Abstract
Adolescence is a time of significant brain development, and exposure to nicotine during this period is associated with higher subsequent rates of dependence. Chronic nicotine exposure alters expression of nicotinic acetylcholine receptors (nAChRs), changing the pattern of nicotine responsiveness. We used quantitative autoradiography to measure three major subtypes of nAChRs after chronic nicotine exposure by osmotic minipump in adult and periadolescent rats. Comparison of control animals at the two different ages revealed that periadolescents express consistently greater numbers of α4β2* nAChRs compared to the same brain regions of adults. Similar but less pronounced increases in α7 nAChRs were found in control periadolescent rats compared to adults. Binding of [125I]α-conotoxin MII (largely to α6* nAChRs) did not systematically differ between adults and periadolescents. The response to chronic nicotine exposure also differed by age. Up-regulation of α4β2* nAChRs was prominent and widespread in adult animals; in periadolescents, α4β2* up-regulation also occurred, but in fewer regions and to a lesser extent. A similar pattern of response was seen with α7 receptors: adults were more responsive than periadolescents to nicotine-induced up-regulation. In adult animals, chronic nicotine exposure did not cause up-regulation of α6* nAChRs; binding was down-regulated in three regions. Unlike the other subtypes, the response of α6* nAChRs to chronic nicotine was greater in periadolescents, with more regions showing greater down-regulation compared to adults. These differences in receptor expression and regulation between age groups are likely to be important given the unique vulnerability of adolescents to nicotine-induced behavioral changes and susceptibility to drug abuse.
Classification terms: Section: 3. Neurophysiology, Neuropharmacology & Other Forms of Cellular Communication
Key words: Nicotine, adolescence, dependence, nicotinic receptor subtypes, autoradiography
1. INTRODUCTION
Adolescence is the most common period for initiation of recreational drug use (Spear 2004). Such use often begins with tobacco products, and recent evidence suggests that adolescents are particularly susceptible to the addictive and adverse affects of tobacco smoke. Adolescent use of tobacco is associated with subsequent higher daily consumption and a lower probability of smoking cessation (Chen and Millar 1998). Furthermore, there is a growing body of evidence suggesting that smokers, particularly those who begin smoking during adolescence, are more vulnerable to subsequent drug abuse (Chambers et al. 2003;Adriani et al. 2006).
Adolescence is characterized by extensive physiological and psychological development. Recent studies have shown that the human brain continues to develop through adolescence (Sowell et al. 2003). There is a significant amount of brain growth in early adolescence followed by a decrease in grey matter during the transition from adolescence to adulthood, coinciding with a gradual loss of synapses and strengthening of remaining synapses (Sowell et al. 2003). This occurs in several regions of the brain and coincides with changes in complex social behaviors. In rats, periadolescence has classically been defined as the time between the earliest detection of diurnal gonadotropin cycling (approximately postnatal day 28 [PN28]) and reproductive maturity (approximately PN38–42) (Spear and Brake 1983). Neurochemical, neuroanatomical and behavioral changes that occur during this period in rats are similar to those seen in human adolescents (Spear and Brake 1983;Slotkin 2002;Adriani et al. 2003).
Nicotine is the major neuroactive and addictive component of tobacco smoke. Nicotine acts on pathways affecting neuronal development and behavior, modulating anxiety, behavioral inhibition, reward, and habit formation. Nicotine has been demonstrated to cause long-lasting adverse effects on adolescent rat brain, including altered proliferation, differentiation, synaptic activity, synaptic maturation, and increased cell damage and cell death (Trauth et al. 2000a;Slotkin 2002;Abreu-Villaca et al. 2003). Adolescent rats differ from adult rats in their nicotine-induced behavioral responses (Belluzzi et al. 2004;Adriani et al. 2003), and the effects of adolescent nicotine exposure can persist into adulthood. Pre-exposure to nicotine during, but not following, adolescence sensitizes rats to nicotine effects on conditioned place preference and locomotion during adulthood (Adriani et al. 2006). Also, several recent studies (although not all; (Kelley and Middaugh 1999)), have shown that pretreatment with nicotine during adolescence sensitizes rats to the rewarding effects of other drugs (Collins and Izenwasser 2004); (McMillen et al. 2005;McQuown et al. 2007).
