Abstract
Rationale
Pharmacological agents used in the treatment of anxiety have been reported to decrease threat relevant processing in patients and healthy controls, suggesting a potentially relevant mechanism of action. However, the effects of the anxiolytic diazepam have typically been examined at sedative doses, which do not allow the direct actions on emotional processing to be fully separated from global effects of the drug on cognition and alertness.
Objectives
The aim of this study was to investigate the effect of a lower, but still clinically effective, dose of diazepam on emotional processing in healthy volunteers.
Materials and methods
Twenty-four participants were randomised to receive a single dose of diazepam (5 mg) or placebo. Sixty minutes later, participants completed a battery of psychological tests, including measures of non-emotional cognitive performance (reaction time and sustained attention) and emotional processing (affective modulation of the startle reflex, attentional dot probe, facial expression recognition, and emotional memory). Mood and subjective experience were also measured.
Results
Diazepam significantly modulated attentional vigilance to masked emotional faces and significantly decreased overall startle reactivity. Diazepam did not significantly affect mood, alertness, response times, facial expression recognition, or sustained attention.
Conclusions
At non-sedating doses, diazepam produces effects on attentional vigilance and startle responsivity that are consistent with its anxiolytic action. This may be an underlying mechanism through which benzodiazepines exert their therapeutic effects in clinical anxiety.
Keywords: Diazepam, Startle, Emotional processing, Healthy volunteers, Facial expression recognition, Attentional bias
Introduction
Cognitive psychological theories of anxiety disorders emphasise the important role that mood congruent biases in the processing of emotional material play in the maintenance of these disorders (e.g. Mathews and MacLeod 1994). For example, patients with anxiety disorders typically show heightened processing of threat-relevant stimuli in their environment (MacLeod et al. 1986; Mogg and Bradley 2002), which is normalised after pharmacological and psychological treatment (Mogg et al. 2004; Mathews et al. 1995). Decreased threat processing after administration of drugs typically used in the management of anxiety has also been reported to affect emotional processing in healthy volunteers. For example, short-term administration of the selective serotonin reuptake inhibitor (SSRI), citalopram, decreased the recognition of the negative facial expressions of fear, anger and disgust, with volunteers being more likely to misclassify these expressions as happy (Harmer et al. 2004). Healthy volunteers administered citalopram also recalled a greater proportion of positive vs negative personality characteristics in an incidental test of emotional memory and demonstrated reduced fear potentiation of the startle reflex in the emotion-potentiated startle task (Harmer et al. 2004). One of the intriguing features of these effects in healthy volunteers is that they appear to occur in the absence of any subjective changes in mood, anxiety or energy levels. This has led to the suggestion that modifying the processing of emotionally relevant material may occur directly and represent an important mechanism through which antidepressant agents exert their effects on mood in depression and anxiety disorders (Harmer et al. 2004).
There have also been some reports of effects of the benzodiazepine anti-anxiety drug, diazepam, on emotional processing in healthy volunteers. For example, a single 15-mg dose of diazepam has been reported to decrease the recognition of facial expressions of anger and, in some studies, fear (Blair and Curran 1999; Zangara et al. 2002). In addition, diazepam has been reported to attenuate fear potentiation of the startle response (Bitsios et al. 1999) and the affective potentiation of the startle response by unpleasant pictures (Patrick et al. 1996), mirroring the effects found in the antidepressant studies. However, a number of more recent studies have failed to replicate these specific effects of diazepam on emotional processing and, rather, have found more global impairments in facial expression recognition and startle reactivity after diazepam administration. For example, Coupland et al. (2003) report that, on two tasks of facial expression recognition, a single 15-mg dose of diazepam produced broad impairments in recognition accuracy, recognition thresholds and response times that were not specific to fear and anger but that were apparent across all facial expressions presented, including happy. Similarly, Baas et al. (2002) report that diazepam does not specifically attenuate the fear potentiation of the startle response but rather produces a generalised decrease in response amplitude.
One possible reason for these discrepant findings lies in the sedative effects of diazepam. In all of the previous studies, the dose of diazepam used (typically 10 or 15 mg) was sufficient to produce subjective changes in energy and alertness and measurable increases in response times on the tasks used. As a result, the specific effects of diazepam have not been fully separated from the global cognitive and sedative effects of the drug, and therefore, emotional processing changes previously reported may represent the indirect influence of attention, motivation or task difficulty.
The present study aimed to elucidate whether there are direct effects of diazepam on emotional processing that are independent from the drug’s sedative effect. To do this, we investigated the effect of a lower, but still clinically effective, dose of diazepam (5 mg) on a number of emotional processing tasks in healthy volunteers. Two non-emotional control tasks for simple reaction times and sustained attention were also used to clarify the interpretation of the results. It was predicted that this low dose of diazepam would have no measurable sedative effect and no effect on these control tasks. Any effects on the emotional tasks could therefore be assumed to represent the direct effect of diazepam on affective processing.
Materials and methods
Twenty-four healthy volunteers aged 19–27 years participated in the study. Exclusion criteria included: history of axis I psychiatric disorder [screened for using the Standard Clinical Interview for DSM-IV (SCID)]; history of alcohol or other substance abuse or dependence (assessed using SCID criteria); pregnancy or lactation; history of significant medical disorder and current usage of any medication other than oral contraception. The study was approved by the local ethics committee, and participants gave written informed consent. All participants undertook to abstain from alcohol for 24 h before and after the experimental session. Participants were required to fast for 3 h before the ingestion of the capsule and were not permitted to eat, smoke or drink caffeine for the duration of the experimental session. Testing did not take place during female participants’ premenstrual week.
A between-groups, double-blind, placebo-controlled design was used. Participants were randomly allocated to receive a single dose of diazepam (5 mg) or placebo. The treatments were administered orally in matching opaque capsules. There were no significant differences between the two groups in terms of age, gender, years of education, verbal IQ (as measured using the National Adult Reading Test), body mass index, and scores on the following subjective measures of personality, mood and cognitive style: Eysenck Personality Questionnaire (Eysenck and Eysenck 1975), Beck Depression Inventory (Beck et al. 1961), Dysfunctional Attitudes Scale (Weisman and Beck 1978), Buss–Durkee Hostility Inventory (Buss and Durkee 1957), see Table 1.
