Abstract
Objective To report the results of a systematic review of randomized controlled trials (RCTs) of psychological interventions for children and adolescents undergoing needle-related procedures. Methods A variety of cognitive-behavioral psychological interventions for managing procedural pain and distress in children and adolescents between 2 and 19 years of age were examined. Outcome measures included pain and distress as assessed by self-report, observer report, behavioral/observational measures, and physiological correlates. Results Twenty-eight trials met the criteria for inclusion in the review and provided the data necessary for pooling the results. Together, the trials included 1,039 participants in treatment conditions and 951 in control conditions. The largest effect sizes for treatment improvement over control conditions were found for distraction, combined cognitive-behavioral interventions, and hypnosis, with promising but limited evidence for several other psychological interventions. Conclusions Recommendations for conducting future RCTs are provided, and particular attention to the quality of trial design and reporting is highlighted.
Keywords: adolescents, children, procedural pain, psychological interventions, systematic review
Children often experience unpredictable and severe procedure-related pain in hospitals that can be associated with negative emotional and psychological implications (Cummings, Reid, Finley, McGrath, & Ritchie, 1996; Kazak & Kunin-Batson, 2001). These medical procedures also cause anxiety, fear, and behavioral distress for children and their families, further intensifying their pain and interfering with the procedure (Broome, Bates, Lillis, & McGahee, 1990). Medical procedures, particularly needles, are among the most feared experiences reported by children (Broome et al., 1990).
Psychological interventions for managing pain and distress in children are primarily cognitive-behavioral treatments (CBT). CBT interventions for pain management assist the child to develop and apply coping skills to manage the pain and distress, and when developmentally appropriate, to help the child comprehend how thoughts and behaviors can alter their experience of pain (Keefe, Dunsmore, & Burnett, 1992).
Several narrative, nonsystematic reviews of psychological interventions for the management of procedural pain and distress in children are available (Blount, Piira, & Cohen, 2003; Chen, Joseph, & Zeltzer, 2000; Christophersen & Mortweet, 2001; Powers, 1999). While these reviews typically conclude that psychological interventions are beneficial, the lack of a systematic and pooled approach to integrating the literature limits conclusions regarding the efficacy of these interventions. There have been a few more systematic approaches to integrating this literature (Kleiber & Harper, 1999; Saile, Burgmeier, & Schmidt, 1988), but these reviews are limited in that they have a narrow focus (e.g., distraction only) and are out of date given the rapid growth in research in this area. Furthermore, recent recommendations for enhancing reviews of psychological treatments in pediatric populations suggest that reviews should include meta-analyses, clinical significance, and theory-guided interventions (Drotar, 2002). The present review follows these recommendations, and summarizes our systematic review and meta-analysis for the Cochrane Collaboration (www.cochrane.org) on the efficacy of cognitive-behavioral interventions for managing needle-related procedural pain and distress in children and adolescents (Uman, Chambers, McGrath, & Kisely, 2006). The Cochrane Collaboration (www.cochrane.org) is an international not-for-profit organization, with the mandate of providing up-to-date information, and translation of systematic reviews related to health care. This article is based on a Cochrane Review published in the Cochrane Library, 2006, Issue 4 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Library should be consulted for the most recent version of this review. Cochrane Reviews are published in the Cochrane Library (an online resource); however, given the rigorous criteria they follow, they are often quite extensive and lengthy. To promote knowledge translation, they are often published in abbreviated formats in relevant academic journals. Thus, this review presents an abbreviated version of the original Cochrane Review, highlighting the main procedures, conclusions, and recommendations. This review extends and expands from previous reviews in this area by: (a) providing a more up-to-date synopsis of the literature, (b) including a meta-analytic component, and (c) evaluating a wide range of psychological interventions.
Methods
Participants and Trials
The protocol and completed review for this project were developed for and approved by the Cochrane Collaboration. We searched published and nonpublished randomized controlled trials (RCTs) of psychological interventions for children and adolescents undergoing needle-related procedures. Following the protocols used in previous meta-analyses examining psychological interventions for pain, only RCTs with at least five children/adolescents aged 2–19 years in each study arm were included in this review (Eccleston, Yorke, Morley, Williams, & Mastroyannopoulou, 2004).
We used a comprehensive list of common medical procedures involving needles derived from reviewing the literature and consulting with clinicians and experts in pediatric health. The list of needle procedures consisted of: immunization, venipuncture, finger prick/pin, injection (subcutaneous, intramuscular), lumbar puncture/spinal tap, bone marrow aspiration, bone marrow biopsy, IV/catheter insertion, central line/central venous catheter, suture/laceration repair, accessing a portacath, arterial puncture, arterial blood gas, arterial line, thoracocentesis, and paracentesis. Participants included healthy children and children with chronic or transitory illnesses from both inpatient and outpatient settings. Children undergoing surgery were not included because factors specific to surgery (e.g., sedation) can complicate and interfere with the accuracy of self-reported accounts of pain and distress.
Types of Interventions
Cognitive interventions were defined as interventions that involve identifying and altering negative thinking styles related to anxiety about the medical procedure, and replacing them with more positive beliefs and attitudes, leading to more adaptive behavior and coping styles (Barlow & Durand, 1999). Behavioral interventions were defined as interventions based on principles of behavioral science and learning, by targeting specific behaviors (Barlow & Durand, 1999). Combined cognitive-behavioral interventions were defined as those including at least one cognitive intervention combined with at least one behavioral intervention. Given the heterogeneity of the different interventions in the cognitive and behavioral categories, the interventions were analyzed and interpreted separately. For a complete list of the interventions included in our search along with their definitions, please refer to Table I.
Table I.
