected motor unit with Eq. 5. The relation between amplitude cancellation and STA size was investigated by comparing the degree of cancellation estimated with Eq. 5 and the STA ratio defined in Eq. 10.
Peak-to-peak amplitude of the AP and muscle fiber conduction velocity were estimated from the STA into the interference EMG for each motor unit and compared across contraction levels with a one-way ANOVA. The amount of cancellation observed in the experimental measurements with Eq. 5 was compared with the Eq. 10 ratio by linear regression analysis.
The amount of motor-unit synchronization was quantified from cross-correlation histograms (±100 ms relative to the reference motor-unit discharge, with bin width of 1 ms) (Nordstrom et al. 1992) of all pairs of motor units identified from separate insertion sites during the 2-min feedback contractions. Histograms with a mean bin count less than four were excluded from further analysis (Semmler and Nordstrom 1997). The cumulative sum function was used to identify the location of the peak in the histogram (Ellaway 1978). A significant peak in the cumulative sum was defined as an increase >3SDs with respect to the mean over the first 50 bins (Davey et al. 1986). Synchronization was quantified with the CIS index (Nordstrom et al. 1992), as for the simulated signals.
RESULTS
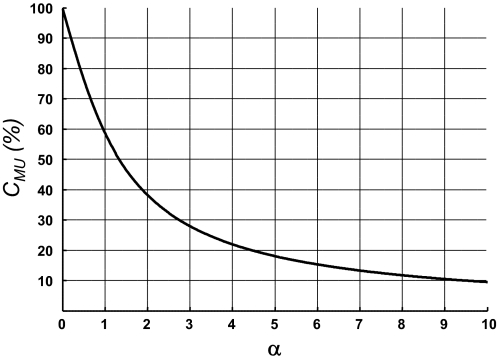
Equation 5 indicates that amplitude cancellation decreases monotonically with an increase in α. Motor-unit APs that are large relative to the amplitude of the interference signal experience less amplitude cancellation, whereas small motor units are almost completely cancelled in the interference signal. Motor-unit APs with an RMS value less than that for the interference EMG (α < 1) are cancelled by >60% (Fig. 2).
FIG. 2.
Theoretical analysis of amplitude cancellation for the APs of single motor units (Eq. 5). The percentage of cancellation decreases monotonically as a function of the ratio α. CMU, amplitude cancellation in individual motor-unit APs; α, ratio between the root mean square (RMS) values of the motor-unit AP and of the interference EMG signal.
Measurement of cancellation in the simulated signals
Figure 3A shows the comparison between the direct computation of cancellation, which is not feasible with experimental recordings, and the estimated cancellation using Eq. 5 for all motor units in the simulated conditions in the absence of synchronization (CIS = 0). There was a strong association (R2 = 0.95; P < 0.001) between the theoretically derived relation and the simulation results, with some variability around the predicted values due to the SD in the estimation of RMS values from 20-s simulated signals. The results indicate that Eq. 5 provides a measure of amplitude cancellation in the absence of motor-unit synchronization, as predicted theoretically.
The presence of synchronized discharges decreased the strength of the association between the theoretically derived and the computed cancellation because synchronization was not included in the analytical analysis. Equation 5 overestimated cancellation for moderate levels of synchronization (CIS = 0.2), especially for small motor-unit APs (Fig. 3B). The same trend was observed when the degree of synchronization was further increased to a CIS value of 1 (Fig. 3C). Accordingly, there was a decrease in the strength of the association between Eq. 5 and the computed cancellation (R2 = 0.42; P < 0.001 for CIS = 0.2; R2 = 0.22; P < 0.01 for CIS = 1). However, the theoretical analysis was inaccurate only for small motor-unit APs (Fig. 3, B and C). For values of α >0.2, the estimate of cancellation from Eq. 5 was reasonable even for large levels of synchronization (Fig. 3). There was a strong association between Eq. 5 and amplitude cancellation for both synchronization levels for α values >0.2 (R2 = 0.92; P < 0.001 for CIS = 0.2; R2 = 0.79; P < 0.001 for CIS = 1), which indicates that the degree of cancellation can be estimated from Eq. 5 even for high levels of motor-unit synchronization when the motor-unit APs are sufficiently large (α > 0.2).