Table 1.
Demographic characteristics and baseline subjective state ratings of 24 healthy volunteers randomly assigned to double-blind intervention with diazepam (5 mg) or placebo
| Diazepam | Placebo | |
|---|---|---|
| Age | 22.9 (2.6) | 22.2 (2.0) |
| Male/female ratio | 6:6 | 6:6 |
| Years of education | 17.8 (2.4) | 17.5 (2.4) |
| Verbal IQ | 119.5 (4.7) | 119.8 (4.0) |
| Body mass index | 21.8 (2.6) | 21.3 (2.5) |
| Eysenck Personality Questionnaire | ||
| Neuroticism | 7.5 (4.3) | 8.7 (4.9) |
| Psychoticism | 2.3 (1.4) | 3.6 (1.7) |
| Extraversion | 15.8 (1.9) | 14.8 (5.1) |
| Beck Depression Inventory | 2.1 (1.8) | 1.9 (2.7) |
| Dysfunctional Attitudes Scale | 124.1 (20.1) | 127.8 (19.1) |
| Buss–Durkee Hostility Inventory | 23 (6.2) | 24.4 (11.4) |
There were no significant differences between the two groups on any of these measures. Values represent mean with standard deviation in parentheses.
On the test day, before receiving active drug or placebo, participants filled out the following subjective measures of mood and energy: Befindlichskeit Scale of Mood and Energy (von Zerssen et al. 1974), Spielberger State Anxiety Inventory (Spielberger et al. 1983) and visual analogue scales measuring alertness, disgust, drowsiness, anxiety, happiness, nausea and sadness. Participants also filled in a side effects checklist. These questionnaires were repeated 50 min after capsule administration and at the end of the experimental session to provide a measure of diazepam’s subjective mood and sedative effects. Sixty minutes after tablet ingestion, participants completed the following tasks in the following order: facial expression recognition, emotional categorisation, Cambridge Neuropsychological Test Automated Battery (CANTAB) simple and five-choice reaction time test, emotional memory, attentional dot probe, affective modulation of the startle reflex and rapid visual information processing (RVIP). The tests were administered in the same order for all participants. At the end of the experimental session, volunteers were asked to report whether they thought they were in the drug or placebo group.
Facial expression recognition task
The facial recognition task featured six basic emotions (anger, disgust, fear, happiness, sadness and surprise) taken from the Pictures of Facial Affect series (Ekman and Friesen 1976). These had been morphed between each prototype and neutral using techniques described by Young et al. (1997), which involved taking a variable percentage of the shape and texture differences between the two standard images 0% (neutral) and 100% (full emotion) in 10% steps. Four examples of each emotion at each intensity were presented (total of ten individuals). Each face was also given in a neutral expression, giving a total of 250 stimuli presentations.
The face stimuli were presented on a computer screen (random order) for 500 ms and replaced by a blank screen. Volunteers indicated which expression they thought the face depicted by pressing a labelled key on the keyboard. Volunteers were asked to respond as quickly and as accurately as possible. Accuracy (number correct out of 40) and response time was recorded for each emotion.
Emotional categorisation and memory
Sixty personality characteristics selected to be disagreeable (e.g. domineering, hostile) or agreeable (e.g. cheerful, generous), taken from Anderson (1968), were presented on a computer screen for 500 ms. These words were matched for length and ratings of frequency and meaningfulness. Participants were asked to categorise these personality characteristics as likeable or dislikeable as quickly and as accurately as possible. Specifically, they were asked to imagine whether they would like or dislike overhearing someone referring to them as possessing this characteristic, so that the judgement was in part self-referential. Classifications and response times for correct identification were recorded.
Ten minutes after the emotional categorisation task, participants were asked to recall as many of the personality characteristics as possible. Recognition memory was then assessed by asking participants to respond with a ‘Yes’ or ‘No’ to each item on a list comprising the 60 targets (previously presented in the emotional categorisation task) and 60 matched distractors (30 positive, 30 negative).
Simple and five-choice reaction time test
This task is part of CANTAB (Cambridge Cognition). The test was in two stages. In the simple reaction time test, participants were required to release a touch-pad when a dot appeared in the centre of the screen and point to the dot as quickly as possible. After this, participants completed the five-choice reaction time test, which was identical except the dot now appeared in one of five locations. There were 10 practice trials and 25 experimental trials for each stage of the experiment.
Accuracy, reaction time (time between target appearing on the screen and the participant’s hand leaving the touch pad) and movement time (time between participant’s hand leaving the touch pad and the target being touched) were measured.
Attentional dot probe
Pairs of photographs of 20 individuals were taken from the JACFEE/JACNeuF sets of facial expressions (Matsumoto and Ekman 1988). Each face pair comprised one emotional and one neutral expression of the same individual or two neutral expressions of the same individual. Half of the emotional faces were fearful and half were happy. Thus, there were three types of face pair: neutral–neutral, fearful–neutral and happy–neutral.
On each trial, one of the faces appeared above and the other below the central fixation position. The emotional faces appeared in the top and bottom location with equal frequency. In the unmasked condition, the face pair was presented for 100 ms, and then, a probe appeared in the location of one of the preceding faces. The probe was two dots presented either vertically (:) or horizontally (··). Participants were required to report the orientation of the dots by pressing a labelled key on a keyboard. Participants were asked to respond as quickly and as accurately as possible. The sequence of events was the same in the masked condition, except the face pair was displayed for 16-ms and followed by a mask (constructed from a jumbled face), which was displayed for 84 ms.