Chronic exposure to nicotine, as well as smoking in humans, is well-known to alter numbers of nicotinic cholinergic receptors (nAChRs) in brain, usually causing up-regulation. Receptor regulation may contribute to behavioral effects of long-term nicotine exposure, including tolerance and dependence. Although extensive work has detailed the effects of chronic nicotine exposure on different subtypes of nAChRs throughout the adult rat brain, there have been relatively few studies of the effects of such exposure on nAChRs in adolescent rat brain.
We have previously employed the chronic nicotine treatment protocol pioneered by Slotkin (Slotkin 2002) coupled with autoradiographic methods to study the response of different subtypes of nAChRs in rats (Nguyen et al. 2003;Nguyen et al. 2004;Rasmussen and Perry 2006;Perry et al. 2007). In the current study, we extend this approach to compare the effects of adult and adolescent chronic nicotine exposure. This method allows simultaneous comparison of the effects on three major nAChR subtypes, α4β2*, α7 and α6*, across a wide range of brain regions. We report distinct differences in the response to nicotine between the two age groups, as well as differences in subtype response. In addition, there were age-related differences in receptor subtype expression seen in saline control animals.
2. RESULTS
The concentrations of nicotine and cotinine achieved in plasma and brain are shown in Table 1. Based on the weight changes that occurred during the 14 day dosing period (+10% in adults, +92% in adolescents), the nominal dose of free base nicotine changed from 6.0 to 3.1 mg/kg/day in adolescents, and to 5.5 mg/kg/day in adults. Accordingly, by the end of the 14 day dosing period, the plasma levels of both nicotine and its primary metabolite cotinine in adult rats were higher than those found in periadolescent rats (1.8-fold for nicotine, 2.2-fold for cotinine). A previous study using this same dosing protocol found similar weight changes, but reported lower absolute plasma levels of both compounds than found in this study, and found a greater discrepancy between levels in adults and adolescents (roughly 3–4 fold greater in adults) (Trauth et al. 2000b). The levels of nicotine were higher in brain than in blood, and were 2.2 times higher in adult brains compared to those of periadolescents. Our results are consistent with a previous study demonstrating that nicotine (but not cotinine) preferentially accumulates in brain versus blood over continual dosing regimens (Ghosheh et al. 2001).
Table 1.
Levels of nicotine and cotinine in rat plasma and brain after 2 weeks treatment
| Plasma | Brain | |||
|---|---|---|---|---|
| Nicotine, µM | Cotinine, µM | Nicotine, µM | Cotinine, µM | |
| Adolescent | 1.08 +/− 0.20 | 2.74 +/− 0.13 | 3.47 +/− 0.70 | 1.09 +/− 0.12 |
| Adult | 1.91 +/− 0.32 | 6.04 +/− 0.59 | 6.04 +/− 0.71 | 2.15 +/− 0.37 |
Representative autoradiographic images are shown for [125I]A-85380 binding in Figure 1, for [125I]αBtx in Figure 2, and for [125I]α-CtxMII binding in Figure 3. Each figure shows total binding in equivalent regions from each of the four treatment groups: A, adult saline; B, adult nicotine; C, periadolescent saline; D, periadolescent nicotine. Non-specific binding in a section adjacent to one of the four total binding sections is shown in E. Adjacent sections from the same animals are shown across the three figures (e.g. 1A, 2A and 3A are all from the same adult saline-treated rat).
Figure 1. Autoradiographic images of [125I]A-85380 binding to α4β2* nAChRs in rat brain sections from animals representing the four treatment groups.