Experimental recordings
The simulation results confirmed the analytical prediction that cancellation can be estimated using Eq. 5. Thus this relation was used in the experimental recordings to analyze the association between amplitude cancellation and the size of the STA from the squared EMG. According to the theoretical derivation and the simulation results reported by Keenan et al. (2006a), the size of the STA from the squared EMG should be predicted by the measure of amplitude cancellation provided by Eq. 5.
The intramuscular recordings yielded 492 motor units identified from the vastus medialis muscle of the 10 subjects at 10 target forces and 184 motor units from the abductor digiti minimi at 5 target forces. Out of the total number of identified motor units, many units were the same motor unit identified at more than one contraction force; this did not affect the analysis of results. The number of identified motor units tended to increase with contraction force, but there was no difference in the number of detected discharges during the 30-s contractions (Table 2). As expected (Hedayatpour et al. 2007), the peak-to-peak amplitude of the surface potentials, muscle fiber conduction velocity, and discharge rate of the identified motor units increased from the low to the high target force (P < 0.05; Table 2).
TABLE 2.
Motor unit characteristics at the low and high target forces for each muscle
| Parameter |
Vastus Medialis |
Abductor Digiti Minimi
|
||
|---|---|---|---|---|
| 2.5% | 25% | 2.5% | 12.5% | |
| Motor units/contraction | 2.1 ± 0.3 | 6.5 ± 2.3* | 2.5 ± 0.4 | 5.1 ± 1.7* |
| Action potentials/contraction | 217.0 ± 47.4 | 250.0 ± 92.5 | 245.0 ± 38.2 | 278.0 ± 45.1 |
| Surface peak-to-peak amplitude, μV | 14.1 ± 5.3 | 38.3 ± 17.9* | 22.2 ± 7.9 | 55.7 ± 21.6* |
| Muscle fiber CV, m/s | 3.78 ± 0.29 | 4.34 ± 0.76* | 3.45 ± 0.22 | 3.79 ± 0.89* |
| Discharge rate, pps | 7.9 ± 1.3 | 10.3 ± 2.1* | 8.1 ± 2.0 | 9.8 ± 2.4* |
Values are means ± SD.
P < 0.05, compared with 2.5%. CV, conduction velocity.
The average force exerted during the 2-min contractions for the measurement of synchronization was 5.4 ± 2.4 and 4.1 ± 2.6% MVC for the vastus medialis and abductor digiti minimi, respectively. These contractions yielded 82 and 94 motor-unit pairs from the vastus medialis and abductor digiti minimi, respectively. Only 35 of the 82 pairs of motor units from the vastus medialis showed significant peaks in the cross-histogram, whereas 90 of the 94 pairs of units had significant cross-histogram peaks in the abductor digiti minimi. The CIS values from the pairs that showed significant peaks were 0.10 ± 0.06 for the vastus medialis and 0.92 ± 0.31 for the abductor digiti minimi. Thus the two muscles presented two substantially different levels of motor-unit synchronization.
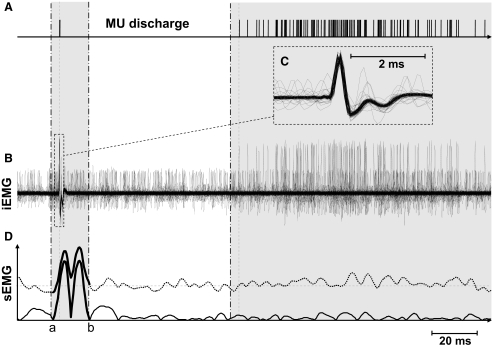
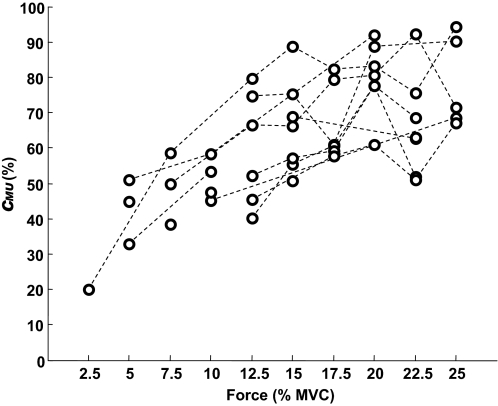
From the 30-s contractions, the times of occurrence of motor-unit APs were used to trigger the averaging of the surface EMG without preprocessing (interference EMG) and after squaring the signal (Fig. 4). The amount of amplitude cancellation for each motor unit was quantified with Eq. 5. As theoretically predicted (Fig. 2), the amount of cancellation for a single motor unit (Fig. 5) and for all motor units in each subject (Fig. 6) increased with target force. For example, the average amount of cancellation for the individual shown in Fig. 6 (vastus medialis muscle) increased from about 20% at 2.5% MVC force to about 80% at 25% MVC force. The average (across all contraction levels and motor units) amount of cancellation varied substantially across subjects: the range across subjects was 49.7–77.8% for the vastus medialis and 45.6–80.1% for the abductor digiti minimi.