On half of the emotional–neutral face trials, the probe appeared in the same position as the emotional face, and on the other half, the probe appeared in the same position as the neutral face. There were 192 trials in total (masked: 32 happy–neutral, 32 fear–neutral, 32 neutral–neutral; unmasked: 32 happy–neutral, 32 fear–neutral, 32 neutral–neutral). There were 8 blocks of unmasked trials (12 trials per block) and 8 blocks of masked trials (12 trials per block), which were presented in an alternating order.
Incorrect trials were excluded from the data analysis. Attentional vigilance scores were calculated for each participant by subtracting the mean reaction time from trials when probes appeared in the same position as the emotional face from trials when probes appeared in the opposite position to the emotional face (incongruent trials minus congruent trials).
Affective modulation of the startle reflex
Picture stimuli from the International Affective Picture System (Lang et al. 1998; Larson et al. 2000) were used, which were designed to elicit positive, negative or neutral emotions. These stimuli had been rated and selected such that the negative and positive pictures were similar in terms of arousal, but opposite in valence, whereas the neutral pictures were low on arousal and average on valence (see Larson et al. 2000). Stimuli were presented for 13 s (intertrial interval: mean = 13 s; range, 11–15 s). There were three experimental blocks of trials each containing 7 pictures of each category (21 pictures per block in total) presented in a fixed pseudo-random order with the constraint that no two of the same type (neutral, positive or negative) were presented successively.
The eye-blink component of the startle response was recorded from the orbicularis oculi using surface electromyography (EMG startle response system, San Diego Instruments, San Diego, CA, USA). Acoustic probes were 50 ms, 100-dB bursts of white noise with a nearly instantaneous rise time delivered binaurally through headphones (generated through the noise generator and amplifier component of the EMG-SR system, San Diego Instruments). Probes were delivered at 1.5, 4.5 and 7.5 s after picture onset. Within each block of 21 pictures, probes were delivered during 5 of each trial type (neutral, positive and negative). To limit expectation of the noise, two trials per valence did not contain any startle probe, and three probes per block were delivered in the intertrial interval. To habituate participants to the startle probes and to orient them to the procedure, participants viewed an introductory set of nine neutral pictures and received nine startle probes (two of which occurred during the intertrial interval).
EMG signals were sampled at a rate of 1,000 samples per second, and the signal was filtered between 1 and 300 Hz and then smoothed with a filter window of 5 ms and rectified. Eye blink magnitudes (in μV) were calculated as the peak amplitude of the eye blink reflex 20–120 ms after probe onset relative to baseline (average EMG signal for 20 ms time frame after probe onset). Trials with no perceptible eye-blink reflex were assigned a magnitude of zero and included in the analysis. Eye-blink reflexes with excessive noise during the 20-ms prestartle baseline period were excluded. These were defined as those traces where baseline levels of activity were higher than identified peaks. Trials were evaluated by an experimenter who was blind to the treatment group allocation, and on average, 3.6 trials were excluded per subject on this basis. Eye-blink magnitudes were analysed both as raw data and also z-transformed within participants to allow direct comparison of the acoustic startle response during neutral, positive and negative pictorial stimuli presentation. To aid visual understanding of the results, the data are presented graphically as t-scores.
Rapid visual information processing
This is a test of sustained attention with a working memory component from the CANTAB (Cambridge Cognition) battery. Digits were presented sequentially on a computer screen at a rate of 100/min. Participants were required to detect and respond as quickly as possible (by depressing a touch-pad) to targets of three consecutive digits (e.g. 2–4–6). There was initially a practice phase with one target sequence, followed by an experimental phase with three target sequences. The experimental phase lasted 3 min, during which time a target sequence was presented 27 times. Number of correct hits, false alarms and response time were measured. The following performance indices, derived from signal detection theory, were also calculated from the results: A′ (sensitivity to difference between targets and non-targets) and B″ (tendency to withhold responding). To avoid infinite values for the calculation of B″, 0.5 was added to each data cell as suggested by Snodgrass and Corwin (1988).
Statistical analyses
Data were analysed using between-groups one-way (subjective state, simple reaction time and RVIP) or split-plot two-way (facial expression recognition, affective modulation of startle reflex, attentional vigilance scores, emotional categorisation and memory) analyses of variance. The between-subjects factor was the treatment group (diazepam or placebo) and the within-subjects factors were facial expression (facial expression recognition and attentional dot probe), picture valence (affective startle) and word valence (emotion categorisation and memory). Where necessary, the interpretation of significant interaction effects was aided by the use of simple main effect analyses.
Results
Subjective state, energy and side effects
There were no main effects of treatment group or interactions between sampling time and treatment group on the state anxiety inventory, the Befindlichskeit scale of mood and energy or the visual analogue scales. There were very few reports of side effects, and these were not significantly different between the diazepam and placebo groups. The majority of the volunteers (17 out of 24) said that they thought they were in the placebo group. Of the seven volunteers who thought they had been given diazepam, four were in the drug group and three were in the placebo group.
Facial expression recognition
There was no main effect of treatment group or interaction between treatment group and facial expression for accuracy or reaction times in this task (all comparisons p > 0.2, see Table 2). Given previous reports of a reduction in the recognition of angry and fearful faces after diazepam treatment, individual t tests were carried out for these two expressions, which confirmed no significant effect of treatment group (anger: t(22) = −0.795, p = 0.435, fear: t(22) = 0.393, p = 0.698).
Table 2.