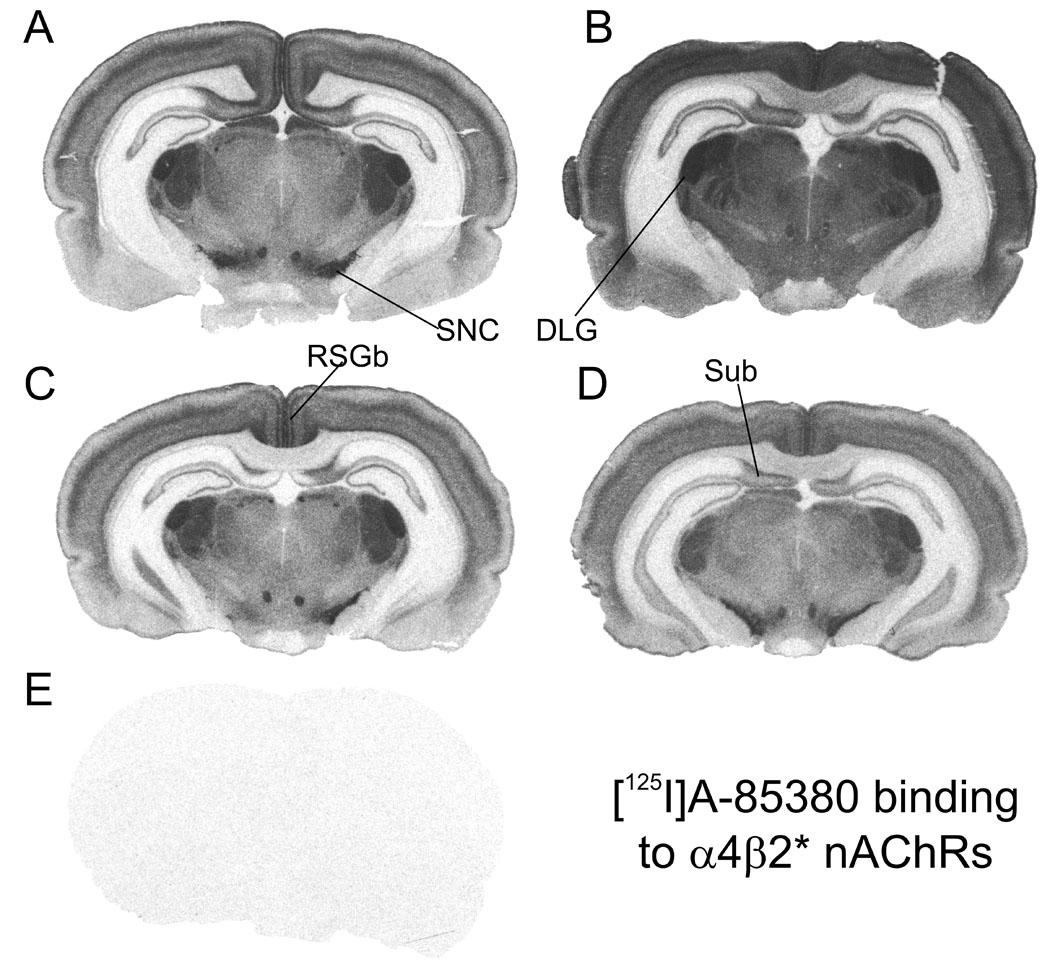
Binding of [125I]A-85380 (0.6 nM) was done in the presence of 100 nM αCtxMII to block binding to α6/α3* subtypes, and is shown in representative sections from four treatment groups: A, adult saline; B, adult nicotine; C; periadolescent saline; D, periadolescent nicotine; E, non-specific binding in the presence of 100 µM nicotine in an adjacent section. DLG, dorsal lateral geniculate nucleus; RSGb, retrosplenial granular cortex; SNC, substantia nigra, pars compacta; Sub, subiculum. Sections cut approximately −5.0 mm from bregma.
