Abstract
Background and aims
The purpose of this study was to determine the accuracy, interobserver variability, timing and discordance with relaparotomy of postoperative radiological examination of colorectal anastomoses.
Patient/methods
From 2000 to 2005, 429 patients underwent an ileocolonic, colo-colonic, or colorectal anastomosis. Radiological examination of the anastomosis was not performed routinely, but only when there were clinically signs of leakage. Radiological imaging was reviewed by an independent radiologist and medical records were retrospectively analyzed. Clinical anastomotic leakage was the standard of reference and defined as leakage confirmed during relaparotomy, drainage of pus per anum or as an anastomotic defect identified at digital examination.
Results
Radiological evaluation of the anastomosis was performed in 91 patients (21%): CT in 27 patients, contrast radiography in 40, and both imaging modalities in 24 patients. The interobserver variability of CT and contrast radiography was 10% and 14%, respectively. The sensitivity and negative predictive value of imaging of the anastomosis was 65% and 73%, respectively. Anastomotic leakage was found in 11 of 21 patients (52%) who underwent relaparotomy despite negative imaging. Three of 36 patients (8%) with a diagnosis of anastomotic leakage based on radiological examination had an intact anastomosis at relaparotomy.
Conclusion
Radiological imaging of the anastomosis after colorectal surgery should be restrictively applied and interpreted with caution because of the high false-negative rate and the substantial interobserver variability.
Keywords: Colorectal surgery, Anastomosis, Leakage, Radiological imaging, Contrast radiography, CT
Introduction
Colorectal surgery may be complicated by anastomotic leakage that initially may present with mild and difficult to interpret symptoms. It is of utmost importance to detect failure of the anastomosis at an early stage to prevent further deterioration of the patient’s clinical condition. Symptoms and signs that should raise the suspicion of anastomotic dehiscence are fever, adynamic ileus, increased fluid collection through abdominal drains, renal failure, leukocytosis, and cardiac symptoms [1–4].
In pronounced cases with clinically apparent leaks, there is no need for radiological imaging to confirm the diagnosis, but urgent relaparotomy should be performed as early intervention in order to avert potential threatening consequences [1]. The diagnostic challenge is to identify anastomotic leakage early in the postoperative period and in those cases with mild or nonspecific symptoms. Because of the relatively low specificity of clinical parameters, additional diagnostic tests are often required [1]. Digital examination is a test that can be simply and effectively applied in patients with a low rectal anastomosis, although some surgeons are reluctant to perform this maneuver as it may interfere with the integrity of the anastomosis [5]. The alternatives in these patients and in those with a more proximal anastomosis are radiological imaging modalities or endoscopy [6–8].
The primary purpose of the present study was to determine the accuracy of radiological imaging (either water-soluble contrast radiography, contrast-enhanced computed tomography (CT) or both modalities) in patients with a postoperative course suggestive of anastomotic leakage after colorectal resection. Secondary, the interobserver variability of radiological imaging of the anastomosis was determined. Finally, the timing of occurrence of clinical symptoms suggestive of leakage, radiological examination and relaparotomy as well as concordance between radiological and operative findings were assessed.
Materials and methods
From January 2000 to October 2005, 429 consecutive patients underwent an ileocolonic, colo-colonic or colorectal anastomosis at the Sint Lucas Andreas hospital, a large community teaching hospital in Amsterdam, the Netherlands. The type of resection in these patients is summarized in Table 1. Type of anastomosis was end-to-end, end-to-side, side-to-end, or side-to-side depending on preferences of the surgeon. Radiological examination of the anastomosis was not performed on a routine basis, but only when leakage was suspected on clinical grounds. In general, left-sided anastomoses were examined using transanal contrast administration with radiographic imaging or CT scanning and the remaining patients underwent CT scanning with oral and intravenous contrast.
Table 1.