Means (SEM) from the facial expression recognition task; emotional categorisation and memory, CANTAB reaction time tasks and Rapid visual information processing (RVIP)
| Diazepam | Placebo | |
|---|---|---|
| Facial expression recognition | ||
| Hits (out of 40) | ||
| Angry | 22.25 (0.8) | 23.58 (1.5) |
| Disgusted | 21.25 (1.3) | 21.08 (1.7) |
| Fearful | 21.67 (1.1) | 21 (1.3) |
| Happy | 27.58 (1.0) | 27.33 (1.1) |
| Sad | 23.25 (1.3) | 19 (1.5) |
| Surprised | 23.67 (1.2) | 23.17 (0.9) |
| Reaction time (ms) | ||
| Angry | 1590.73 (222.7) | 1307.08 (101.9) |
| Disgusted | 1394.71 (112) | 1479.79 (153.7) |
| Fearful | 1749.09 (152.4) | 1728.28 (89.7) |
| Happy | 1382.96 (131.1) | 1262.92 (77.9) |
| Sad | 1415.33 (131.8) | 1447.9 (92.0) |
| Surprised | 1624.79 (149.6) | 1484.19 (73.1) |
| Emotional categorisation | ||
| Hits (out of 30) | ||
| Positive | 29 (0.3) | 29.3 (0.3) |
| Negative | 29.3 (0.3) | 28.9 (0.4) |
| Reaction Time (ms) | ||
| Positive | 958 (36.7) | 1001.1 (37.9) |
| Negative | 992.6 (38.8) | 1066.5 (49.7) |
| Emotional memory | ||
| Recall hits | ||
| Positive | 5.5 (0.8) | 4.8 (0.7) |
| Negative | 3.3 (0.7) | 3.5 (0.6) |
| Recall false alarms | ||
| Positive | 2.3 (0.5) | 3 (0.8) |
| Negative | 1.6 (0.5) | 1.6 (0.4) |
| Recognition hits | ||
| Positive | 24 (1.0) | 23.8 (1.0) |
| Negative | 21.8 (1.0) | 22.8 (1.0) |
| Recognition false alarms | ||
| Positive | 9.7 (1.8) | 10.7 (1.3) |
| Negative | 5 (1.2) | 5.4 (0.9) |
| Simple reaction time | ||
| Hits (out of 25) | 24.6 (0.2) | 24.6 (0.2) |
| Movement time (ms) | 389.8 (37.1) | 374.9 (27.2) |
| Reaction time (ms) | 279 (7.7) | 317.3 (19.3) |
| Five-choice reaction time | ||
| Hits (out of 25) | 24.9 (0.1) | 24.8 (0.2) |
| Movement time (ms) | 391.3 (31.1) | 403.6 (14.3) |
| Reaction time (ms) | 308.7 (10.6) | 361 (23.9) |
| RVIP | ||
| Hits (out of 27) | 22.5 (1.1) | 22.25 (0.9) |
| Reaction time (ms) | 410.2 (24.3) | 440.8 (26.5) |
| False alarms | 0.1 (0.05) | 0.3 (0.06) |
| A′ | 0.96 (0.01) | 0.96 (0.01) |
| B″ | 0.86 (0.02) | 0.87 (0.01) |
Emotion categorisation and memory
There was no main effect of word valence or treatment group and no interaction between word valence and treatment group on accuracy or reaction time in the categorisation of personality characteristics as positive or negative (see Table 2). Recall and recognition of positive and negative personality characteristics were also not significantly affected by treatment group [recall: main effect of group F(1,22) = 0.08, p = 0.78; group × valence F(1,22) = 1.24, p = 0.28; recognition: main effect of group F(1,22) = 0.11, p = 0.74; group × valence F(1,22) = 0.9, p = 0.35).
CANTAB reaction time
Simple reaction time
There was no significant effect of treatment group on accuracy [F(1,23) = 0.000, p = 1] or movement time [F(1,23) = 0.256, p = 0.62]. There was a borderline significant effect of treatment group on reaction time [F(1,23) = 3.931, p = 0.06] reflecting that the diazepam group was faster to react than the placebo group (see Table 2).
Five-choice reaction time
There was no significant effect of treatment group on accuracy [F(1,23) = 1.088 p = 0.31] or movement time [F(1,23) = 0.040, p = 0.84]. There was a significant effect of treatment group on reaction time [F(1,23) = 4.292, p = 0.05], again reflecting faster reaction times in the diazepam group compared with the placebo group (see Table 2).
Attentional dot probe
Accuracy rates were very high in this task (placebo mean, 93%; diazepam mean, 94%), and there were no significant effects of treatment group on accuracy across any of the task conditions. Data from trials with errors were discarded and not analysed further.
In the masked condition, there was a significant interaction between treatment group and facial expression [F(1,22) = 5.254, p = 0.032]. This significant interaction was further corroborated with independent samples t tests, which revealed that attentional vigilance to happy faces was significantly increased in the diazepam group compared with the placebo group [t(22) = 2.790, p = 0.01] but that the difference between the two groups in the fearful face condition was not significant [t(22) = −1.040, p = 0.3]. In the unmasked condition, there was a significant main effect of facial expression [F(1,22) = 5.092, p = 0.034], reflecting that both groups showed relatively increased attentional vigilance towards the fearful faces relative to the happy faces (see Fig. 1). However, there was no significant effect of treatment group in the unmasked condition [F(1,22) = 0.262, p = 0.614], and no significant group by facial expression interaction [F(1,22) = 0.031, p = 0.863].
Fig. 1.
Attentional vigilance in the attentional probe task in the masked condition (upper) and the unmasked condition (lower). Vigilance is calculated by subtracting mean reaction time to respond when probe replaces emotional face (fearful or happy) from the reaction time when the probe replaces the neutral face. Thus, the higher the vigilance score, the greater the attentional bias towards the emotional face. Error bars represent standard error of mean. Only values from correct trials are displayed. *p < 0.05
One-sample t tests were used to compare attentional bias scores to zero within each group to clarify where an absolute bias was present. These analyses revealed that the placebo group showed a significant bias away from masked happy faces [t(11) = −2.379, p = 0.037], which was not present in the diazepam group [t(11) = 1.604, p = 0.137]. Neither group showed a significant bias towards or away from fearful faces in the masked condition (all p’s > 0.2) or to fearful or happy faces in the unmasked condition.
Affective modulation of the startle reflex
One participant’s data from the diazepam treatment group was excluded from the analysis, as it was considered to be an extreme outlier, falling more than 2 standard deviations above the group mean across all conditions. Data is also missing from a second participant from the placebo group who did not complete the affective startle task because they found the procedure distressing.