Psychological Intervention Definitions
| Cognitive Interventions | Definitions |
|---|---|
| Cognitive distraction | Cognitive techniques to shift attention away from procedure (e.g., counting, nonprocedural talk). |
| Imagery | Techniques to encourage child to cope with the pain/distress of the procedure by having them imagine a pleasant object or experience (e.g., enchanted forest). |
| Hypnosis | Dissociation from painful experience and distress via hypnotic induction, suggestions, and imagined fantasy; similar to but more involved than imagery. |
| Preparation/information | Explaining the steps of the procedures and/or providing sensory information associated with the procedure (e.g., providing instructions about what the procedure will involve). |
| Thought-stopping | Child repeats “stop” or a similar statement during times of distress/pain, to block out negative thoughts. |
| Coping self-statements | Child repeats a set of positive thoughts (e.g., “I can do this”; “This will be over soon”). |
| Suggestion | Providing verbal or nonverbal cues to the child suggesting that the administered intervention will or can reduce pain and/or distress. |
| Memory change | Helping child to reframe negative memories of the procedure into positive ones. |
| Parent training | Training the parent (not the child) to engage in one of the above cognitive strategies. The goal is to decrease the parent's distress that in turn may decrease the child's distress or pain, or both. |
| Behavioral Interventions | Definitions |
|---|---|
| Behavioral distraction | Behavioral techniques to shift attention away from procedure (e.g., videotapes, games). |
| Muscle relaxation | Tensing and relaxing various muscle groups of the body. |
| Breathing exercises | Deep breathing or breathing from the diaphragm rather than the chest (e.g., using party blowers, blowing bubbles, pretending to inflate or deflate a tire through inhaling/exhaling). |
| Modeling | Demonstration of positive coping behaviors during a mock procedure by another child or adult. |
| Rehearsal | Practice using positive coping behaviors demonstrated during modeling. |
| Desensitization | Gradual systematic exposure to the feared stimuli, generally involving a hierarchy of feared stimuli. |
| Positive reinforcement | Providing positive statements and/or tangible rewards (e.g., toys) to the child following the procedure. |
| Parent training | Training the parent (not the child) to engage in one of the above behavioral strategies. |
| Parent/staff coaching | Training the parent or medical staff to actively coach the child to use one of the above strategies. |
| Virtual reality | Using technology and equipment (e.g., goggles, headphones) to absorb the child's attention; more involved than distraction. |
| Combined Cognitive- Behavioral Therapy (CBT) | Definition |
|---|---|
| Combined CBT | Any intervention using at least one of the above cognitive interventions in combination with at least one of the above behavioral interventions. |
In addition, for trials to be included in our review, the treatment conditions needed to be compared with a control or comparison group, which could include nonspecific treatment (i.e., attention placebo) or routine/standard care. Finally, while not the focus of the present review, it is important to acknowledge that although all of these interventions fall under the cognitive-behavioral umbrella, they may have different theoretical underpinnings, and may therefore target different underlying mechanisms.
Types of Outcome Measures
The two outcome measures of interest were pain and distress, assessed using scales or measures with established reliability and validity (i.e., as evidenced in at least one prior published study in a peer-reviewed journal). Pain and distress are associated but distinct constructs, therefore, we felt that it was important to include them as separate outcomes while acknowledging their overlap. For the purpose of this review, pain was defined using the definition recommended by the International Association for the Study of Pain (IASP) as: “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (IASP, 2004). Distress was broadly defined as any type of negative affect associated with the procedure (e.g., anxiety, stress, fear). Outcome measures for both pain and distress included self-report (e.g., visual analog scales, numerical rating scales, faces scales), observer reports, behavioral/observational measures, and physiological correlates (e.g., heart rate).
Search Strategy and Trial Selection
We included published and unpublished RCTs and dissertations in our search. Unpublished RCTs were included to reduce potential publication bias often associated with null findings. Published studies and dissertations were identified by conducting electronic searches of seven databases from their inception until 2005: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PsycINFO, EMBASE, Cumulative Index to Nursing and Allied Health Literature, Web of Science, and Dissertation Abstracts International. We also searched for additional published and unpublished RCTs by directly contacting experts in the area of pediatric pain, and by posting messages on the following electronic mailing lists (i.e., listservs): Pediatric Pain, Pain in Child Health (PICH), the American Psychological Association's (APA) Society of Pediatric Psychology Division 54, and the APA's Health Psychology Division 38. In addition, we consulted an updated reference of empirically supported treatments for procedural pain, published by the APA's Society of Pediatric Psychology Division 54 (www.societyofpediatricpsychology.org), as an addendum to the review by Powers (1999). Finally, reference and citation lists from papers identified as reviews, meta-analyses, or RCTs meeting inclusion criteria for this review were searched. No language restrictions were imposed.
Once a comprehensive list of citations was derived, two reviewers independently screened all trial titles and abstracts. All relevant full articles were retrieved and reviewed independently by the same two reviewers to determine whether they met the inclusion criteria for the present review. Reviewers were not blind to authors, institutions, journals, or results. In order to be included in our meta-analysis, each trial had to provide means and standard deviations for relevant outcomes. Attempts were made to obtain missing data from the authors.
Study Quality
Each study included in the review was scored for quality independently by two reviewers using the Oxford Quality Scale created by Jadad and colleagues (1996). The scale is comprised of five questions for a maximum score of five points. Each of the following questions can be allotted or subtracted one point: (a) Is the study randomized? If “yes,” give one point; (b) Is the randomization procedure reported and appropriate? If “yes,” give one point. If “no,” deduct one point; (c) Is the study double blind? If “yes,” give one point; (d) Is the blinding procedure appropriate and adequate? If “yes,” give one point. If “no” deduct one point; and (e) Are withdrawals and dropouts described? If “yes,” give one point. It is often not feasible for studies examining psychological management of pain and distress to be double-blinded. Despite this limitation of the scale for studies of psychological interventions, it is a validated and internationally recognized measure and was, therefore, used to assess study quality in this review. In order to compensate for this limitation of the scale, we also coded all trials on: (a) whether study coders were blind to the interventions (e.g., researchers coding child reactions from videotapes where the intervention is not visible), and (b) whether treatment fidelity was reported. Because of differences in the nature of the interventions (e.g., active versus passive), and the person administering them (e.g., child, parent, healthcare professional), we included the broadest definition of treatment fidelity to allow a maximal chance of receiving credit for this item. We considered a trial to have met this criterion if the trial provided information about: (a) whether the children actually adhered to the administered intervention (e.g., watched the television if that was used as distraction), or (b) whether the person administering the intervention followed the study protocol (e.g., parent positioning).
Statistical Analyses
Statistical analyses were conducted using RevMan 4.2 software, the statistical software recommended by the Cochrane Collaboration. Differences between the results of each included trial were analyzed using tests of heterogeneity (chi-square) in order to determine whether the results were statistically similar enough to combine. Where statistically significant heterogeneity was detected, the data were still pooled; however, these results should be interpreted with caution. Results were analyzed using random effects models for combining effect sizes. Compared with fixed effects models, which assume that there is no heterogeneity among study results, random effects models are more appropriate to use when studies may be heterogeneous as they assume that the effect sizes are not derived from the same distribution (Petticrew & Roberts, 2006). We computed standardized mean differences (SMD) with 95% confidence intervals (CI) that allowed us to combine the results from different scales measuring the same construct (e.g., pain). For each outcome assessed, the intervention is considered efficacious only if the SMD and both anchors of the CI fall in the negative range (Petticrew & Roberts, 2006). That is, the intervention for a particular outcome is not considered efficacious if the CI includes zero.