FIG. 4.
STA from the discharge of a single motor unit into the surface EMG. A: the discharge times of a single motor unit have been aligned (leftmost tic mark), and subsequent discharges of the unit are shown in the same trace. B: superimposed intramuscular EMG recordings aligned with respect to the identified APs. C: the shape of the intramuscular AP. D: STAs from the interference (solid line) and the squared (dotted line) EMG signals.
FIG. 5.
Estimation of amplitude cancellation for a motor unit as a function of contraction force with the vastus medialis muscle. A: the motor unit was identified from the intramuscular recordings obtained during 6 contractions (2.5–15% MVC force). The similarity in shape of the intramuscular APs was used to ensure that the same motor unit was identified at all target forces. B: corresponding surface EMG signals with the discharge times of the identified motor unit indicated with circles. The surface APs can be distinguished in the surface EMG for the contraction at 2.5% MVC force, but this was more difficult with an increase in the amplitude of the interference EMG. C: the STAs from the interference and the squared EMGs were computed for the identified motor unit and the amount of cancellation was estimated with Eq. 5. At the target force of 15% MVC, the APs of the identified motor unit are almost completely cancelled (∼90% cancellation). The numerator in Eq. 10 is obtained by subtraction of the baseline value from the RMS of the STA into the squared EMG (with subsequent square root). The ratio between the result of this subtraction and the RMS of the STA into the interference EMG is proportional to cancellation (Eq. 10).
FIG. 6.
Amount of amplitude cancellation for all motor units identified in the vastus medialis muscle during the 10 contractions of one subject. The dashed lines connect values for the same motor unit.
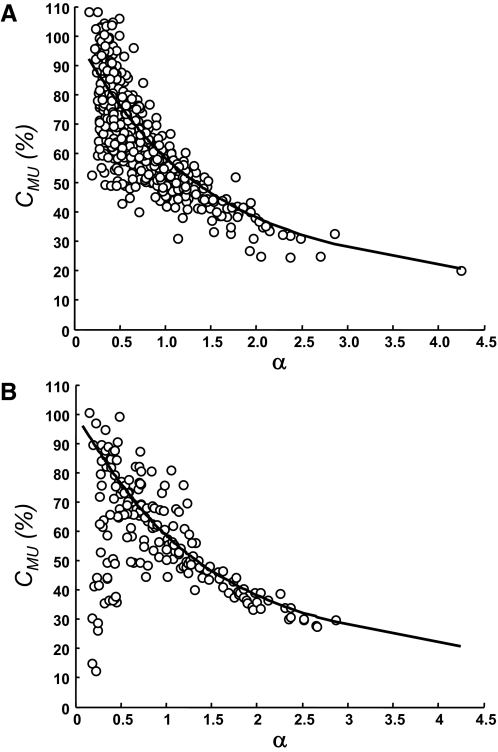
The amount of amplitude cancellation obtained from Eq. 5 was compared with the ratio in Eq. 10, which involves the size of the STA relative to the squared EMG. Within the limits of experimental accuracy in estimating the STA from a limited number of triggers, the ratio in Eq. 10 approximated the expected amount of amplitude cancellation in both muscles (Fig. 7). For small values of α, however, the estimated size of the STA obtained from the squared EMG signal was rather variable because the AP was almost completely cancelled in the summed signal. Moreover, according to the simulation analysis, synchronization of motor units may increase the variability for small values of α, as observed experimentally, especially for the abductor digiti minimi (Fig. 7). The experimental measurements of cancellation with Eq. 5 and the STA ratio in Eq. 10 were linearly correlated, although the strength of the correlation was greater for the vastus medialis muscle (R2 = 0.65; P < 0.001) compared with abductor digiti minimi (R2 = 0.26; P < 0.05; Fig. 7). When considering only α values >0.5 (n = 129), however, the strength of the correlation increased substantially in the abductor digiti minimi (R2 = 0.63; P < 0.001). The correlation between Eq. 5 and Eq. 10 experimentally demonstrated that the size of the STA from the squared EMG is associated with the amount of cancellation.