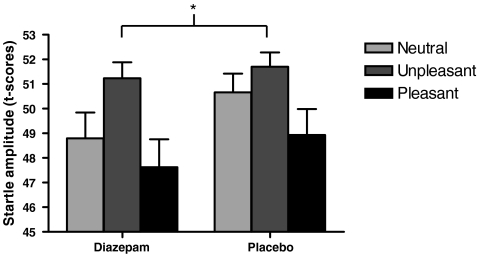
There was a significant modulation of the startle amplitude by the valence of the picture (main effect of picture valence [F(2,40) = 4.047, p = 0.028]), with increased startle amplitude in both groups in the unpleasant condition relative to the pleasant and neutral conditions (see Table 3). There was also a significant main effect of treatment group [F(1,20) = 6.856, p = 0.016], reflecting decreased startle amplitude across all conditions in the diazepam group compared to the placebo group (see Table 3). There was no interaction between picture valence and treatment group [F(2,40) = 0.318, p = 0.714]. Converting the startle amplitudes to standard (z) scores revealed the same pattern of effects with a potentiated startle response in the negative picture condition in both groups [main effect of picture valence: F(2,40) = 5.239, p = 0.01] but no interaction between picture valence and treatment group [F(2,40) = 0.259, p = 0.773]. Hence, startle responses were generally reduced in the diazepam group, irrespective of the picture stimuli presented. This pattern of results can be seen graphically in Fig. 2, which depicts the t-scores for each picture valence and each treatment group. The mean startle peak latency was 61.04 ms, and this was not significantly affected by treatment group or picture valence (see Table 3).
Table 3.
Startle amplitude and startle peak latency for the affective modulation of the startle reflex paradigm
| Startle amplitude (μV) | Startle peak latency (ms) | |||||
|---|---|---|---|---|---|---|
| Negative | Neutral | Positive | Negative | Neutral | Positive | |
| Placebo | 3063.8 | 3001.8 | 2537.5 | 61.15 (1.4) | 61.85 (1.5) | 62.52 (1.9) |
| Diazepam | 2063.8 | 1744.5 | 1698.3 | 60.18 (1.6) | 60.26 (1.5) | 60.04 (1.8) |
Values represent mean with standard error in parentheses. There was significantly decreased startle amplitude across all picture valence conditions in the diazepam group compared with the placebo group. There was no significant effect of diazepam on startle peak latency.
Fig. 2.
Startle eye-blink responses to a burst of white noise presented during the presentation of neutral (light grey), pleasant (black) and unpleasant (dark grey) pictures. Both groups demonstrated increased startle amplitude in the unpleasant condition relative to the pleasant and neutral conditions. Startle responses were significantly reduced in the diazepam group compared to the placebo group, irrespective of the picture stimuli presented. Error bars represent standard error of mean. *p < 0.05
Rapid visual information processing
There were no significant differences between the two treatment groups for accuracy [F(1,23) = 0.248, p = 0.624], reaction time [F(1,23) = 1.259, p = 0.274] or false alarms [F(1,23) = 2.008, p = 0.170] in this task. Diazepam also did not significantly affect the sensitivity measure, A′ [F(1,23) = 0.275, p = 0.605] or the response bias measure, B″ [F(1,23) = 1.161, p = 0.293] on this task (see Table 2).
Discussion
This study investigated the effect of a single, low dose of diazepam on a number of measures of emotional processing in healthy volunteers. It was found that diazepam significantly modulated attentional vigilance to masked emotional faces and significantly decreased overall startle reactivity. These effects occurred in the absence of any measurable sedative effects of the drug, suggesting that they represent a direct effect of the drug. There was no effect of diazepam on emotional memory or the recognition of facial expressions of fear or anger.
Diazepam has previously been reported to have effects on emotional processing (e.g. Blair and Curran 1999; Zangara et al. 2002; Patrick et al. 1996). However, in these earlier studies, a higher dose of diazepam was used, and there were measurable sedative effects evident in longer reaction times and subjective reports of increased drowsiness. The present study used a lower dose of diazepam to reduce such confounding sedative effects. Three lines of evidence suggest that, at this lower dose, the participants were not measurably sedated by the diazepam. First, unlike the previous studies, there were no differences between the drug and placebo groups on subjective reports of alertness, mood and energy. Second, the response times across all tasks were broadly equivalent in the two groups. There was a marginal decrease in reaction times on the CANTAB reaction time tasks in the diazepam group, further supporting the notion that diazepam did not have a sedative effect at this dose. Third, the participants were unable to accurately guess which group they had been allocated to. Thus, at this dose, it seems that the direct effect of diazepam on emotional processing could be investigated, unconfounded by global effects on cognition and alertness. It would have been useful, however, to have an additional treatment group with a higher, sedative dose of diazepam, to more closely distinguish the sedative effects of diazepam from the direct effects of the drug on emotional processing.
As higher doses of diazepam have well-documented sedative effects, it is surprising that we found marginally reduced reaction times in the diazepam group compared with the placebo group on the CANTAB simple and five choice reaction time tasks. The order of test administration was kept the same across all participants, so this difference cannot be explained by different order effects in the two treatment groups. One possibility is that, given the equivalent response times across the two groups in the other tasks, the reduced reaction times in the present study may represent a Type I error. An alternative explanation is that the decreased reaction times in the diazepam group may be reflective of an effect of the drug on inhibitory function. Deakin et al (2004) reported that whilst diazepam has detrimental effects on cognitive tasks that tap planning and decision-making processes, 20 mg of diazepam also reduced the response bias measure B″ on the RVIP task, consistent with the drug reducing the tendency to withhold or inhibit a response. However, both in this previous study and in the present study, a 5-mg dose was not found to significantly affect this measure on the RVIP, suggesting that the reduced reaction times seen in the present study are not mediated by such a disinhibitory mechanism. Nonetheless, this increased speed of response relative to placebo further supports the absence of general deficits in motor output systems after diazepam administration, which could otherwise, for example, contribute to the reduced startle responses also seen in this study.