Figure 2. Autoradiographic images of [125I]αBtx binding to α7 nAChRs in rat brain sections from animals representing the four treatment groups.
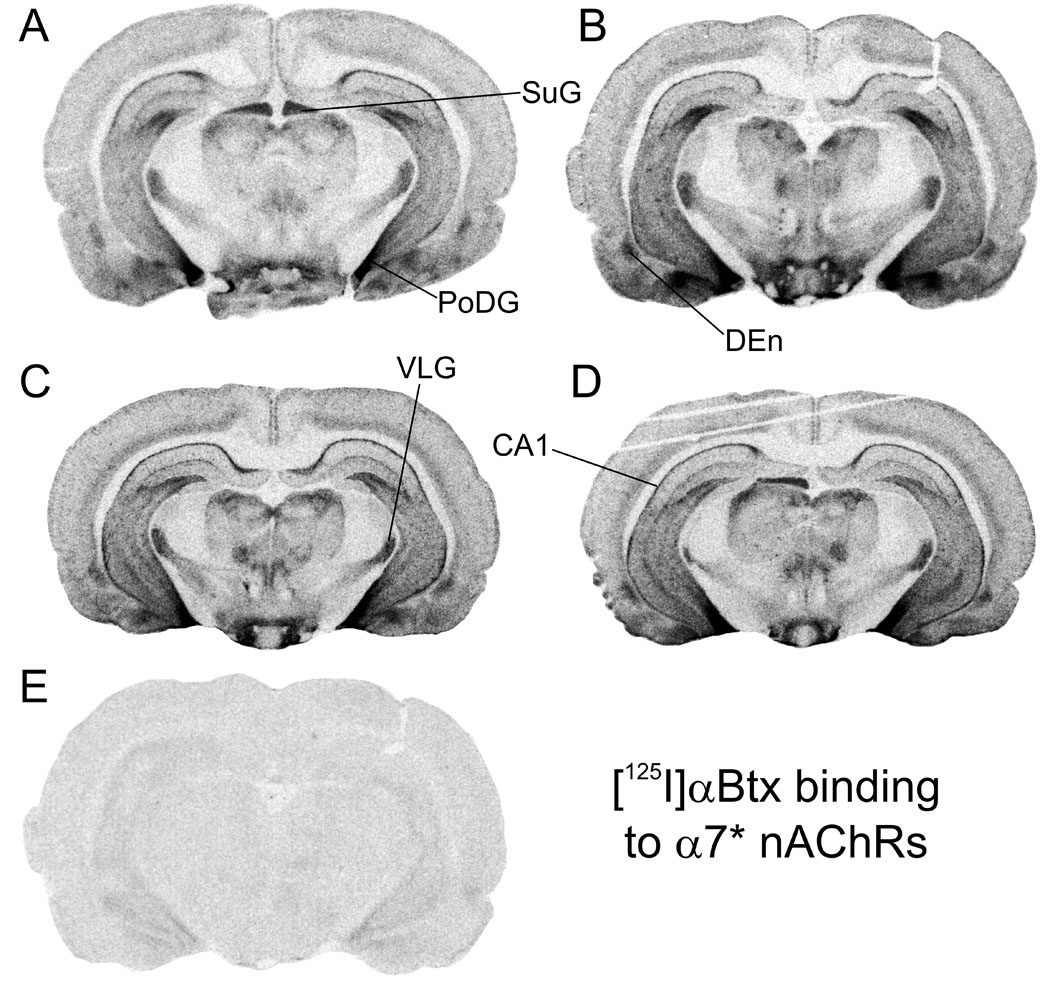
Binding of 0.72 nM [125I]αBtx is shown in representative sections from four treatment groups: A, adult saline; B, adult nicotine; C; periadolescent saline; D, periadolescent nicotine; E, non-specific binding in the presence of 100 µM nicotine in an adjacent section. CA1, stratum oriens, hippocampus; DEn, endopiriform nucleus; PoDG, posterior dentate gyrus; SuG, superior colliculus, superficial grey layer; VLG, ventral lateral geniculate nucleus. Sections cut approximately −5.0 mm from bregma.
