Abstract
Introduction
Anorexia nervosa is a psychiatric illness that results in significant bone loss. Studies examining the neuroendocrine dysregulation that occurs in AN may increase understanding of endocrine systems that regulate bone mass. PYY is an anorexigenic peptide derived primarily from the intestine, with actions mediated via activation of Y-receptors. We have previously shown that PYY levels are elevated in adolescents with AN. Y2 receptor knockout mice have increased bone mineral density (BMD) and thus PYY may play a role in regulating bone mass. We hypothesized that PYY levels would be inversely associated with BMD in women with AN.
Methods
This was a cross-sectional study performed in a General Clinical Research Center of 12 adult women with AN, (mean ± SEM) mean age 30.9 ± 1.8 years, BMI 17.1 ± 0.4 kg/m2, and % ideal body weight 77.5 ± 1.7%. PYY concentrations were measured hourly from 20:00 h to 08:00 h. BMD was measured using dual x-ray absorptiometry (DXA).
Results
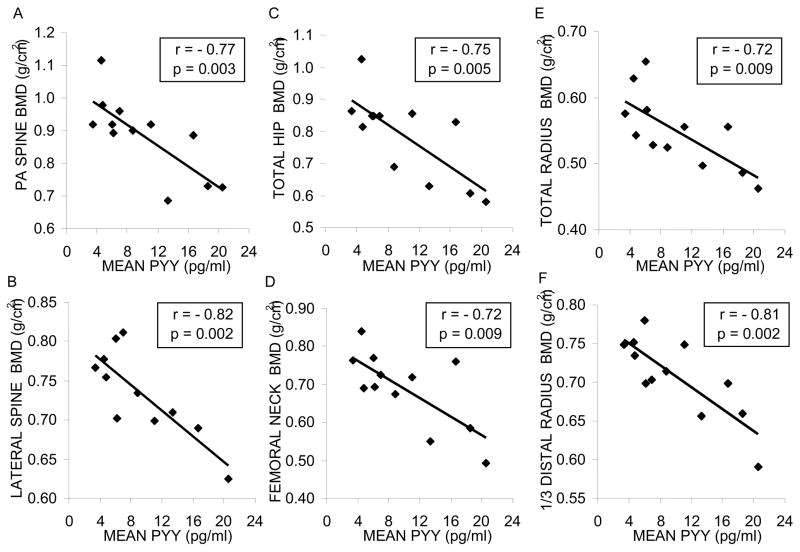
In women with AN, mean overnight PYY levels strongly inversely correlated with BMD at the PA spine (r = −0.77, p = 0.003), lateral spine (r = −0.82, p = 0.002), total hip (r = −0.75, p = 0.005), femoral neck (r = −0.72, p = 0.009), total radius (r = −0.72, p = 0.009) and 1/3 distal radius (r = −0.81, p = 0.002). Body mass index was inversely correlated with PYY level (r = −0.64, p = 0.03). Multivariate stepwise regression analysis was performed to determine the contribution of age, duration of AN, BMI, fat-free mass, and PYY to BMD. For PA and lateral spine, PYY was the primary determinant of BMD, accounting for 59% and 67% of the variability, respectively. Fat-free mass and duration of anorexia nervosa were the primary determinants of BMD at other skeletal sites.
Conclusions
In women with anorexia nervosa, an elevated PYY level is strongly associated with diminished BMD, particularly at the spine. Therefore further investigation of the hypothesis that PYY may contribute to the prevalent bone pathology in this disorder is merited.
Keywords: peptide YY (PYY), anorexia nervosa, bone mineral density (BMD), osteoporosis, body composition
Introduction
Recent data suggest that the hypothalamus plays a direct role in the regulation of BMD [1–5]. The discovery of the molecular circuitry that links leptin signaling to alterations in BMD initiated an active area of investigation [1]. It has been established that leptin receptors are expressed in high density on neuropeptide Y (NPY)-secreting neurons in the arcuate nucleus [6] and that suppression of NPY release by leptin mediates anorexigenic effects [7–9]. However, a decline in NPY does not appear to be the mechanism for leptin’s negative effect on BMD, as intracerebroventricular infusion of NPY also has a deleterious impact on BMD [1]. These data have inspired studies, primarily via knockout mouse models, to examine the role of NPY neuron signaling in the regulation of BMD [3, 4].