Results
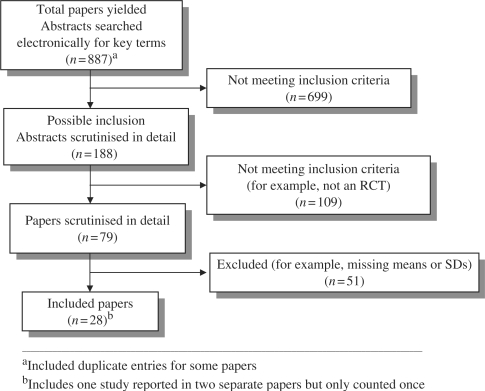
Our search retrieved 887 citation abstracts. Of these, 79 papers were potentially relevant and were reviewed in further detail. Of these, we excluded 51 because they did not meet our inclusion criteria (Fig. 1). Primary reasons for exclusion fell into the following categories: (a) met inclusion criteria but missing means, standard deviations, or both (n = 21 trials); (b) not a randomized controlled trial (n = 4); (c) no control/comparison group (n = 6); (d) surgical procedure (n = 5); (e) inappropriate randomization procedure (e.g., alternation) (n = 6); (f) failed randomization (n = 2); (g) inappropriate outcome measures (n = 2); (h) exceeded age range (n = 1); (i) inappropriate intervention (n = 1); (j) inappropriate control/comparison group (n = 1); (k) no needle procedure (n = 1); and (l) fewer than five participants per condition (n = 1). In total, 29 papers representing 28 separate studies were included (N = 1,951 participants in total). Twenty-six studies were retrieved from the original database search, and two more trials were identified from a secondary search conducted in 2006 during the period it took for the review to be completed. These studies met all inclusion criteria and provided the data necessary for pooling. The 28 included studies involved investigators from eight countries (USA, Canada, Australia, UK, Greece, Kuwait, Israel, and The Netherlands). Trial characteristics are provided in Table II.
Figure 1.
Number of papers yielded by search strategy in systematic review.
Table II.
Twenty-eight Trials Included in Meta-analysis
| Study | N | Age (yrs) | Procedure | Psychological intervention(s) |
|---|---|---|---|---|
| Blount et al. (1992) | 60 | 3–7 | Immunization | Distraction + coping skills + deep breathing |
| Cassidy et al. (2002) | 62 | 5 | Immunization | Distraction using TV cartoon |
| Cavender, Goff, Hollen, and Guzzetta, (2004) | 43 | 4–11 | Venipuncture/IV insertion | Parental positioning + distraction |
| Chen, Zeltzer, Craske, and Katz (1999) | 50 | 3–18 | Lumbar puncture (LP) | Memory reframing |
| Cohen, Blount, and Panopoulos (1997) | 92 | 4–6 | Immunization | Nurse coaching ± parent and child training |
| Cohen, Blount, Cohen, Schaen, and Zaff (1999) | 39 | 8.8–11.1 | Immunization | Nurse coaching + movie distraction |
| Cohen, Bernard, Greco, and McClellan (2002) | 61 | 3.7–6.9 | Immunization | Coping skills |
| Eland (1981) | 40 | 4.9–5.9 | Intramuscular injection | Cognitive information |
| Fanurik, Koh, and Schmitz (2000) | 160 | 2–16 | IV insertion | Distraction |
| Fowler-Kerry and Lander (1987) | 200 | 4.6–6.2 | Immunization | Distraction; suggestion (separate and together) |
| French, Painter, and Coury (1994) | 149 | 4–7 | Immunization | Blowing out air |
| Gonzalez, Routh, and Armstrong (1993) | 42 | 3–7 | Routine injections | Distraction |
| Goodenough et al. (1997) | 117 | 3.5–17.7 | Venipuncture | Suggestion |
| Harrison (1991) | 100 | 6–12 | Venous blood sampling | Preparation |
| Katz, Kellerman, and Ellenberg (1987) | 36 | 6–11 | Bone marrow aspiration (± LP) | Hypnosis |
| Kleiber, Craft-Rosenberg, and Harper (2001) | 44 | 4–7 | IV insertion | Distraction |
| Krauss (1998)* | 50 | 4–7 | Immunization | Videotape modeling + parent participation |
| Kuttner (1987) | 25 | 3–6.1 | Bone marrow aspiration | Hypnosis; distraction |
| Liossi and Hatira (1999) | 30 | 5–15 | Bone marrow aspiration | Cognitive-behavioral intervention; hypnosis |
| Liossi and Hatira (2003) | 80 | 6–16 | LP | Hypnosis |
| Liossi, White, and Hatira (2006) | 45 | 6–16 | LP | Hypnosis |
| Posner (1998)* | 20 | 3.3–10.5 | Venipuncture | Parent-assisted behavioral intervention |
| Press et al. (2003) | 94 | 6–16 | Venipuncture | Distraction |
| Tak, and van Bon (2005) | 136 | 3–12 | Venipuncture | Distraction; providing information |
| Tyc, Leigh, Mulhern, Srivastava, and Bruce (1997) | 55 | 6.3–18.6 | IV insertion | Cognitive-behavioral intervention |
| Vessey, Carlson, and McGill (1994) | 100 | 3.6–12.11 | Routine blood draws | Distraction |
| Wint, Eshelman, Steele, and Guzzetta (2002) | 30 | 10–19 | LP | Virtual reality distraction |
| Zabin (1982)* | 48 | 6–11 | Finger capillary puncture | Distraction; modeling |
*Represents unpublished doctoral dissertation.
Descriptions of the included trials are described here, where numbers (n) refer to number of trials rather than sample sizes. The diagnostic status of the children in the included studies was the following: (a) healthy children (n = 15); (b) oncology patients with Leukemia/Lymphoma (n = 9); (c) children without a current diagnosis who were being evaluated for various medical conditions (n = 4); and (d) children being treated for a variety of other conditions (e.g., surgical referral; trauma; vomiting; chronic urinary tract infections; chronic constipation; n = 2). The treatment settings were described as: (a) community health center/clinic (n = 8); (b) hospital (n = 9); (c) health department clinic (n = 4); (d) emergency department of a pediatric medical center/hospital (n = 2); (e) treatment/surgery room of a clinic (n = 2); (f) school health center/clinic (n = 1); (g) urban pediatric practice (n = 1); and (h) private pediatrician's office (n = 1).
The Kappa coefficient for the inter-rater reliability using the 5-point Oxford Quality Scale was 0.93. The two raters also independently coded all of the included studies to assess whether: (a) coders were blind to treatment conditions, and (b) treatment fidelity was reported. Inter-rater reliabilities calculated using Kappa coefficients for coder blinding and treatment fidelity were 0.76 and 0.91, respectively. As expected, none of the trials were double-blind; thus the highest possible attainable score was three out of five. Only three studies achieved a score of 3, five achieved a score of 2, four achieved a score of 1, and the remaining 16 achieved scores of zero.