FIG. 7.
Comparison of the ratio in Eq. 10 derived from the STAs into the interference and the squared EMGs (circles) with the amount of cancellation measured experimentally by Eq. 5 (solid line). A: vastus medialis muscle. B: abductor digiti minimi muscle. The motor-unit data (n = 492 in A and n = 184 in B) are shown as a function of the ratio α between the RMS of the motor-unit AP and the RMS of the interference signal.
DISCUSSION
The amount of amplitude cancellation experienced by the APs of individual motor units has been theoretically proven to depend only on the ratio between the RMS of the motor-unit AP and the RMS of the interference signal. According to this theoretical derivation, small surface APs are cancelled more than large APs in the interference signal. The derivation indicates that the amplitude cancellation of individual motor units can be experimentally estimated by the STA of the interference and squared EMGs and that the relative reduction in the size of the STA extracted from the squared EMG is attributable to amplitude cancellation.
The analytical derivation of cancellation provides the means for predicting the amount of amplitude cancellation in a population of motor units. The amount of cancellation at each amplitude of the interference EMG depends on the size of the motor-unit AP. Because low-threshold motor units tend to produce smaller surface APs than high-threshold units (Keenan et al. 2006b), it is the surface APs of the low-threshold motor units that are reduced more by amplitude cancellation (Fig. 2). As a consequence of this effect, the surface EMG amplitude can be relatively insensitive to changes in the activity of low-threshold motor units. For example, Mottram et al. (2005) found that the discharge rate of low-threshold motor units decreased more rapidly during one type of fatiguing contraction compared with another type, whereas the change in the amplitude of the surface EMG was similar during the two tasks. Similarly, indexes of neural drive to the muscle based on EMG amplitude, such as neuromuscular efficiency (Deschênes et al. 2002), are largely biased toward changes in the activity of high-threshold motor units.
The sensitivity of EMG amplitude to the recruitment of motor units depends on the ratio (α) of the RMS values for the AP and the interference EMG. Due to this relation, a doubling of the amplitude of the interference EMG will reduce the index α for a motor-unit AP by one half. For a motor unit with α = 1, a doubling of the interference EMG amplitude will increase the amplitude cancellation of its surface AP from about 60 to 75% (Fig. 2). If α is reduced to one third of the initial value, amplitude cancellation will increase further to about 85%. The relation between the ratio α and amplitude cancellation explains why simulation results (Keenan et al. 2005) indicated that a doubling in the number of motor units in a muscle produced only a limited increase in surface EMG amplitude. Furthermore, the theoretical relation suggests that surface EMG amplitude is relatively insensitive to the changes in motor-unit activity at high levels of excitation: when α is ≪1, cancellation is close to 100% (Eq. 5; Fig. 2).
When an individual sustains a submaximal contraction, the amplitude of the surface EMG increases due to the recruitment of additional motor units (Garland et al. 1994; Person and Kudina 1972; Riley et al. 2008), the decrease in muscle fiber conduction velocity (Bigland-Ritchie et al. 1981; Merletti et al. 1990), and the change in the shape of the intracellular AP (Dimitrova and Dimitrov 2002; Hanson and Persson 1971). According to the relation in Eq. 5, the amount of amplitude cancellation for a motor-unit AP will increase with the progressive increase in the amplitude of the surface EMG. Because the relation represented in Eq. 5 is nonlinear (Fig. 2), however, an increase in the amplitude of the interference signal has a different influence on the amount of amplitude cancellation experienced by each motor unit. The relative contribution of different motor units to the signal amplitude changes with increases in EMG amplitude. Similarly, the amount of cancellation in motor-unit APs may differ in fatigued and unfatigued states due to differences in the RMS of the APs (Dimitrova and Dimitrov 2002; Hanson and Persson 1971), which is one of the factors that may contribute to the surface EMG at the end of a sustained submaximal contraction not reaching the amplitude achieved during a control MVC (Fuglevand et al. 1993b).