Type of resection of all patients (N = 429) who underwent an ileocolonic, colo-colonic or colorectal anastomosis
| Type of surgery | No. |
|---|---|
| Ileocolonic resection | 36 (8%) |
| Right hemicolectomy | 144 (34%) |
| Transverse colonic resection | 13 (3%) |
| Left hemicolectomy | 35 (8%) |
| Sigmoidal resection | 93 (22%) |
| Subtotal or total colectomy | 9 (2%) |
| Low anterior resection | 82 (19%) |
| Restore colonic continuity after previous colostomy | 17 (4%) |
Postoperative contrast radiography was performed with water-soluble contrast (Iohexol 140 mg I/ml, Omnipaque® GE Healthcare, Salt Lake City, Utah, USA). After introduction of a rectum cannula or a Foley catheter contrast was carefully administered under fluoroscopic control. Patients were in a left lateral or supine position at the start of the investigation and images were taken at different angles. CT imaging was performed on a 4-row multidetector helical CT scanner (Aquilion 4S, Toshiba Medical Systems Europe, Zoetermeer, Netherlands). Consecutive 3 mm slices were obtained and digitally archived after reconstruction at 2 mm interval to obtain adequate multiplanar reconstruction interpretation. Patients were prepared with 1 l of oral contrast fluid (30 ml megluminejoxitalamaat 300 mg I/ml, Guerbet, France, diluted in 1 l of tapwater) in 1 h and intravenous contrast fluid (Iohexol 30 mg I/ml, GE Healthcare), 100 ml in 50 s. Scanning started with 80 s delay. In patients with distal anastomoses, 500 to 1,000 ml contrast (30 ml megluminejoxitalamaat, diluted in 1 l of tap water) was administrated through a transanal Foley catheter.
All images were reviewed by a radiologist (AW) blinded for the initial report. Evaluation by the independent radiologist was compared with the original reports. In case of discrepancies, a final decision was made by concensus.
All medical records of the patients in whom radiological imaging of the anastomosis was performed were retrospectively reviewed. The presence or absence of anastomotic leakage was determined. Standard of reference was clinical anastomotic leakage, which was defined as leakage confirmed during relaparotomy, as drainage of pus per anum or as an anastomotic defect identified at digital examination. Radiological anastomotic leakage was defined as radiological features suggestive for leakage in patients who did not develop clinical leakage. These radiological features were the presence of contrast outside the bowel lumen, perianastomotic fluid collections and when air was noted directly near the anastomosis or when a pneumoperitoneum was seen more than 1 week postoperatively according to Zissin and Gayer [8].
The number of clinical parameters suggestive of anastomotic leakage were retrospectively determined. These parameters included tachycardia (heart rate >100 beats per minute), fever (body temperature >38°C), local or generalized peritoneal reaction during physical examination, leukocytosis (>10 × 103/ml), prolonged adynamic ileus (>2 days postoperatively), and delayed gastric emptying (nasogastric tube production of more than 200 ml per day or vomiting necessitating tube reinsertion) [2]. In addition, the timing of occurrence of two, three or four clinical parameters, the timing of radiological imaging, and the timing of relaparotomy were determined.
To compare sensitivity and negative predictive value of contrast radiography and CT scan, 95% confidence intervals of the differences were determined. If the confidence interval did not include zero, the difference between two percentages was considered to be statistically significant.
Results
Radiological imaging of the anastomosis was performed in 91 of the 429 patients (21%), whereas the anastomosis was not radiologically evaluated in 25 patients with clinically overt anastomotic leakage. The imaging modality was CT in 27 patients (30%), contrast radiography in 40 (44%) and both imaging modalities were performed in 24 patients (26%). No complications of rectally administered contrast were observed. One of the contrast radiographies could not be reviewed because of insufficient archiving. The initial evaluation and the review by the independent radiologist differed in eight of 63 valid contrast radiographies (interobserver variability, 13%) and in five of 51 CT scans (interobserver variability, 10%) as shown in Table 2.
Table 2.