Seven of the 28 included studies provided information describing how many participants withdrew after consenting to participate, and provided the reasons for withdrawals when they occurred. In addition, only 10 of the trials reported that coders were blind for at least one measure. Treatment fidelity was addressed in 8 out of the 28 included studies.
Meta-analysis
Each psychological intervention was analyzed separately into the following categories based on the outcome measures with available data: (a) self-reported pain; (b) observer-reported child pain; (c) self-reported distress; (d) observer-reported child distress; (e) behavioral measures of pain; (f) behavioral measures of distress; and (g) physiological measures (e.g., heart rate). When a study provided more than one observer rating of the same construct (e.g., parent and nurse ratings) or used more than one behavioral measure to assess the same construct, these measures were pooled together in order to summarize the large amount of data reported in these studies. Results based on one or two studies, and results with significant heterogeneity, should be interpreted with caution. Interventions are presented below in alphabetical order (see Table III for a summary).
Table III.
Efficacy of Psychological Interventions Assessed in Meta-analysis
| Intervention | SR-P | OR-P | BM-P | SR-D | OR-D | BM-D | PHY |
|---|---|---|---|---|---|---|---|
| Blowing out air | ? | – | – | – | – | ? | – |
| Combined CBT | ? | ? | – | ? |  |
✓ | ? |
| Distraction | ✓ | ? | ? | ? | ? | ? | – |
| Distraction + suggestion | ✓ | – | – | – | – | – | – |
| Filmed modeling | – | – | – | ? | ? | – | – |
| Hypnosis |  |
– | – |  |
? |  |
– |
| Memory alteration | ? | ? | – | – | ? | ? | ? |
| Nurse coach + distraction | ? | ? | – | ? | ? | ✓ | ? |
| Parent coach + distraction | ? | – | – | – | ? | ? | – |
| Parent positioning + distraction | ? | – | – | ? | ✓ | ? | – |
| Providing information/preparation | ? | ✓ | – | – | ? | ? | ✓ |
| Suggestion | ? | ? | – | ? | ? | – | – |
| Videotaped modeling + parent coaching | – | – | – | – | ? | – | – |
| Virtual reality | ? | – | – | – | – | – | – |
SR-P, self-reported pain; OR-P, observer-reported pain; BM-P, behavioral measures of pain; SR-D, self-reported distress; OR-D, observer-reported distress; BM-D, behavioral measures of distress; PHY, physiological correlates of pain/distress.
✓ represents evidence that the intervention is efficacious.
? represents insufficient data to support the efficacy of this intervention.
– represents that no trials assessing this outcome were available.
(Underlined symbols indicate significant heterogeneity based on Chi-square testing (P < 0.01).
Note: This table includes interventions for which data was available for pooling.
Blowing Out Air
The efficacy of blowing out air was assessed in one study with 75 participants (French et al., 1994). The results of this meta-analysis indicate that this intervention was not efficacious in reducing self-reported pain (SMD = −0.38, CI = −0.84, 0.08) or behavioral distress (SMD = −0.32, CI = −0.77, 0.14), as the CIs for these outcomes include zero.
Combined Cognitive Behavioral Intervention/Treatment (CBT)
Six RCTs involving a total of 277 participants, examined the efficacy of combined cognitive behavioral interventions (Blount et al., 1992; Cohen et al. 1997, 2002; Liossi & Hatira, 1999; Posner, 1998; Tyc et al., 1997). The interventions in this category were heterogeneous, as they involved different combinations of cognitive and behavioral components. However, the six trials were analyzed together because subgroups were small, thereby preventing meaningful subanalyses. Taken together, the evidence for these interventions shows that they were generally not efficacious in reducing self-reported pain (SMD = −0.87, CI = −1.90, 0.16), observer-reported pain (SMD = −0.10, CI = −0.54, 0.34), self-reported distress (SMD = −0.75, CI = −1.75, 0.25), or heart rate (SMD = −0.62, CI = −1.52, 0.28), since the CIs for these outcomes include zero. However, combined CBT was efficacious at reducing other-reported distress (SMD = −0.88, CI = −1.65, −0.12) and behavioral measures of distress (SMD = −0.67, CI = −0.95, −0.38). Thus, combined CBT appeared to be efficacious at reducing some, but not all, of the assessed outcome measures. However, these results need to be interpreted cautiously as they combined a heterogeneous group of interventions, which is also reflected in the significant heterogeneity tests. Furthermore, when multiple interventions are included in a package format, it is difficult to tease apart which specific components are beneficial, unless they are also assessed separately.
Distraction
Eleven trials (N = 682) examined the efficacy of distraction on pain and distress (Cassidy et al., 2002; Cavender et al., 2004; Fanurik et al., 2000; Fowler-Kerry & Lander, 1987; Gonzalez et al., 1993; Kleiber et al., 2001; Kuttner, 1987; Press et al., 2003; Tak & van Bon, 2005; Vessey et al., 1994; Zabin, 1982). Of those, nine trials (N = 634) examined the efficacy of distraction on self-reported pain, resulting in a SMD of −0.24 (CI = −0.45, −0.04), demonstrating that distraction was efficacious at reducing self-reported pain. Although SMDs for observer-reported distress, behavioral measures of pain, and behavioral measures of distress all fell in the negative range (−0.09, −0.15, and −0.05, respectively), their CIs include zero, suggesting that distraction was not efficacious as assessed across these outcomes. In addition, for the outcomes of observer-reported pain and self-reported distress, the SMDs were positive (0.07 and 0.00, respectively), suggesting that distraction was not efficacious in reducing ratings using these measures.
Distraction + Suggestion
One study with 120 participants assessed the impact of distraction + suggestion on self-reported pain (Fowler-Kerry & Lander, 1987). The results of this meta-analysis indicate that this combined intervention was efficacious in reducing self-reported pain (SMD = −0.64, CI = −1.03, −0.25). However, given that the two components of this intervention (distraction and suggestion) were delivered together, the impact of each component separately is unclear. Future trials comparing both components separately and together are necessary before we can make firmer conclusions.
Filmed Modeling
Filmed modeling was assessed in one study (N = 32; Zabin, 1982) and was found not to be efficacious in reducing self-reported distress (SMD = −0.03, CI = −0.73, 0.66) or observer-reported distress (SMD = 0.10, CI = −0.59, 0.80).