Heightened anxiety has been associated with a selective attentional bias towards threat cues in the environment (MacLeod et al. 1986; Mogg and Bradley 1998). Such attentional orienting towards threat has been proposed to play an important role in the maintenance of a number of clinical anxiety disorders (Eysenck 1992; Mathews and MacLeod 1994). Consistent with this, these attentional biases have been shown to be reduced after psychological treatment (Mathews et al. 1995; Mogg et al. 2004). It is therefore of great interest that diazepam also modulated attentional vigilance towards emotional faces. The significant interaction between face expression and treatment group on the attentional probe task reflects increased attentional vigilance to masked positive vs threatening faces in the diazepam group compared with the placebo group. Such a change in attentional orienting may represent an important mechanism through which anxiolytic drugs such as diazepam exert their therapeutic effects on clinical anxiety.
Subsequent analyses revealed that this interaction was mainly driven by increased attentional vigilance to happy faces in the diazepam group. It has previously been suggested that attentional vigilance effects in the happy condition of this paradigm are relevant to threat-related processing biases (Cooper and Langton 2006). These authors argue that, in this condition, the neutral face is relatively more hostile or threatening than the happy face, and therefore, at short presentation lengths, attention is automatically biased towards the neutral face, reflected in a relative ‘avoidance’ of the happy face. Consistent with this suggestion, in the present study, the placebo group showed a significant bias away from happy faces in the masked condition, an effect that was abolished by diazepam administration. Taken in light of Cooper and Langton’s (2006) interpretation, this pattern of effects is consistent with the idea that diazepam is reducing attentional vigilance to mildly threatening or ambiguous (neutral) faces. It is perhaps surprising that there is not a similar reduced attentional bias to threat-related stimuli in the fearful face condition after diazepam. However, this is, in part, accountable by the lack of significant absolute bias to fearful faces in the placebo group.
There is evidence from clinical studies in which threat cues are presented very briefly or under conditions of restricted awareness that the anxiety-related attentional bias towards threat may operate at a very early, pre-attentive stage of processing (e.g. Mogg and Bradley 2002; van den Hout et al. 1997). Consistent with this, we found that diazepam specifically affected attentional vigilance to emotional faces when the stimuli were presented very briefly (16 ms) in a masked paradigm. One possible explanation for these effects of diazepam that must be considered is that the effect of the drug was simply to reduce the perception of very briefly presented stimuli and thus reduce the basic effect of such a stimulus. Consistent with this explanation, a previous study has demonstrated that a different benzodiazepine, lorazepam, significantly increases the threshold for extracting information from briefly presented stimuli (Giersch and Herzog 2004). However, in the latter study, the effect of diazepam was also examined when administered at a dose that was approximately three times that used in the present study. Although this dose had a measurable sedative action, it had no significant effect on the threshold for extracting information from briefly presented stimuli. This suggests that a purely perceptual explanation of the effects of diazepam on attentional vigilance to emotional faces in the present study is unlikely.
We did not find a significant effect of diazepam on attentional vigilance to emotional faces in the unmasked condition. However, in the absence of a significant bias in the placebo group to unmasked fearful or happy faces, this lack of effect is difficult to interpret. Future studies are needed to assess whether the effects of diazepam on emotional processing are limited to stimuli presented subliminally; this could have important implications for our theoretical understanding of how anxiolytic drugs may work compared to antidepressant drugs. Indeed, we have recently reported broader actions of citalopram on attention to both subliminal and supraliminal emotional information in a dot-probe task (Browning et al. 2007).
Diazepam also reduced baseline startle reactivity in the affective modulation of the startle reflex paradigm. This paradigm is based on the animal fear-potentiated startle, which is a well-established model of fear and anxiety and is sensitive to a range of pharmacological manipulations. In humans, the startle reflex is potentiated by aversive stimulation, for example, in contexts that are associated with shock (e.g. Greenwald et al. 1998) and when people are viewing unpleasant pictures (e.g. Vrana et al. 1988). Drugs that increase anxiety have been shown to increase startle responses in both animal and human models (e.g. Davis et al. 1979; Morgan et al. 1993). Conversely, reduced startle responses have been reported after the administration of a range of agents used in the treatment of anxiety disorders (see Davis et al. 1993 for a review). Further evidence to support the face validity of the startle reflex as a model of anxiety comes from clinical reports of elevated startle reactivity in anxiety disorders, such as post-traumatic stress disorder (see Grillon and Baas 2003 for a review).
Some previous studies have reported that diazepam blocks the potentiation of the startle response by threat (Patrick et al. 1996; Bitsios et al. 1999). However, the most consistent finding from human studies is that, as was found in the present study, diazepam reduces overall startle amplitude (Abduljawad et al. 1997; Baas et al. 2002; Scaife et al. 2005). The pattern of results found in the current study could reflect a general emotional dampening effect, such that the pictures were less emotionally salient after diazepam administration. However, the affective content of the pictures modulated the startle response in both groups, suggesting that the pictures continued to hold emotional significance in both groups. Instead, Grillon (2002) has proposed that baseline startle reactivity reflects contextual anxiety induced by the negative context of the startle experiment, whereas the selective potentiation of the startle response to threat is an index of cue-specific fear. Accordingly, the decrease in baseline startle reactivity after diazepam treatment may reflect a reduced sensitivity to the anxiogenic context of the startle experiment.