NPY is a member of the neuropeptide Y family of molecules, which additionally includes peptide YY (PYY) and pancreatic polypeptide (PP). These peptides bind with differing affinities to receptors of the Y-receptor family (Y1, Y2, Y3, Y4, and Y5 in humans, plus y6 in rodents) [10]. Whereas Y1 and Y5 receptors are believed to primarily mediate feeding behavior [11, 12], studies of Y2 and Y4 receptors suggest a role in controlling BMD [3, 4]. Y2 receptors are expressed on NPY-secreting neurons in the arcuate nucleus and are believed to act as auto-receptors to decrease the release of NPY [13–15]. Peptide YY (PYY) is a gut-derived hormone that is released from intestinal endocrine cells in response to food intake [16]. PYY circulates in two forms, PYY1–36 and PYY3–36, with PYY3–36 predominating [17]. Whereas PYY1–36 has affinity for Y1, Y2, and Y5 receptors, PYY3–36 is selective for Y2 receptors [18, 19]. As Y2 receptor knockout mice demonstrate an increase in trabecular bone volume and BMD by increased formation via hypothalamic signaling [3], we hypothesized that an increase in Y2 signaling may lead to a decline in BMD and that Y-receptor ligands may impact bone dynamics in humans. Thus conditions that increase or are associated with elevated levels of PYY may lead to detrimental effects on BMD.
Anorexia nervosa is a disease of severe caloric restriction that is commonly associated with low BMD, such that almost one-third of adults have osteoporosis [21]. Therefore mechanisms leading to bone loss are important to identify. Several previous studies have suggested that females with anorexia nervosa have elevated PYY levels relative to normal weight or obese individuals [22–25]. This is the first study to demonstrate a strong association between elevated PYY values and lower BMD in women with anorexia nervosa, suggesting a possible contribution of PYY to the severely low BMD found in this disorder. These data demonstrate an inverse correlation between body mass index and PYY values and suggest that further studies are needed to determine whether PYY has an independent deleterious effect on BMD.
Materials and Methods
Subjects
Twelve women with anorexia nervosa, as defined by DSM-IV criteria, were recruited for this study. Inclusion criteria were a weight less than 85% of ideal body weight as defined by the 1983 Metropolitan Life Insurance Tables, age 18 to 45 years old, normal TSH or free T4 levels, and BMD T-score < −1.0. Women were excluded if they had a condition known to affect bone, such as renal failure or alcohol abuse. All but one woman had previously used combination oral contraceptive pills, and two women were actively taking the medications at the time of the study. One woman had previously taken a bisphosphonate for 4 months but discontinued the medication greater than one year prior to the start of the study. Methods of recruitment included advertisements in local community newspapers, posted advertisements on local college campuses, and by referral from local physicians. The study was approved by the Partners Human Research Committee Institutional Review Board, and all subjects provided informed consent prior to participation.
Experimental protocol
Eligible subjects underwent an overnight stay at the Massachusetts General Hospital General Clinical Research Center. Height was measured in triplicate using a stadiometer, and weight was measured on an electronic scale. BMI was calculated as [weight (kg)/(height (m))2]. Frame size (small, medium, or large) was determined from a standardized measurement of elbow width, gender, and age [26]. Blood samples were obtained every hour beginning at 20:00 h and continuing until 08:00 h the following morning via an indwelling intravenous catheter. BMD [posterior-anterior (PA) lumbar spine, lateral lumbar spine, total hip, femoral neck, total radius, and 1/3 distal radius] and fat and fat-free mass measurements were obtained by dual energy x-ray absorptiometry (DXA) (Hologic 4500, Waltham, MA). Volumetric BMD was calculated using the following formulas for bone mineral apparent density (BMAD): L2 – L4 spine BMAD = bone mineral content (BMC) / projected area (Ap)3/2 and femoral neck BMAD = BMC / (Ap)2 [27].
Biochemical measurements
Serum PYY was measured using a human PYY radioimmunoassay (RIA) (Phoenix Pharmaceuticals, Inc., Belmont, CA). This assay detects both PYY1–36 and PYY3–36 peptides. Intra-assay coefficient of variation (CV) of the assay is 6 – 10% and inter-assay CV is 14 – 21%. Serum IGF-I was measured using a solid-phase, enzyme-labeled chemiluminescent immunometric assay (Immulite; Diagnostic Products Corp.). The intra-assay CV is 3.8% and inter-assay CV is 5.4% at 169 ng/ml. IGF-I can be converted to SI units (μg/liter) by multiplying by 1.