Hypnosis
Five studies (N = 163) assessed the efficacy of hypnosis (Katz et al., 1987; Kuttner, 1987; Liossi & Hatira, 1999, 2003; Liossi et al., 2006). Of all the interventions assessed in this review, there was the most positive evidence in support of hypnosis across several outcomes. SMDs and CIs fell in the negative range for self-reported pain (SMD = −1.47, CI = −2.67, −0.27), self-reported distress (SMD = −2.20, CI = −3.69, −0.71), and behavioral measures of distress (SMD = −1.07, CI = −1.79, −0.35). These two first outcomes were based on four studies (N = 146), while the latter outcome is based on all five studies. One study (N = 36) assessed observer-reported distress, and although the SMD was negative (−0.39), the CI included zero (−1.05, 0.27) suggesting that hypnosis was not efficacious in reducing observer-reported distress. Observer-reported pain, behavioral measures of pain, and physiological correlates were not assessed in any of these trials. Overall, given the relatively large effect sizes of the other hypnosis outcomes (i.e., self-reported pain, self-reported distress, and behavioral measures of distress), hypnosis appears to be an efficacious intervention for reducing both pain and distress during needle procedures. However, the tests for heterogeneity were significant for these outcomes. One possible interpretation for this heterogeneity is that the study samples or hypnosis techniques included in these analyses may not be similar enough to combine.
Memory Alteration
Only one study (N = 50) examined memory alteration (i.e., suggestions provided to encourage positive memories of previous needle procedures) on pain and distress, using eight different outcome measures (Chen et al., 1999). Seven of the outcomes for memory alteration had SMDs and CIs falling in the positive range (i.e., including zero). These include self-reported pain (SMD = −0.01, CI = −0.84, 0.82), observer-reported pain (SMD = 0.20, CI = −0.41, 0.80), observer-reported distress (SMD = 0.13, CI = −0.43, 0.68), behavioral measures of distress (SMD = −0.05, CI = −0.60, 0.51), heart rate (SMD = −0.20, CI = −0.40, 0.79), cortisol level (SMD = 0.00, CI = −0.59, 0.59), and systolic blood pressure (SMD = 0.47, CI = −0.15, 1.09). Diastolic blood pressure was the only outcome measure with an SMD (−0.65) and CI (−1.27, −0.02) falling in the negative range. Since diastolic blood pressure was the only outcome to demonstrate efficacy, and is based on one study, this finding should be interpreted cautiously and requires replication. Despite this one positive outcome, the overall pattern of results suggests that memory alteration was not efficacious in reducing pain or distress across self-report, observer-report, and behavioral measures of pain and distress.
Nurse Coaching + Distraction
Only two studies (N = 138) assessed nurse coaching + distraction (Cohen et al., 1997; Cohen et al., 1999). The results of these trials indicate that this intervention was not efficacious in reducing any of the following measures of pain or distress: self-reported pain (SMD = −1.13, CI = −3.52, 1.25), observer-reported pain (SMD = 0.07, CI = −0.38, 0.51), self-reported distress (SMD = 0.08, CI = −0.36, 0.53), observer-reported distress (SMD = −0.79, CI = −2.73, 1.14), or the physiological correlate of heart rate (SMD = −0.15, CI = −0.59, 0.29). The only outcome that demonstrated efficacious results for this intervention was behavioral measures of distress (SMD = −0.53, CI = −0.87, −0.19). Behavioral measures of pain were not assessed. Taken together, this overall pattern of results suggests that nurse coaching + distraction is not efficacious based on these two trials.
Parent Coaching + Distraction
The evidence for parent coaching + distraction is based on two trials (N = 104; Blount et al., 1992; Kleiber et al., 2001). The results of this meta-analysis indicate that this intervention was not efficacious at reducing any of the assessed outcome measures including: self-reported pain (SMD = 0.31, CI = −0.28, 0.91), observer-reported distress (SMD = 0.22, CI = −0.38, 0.81), or behavioral measures of distress (SMD = −0.58, CI = −1.48, 0.32). In addition, the test for heterogeneity was significant for behavioral measures of distress. Observer-reported pain, behavioral measures of pain, and physiological correlates were not assessed in these trials.
Parent Positioning + Distraction
The effects of parent positioning + distraction were assessed in one study that included 43 participants (Cavender et al., 2004). The results of this meta-analysis indicate that this intervention was efficacious at reducing observer-reported distress (SMD = −0.70, CI = −1.32, −0.08) but was not efficacious at reducing self-reported pain (SMD = −0.25, CI = −0.85, 0.35), self-reported distress (SMD = −0.32, CI = −0.92, 0.29), or behavioral measures of distress (SMD = −0.32, CI = −0.92, 0.29). However, no firm conclusions can be made since only one study was included.
Providing Information/Preparation
Two trials (N = 154; Harrison, 1991; Tak & van Bon, 2005) assessed the efficacy of providing information and preparing the child (e.g., explaining what will happen during the needle-procedure). Information/preparation was efficacious in reducing observer-reported pain (SMD = −0.77, CI = −0.17, −0.36) and pulse rates (SMD = −0.47, CI = −0.87, −0.07). Although the SMDs for self-reported pain and observer-reported distress both fell in the negative range (−0.22 and −0.15), their CIs included zero (−1.20, 0.76 and −0.88, 0.57, respectively), suggesting that this intervention was not efficacious at reducing pain and distress across these two outcomes. Furthermore, providing information/preparation was not efficacious in reducing distress as assessed by behavioral measures of distress (SMD = 0.24, CI = −0.30, 0.78), as the SMD was positive and the CI included zero. In addition, the test for heterogeneity was significant for self-reported pain and observer-reported distress, suggesting that these results should be interpreted with caution.
Suggestion
Three studies (N = 238; Eland, 1981; Fowler-Kerry & Lander, 1987; Goodenough et al., 1997) examined the efficacy of providing suggestions (e.g., suggesting that the procedure will not be painful). Based on the results of our analysis, suggestion was not efficacious at reducing any of the measures assessed, including self-reported pain (SMD = −0.20, CI = −0.55, 0.15), observer-reported pain (SMD = −0.40, CI = −0.85, 0.05), self-reported distress (SMD = −0.33, CI = −0.78, 0.12), and observer-reported distress (SMD = 0.00, CI = −0.62, 0.62). Self-reported pain was based on the findings of three studies, while the other outcomes were based on the results of one study. Behavioral measures and physiological correlates were not assessed. Taken together, this pattern of findings indicates that this intervention was not efficacious.
Videotaped Modeling + Parent Coaching
Only one study (N = 50) assessed the efficacy of this intervention on observer-reported distress (Krauss, 1998). The results of this meta-analysis indicate that it was not efficacious (SMD = −0.54, CI = −1.11, 0.02). No other outcome measures were assessed in this trial. Given that this outcome was based on only one trial, further research is required before stronger conclusions can be made.
Virtual Reality
Only one study with 30 participants provided data on the impact of virtual reality on self-reported pain (Wint et al., 2002). While the SMD was negative (−0.29), the CI included zero (−1.02, 0.43). Given that this outcome was based on only one small study, definitive conclusions cannot be made until further trials are conducted.