Discrepancies between review of independent radiologist and initial report of contrast radiography and CT scanning for suspected anastomotic leakage
| Contrast radiography (N = 63a) | CT (N = 51) | |
|---|---|---|
| Discrepancies with initial report | 8 (13%) | 5 (10%) |
| No contrast leakage | 3 | 1 |
| Contrast leakage | 1 | 1 |
| Presacral abscess | – | 3 |
| Visualization of side-to-end anastomosis instead of contrast leakage | 4 | – |
| Concordance (% (95% confidence interval)) | 87 (77–95) | 90 (82–98) |
aOne missing value because of insufficiently printed imaging
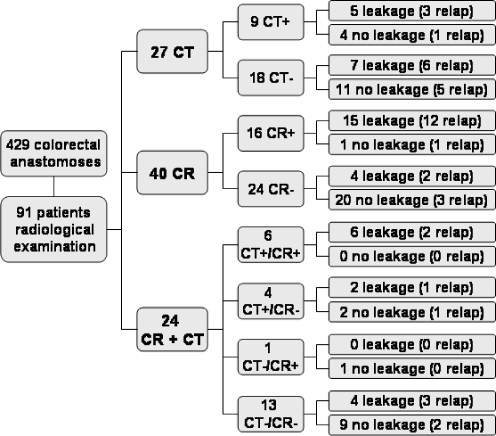
Forty-three of 91 patients who underwent radiological examination of the anastomosis had clinical anastomotic leakage based on relaparotomy in 29 patients and on transanal drainage of pus or a palpable defect at the level of the anastomosis in 14 patients. Adding the 25 patients with anastomotic leakage who did not undergo radiological imaging results in an overall leakage rate of 16% (68/429). The sensitivity and negative predictive value of imaging of the anastomosis in the whole group of 91 patients was 65% and 73%, respectively (Table 3). The false negative rate of radiological examination was 35% (15/43). Three of 36 patients (8%) with a diagnosis of anastomotic leakage based on radiological examination had an intact anastomosis at relaparotomy. The sensitivity of contrast radiography was 14% higher than the sensitivity of CT scan (Table 3) but this was not statistically significant based on the 95% confidence interval (−12% to 40%). Similarly, the negative predictive value was not significantly different between contrast radiography and CT scan (difference 10% (−11% to 40%)). Relaparotomy was performed in 21 of 55 patients (38%) without features of anastomotic leakage on radiological imaging. Anastomotic leakage was found in 11 of those 21 patients (52%). The correlation between radiological imaging and clinical presence or absence of anastomotic leakage is depicted in Fig. 1. Table 4 shows the sensitivity and negative predictive value of radiological imaging depending on timing (<7 or ≥7 days postoperatively) and on the level of the anastomosis (proximal or distal).
Table 3.
Correlation between results of radiological examination of the anastomosis and the presence or absence of clinical anastomotic leakage for each imaging modality separately and for the whole group of patients (only contrast radiography in 40, only CT in 27, and both imaging modalities in 24 patients)
| Clinical anastomotic leakage | Sensitivity % (CI) | Negative predictive value % (CI) | ||
|---|---|---|---|---|
| Yes | No | |||
| All patients (N = 91) | 65 (51–79) | 73 (61–84) | ||
| Leakagea | 28 | 8b | ||
| No leakage | 15 | 40 | ||
| Contrast radiography (N = 64) | 68 (51–84) | 76 (62–89) | ||
| Leakage | 21 | 2c | ||
| No leakage | 10 | 31 | ||
| CT(N=51) | 54 (34–74) | 66 (49–82) | ||
| Leakage | 13 | 6d | ||
| No leakage | 11 | 21 | ||
CI = 95% confidence interval
aFive patients with leakage by only one of both imaging modalities
bThree negative relaparotomy
cOne negative relaparotomy
dTwo negative relaparotomy
Fig. 1.
Flow chart showing the type and result of radiological examination for suspected anastomotic leakage (N = 91) in a group of 429 patients who underwent an ileocolonic, colo-colonic or colorectal anastomosis. Radiological results are correlated with clinical presence or absence of anastomotic dehiscence. CT computed tomography, CR contrast radiography, plus sign radiological signs of leakage, minus sign no radiological signs of leakage, relap relaparotomy
Table 4.