Figure 3. Autoradiographic images of [125I]αCtxMII binding to α6* nAChRs in rat brain sections from animals representing the four treatment groups.
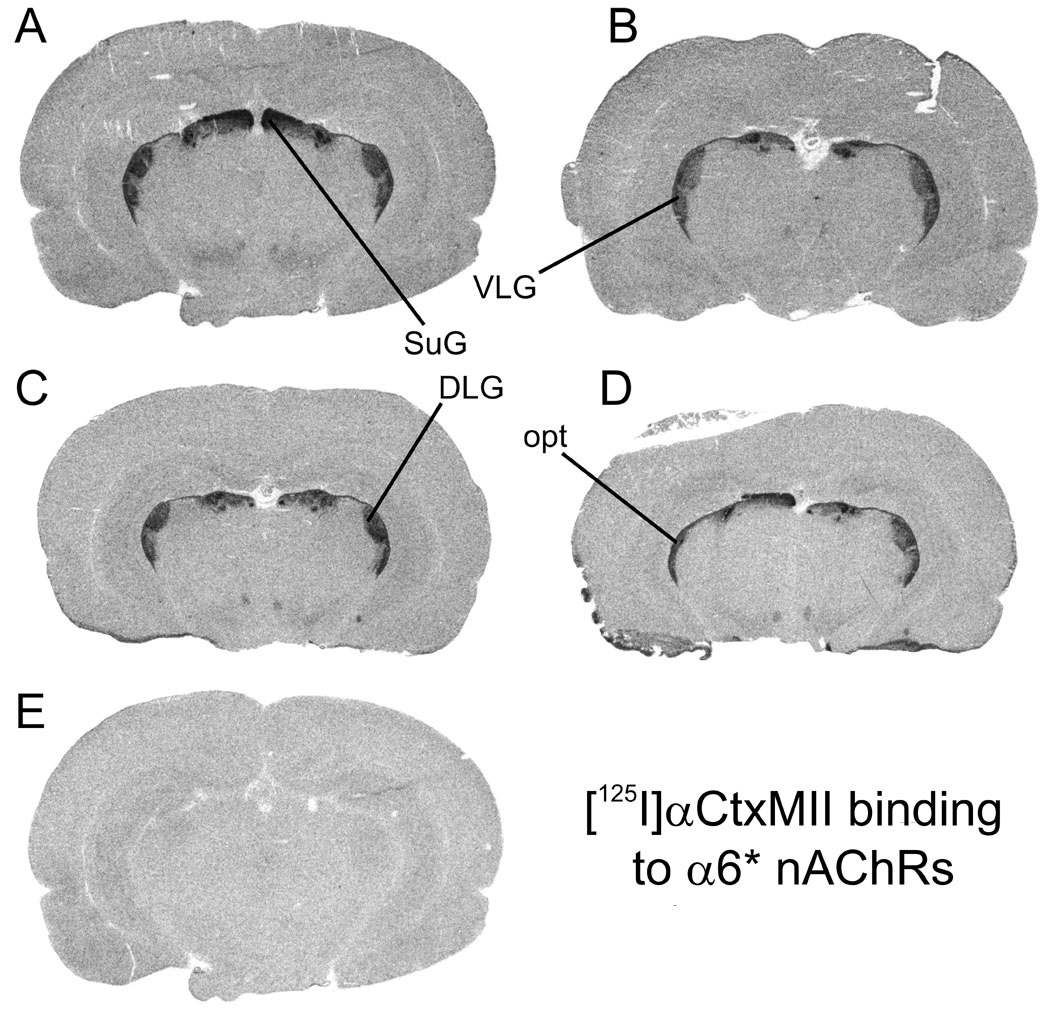
Binding of 0.8 nM [125I]αCtxMII is shown in representative sections from four treatment groups: A, adult saline; B, adult nicotine; C; periadolescent saline; D, periadolescent nicotine; E, non-specific binding in the presence of 100 µM nicotine in an adjacent section. DLG, dorsal lateral geniculate nucleus; opt, optic tract; SuG, superior colliculus, superficial grey layer; VLG, ventral lateral geniculate nucleus. Sections cut approximately −5.0 mm from bregma.