The simulation results demonstrated the validity of the theoretical derivations in the absence of motor-unit synchronization. The simulations also allowed the inclusion of synchronization, which was not investigated theoretically. The results indicated that the presence of synchronization has the greatest influence on the cancellation experienced by small motor units (Fig. 3, B and C), consistent with the observation that the summing of motor-unit APs in the presence of synchronization depends on the duration of the APs (Zhou and Rymer 2004). The amount of cancellation is, in general, smaller than predicted by Eq. 5 when motor units are synchronized (Fig. 3, B and C); however, the difference is relatively small for α values >0.2. The presence of synchronization reduces, on average, amplitude cancellation for small motor units, although it increases the total cancellation in the signal by decreasing the α value (compare the α axis in Fig. 3A with Fig. 3, B and C), as observed in a previous simulation study (Keenan et al. 2006a). The two muscles investigated experimentally exhibited different degrees of synchronization: it was low in vastus medialis, as previously reported (Kim et al. 2001), and high in abductor digiti minimi as reported for other hand muscles (Semmler and Nordstrom 1999). The simulation analysis indicated a weaker correlation between the STA-derived and the analytical estimates of synchronization for abductor digiti minimi (Fig. 7).
Extraction of the STA from the squared EMG followed by taking the square root of the signal is a similar operation to obtaining a STA from the rectified EMG; the latter has been used to assess the amount of motor-unit synchronization (Milner-Brown et al. 1973, 1975). However, the theoretical analysis proved that the size of the STA from the squared EMG is fully explained by the amount of amplitude cancellation even in the absence of motor-unit synchronization: the larger the cancellation, the smaller the size of the STA. This association was experimentally proved by predicting the reduction in size of the STA from the squared EMG with Eq. 5 (Fig. 7). Because cancellation depends on the size of the motor-unit AP, the size of the STA from the squared EMG relative to that from the interference EMG differs among motor units in a predictable way. Moreover, the STA from the squared EMG will change with the amplitude of the surface EMG because this influences cancellation, which explains previous results (Yue et al. 1995).
In summary, amplitude cancellation of motor-unit APs in the surface EMG can be analytically predicted and experimentally measured, within the limitations of the STA approach (Keenan et al. 2006a) and for low levels of motor-unit synchronization. The size of the STA extracted from the squared EMG can be largely predicted from the size of the surface AP of the motor unit and the amplitude of the interference EMG. The amount of amplitude cancellation is greatest for low-threshold motor units that produce small surface APs, although the presence of synchronization may decrease the amount of cancellation experienced by these motor units. These effects confound the interpretation of changes in the amplitude of the surface EMG as an index of the activation of a motor-unit population.
GRANTS
This work was partly supported by the European Project “Cybernetic Manufacturing Systems” under Contract CyberManS 016712, Danish Technical Research Council Project “Centre for Neuroengineering (CEN),” under Contract 26-04-0100 to D. Farina, and National Institute of Neurological Disorders and Stroke Grant NS-43275 to R. M. Enoka.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Andreassen and Arendt-Nielsen 1987.Andreassen S, Arendt-Nielsen L. Muscle fibre conduction velocity in motor units of the human anterior tibial muscle: a new size principle parameter. J Physiol 391: 561–571, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong et al. 1988.Armstrong JB, Rose PK, Vanner S, Bakker GJ, Richmond FJ. Compartmentalization of motor units in the cat neck muscle, biventer cervicis. J Neurophysiol 60: 30–45, 1988. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie et al. 1981.Bigland-Ritchie B, Donovan EF, Roussos CS. Conduction velocity and EMG power spectrum changes in fatigue of sustained maximal efforts. J Appl Physiol 51: 1300–1305, 1981. [DOI] [PubMed] [Google Scholar]