Statistical analysis
Data were analyzed using the JMP program (version 4, SAS Institute, Inc., Cary, NC). Univariate regression models were constructed to determine whether there was an association between the following variables and BMD at each regional skeletal site: PYY, IGF-1, BMI, fat-free mass, age, and duration of anorexia nervosa. The three variables with the strongest association with BMD at each site were entered into a stepwise regression model to investigate factors associated with the variability of BMD at each site among the group. Because BMI and fat-free mass were co-linear (r=0.76, p=0.0039), when both variables were among the strongest correlates with BMD, only the most strongly associated with BMD of the two was entered into the model. Multivariate regression modeling was used to control for BMI.
Results
Twelve women with anorexia nervosa, as defined by DSM-IV criteria, were studied. Clinical characteristic data are presented in Table 1.
Table 1.
Clinical characteristics of women with anorexia nervosa
| Mean ± SEM | |
|---|---|
| Age (y) | 30.9 ± 1.8 |
| Age at diagnosis (y) | 18.1 ± 1.6 |
| Weight (kg) | 44.8 ± 1.6 |
| BMI (kg/m2) | 17.0 ± 0.4 |
| % IBW (%) | 77.5 ± 1.7 |
| Fat-free mass (kg) | 35.3 ± 1.1 |
| Fat mass (kg) | 8.7 ± 0.7 |
| IGF-1 (ng/ml) | 144.7 ± 13.1 |
BMI, body mass index; IBW, ideal body weight; IGF-I, insulin-like growth factor-I. IGF-1 can be converted to SI units (μg/liter) by multiplying by 1
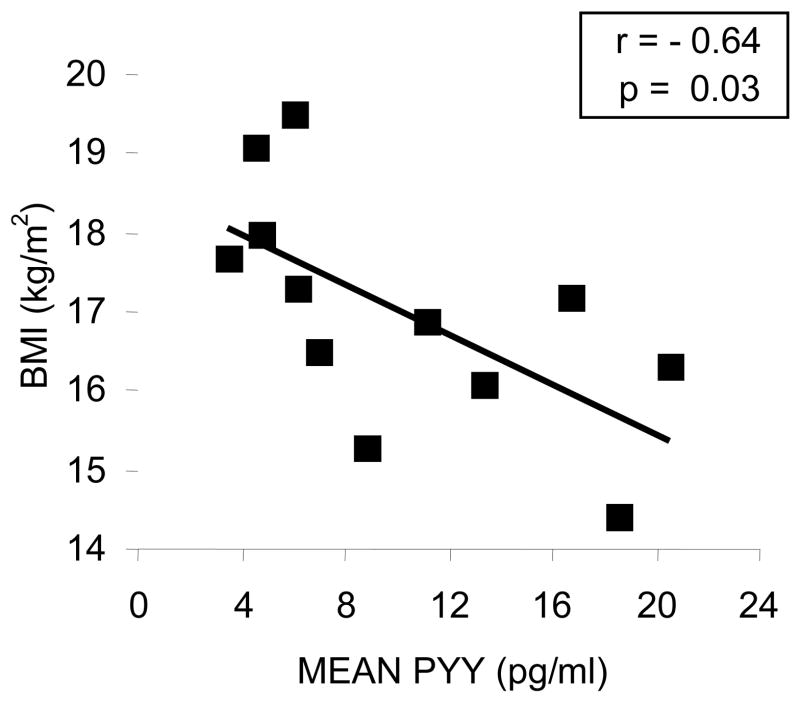
PYY levels were measured hourly beginning at 20:00 h and ending at 08:00 h. Hourly sampling enables an integrated measure of PYY levels overnight. A mean PYY value was calculated over the 12-hour sampling period for each subject. Mean PYY levels were inversely correlated with BMI (r = −0.64, p = 0.03, n = 12) (Figure 1) and fat-free mass (r=−0.71, p=0.01), but not fat mass (r=−0.36, p=0.25).
Figure 1. PYY is inversely associated with BMI in women with anorexia nervosa.
There was a strong inverse correlation between body mass index and mean PYY concentration over a 12-hour sampling period in this group of women with anorexia nervosa. PYY, peptide YY; BMI, body mass index.