Sensitivity and negative predictive value of imaging of the anastomosis (CT, contrast radiography or both modalities) depending on timing postoperatively and level of the anastomosis
| N | Sensitivity % (CI) | Negative predictive value % (CI) | |
|---|---|---|---|
| Timing of imaging | |||
| 7<days postop | 43 | 53 (29–75) | 92 (72–99) |
| 7≥days postop | 48 | 75 (53–89) | 75 (53–89) |
| Level of anastomoses | |||
| distala | 65 | 69 (51–83) | 83 (65–94) |
| proximalb | 26 | 50 (17–83) | 83 (58–96) |
CI = 95% confidence interval
aSigmoidresection, low anterior resection and subtotal or total colectomy
bIleocoecal resection, right hemicolectomy and left hemicolectomy
Twenty-four patients underwent both CT scanning and contrast radiography and discordancy was found in five patients (21%). Both imaging modalities were positive for anastomotic leakage in six patients and all these patients did fulfil the criteria of clinical anastomotic leakage. Four of 13 patients (31%) without signs of anastomotic leakage by contrast radiography as well as CT scanning did have a clinical anastomotic leakage, based on relaparotomy in three of them.
The timing of radiological examination of the anastomosis and relaparotomy is displayed in Table 5. The time interval between the occurrence of clinical parameters suggestive of anastomotic leakage and radiological imaging decreased with an increasing number of clinical parameters: median, two and less than 24 h for two and four clinical parameters, respectively. The median time interval between imaging and relaparotomy was less than 24 h for patients with anastomotic leakage based on contrast radiography or CT scan (N = 20) as well as for patients who did not have radiological features of leakage (N = 22).
Table 5.
Timing of radiological examination of the anastomosis and relaparotomy
| No. of patients | Median time interval in days (range) | |
|---|---|---|
| Primary laparotomy—first imaging modality | 91 | 7 (1–49) |
| Primary laparotomy—second imaging modality | 24 | 12 (4–44) |
| Primary laparotomy—relaparotomy | 42 | 7 (2–24) |
| 2 clinical parameters—first imaging | 82 | 2 (0–47) |
| 3 clinical parameters—first imaging | 65 | 1 (0–46) |
| 4 clinical parameters—first imaging | 38 | 0 (0–26) |
| Imaging—relaparotomy | 42 | 0 (0–8)a |
| Negative imaging—relaparotomy | 22 | 0 (0–8)a |
| Positive imaging—relaparotomy | 20 | 0 (0–3)a |
aCalculated from second imaging (nine patients with both imaging modalities before relaparotomy)
Discussion
The false-negative rate of radiological imaging of the anastomosis in colorectal surgery was 35% in the present study with a negative predictive value of 73% and these percentages seem to be lower in the early postoperative period (<7 days) and in proximal anastomoses. The limited accuracy restricts their usefulness in clinical decision making if anastomotic leakage is suspected. This is illustrated by the fact that relaparotomy was performed shortly after negative imaging in 22 patients, with half of these patients having an anastomotic leak. Three studies reported the accuracy of routine water-soluble contrast radiography. The false negative rates were 49% (11/23) in a series of 233 colorectal and left-sided colonic anastomoses, 29% (4/14) in 117 left-sided colonic anastomoses, and 23% (7/31) in 202 contrast radiographies of low rectal anastomoses [5, 9, 10]. Four other studies described results of radiological imaging in the subgroup of patients with clinical anastomotic dehiscence. CT was able to confirm clinical leakage in 48% to 100% and contrast radiography was positive in 40% to 83% of the patients [1, 11–13]. Our findings fit well within these rather wide ranges, but interpretation is hampered by the different clinical circumstances in which the radiological techniques were applied.
Timing of imaging may be related to false-negative findings, as the anastomotic defect may be initially too small to allow easy flow of contrast outside the intestinal lumen (Fig. 2). This is illustrated by the finding that contrast leakage was visualized only after repeated CT scanning the next day in one of 13 patients as reported by DuBrow et al. [11]. In distal anastomoses, inflating the balloon of the transanal catheter for contrast administration may lead to sealing of a defect, also resulting in false-negative imaging [3]. In more proximal anastomoses, the rectally administered contrast has been diluted at this level and there may be not enough remaining pressure to induce contrast leakage [13]. Our median time interval between index laparotomy and first imaging of 7 days (Table 5) is comparable to data in the literature, although the range was rather wide [9, 10, 14].