- Datta et al. 1991.Datta AK, Farmer SF, Stephens JA. Central nervous system pathways underlying synchronization of human motor unit firing studies during voluntary contractions. J Physiol 432: 401–425, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey et al. 1986.Davey NJ, Ellaway PH, Stein RB. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods 17: 153–166, 1986. [DOI] [PubMed] [Google Scholar]
- Day and Hulliger 2001.Day SJ, Hulliger M. Experimental simulation of cat electromyogram: evidence for algebraic summation of motor-unit action-potential trains. J Neurophysiol 86: 2144–2158, 2001. [DOI] [PubMed] [Google Scholar]
- De Luca et al. 1982.De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca et al. 1993.De Luca CJ, Roy AM, Erim Z. Synchronization of motor-unit firings in several human muscles. J Neurophysiol 70: 2010–2023, 1993. [DOI] [PubMed] [Google Scholar]
- Deschênes et al. 2002.Deschênes MR, Giles JA, McCoy RW, Volek JS, Gomez AL, Kraemer WJ. Neural factors account for strength decrements observed after short-term muscle unloading. Am J Physiol Regul Integr Comp Physiol 282: R578–R583, 2002. [DOI] [PubMed] [Google Scholar]
- Dimitrova and Dimitrov 2002.Dimitrova NA, Dimitrov GV. Amplitude-related characteristics of motor unit and M-wave potentials during fatigue. A simulation study using literature data on intracellular potential changes found in vitro. J Electromyogr Kinesiol 12: 339–349, 2002. [DOI] [PubMed] [Google Scholar]
- Elek et al. 1992.Elek JM, Kossev A, Dengler R, Schubert M, Wohlfahrt K, Wolf W. Parameters of human motor unit twitches obtained by intramuscular microstimulation. Neuromuscul Disord 2: 261–267, 1992. [DOI] [PubMed] [Google Scholar]
- Ellaway 1978.Ellaway PH Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978. [DOI] [PubMed] [Google Scholar]
- Farina et al. 2002.Farina D, Arendt-Nielsen L, Merletti R, Graven-Nielsen T. Assessment of single motor unit conduction velocity during sustained contractions of the tibialis anterior muscle with advanced spike triggered averaging. J Neurosci Methods 115: 1–12, 2002. [DOI] [PubMed] [Google Scholar]
- Farina et al. 2003.Farina D, Arendt-Nielsen L, Merletti R, Indino B, Graven-Nielsen T. Selectivity of spatial filters for surface EMG detection from the tibialis anterior muscle. IEEE Trans Biomed Eng 50: 354–364, 2003. [DOI] [PubMed] [Google Scholar]
- Farina et al. 2000.Farina D, Fortunato E, Merletti R. Noninvasive estimation of motor unit conduction velocity distribution using linear electrode arrays. IEEE Trans Biomed Eng 47: 380–388, 2000. [DOI] [PubMed] [Google Scholar]
- Farina et al. 2004.Farina D, Mesin L, Martina S, Merletti R. A surface EMG generation model with multilayer cylindrical description of the volume conductor. IEEE Trans Biomed Eng 51: 415–426, 2004. [DOI] [PubMed] [Google Scholar]
- Fuglevand et al. 1993a.Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70: 2470–2488, 1993a. [DOI] [PubMed] [Google Scholar]
- Fuglevand et al. 1993b.Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572, 1993b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland et al. 1994.Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol 76: 2411–2419, 1994. [DOI] [PubMed] [Google Scholar]
- Hanson and Persson 1971.Hanson J, Persson A. Changes in the action potential and contraction of isolated frog muscle after repetitive stimulation. Acta Physiol Scand 81: 340–348, 1971. [DOI] [PubMed] [Google Scholar]
- Heckman and Binder 1991.Heckman CJ, Binder MD. Computer simulation of the steady-state input-output function of the cat medial gastrocnemius motoneuron pool. J Neurophysiol 65: 952–967, 1991. [DOI] [PubMed] [Google Scholar]
- Hedayatpour et al. 2007.Hedayatpour N, Arendt-Nielsen L, Farina D. Motor unit conduction velocity during sustained contraction of the vastus medialis muscle. Exp Brain Res 180: 509–516, 2007. [DOI] [PubMed] [Google Scholar]
- Kakuda et al. 1991.Kakuda N, Nagaoka M, Tanaka R. Discrimination of different motor units by spike-triggered averaging of surface electromyograms. Neurosci Lett 122: 237–240, 1991. [DOI] [PubMed] [Google Scholar]
- Keenan et al. 2005.Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated electromyogram. J Appl Physiol 98: 120–131, 2005. [DOI] [PubMed] [Google Scholar]
- Keenan et al. 2006a.Keenan KG, Farina D, Merletti R, Enoka RM. Amplitude cancellation reduces the size of motor unit potentials averaged from the surface EMG. J Appl Physiol 100: 1928–1937, 2006a. [DOI] [PubMed] [Google Scholar]