Correlation coefficients were calculated to determine the association between mean PYY values and regional BMD. At all sites measured, including PA spine, lateral spine, total hip, femoral neck, total radius, and 1/3 distal radius, there was a significant inverse correlation between mean PYY level and BMD (Figure 2). After controlling for BMI, negative correlations of PYY with PA spine (p=0.05), lateral spine (p=0.01) and distal 1/3 radius (p=0.03) remained significant. There was a trend toward a correlation at the total hip (p=0.06). Associations between PYY and both femoral neck (p=0.12) and total radius (p=0.19) were no longer significant. In addition, correlations remained significant (p < 0.02 for spine and radius and p < 0.04 for hip measurements) after controlling for exogenous estrogen/progesterone use in two subjects. Linear regression models were constructed to determine the relationship between BMD and the following variables: age, duration of anorexia nervosa, BMI, fat-free mass, fat mass and IGF-1 concentrations. There were no significant associations between BMD at any site and either fat mass or IGF-1. Linear regression models for all other variables are presented in Table 2. Table 3 presents the results of stepwise regression modeling to determine the relative contribution of the strongest correlates on linear regression modeling to the variability of BMD among the group at each skeletal site (n = 12 for all bone regions except n = 11 for lateral spine). Mean PYY levels accounted for the largest proportion of the variability in BMD at the PA and lateral spine, whereas fat-free mass was the most important determinant of the variability of BMD at the femoral neck and distal radius, BMI accounted for the largest percent of the variability at the total radius, and the duration of anorexia nervosa was the primary determinant of BMD at the total hip.
Figure 2. PYY is inversely associated with bone mineral density in women with anorexia nervosa.
In women with anorexia nervosa, mean PYY is inversely associated with BMD at each of the following sites: (A) PA spine, (B) lateral spine, (C) total hip, (D) femoral neck, (E) total radius, and (F) 1/3 distal radius. BMD, bone mineral density; PA, posterior-anterior; PYY, peptide YY.
Table 2.
Linear regression models: determinants of regional BMD
| BMD region | Age | Duration of AN | BMI | Fat-free mass | PYY | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | P | R | p | R | p | |
| PA spine | −0.43 | 0.17 | −0.65 | 0.02 | 0.68 | 0.02 | 0.74 | 0.006 | −0.77 | 0.003 |
| Lateral spine | −0.63 | 0.04 | −0.68 | 0.02 | 0.50 | 0.11 | 0.52 | 0.10 | −0.82 | 0.002 |
| Total hip | −0.52 | 0.08 | −0.75 | 0.005 | 0.78 | 0.003 | 0.84 | 0.0007 | −0.75 | 0.005 |
| Femoral neck | −0.56 | 0.06 | −0.65 | 0.02 | 0.70 | 0.01 | 0.82 | 0.001 | −0.72 | 0.009 |
| Total radius | −0.55 | 0.06 | −0.58 | 0.05 | 0.86 | 0.0003 | 0.77 | 0.003 | −0.72 | 0.009 |
| Distal 1/3 radius | −0.41 | 0.19 | −0.55 | 0.06 | 0.67 | 0.02 | 0.86 | 0.0003 | −0.81 | 0.002 |
| PA spine BMAD | −0.49 | 0.10 | −0.70 | 0.01 | 0.68 | 0.02 | 0.56 | 0.06 | −0.78 | 0.003 |
| Femoral neck BMAD | −0.64 | 0.02 | −0.68 | 0.01 | 0.50 | 0.10 | 0.60 | 0.04 | −0.65 | 0.02 |
Table 3.