Discussion
To our knowledge, this is the most comprehensive meta-analysis of psychological interventions for the management of procedural pain and distress in children. In the present review, sufficient evidence for an intervention was determined if the intervention was efficacious across multiple outcome domains, and/or if the number of analyzed trials and included participants was large. Overall, the results of this review suggest that there is sufficient evidence to support the efficacy of: (a) combined CBT for observer-reported distress and behavioral measures of distress, (b) distraction for self-reported pain, (c) distraction + suggestion for self-reported pain, (d) hypnosis for self-reported pain, self-reported distress, and behavioral measures of distress, (e) providing information/preparation for observer-reported pain and improving pulse rates, (f) nurse coaching + distraction for behavioral measures of distress, and (g) parent positioning + distraction for observer-reported distress. There was insufficient evidence to support the efficacy of the other interventions assessed in this review. However, while our stringent criteria did not find support for the efficacy of these interventions on the remaining outcomes, we acknowledge that in several cases, these results were based on limited data. Thus, although the findings from our review suggest that there is currently not enough evidence to support the efficacy of these interventions, we acknowledge that further trials are needed to provide more definitive conclusions. To reflect this important distinction in Table III, we opted to symbolize nonefficacious outcomes using question marks, to convey the fact that more research is needed in these areas.
In order to make overall conclusions about the efficacy of interventions across outcomes, we considered an intervention efficacious: (a) if all of the outcomes demonstrated efficacy, or (b) if several outcomes were assessed and the majority of them demonstrated efficacy. We also gave more weight to conclusions based on several studies with a larger combined sample, compared with conclusions based on one or two studies with a smaller combined sample. Taken together, it appears that there is the most positive support for the efficacy of combined CBT, distraction, and hypnosis. There is also preliminary support for the efficacy of providing information/preparation, although this intervention was only based on the results of two studies. Overall, the conclusions derived from this review are generally consistent with previous reviews (Blount et al., 2003; Chen et al., 2000; Christophersen & Mortweet, 2001; Powers, 1999), but to the best of our knowledge, this review provides the only recent meta-analysis covering a broad range of different psychological interventions.
In addition to effect sizes, trials examining pharmacological interventions for pain management often report the percentage in pain reduction that can be attributed to the intervention ([pain in treatment group – pain in control group] / pain in control group) × 100. Although this was not one of our primary analyses, we calculated the mean percentage in pain reduction across interventions for the most common outcome in our review: self-reported pain. Out of the 13 interventions that provided data for this outcome, there was an average pain reduction of 20.65% that could be attributed to the psychological interventions. In comparison, for topical anesthetics, the percent reduction using meta-analytic techniques has been estimated to range from 20% to 50%, depending on the outcome measure used (Shah, Rieder, & Taddio, 2008). Thus, calculating the average pain reduction for the psychological interventions analyzed in our review contributes to the interpretation of our results, and also allows for comparability and consistency with other procedural pain management studies.
It is important to note that sometimes the conclusions derived from a meta-analysis may differ from those stated in the original, individual studies (Petticrew & Roberts, 2006). This discrepancy can often be accounted for by examining the statistical procedures and criteria used. Specifically, meta-analyses typically rely on effect sizes while individual studies may rely on statistical significance (e.g., p-values). Even though groups may be statistically different, the magnitude (i.e., effect size) of the differences may be minimal. In addition, in the present meta-analysis we used the more stringent criteria recommended by the Cochrane Collaboration for judging intervention efficacy, whereby both the SMD and CIs had to fall in the negative range. While a study may conclude that the intervention was efficacious based on statistical significance, if the trial has a small sample size or low power, this may be insufficient to meet the more stringent criteria of a meta-analysis.
While the results of this review help summarize the large body of literature on psychological interventions for needle-related pain and distress in children, there are several limitations that must be addressed. First of all, the results of a meta-analysis are only as strong as the studies included. Given that we included only true RCTs, we excluded studies that used less stringent designs. Second, we used SMD because of the variability in outcome measures employed by the various studies. While various measures may claim to assess the same constructs, this may not always be the case as no two measures are exactly alike. Third, although attempts were made to retrieve unreported data by contacting study authors, we were not able to include 23 studies for which no means or standard deviations were provided. Had data for these studies been available, the results of this review would be more powerful and informative. Fourth, although we conducted a thorough search for studies, it is possible that we did not locate all relevant studies, particularly those that were published in more remote sources or were unpublished. Given that studies with positive results favoring treatment may be more likely to be submitted for publication and ultimately published, this could introduce bias into the results. Fifth, no two studies used the exact same intervention that followed the same manualized protocol. Thus, we restricted our pooling to interventions that were very similar, and could appropriately be pooled. Finally, it is also important to note that the overall study quality ratings fell in the low range. As mentioned earlier, the Oxford Quality Scale is not the most appropriate scale for psychological interventions because non-double-blind trials are penalized, and, therefore, the information provided by this scale is limited in its utility.
Based on the results of this review, several recommendations can be made for conducting and reporting RCTs, in order to facilitate future systematic reviews and meta-analyses. First, study authors should include the means and standard deviations (or comparable measures of variability such as standard errors) for all of the group outcome measures, regardless of whether or not there are statistically significant differences between the groups.
Second, another challenge of conducting a systematic review occurs when various studies assessing the same constructs use different outcome measures. This challenge could be alleviated by having a set of recommended outcome measures for various age groups that could be used as the “gold standard” in pain studies. Authors could include additional measures of their choice; however, the implementation of this standard set of outcome measures would facilitate comparisons between studies and reduce the bias introduced by allowing authors to selectively choose which outcomes they employ. This issue has been recently addressed by the Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (Ped-IMMPACT) task force, which called for a set of standard pediatric pain assessment measures (www.immpact.org). To date, two systematic reviews have been published from this task force. These reviews provide evidence-based and developmentally appropriate recommendations for self-report measures (Stinson, Kavanagh, Yamada, Gill, & Stevens, 2006) and observational measures (von Baeyer & Spagrud, 2007) for assessing pain and distress in children and adolescents.
Third, we were unable to test for age effects as originally planned because of the varying and broad age ranges used in the various trials. Thus, the development of a set of standard developmental age ranges for studies would facilitate comparisons across trials. Also, it would be helpful for study authors to provide break-downs of their findings by age groups, to enable others to differentiate the effects across different developmental periods.
Finally, our last recommendation advocates that future systematic reviews should include comprehensive quality assessments of all included trials, using quality scales appropriate for psychological interventions. The inclusion of quality scale ratings helps provide an objective measure of the internal and external validity of the included trials, and is critical for making well-informed interpretations of review findings.