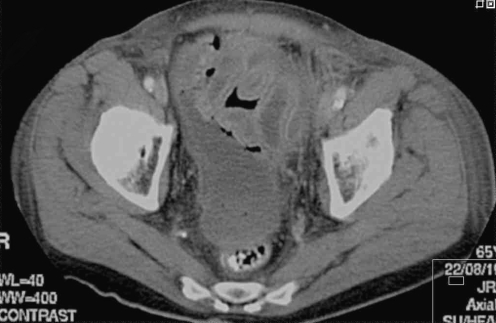
Fig. 2.
CT scan with rectally administered contrast in a patient who underwent a left hemicolectomy for colonic cancer and a negative contrast radiography postoperatively. A fluid collection was found near the anastomosis, but without contrast outside the intestinal lumen. Anastomotic leakage because of ischemia was found at relaparotomy the same day
Another factor determining sensitivity of radiological examination of the anastomosis is quality of the radiological technique. The higher spatial resolution of CT enables visualization of small contrast leakage that may have been missed with conventional radiology, especially with the more recently introduced helical and multidetector row CT scanners [6]. Furthermore, patient selection (routinely, based on a certain degree of clinical suspicion, or confirming clinical leakage) influences the a priori chance of leakage and thereby determines the accuracy of the imaging modality. Most patients ultimately found to have an anastomotic leak have an insidious clinical course, with low-grade fever, prolonged ileus or failure to thrive [12]. Alves et al. showed that anastomotic leakage was found in only 18% of the patients with two clinical parameters suggestive of leakage [1].
Finally, the definition of the ‘gold standard’ may explain the reported differences in sensitivity. Considerable variation in defining anastomotic leakage exists in the literature due to the lack of consensus. In a systematic review by Bruce et al., 29 separate definitions were used for leakage of lower gastrointestinal anastomoses [15]. Lower reported incidences may be due to, for example, not relating an intra-abdominal abscess to an anastomotic dehiscence.
Only in case of a negative relaparotomy, radiological signs of leakage were defined as a false-positive result in our study. The remaining patients with positive imaging and mild signs or symptoms suggestive of anastomotic dehiscence where defined as a radiological leakage, although this could have been a false-positive result either. Therefore, we did not calculate the specificity of radiological imaging. Actually, the specificity is of only minor clinical importance, because the consequences of positive imaging are determined by the patient’s clinical condition. In other words, a patient with radiological signs of anastomotic leakage and a good and stable clinical condition will generally be treated conservatively anyway. Analysis of 135 consecutive patients from the St Mark’s hospital demonstrated that a radiological leak did not alter clinical management in the majority of cases [16]. Similarly, DuBrow et al. concluded that the presence of radiologic abnormalities indicating leaks did not invariably lead to therapeutic intervention [11].
The literature is not conclusive in what is the best imaging modality in patients with suspected anastomotic leakage. Water-soluble contrast radiography and contrast-enhanced CT scanning are the most frequently used diagnostic tools and are probably complimentary [12]. These imaging modalities are sometimes elusive or at least uncertain as a spectrum of findings due to anastomotic leakage can be seen [5, 6, 8]. This may explain the interobserver variability in the present study (Table 2), which is similar to the 13% disagreement as reported by Haynes et al. [10]. The advantage of CT imaging is the ability to detect other causes of the clinical symptoms, such as an intra-abdominal abscess, which offers the possibility of percutaneous drainage avoiding surgery [17].
In distal anastomoses not accessible for digital examination, water-soluble contrast radiography may have additional diagnostic value, although the insertion of a contrast injection catheter has to be done carefully and excessive pressure used during the examination can both precipitate and aggravate pelvic sepsis [5, 10, 16]. When there is an ongoing clinical suspicion of leakage in more proximal anastomoses, CT imaging after oral administration of water-soluble contrast is the method of choice which also visualizes subtle suggestion of leakage, such as perianastomotic collections [6]. Finally, a plain film of the pelvis is suggested to be a sensitive method in detecting disrupture of a staple ring [10, 14].