An anxiolytic effect of diazepam on baseline startle magnitude can be difficult to disentangle from the more general sedative or muscle relaxant properties of the drug. However, there is some evidence to suggest that sedation per se is not sufficient to produce reductions in baseline startle reactivity. For example, a recent study demonstrated that a sedative drug (diphenhydramine) that is not anxiolytic had no effect on startle amplitude (Grillon et al. 2006). Furthermore, Guscott and colleagues (2000) have demonstrated in rodents that the effect of benzodiazepines on baseline startle is dependent on the presence of an anxiogenic context. Differences in state anxiety scores have also been shown to account for a large part of the variance in startle magnitude, further suggesting that baseline startle changes are not simply reflective of sedative effects (Baas et al. 2002). The demonstration in the present study of reduced baseline startle reactivity after a low dose of diazepam and in the absence of measurable sedative effects lends support to this notion. However, there are also some studies demonstrating non-specific effects of benzodiazepines on baseline startle in the absence of any threatening context or manipulation with a higher dose than the one used in this study (Abduljawad et al. 1997; Rodriguez-Fornells et al. 1999). Benzodiazepine effects on baseline startle reactivity are therefore generally recognised to reflect an interplay between non-specific sedative effects and more direct anxiolytic action (Baas et al. 2002).
There was no effect of diazepam on the recognition of facial expression in the present study. This is in contrast to two previous studies that have reported a selective decrease in recognition of the facial expressions of anger (Blair and Curran 1999) and fear and anger (Zangara et al. 2002) after a higher dose of diazepam. One explanation for this discrepancy is that the previously reported deficits in facial expression recognition reflect a difficulty effect rather than a specific deficit in anger and fear perception. In support of this, cross-cultural studies have consistently demonstrated that negative expressions such as anger, disgust and fear are harder to recognise than expressions such as happy (Russell 1994; Biehl et al. 1997). Further, Coupland et al. (2003) report that in a healthy volunteer study, a relatively high dose of diazepam (15 mg) produced broad impairments in the recognition of all facial expressions that were not specific to fear or anger. However, consistent with the present findings, a recent study reported no effect of the benzodiazepine, lorazepam, on the recognition of facial expressions of emotion despite the drug having a measurable sedative effect (Kamboj and Curran 2006). This suggests that even at sedative doses, benzodiazepines do not necessarily impair the recognition of threat-related emotional expressions, and this may not therefore represent a central mechanism through which this class of drugs exert their anxiolytic effects.
Administration of serotonin-promoting antidepressant agents, such as the SSRI citalopram (Harmer et al. 2004) and the serotonin precursor tryptophan (Murphy et al. 2006) have been shown to exert effects on emotional processing that are reminiscent of those seen in the present study and consistent with their therapeutic use in both depression and anxiety. Specifically, subchronic (7 day) administration of citalopram and tryptophan to healthy volunteers has been shown to reduce potentiation of the startle reflex by unpleasant pictures and baseline startle responses, respectively (Harmer et al. 2004; Murphy et al. 2006). Tryptophan and citalopram have also both been shown to modulate attentional vigilance to emotional stimuli (Murphy et al. 2006; Browning et al. 2007), although this was not restricted to subliminal presentation as seen here. This raises the intriguing possibility that there may be common pathways through which different classes of anxiolytic agents exert their effects on clinical anxiety and that the modulation of emotional processing may be an important component of such therapeutic effects.
However, we found previously that repeated administration of citalopram also reduced the recognition of negative facial expressions of emotion and increased positive emotional memory (Harmer et al. 2004), effects not seen after diazepam treatment in the present study. One possible explanation for this is that these tasks tap into different aspects of emotional processing that are less relevant to the anxiolytic action of diazepam. Anxiety has been particularly linked with pre-attentive, early biases in emotional processing, such as initial orienting to threatening stimuli, whereas depression has been more closely associated with a bias in later, more strategic aspects of emotional processing (Williams et al. 1988; Bradley et al. 1997). Facial expression recognition and emotional memory involve more strategic or elaborative processing than the automatic processing involved in the emotion-potentiated startle and the masked condition of the attentional probe task. It therefore seems reasonable to suggest that the affective modulation of the startle reflex and attentional probe tasks are measures of the anxiolytic action of drugs on emotional processing, whereas the facial expression recognition and emotional memory are rather measures of the antidepressant action. In support of this notion, a noradrenergic antidepressant that is not typically used in the treatment of anxiety, reboxetine, showed the opposite pattern to diazepam on these tasks with effects on the recognition of facial expressions and emotional memory but no effect on the emotion potentiation of the startle response in the presence of negative pictures (Harmer et al. 2004). Future patient and imaging studies are needed to assess the clinical validity and to characterise the neural substrates of this distinction.
In summary, this study demonstrates a modulation of attentional bias to masked emotional faces and decreased startle responsivity in healthy volunteers after acute diazepam administration, in the absence of the global sedative effects of the drug. These effects on emotional processing are reminiscent of the effects of other anxiolytic agents, such as SSRIs, and are opposite to the biases seen in anxiety disorders. This gives important insight into a potential mechanism through which benzodiazepines may exert their therapeutic effects on clinical anxiety.
Acknowledgements
SEM is supported by a Wellcome Trust studentship.