Regional BMD variability (stepwise regression modeling)
| BMD Region | Variable | Variability explained by model
(r2) |
Cumulative variability explained by model
(Cumulative r2) |
F ratio | p value |
|---|---|---|---|---|---|
| PA Spine | |||||
| PYY | 59.3% | 59.3% | 1.8 | 0.003 | |
| Fat-free mass | 7.5% | 66.8% | 1.6 | 0.19 | |
| Duration of AN | 0.2% | 67.0% | 0.04 | 0.84 | |
| Lateral Spine | |||||
| PYY | 67.1% | 67.1% | 5.8 | 0.002 | |
| Age | 4.8% | 71.9% | 0.5 | 0.27 | |
| Duration of AN | 0.02 | 72.1% | 0.04 | 0.85 | |
| Total Hip | |||||
| Duration of AN | 56.5% | 56.5% | 1.2 | 0.005 | |
| PYY | 8.0% | 64.5% | 1.8 | 0.19 | |
| Age | 0.01% | 64.6% | 0.006 | 0.94 | |
| Femoral Neck | |||||
| Fat-free mass | 67.0% | 67.0% | 4.7 | 0.001 | |
| PYY | 3.7% | 70.7% | 0.5 | 0.31 | |
| Duration of AN | 0.5% | 71.2% | 0.1 | 0.73 | |
| Total Radius | |||||
| BMI | 74.4% | 74.4% | 10.4 | 0.0003 | |
| PYY | 4.7% | 79.1% | 1.2 | 0.19 | |
| Duration of AN | 0% | 79.1% | 0.001 | 0.98 | |
| 1/3 Distal Radius | |||||
| Fat-free mass | 74.0% | 74.0% | 10.4 | 0.0003 | |
| PYY | 7.7% | 81.7% | 5.8 | 0.08 | |
| Duration of AN | 3.3% | 85.0% | 1.8 | 0.22 | |
| PA Spine BMAD | |||||
| PYY | 60.9% | 60.9% | 1.8 | 0.003 | |
| BMI | 5.6% | 66.5% | 1.2 | 0.25 | |
| Duration of AN | 2.2% | 68.7% | 0.6 | 0.47 | |
| Femoral Neck BMAD | |||||
| Duration of AN | 46.9% | 46.9% | 0.2 | 0.01 | |
| Age | 5.8% | 52.7% | 1.2 | 0.32 | |
| PYY | 4.9% | 57.6% | 0.9 | 0.36 | |
AN, anorexia nervosa; BMI, body mass index
Bone size in individuals with anorexia nervosa may be smaller than in a control population and thus standard areal BMD may underestimate BMD [28]. To control for potential decreased bone size in this AN population, volumetric BMD was calculated and compared to mean PYY levels. At the femoral neck the correlations for areal and volumetric BMD versus mean PYY were r = −0.72, p = 0.009 and r = −0.65, p =0.02, respectively. At L2 – L4 PA spine, the correlations for areal and volumetric BMD versus mean PYY were r = −0.81, p = 0.002 and r = −0.78, p = 0.003, respectively. In stepwise regression analysis, only mean PYY was significantly associated with the variability of L2 – L4 PA spine volumetric BMD, accounting for 61% of the variability.
Discussion
While the role of hypothalamic hormones in regulating eating behaviors has received significant attention over the last decade, the dual function of these hormones in controlling BMD is a new area of investigation. Previous studies in mice have revealed that leptin signaling and Y receptor activation are involved in the central nervous system control of BMD [1]. Leptin has been proposed to activate the sympathetic nervous system and adrenergic activity, via β2 receptors, which in turn inhibits osteoblast activity [2]. The downstream mechanism connecting Y receptor activation to decreased BMD has yet to be elucidated.
Y receptors are activated by NPY, PYY, and pancreatic polypeptide (PP) and the specificity of receptor activation is dictated by the affinity of the peptides for each receptor subtype [10]. NPY and PYY1–36 have similar affinities for Y1, Y2, and Y5 receptors, whereas PYY3–36 has selective affinity for Y2 receptors [18, 19]. Pancreatic polypeptide has highest affinity for Y4 receptors [10]. NPY is a neurotransmitter that is synthesized and released from neurons, whereas PYY and PP are gut-derived hormones that circulate with effects at distant central and peripheral sites [10]. As NPY and PYY have similar receptor affinity profiles, PYY may be a link between the periphery and central nervous system for regulating eating behavior and BMD.
Much of the previous research concerning PYY has focused on its role in regulating energy intake. PYY is released from intestinal cells following caloric intake [16]. Obese individuals have lower fasting PYY levels and a suppressed rise in PYY following a meal, compared to normal weight individuals [29]. Studies in humans and rodents have shown that systemic PYY3–36 infusion decreases caloric intake, even in obese subjects [29, 30]. However, others have been unable to reproduce the anorexic effects of PYY3–36 central or peripheral infusion [31]. PYY3–36 binds with high affinity to the Y2 receptor and activation of auto-inhibitory Y2 receptors on NPY neurons potentially provides negative feedback for NPY release [13–15]. The suppression of NPY release is the proposed mechanism for the anorectic effects of PYY3–36 infusion as Y2 receptor knockout mice do not have appetite suppression with PYY infusion [30].