In conclusion, radiological imaging may be of value in case of clinical suspicion of anastomotic leakage with mild clinical symptoms, but our data suggest that the results should be interpreted with caution because of the high false-negative rate and the substantial interobserver variability. CT scanning can help to indicate alternative diagnoses and the possibility of minimally invasive percutaneous treatment.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999;189:554–559. doi: 10.1016/S1072-7515(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 2.Doeksen A, Tanis PJ, Vrouenraets BC, van Lanschot JJB, van Tets WF. Factors determining delay in relaparotomy for anastomotic leakage after colorectal resection. World J Gastroenterol. 2007;13:3721–3725. doi: 10.3748/wjg.v13.i27.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim M, Akhtar S, Sasapu K, Harris K, Burke D, Sagar P, Finan P. Clinical and subclinical leaks after low colorectal anastomosis: a clinical and radiologic study. Dis Colon Rectum. 2006;49:1611–1619. doi: 10.1007/s10350-006-0663-6. [DOI] [PubMed] [Google Scholar]
- 4.Sutton CD, Marshall LJ, Williams N, Berry DP, Thomas WM, Kelly MJ. Colo-rectal anastomotic leakage often masquerades as a cardiac complication. Colorectal Dis. 2004;6:21–22. doi: 10.1111/j.1463-1318.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang CL, Seow-Choen F. Digital rectal examination compares favourably with conventional water-soluble contrast enema in the assessment of anastomotic healing after low rectal excision: a cohort study. Int J Colorectal Dis. 2005;20:262–266. doi: 10.1007/s00384-004-0652-y. [DOI] [PubMed] [Google Scholar]
- 6.Scardapane A, Brindicci D, Fracella MR, Angelelli G. Post colon surgery complications: imaging findings. Eur J Radiol. 2005;53:397–409. doi: 10.1016/j.ejrad.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann D, Ruckauer K, Salm R. Early post-operative endoscopy of the operated intestine. Endoscopy. 1981;13:108–112. doi: 10.1055/s-2007-1021660. [DOI] [PubMed] [Google Scholar]
- 8.Zissin R, Gayer G. Postoperative anatomic and pathologic findings at CT following colonic resection. Semin Ultrasound CT MR. 2004;25:222–238. doi: 10.1053/j.sult.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Akyol AM, McGregor JR, Galloway DJ, George WD. Early postoperative contrast radiology in the assessment of colorectal anastomotic integrity. Int J Colorectal Dis. 1992;7:141–143. doi: 10.1007/BF00360354. [DOI] [PubMed] [Google Scholar]
- 10.Haynes IG, Goldman M, Silverman SH, exander-Williams J, Keighley MR. Water-soluble contrast enema after colonic anastomosis. Lancet. 1986;1:675–676. doi: 10.1016/S0140-6736(86)91743-5. [DOI] [PubMed] [Google Scholar]
- 11.DuBrow RA, David CL, Curley SA. Anastomotic leaks after low anterior resection for rectal carcinoma: evaluation with CT and barium enema. Am J Roentgenol. 1995;165:567–571. doi: 10.2214/ajr.165.3.7645472. [DOI] [PubMed] [Google Scholar]
- 12.Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicksa GA, Dring RV, Johnson KH, Sardella WV, Vignati PV, Cohen JL. Anastomotic leaks: what is the best diagnostic imaging study? Dis Colon Rectum. 2007;50:197–203. doi: 10.1007/s10350-006-0708-x. [DOI] [PubMed] [Google Scholar]
- 14.Williams CE, Makin CA, Reeve RG, Ellenbogen SB. Over-utilisation of radiography in the assessment of stapled colonic anastomoses. Eur J Radiol. 1991;12:35–37. doi: 10.1016/0720-048X(91)90129-J. [DOI] [PubMed] [Google Scholar]
- 15.Bruce J, Krukowski ZH, Al-Khairy G, Russell EM, Park KG. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88:1157–1168. doi: 10.1046/j.0007-1323.2001.01829.x. [DOI] [PubMed] [Google Scholar]
- 16.Shorthouse AJ, Bartram CI, Eyers AA, Thomson JP. The water soluble contrast enema after rectal anastomosis. Br J Surg. 1982;69:714–717. doi: 10.1002/bjs.1800691210. [DOI] [PubMed] [Google Scholar]
- 17.Lucey BC, Maher MM, Boland GW, Gervais DA, Mueller PR. Percutaneous treatment by interventional radiologists of anastomotic leaks: basic concepts. Am J Roentgenol. 2002;179:365–369. doi: 10.2214/ajr.179.2.1790365. [DOI] [PubMed] [Google Scholar]