In terms of clinical implications, it is important that health professionals and families be aware of the value of incorporating psychological strategies for procedural pain and distress into practice with children. However, the effectiveness of these interventions likely depends on numerous factors including the age of the child and the nature of the procedure. Future research will hopefully provide a clearer picture of which interventions work best for children of various ages who are undergoing different medical procedures. Furthermore, the results of this review also highlight the importance and utility of using self-report measures of pain and distress, as the ratings obtained via self-report were not always congruent with observer-ratings, behavioral measures, or physiological correlates.
In sum, our review suggests that various psychological interventions, particularly distraction, hypnosis, and combined cognitive-behavioral interventions, can help children by reducing the pain and distress that accompany needle-related procedures. The effectiveness of these interventions likely depends on numerous factors including the age of the child and the nature of the procedure, which need to be addressed further in future trials. This review highlights the need for health professionals and caregivers to be aware of the value of incorporating evidence-based psychological interventions into the standard management of pediatric procedural pain.
Acknowledgments
L.S.U. was funded by the Nova Scotia Health Research Foundation (NSHRF). C.T.C. and P.J.McG. are supported by Canada Research Chairs (CRCs). This research was supported by an IWK Establishment Award awarded to C.T.C. We thank all the members of C.T.C.'s research team who helped with different aspects of this project: Darby Eakins, Jessica Ferguson, Kelly Hayton, Crystal Holly, and Sarah Peddle. We also thank the Dalhousie Cochrane Group, and the Cochrane Pain, Palliative, and Supportive Care (PAPAS) group for their assistance with various parts of this review. The results reported in this article are part of a systematic review completed for the Cochrane Collaboration (Issue 4, 2006). Portions of this review were presented at the 12th Cochrane Colloquium (Ottawa, ON, October 2004), the 5th Biennial International Forum on Pediatric Pain (White Point, NS, May 2005) and the International Association for the Study of Pain 11th World Congress on Pain (Sydney, Australia, August 2005).
Conflict of interest: None declared.
Footnotes
*This article is an abbreviated version of a larger review first published with the Cochrane Collaboration: Uman, L.S., Chambers, C.T., McGrath, P.J., Kisely, S. (2006). Psychological interventions for needle-related procedural pain and distress in children and adolescents (Review). The Cochrane Database of Systematic Reviews, 4.
References
*References marked with an asterisk indicate studies included in the meta-analysis
- Barlow DH, Durand VM. [Google Scholar]
- Blount RL, Bachanas PJ, Powers SW, Cotter MW, Franklin A, Chaplin W, et al. Training children to cope and parents to coach them during routine immunizations: Effects on child, parent, and staff behaviors. Behavior Therapy. 1992;23:689–705. [*] [Google Scholar]
- Blount RL, Piira T, Cohen LL. Management of pediatric pain and distress due to medical procedures. In: Roberts MC, editor. Handbook of pediatric psychology. 3rd. New York: Guilford Press; 2003. pp. 216–233. [Google Scholar]
- Broome ME, Bates TA, Lillis PP, McGahee TW. Children's medical fears, coping behaviors, and pain perceptions during a lumbar puncture. Oncology Nursing Forum. 1990;17:361–367. [PubMed] [Google Scholar]
- Cassidy K-L, Reid GJ, McGrath PJ, Finley GA, Smith DJ, Morley C, et al. Watch needle, watch TV: Audiovisual distraction in preschool immunization. American Academy of Pain Medicine. 2002;3:108–118. doi: 10.1046/j.1526-4637.2002.02027.x. [*] [DOI] [PubMed] [Google Scholar]
- Cavender K, Goff MD, Hollen EC, Guzzetta CE. Parents’ positioning and distracting children during venipuncture: Effects on children's pain, fear, and distress. Journal of Holistic Nursing. 2004;22:32–56. doi: 10.1177/0898010104263306. [*] [DOI] [PubMed] [Google Scholar]
- Chen E, Joseph MH, Zeltzer LK. Acute pain in children: Behavioral and cognitive interventions in the treatment of pain in children. Pediatric Clinics of North America. 2000;47:1–13. doi: 10.1016/s0031-3955(05)70223-6. [DOI] [PubMed] [Google Scholar]
- Chen E, Zeltzer LK, Craske MG, Katz ER. Alterations of memory in the reduction of children's distress during repeated aversive medical procedures. Journal of Consulting and Clinical Psychology. 1999;67:481–490. doi: 10.1037//0022-006x.67.4.481. [*] [DOI] [PubMed] [Google Scholar]
- Christophersen, E. R. & Mortweet SL. Treatments that work with children: Empirically supported strategies for managing childhood problems. Washington, DC: American Psychological Association; 2001. Assessment and management of pain. (pp. 159–199) [Google Scholar]
- Cohen LL, Bernard RS, Greco LA, McClellan CB. A child-focused interventions for coping with procedural pain: Are parent and nurse coaches necessary? Journal of Pediatric Psychology. 2002;27:749–757. doi: 10.1093/jpepsy/27.8.749. [*] [DOI] [PubMed] [Google Scholar]
- Cohen LL, Blount RL, Cohen RJ, Schaen ER, Zaff JF. A comparative study of distraction versus topical anesthesia for pediatric pain management during immunizations. Health Psychology. 1999;18:591–598. doi: 10.1037//0278-6133.18.6.591. [*] [DOI] [PubMed] [Google Scholar]
- Cohen LL, Blount RL, Panopoulos G. Nurse coaching and cartoon distraction: An effective and practical intervention to reduce child, parent, and nurse distress during immunizations. Journal of Pediatric Psychology. 1997;22:355–370. doi: 10.1093/jpepsy/22.3.355. [*] [DOI] [PubMed] [Google Scholar]
- Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain. 1996;68:25–31. doi: 10.1016/S0304-3959(96)03163-6. [DOI] [PubMed] [Google Scholar]
- Drotar D. Enhancing reviews of psychological treatments with pediatric populations: Thoughts on next steps. Journal of Pediatric Psychology. 2002;27:167–176. doi: 10.1093/jpepsy/27.2.167. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Yorke L, Morley S, Williams A de C., & Mastroyannopoulou, K. In The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd; 2004. Psychological therapies for the management of chronic and recurrent pain in children and adolescents (Cochrane Review). (issue 2) [DOI] [PubMed] [Google Scholar]
- Eland ME. Minimizing pain associated with prekindergarten intramuscular injections. Issues in Comprehensive Pediatric Nursing. 1981;5:361–371. doi: 10.3109/01460868109106351. [*] [DOI] [PubMed] [Google Scholar]