Most previous studies have reported elevated PYY levels in subjects with anorexia nervosa relative to normal weight individuals [22, 24, 25]. Changes in PYY levels with weight gain are conflicting, with one reporting a decrease [24] and the other no change [25] in PYY levels following an increase in weight. Our group has shown that in adolescent girls with anorexia nervosa, BMI and PYY levels were inversely correlated, and a trend toward a decrease in PYY levels followed weight recovery [22]. Our data show an inverse association between BMI and PYY levels in adult women with anorexia nervosa. By examining a time-course of PYY over 12 hours, our data provide a more integrated assessment of PYY values in this population. In comparing secretion patterns between the extremes of BMI, we found that PYY levels were persistently elevated throughout the sampling period. Whether PYY plays a role in initiation or perpetuation of the food-restriction in anorexia nervosa has yet to be determined.
Both leptin and PYY are peripherally-released molecules which are long-term and short-term signals, respectively, of the energy-replete state [16, 32]. Their effects in the central nervous system are to decrease subsequent caloric intake. Additionally, leptin has been shown to be catabolic with respect to BMD [1]. As suggested by our data, the elevated levels of PYY in anorexia nervosa and the associated low BMD characteristic of this disorder suggest that PYY may act similarly to leptin, as a catabolic signal to bone. Assuming that leptin and PYY both relay a catabolic signal to bone, the magnitude of their effect may be highlighted by examining human phenotypes at opposite extremes, obesity and anorexia nervosa. Obese individuals have elevated leptin and low PYY levels, whereas individuals with anorexia nervosa have low leptin and elevated PYY levels. Because obesity is associated with increased BMD [33] and anorexia nervosa with decreased BMD [21], this may imply that, if these peptides exert independent effects on bone, PYY may have a dominant effect on bone compared to leptin. To our knowledge, our data are the first to show a significant association between PYY levels and BMD in osteopenic women. The strong correlation between PYY levels and BMD suggest the possibility that PYY plays a critical role in the central nervous system regulation of BMD, and further studies in larger populations are warranted.
Y2 receptor knockout mice have a two-fold increase in trabecular bone volume at the distal femoral metaphysis with increased trabecular number and thickness, and a trend, but no statistically significant increase, in cortical bone. Osteoclast surface was not affected but osteoclast number was reduced. Osteoblast surface, osteoblast number, osteoid surface, and mineralizing surface were all unaffected but rates of mineral apposition and bone formation were increased. As there is no detectable Y receptor expression in bone, this was believed to represent an effect mediated within the central nervous system. These data suggest that signaling through the Y2 receptor suppresses bone formation, particularly trabecular bone [3]. As PYY3–36 is a preferential Y2 receptor agonist, we hypothesized that states of PYY excess, such as anorexia nervosa, may be detrimental to BMD. Our data are in agreement with this hypothesis, as they demonstrate strong inverse correlations between PYY and BMD in the spine, a region with an increased proportion of trabecular bone. In addition, in a study by Misra et al., PYY was found to negatively correlate with markers of bone formation and resorption [22] supporting a role for PYY in the regulation of BMD. However, in contrast with the data regarding the effects of the Y receptor knock-out effects on bone, Wortley et al. have recently reported a PYY-knock-out mice model with an osteopenic phenotype, including a reduction in trabecular bone mass and decrease in bone strength [20]. Therefore, an alternative plausible hypothesis is that the high PYY levels in anorexia nervosa may reflect resistance at the level of bone to the positive effects of the PYY. Interestingly, in multivariate stepwise analyses, PYY was more strongly associated with the variability in spine BMD in anorexia nervosa than any other variables entered into the model. However, the use of regression modeling does not prove a causal relationship between PYY levels and BMD, and we cannot rule out that the association observed may simply reflect the strong inverse relationship between PYY and BMI. However, the fact that such strong associations were seen, even within a relatively small group, suggests that further research into a possible role for PYY in reducing BMD is warranted.
Acknowledgments
We would like to thank our subjects for their participation in this study. Additionally, we would like to thank the nursing, bionutrition, and laboratory staff of the Massachusetts General Hospital General Clinical Research Center and the Massachusetts Institute of Technology Clinical Research Center.