Disclosure/Conflict of interest
There are no conflicts of interest for any of the authors relating to this manuscript. CJH has acted as a consultant for p1vital, Lundbeck and Merck, Sharpe & Dohmne. PJC has served on advisory boards for Eli Lilly, Sevier and Wyeth.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of clonidine and diazepam on the acoustic startle response and on its inhibition by ‘prepulses’ in man. J Psychopharmacol. 1997;11:29–34. doi: 10.1177/026988119701100110. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. J Pers Soc Psychol. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Baas JM, Grillon C, Bocker KB, Brack AA, Morgan CA, III, Kenemans JL, Verbaten MN. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology (Berl) 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Biehl M, Matsumoto D, Ekman P, Hearn V, Heider K, Kudoh T, Ton V. Matsumoto and Ekman's Japanese and Caucasian facial expressions of emotion (JACFEE): Reliability data and cross-national differences. J Nonverbal Behav. 1997;21:3–21. doi: 10.1023/A:1024902500935. [DOI] [Google Scholar]
- Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E. Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. J Psychopharmacol. 1999;13:226–234. doi: 10.1177/026988119901300303. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Curran HV. Selective impairment in the recognition of anger induced by diazepam. Psychopharmacology (Berl) 1999;147:335–338. doi: 10.1007/s002130051177. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behav Res Ther. 1997;35:911–927. doi: 10.1016/S0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer C. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cooper RM, Langton SR. Attentional bias to angry faces using the dot-probe task? It depends when you look for it. Behav Res Ther. 2006;44:1321–1329. doi: 10.1016/j.brat.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Coupland NJ, Singh AJ, Sustrik RA, Ting P, Blair R. Effects of diazepam on facial emotion recognition. J Psychiatry Neurosci. 2003;28:452–463. [PMC free article] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-V. [DOI] [PubMed] [Google Scholar]
- Deakin JB, Aitken MR, Dowson JH, Robbins TW, Sahakian BJ. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl) 2004;173:88–97. doi: 10.1007/s00213-003-1695-4. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial effect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Eysenck MW. Anxiety: the cognitive perspective. Hove: Erlbaum; 1992. [Google Scholar]
- Eysenck SBG, Eysenck HJ. Manual of the EPQ (Eysenck Personality Questionnaire) London: University of London Press; 1975. [Google Scholar]
- Giersch A, Herzog MH. Lorazepam strongly prolongs visual information processing. Neuropsychopharmacology. 2004;29:1386–1394. doi: 10.1038/sj.npp.1300429. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Bradley MM, Cuthbert BN, Lang PJ. Startle potentiation: shock sensitization, aversive learning, and affective picture modulation. Behav Neurosci. 1998;112:1069–1079. doi: 10.1037/0735-7044.112.5.1069. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/S0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/S1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Guscott MR, Cook GP, Bristow LJ. Contextual fear conditioning and baseline startle responses in the rat fear-potentiated startle test: a comparison of benzodiazepine/gamma-aminobutyric acid-A receptor agonists. Behav Pharmacol. 2000;11:495–504. doi: 10.1097/00008877-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Curran HV. Scopolamine induces impairments in the recognition of human facial expressions of anger and disgust. Psychopharmacology (Berl) 2006;185:529–535. doi: 10.1007/s00213-006-0332-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainsville: University of Florida, Center for Research in Psychophysiology; 1998. [Google Scholar]
- Larson CL, Ruffalo D, Nietert JY, Davidson RJ. Temporal stability of the emotion-modulated startle response. Psychophysiology. 2000;37:92–101. doi: 10.1017/S0048577200981344. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mogg K, Kentish J, Eysenck M. Effect of psychological treatment on cognitive bias in generalized anxiety disorder. Behav Res Ther. 1995;33:293–303. doi: 10.1016/0005-7967(94)E0022-B. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion (JACFEE). Intercultural and Emotion Research Laboratory. San Francisco, CA: Department of Psychology, San Francisco State University; 1988. [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36:809–848. doi: 10.1016/S0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40:1403–1414. doi: 10.1016/S0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Baldwin DS, Brodrick P, Bradley BP. Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology (Berl) 2004;176:466–470. doi: 10.1007/s00213-004-1902-y. [DOI] [PubMed] [Google Scholar]
- Morgan CAI, Southwick SM, Grillon C, Davis M, Krystal JH, Charney DS. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology (Berl) 1993;110:342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Longhitano C, Ayres RE, Cowen PJ, Harmer CJ. Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology (Berl) 2006;187:121–130. doi: 10.1007/s00213-006-0401-8. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Berthot BD, Moore JD. Diazepam blocks fear-potentiated startle in humans. J Abnorm Psychol. 1996;105:89–96. doi: 10.1037/0021-843X.105.1.89. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Riba J, Gironell A, Kulisevsky J, Barbanoj MJ. Effects of alprazolam on the acoustic startle response in humans. Psychopharmacology (Berl) 1999;143:280–285. doi: 10.1007/s002130050948. [DOI] [PubMed] [Google Scholar]
- Russell JA. Is there universal recognition of emotion from facial expression? A review of the cross-cultural studies. Psychol Bull. 1994;115:102–141. doi: 10.1037/0033-2909.115.1.102. [DOI] [PubMed] [Google Scholar]
- Scaife JC, Langley RW, Bradshaw CM, Szabadi E. Diazepam suppresses the acquisition but not the expression of ‘fear-potentiation’ of the acoustic startle response in man. J Psychopharmacol. 2005;19:347–356. doi: 10.1177/0269881105053285. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol: General. 1988;117:34–50. doi: 10.1037/0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RD. Manual for the state-trait anxiety inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- van den Hout M, Tenney N, Huygens K, de JP. Preconscious processing bias in specific phobia. Behav Res Ther. 1997;35:29–34. doi: 10.1016/S0005-7967(96)00080-0. [DOI] [PubMed] [Google Scholar]
- von Zerssen D, Strian F, Schwarz D. Evaluation of depressive states, especially in longitudinal studies. Mod Probl Pharmacopsychiatry. 1974;7:189–202. doi: 10.1159/000395076. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? J Abnorm Psychol. 1988;97:487–491. doi: 10.1037/0021-843X.97.4.487. [DOI] [PubMed] [Google Scholar]
- Weisman AN, Beck AT (1978) Development and validation of the dysfunctional attitudes scale: A preliminary investigation. Paper presented at the American Education Research Association. Toronto, Canada. Ref Type: Conference Proceeding
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Chichester: Wiley; 1988. [Google Scholar]
- Young AW, Rowland D, Calder AJ, Etcoff NL, Seth A, Perrett DI. Facial expression megamix: tests of dimensional and category accounts of emotion recognition. Cognition. 1997;63:271–313. doi: 10.1016/S0010-0277(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Zangara A, Blair RJ, Curran HV. A comparison of the effects of a beta-adrenergic blocker and a benzodiazepine upon the recognition of human facial expressions. Psychopharmacology (Berl) 2002;163:36–41. doi: 10.1007/s00213-002-1120-4. [DOI] [PubMed] [Google Scholar]