- Keenan et al. 2006b.Keenan KG, Farina D, Merletti R, Enoka RM. Influence of motor unit properties on the size of the simulated evoked surface EMG potential. Exp Brain Res 169: 37–49, 2006b. [DOI] [PubMed] [Google Scholar]
- Kernell 1965.Kernell D Synaptic influence on the repetitive activity elicited in cat lumbosacral motoneurones by long-lasting injected currents. Acta Physiol Scand 63: 409–410, 1965. [DOI] [PubMed] [Google Scholar]
- Kim et al. 2001.Kim MS, Masakado Y, Tomita Y, Chino N, Pae YS, Lee KE. Synchronization of single motor units during voluntary contractions in the upper and lower extremities. Clin Neurophysiol 112: 1243–1249, 2001. [DOI] [PubMed] [Google Scholar]
- Masuda et al. 1985.Masuda T, Miyano H, Sadoyama T. The position of innervation zones in the biceps brachii investigated by surface electromyography. IEEE Trans Biomed Eng 32: 36–42, 1985. [DOI] [PubMed] [Google Scholar]
- McComas et al. 1971.McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry 34: 121–131, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill et al. 2005.McGill KC, Lateva ZC, Marateb HR.EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005. [DOI] [PubMed] [Google Scholar]
- Merletti et al. 2003.Merletti R, Farina D, Gazzoni M. The linear electrode array: a useful tool with many applications. J Electromyogr Kinesiol 13: 37–47, 2003. [DOI] [PubMed] [Google Scholar]
- Merletti et al. 1990.Merletti R, Knaflitz M, De Luca CJ. Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol 69: 1810–1820, 1990. [DOI] [PubMed] [Google Scholar]
- Milner-Brown et al. 1975.Milner-Brown HS, Stein RB, Lee RG. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol 38: 245–254, 1975. [DOI] [PubMed] [Google Scholar]
- Milner-Brown et al. 1973.Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol 228: 285–306, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz et al. 2005.Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. [DOI] [PubMed] [Google Scholar]
- Mottram et al. 2005.Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392, 2005. [DOI] [PubMed] [Google Scholar]
- Nordstrom et al. 1992.Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453: 547–574, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person and Kudina 1972.Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972. [DOI] [PubMed] [Google Scholar]
- Riley et al. 2008.Riley ZA, Terry ME, Mendez-Villaneuva A, Litsey JC, Enoka RM. Motor unit recruitment and bursts of activity in the surface electromyogram during a sustained contraction. Muscle Nerve (February 20, 2008) doi: 10.1002/mus.2008.20978. [DOI] [PubMed]
- Rosenfalck 1969.Rosenfalck P Intra- and extracellular potential fields of active nerve and muscle fibers. Acta Physiol Scand Suppl 47: 239–246, 1969. [PubMed] [Google Scholar]
- Semmler and Nordstrom 1999.Semmler JG, Nordstrom MA. A comparison of cross-correlation and surface EMG techniques used to quantify motor unit synchronization in humans. J Neurosci Methods 90: 47–55, 1999. [DOI] [PubMed] [Google Scholar]
- Semmler et al. 1997.Semmler JG, Nordstrom MA, Wallace CJ. Relationship between motor unit short-term synchronization and common drive in human first dorsal interosseous muscle. Brain Res 767: 314–320, 1997. [DOI] [PubMed] [Google Scholar]
- Slawnych et al. 1990.Slawnych MP, Laszlo CA, Hershler C. A review of techniques employed to estimate the number of motor units in a muscle. Muscle Nerve 13: 1050–1064, 1990. [DOI] [PubMed] [Google Scholar]
- Stein and Yang 1990.Stein RB, Yang JF. Methods for estimating the number of motor units in human muscles. Ann Neurol 28: 487–495, 1990. [DOI] [PubMed] [Google Scholar]
- Troni et al. 1983.Troni W, Cantello R, Rainero I. Conduction velocity along human muscle fibers in situ. Neurology 33: 1453–1459, 1983. [DOI] [PubMed] [Google Scholar]
- Yao et al. 2000.Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol 83: 441–452, 2000. [DOI] [PubMed] [Google Scholar]
- Yue et al. 1995.Yue G, Fuglevand AJ, Nordstrom MA, Enoka RM. Limitations of the surface electromyography technique for estimating motor unit synchronization. Biol Cybern 73: 223–233, 1995. [DOI] [PubMed] [Google Scholar]
- Zhou and Rymer 2004.Zhou P, Rymer WZ. Factors governing the form of the relation between muscle force and the EMG: a simulation study. J Neurophysiol 92: 2878–2886, 2004. [DOI] [PubMed] [Google Scholar]