Support: This work was supported in part by National Institutes of Health Grants R01-DK52625 and M01-RR-01066 National Center for Research Resources General Clinical Research Centers Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 2.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 3.Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. Journal of Clinical Investigation. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sainsbury A, Baldock PA, Schwarzer C, Ueno N, Enriquez RF, Couzens M, Inui A, Herzog H, Gardiner EM. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Molecular and Cellular Biology. 2003;23:5225–5233. doi: 10.1128/MCB.23.15.5225-5233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Ae Kim C, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. Journal of Neuroendocrinology. 1996;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- 7.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, Mackellar W, Rosteck PF, Schoner B, Smith D, Tinsley FC, Zhang X-Y, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;12:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 10.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacological Reviews. 1998;50:143–150. [PubMed] [Google Scholar]
- 11.Marsh DJ, Hollopeter G, Kafer KE, Palmiter Rd. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nature Medicine. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- 12.Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nature Medicine. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- 13.Broberger C, Landry M, Wong H, Walsh JN, Hokfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, DiMaggio DA, Han SP, Westfall TC. Autoreceptor-induced inhibition of neuropeptide Y release from PC-12 cells is mediated by Y2 receptors. American Journal of Physiology. 1997;273:H1737–1744. doi: 10.1152/ajpheart.1997.273.4.H1737. [DOI] [PubMed] [Google Scholar]
- 15.King PJ, Williams G, Doods H, Widdowson PS. Effect of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246 on neuropeptide Y release. European Journal of Pharmacology. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- 16.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 17.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regulatory Peptides. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 18.Dumont Y, Fournier A, St-Pierre S, Quirion R. Characterization of neuropeptide Y binding sites in rat brain membrane preparation using [125I][Leu31, Pro34]Peptide YY and [125I]Peptide YY3-36 as selective Y1 and Y2 radioligands. Journal of Pharmacology and Experimental Therapeutics. 1995;272:673–680. [PubMed] [Google Scholar]
- 19.Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Annals of the New York Academy of Sciences. 2003;994:162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 20.Wortley KE, Garcia K, Okamoto H, Thabet K, Anderson KD, Shen V, Herman HP, Valenzuela D, Yancopoulos GD, Tschop MH, Murphy A, Sleeman MW. Peptide YY regulates bone turnover in rodents. Gastroenterology. 2007 doi: 10.1053/j.gastro.2007.08.024. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Annals of Internal Medicine. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. Journal of Clinical Endocrinology & Metabolism. 2006;91:1027–1033. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- 23.Otto B, Rochlitz H, Mohlig M, Burget L, Kampe J, Pfluger P, Castenada T, Krishna R, Bodani U, Mehta K, Cuntz U, Tschop M, Bidlingmaier M, Pfeiffer A, Spranger J. Circulating concentrations of human peptide YY: influence of acute and chronic energy balance changes. Experimental and Clinical Endocrinology and Diabetes. 2005;113:S1 V9–72. [Google Scholar]
- 24.Nakahara R, Kojima S, Tanaka M, Yasuhara D, Harada T, Sagiyama K, Muranaga T, Nagai N, Nakazato M, Nozoe S, Naruo T, Inui A. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J Psychiatr Res. 2007;41:814–20. doi: 10.1016/j.jpsychires.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Pfluger PT, Kampe J, Castaneda T, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AFH, Koebnick C, Weickert MO, Otto B, Spranger J, Tschop MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3–36. J Clin Endocrinol Metab. 2007;92:583–8. doi: 10.1210/jc.2006-1425. [DOI] [PubMed] [Google Scholar]
- 26.Frisancho AR, Flegel PN. Elbow breadth as a measure of frame size for US males and females. American Journal of Clinical Nutrition. 1983;37:311–314. doi: 10.1093/ajcn/37.2.311. [DOI] [PubMed] [Google Scholar]
- 27.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. Journal of Clinical Endocrinology and Metabolism. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 28.Duan Y, Parfitt A, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. Journal of Bone and Mineral Research. 1999;14:1796–1802. doi: 10.1359/jbmr.1999.14.10.1796. [DOI] [PubMed] [Google Scholar]
- 29.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. New England Journal of Medicine. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 30.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatel MA, Cone RD, Bloom SR. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 31.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-Sickinger A, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Withcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004;430:1–3. doi: 10.1038/nature02665. [DOI] [PubMed] [Google Scholar]
- 32.Ahima RS, Flier JS. Leptin. Annual Review of Physiology. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 33.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. Journal of Bone and Mineral Research. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]