- Fanurik D, Koh JL, Schmitz ML. Distraction techniques combined with EMLA: Effects on IV insertion, pain and distress in children. Children's Health Care. 2000;29:87–101. [*] [Google Scholar]
- Fowler-Kerry S, Lander JR. Management of injection pain on children. Pain. 1987;30:169–175. doi: 10.1016/0304-3959(87)91072-4. [*] [DOI] [PubMed] [Google Scholar]
- French GM, Painter EC, Coury DL. Blowing away shot pain: A technique for pain management during immunization. Pediatrics. 1994;93:384–388. [*] [PubMed] [Google Scholar]
- Gonzalez JC, Routh DK, Armstrong FD. Effects of maternal distraction versus reassurance on children's reactions to injections. Journal of Pediatric Psychology. 1993;18:593–604. doi: 10.1093/jpepsy/18.5.593. [*] [DOI] [PubMed] [Google Scholar]
- Goodenough B, Kampel L, Champion GD, Laubreaux L, Nicholas MK, Ziegler JB, et al. An investigation of the placebo effect and age-related factors in the report of needle pain from venipuncture in children. Pain. 1997;72:383–391. doi: 10.1016/s0304-3959(97)00062-6. [*] [DOI] [PubMed] [Google Scholar]
- Harrison A. Preparing children for venous blood sampling. Pain. 1991;45:299–306. doi: 10.1016/0304-3959(91)90054-2. [*] [DOI] [PubMed] [Google Scholar]
- IASP Task Force on Taxonomy. Classification of chronic pain. 2nd. Seattle: IASP Press; 2004. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinicial Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Katz ER, Kellerman J, Ellenberg L. Hypnosis in the reduction of acute pain and distress in children with cancer. Journal of Pediatric Psychology. 1987;12:379–394. doi: 10.1093/jpepsy/12.3.379. [*] [DOI] [PubMed] [Google Scholar]
- Kazak AE, Kunin-Batson A. Psychological and integrative interventions in pediatric procedure pain. In: Finley GA, McGrath PJ, editors. Acute and procedure pain in infants and children. Seattle, WA: IASP Press; 2001. pp. 77–100. [Google Scholar]
- Keefe FJ, Dunsmore J, Burnett R. Behavioral and cogntive-behavioral approaches to chronic pain: Recent advances and future directions. Journal of Consulting and Clinical Psychology. 1992;60:528–536. doi: 10.1037//0022-006x.60.4.528. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Craft-Rosenberg M, Harper DC. Parents as distraction coaches during IV insertion: A randomized study. Journal of Pain and Symptom Management. 2001;22:851–861. doi: 10.1016/s0885-3924(01)00316-5. [*] [DOI] [PubMed] [Google Scholar]
- Kleiber C, Harper DC. Effects of distraction on children's pain and distress during medical procedures: A meta-analysis. Nursing Research. 1999;48:44–49. doi: 10.1097/00006199-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Krauss WJ. Videotape modeling and parent participants: Effects on reducing distress behavior in children undergoing immunization procedures. Fresno: Unpublished doctoral dissertation, California School of Professional Psychology; 1998. [*] [Google Scholar]
- Kuttner L. Favorite stories: A hypnotic pain-reduction technique for children in acute pain. American Journal of Clinical Hypnosis. 1987;30:289–295. doi: 10.1080/00029157.1988.10402752. [*] [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis versus cognitive behavioral training for pain management with pediatric cancer patients undergoing bone marrow aspirations. International Journal of Clinical and Experimental Hypnosis. 1999;47:104–116. doi: 10.1080/00207149908410025. [*] [DOI] [PubMed] [Google Scholar]
- Liossi C, Hatira P. Clinical hypnosis in the alleviation of procedure-related pain in pediatric oncology patients. International Journal of Clinical and Experimental Hypnosis. 2003;51:4–28. doi: 10.1076/iceh.51.1.4.14064. [*] [DOI] [PubMed] [Google Scholar]
- Liossi C, White P, Hatira P. Randomized clinical trial of local anesthetic versus a combination of local anesthetic with self-hypnosis in the management of pediatric procedure-related pain. Health Psychology. 2006;25:307–315. doi: 10.1037/0278-6133.25.3.307. [*] [DOI] [PubMed] [Google Scholar]
- Petticrew M, Roberts H. Systematic reviews in the social sciences: A practical guide. Oxford: Blackwell Publishing; 2006. [Google Scholar]
- Posner KL. A pharmacobehavioral intervention to reduce child cancer distress. 1998. [*] [Google Scholar]
- Powers SW. Empirically supported treatments in pediatric psychology: Procedure-related pain. Journal of Pediatric Psychology. 1999;24:131–145. doi: 10.1093/jpepsy/24.2.131. [DOI] [PubMed] [Google Scholar]
- Press J, Gidron Y, Maimon M, Gonen A, Goldman V, Buskila D. Effects of active distraction on pain of children undergoing venipuncture: Who benefits from it? The Pain Clinic. 2003;15:261–269. [*] [Google Scholar]
- Saile H, Burgmeier R, Schmidt LR. A meta-analysis of studies on psychological preparation of children facing medical procedures. Psychology and Health. 1988;2:107–32. [Google Scholar]
- Shah V, Rieder M. Systematic review of analgesics for immunization pain in infants and children. Quebec: Abstract submitted to the IXth World Conference on Clinical Pharmacology and Therapeutics, Quebec City; 2008. [Google Scholar]
- Stinson JN, Kavanagh T, Yamada J, Gill J, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Tak JH, van Bon WHJ. Pain- and distress-reducing interventions for venepuncture in children. Child: Care, Health, & Development. 2005;32:257–268. doi: 10.1111/j.1365-2214.2006.00578.x. [*] [DOI] [PubMed] [Google Scholar]
- Tyc VL, Leigh L, Mulhern RK, Srivastava DK, Bruce D. Evaluation of a cognitive-behavioral intervention for reducing distress in pediatric cancer patients undergoing magnetic resonance imaging procedures. International Journal of Rehabilitation and Health. 1997;3:267–279. [*] [Google Scholar]
- Uman LS, Chambers CT, McGrath PJ, Kisely S. Psychological interventions for needle-related procedural pain in children and adolescents. Cochrane Database of Systematic Reviews. 2006;4 doi: 10.1002/14651858.CD005179.pub2. [DOI] [PubMed] [Google Scholar]
- Vessey JA, Carlson KL, McGill J. Use of distraction with children during an acute pain experience. Nursing Research. 1994;43:369–372. [*] [PubMed] [Google Scholar]
- von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain. 2007;127:140–150. doi: 10.1016/j.pain.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Wint SS, Eshelman D, Steele J, Guzzetta CE. Effects of distraction using virtual reality glasses during lumbar punctures in adolescents with cancer. Oncology Nursing Forum. 2002;29:E8–E15. doi: 10.1188/02.ONF.E8-E15. [*] [DOI] [PubMed] [Google Scholar]
- Zabin MA. The modification of children's behavior during blood work procedures. 1982. [*] [Google Scholar]