A-85380 has been shown to bind selectively to β2-containing nAChRs (Sullivan et al. 1996). The concentration of α-CtxMII included in the incubation (100 nM) should effectively block binding to α6β2* (Whiteaker et al. 2000;Champtiaux et al. 2002). The affinity of αCtxMII at α3β2* sites may be somewhat lower (Gotti et al. 2006) and the affinity at α2β2* sites is presently unknown. We will refer to the binding of [125I]A-85380 under these conditions as α4β2*, with the understanding that there may also be small contributions in some regions from these other two relatively minor subtypes. Visual evidence of up-regulated α4β2* binding is apparent in adults by comparing Figures 1A and 1B; in contrast, up-regulation of α4β2* binding in periadolescents is not as readily apparent by visual inspection of Figures 1C and 1D. [125I]αBtx labeling is widespread in mammalian brain, and is thought to be limited largely or entirely to α7 homomers. Similar distributions in adult and periadolescent brains are seen in Figure 2. Although some regions express high levels of both α4β2* and α7 nAChRs (e.g. superior colliculus, cerebral cortex), overall the pattern of α7 binding is very different than that of α4β2*, with relatively high densities in hippocampus, amygdala and hypothalamus, and lower levels in striatum and thalamus compared to α4β2*.
At the concentration employed, [125I]α-CtxMII binding is selective for α6β2* nAChRs (Whiteaker et al. 2000;Champtiaux et al. 2002). Although αCtxMII was originally described in functional studies as selective for α3β2* nAChRs (Cartier et al. 1996), recent evidence suggests that the binding affinity is in the order of 50 nM (Gotti et al. 2006), which means that there would be very little contribution from α3β2* nAChRs in these studies, given the concentration of radioligand employed. Therefore, we will refer to the sites labeled by [125I]αCtxMII under the conditions employed here as α6* nAChRs. α6* nAChRs are expressed in visual systems from the retina to the superior colliculus, and in dopamine systems in rat brain (Whiteaker et al. 2000;Le Novere et al. 1996;Champtiaux et al. 2002). This distribution is reflected in the images shown in Figure 3, for both adult and periadolescent brains. No differences with nicotine treatment are readily apparent from comparing images.
Binding was quantified by digital densitometry. Means of [125I]A-85380 binding to α4β2* receptors in 38 brain regions for the four treatment groups are shown in Table 2. (Note that the individual images shown here may not always reflect the quantitative results obtained by densitometry across multiple images.) Comparison of binding in adult versus periadolescent saline-treated controls reveals a distinct age effect: binding was uniformly higher in periadolescents. The mean binding across all 38 regions was 50% higher in periadolescents, with 33 of 38 regions demonstrating significantly higher binding in the younger rats. The comparison of binding in saline controls for adults and adolescents is presented graphically in Figure 4, demonstrating the age effects: adolescent binding as a percent of that in the adult. A similar but less pervasive pattern was seen when comparing nicotine-treated adults and periadolescents: higher binding in younger rats. As reported in previous studies, there was widespread up-regulation of α4β2* binding in adult rats: the average binding across 38 regions was 55% higher after nicotine treatment, and 28 of 38 regions showed a statistically significant increase (none were lower). The increases in α4β2* binding with chronic nicotine were not uniform across regions: large and consistent increases were detected across cerebral cortex, and in many forebrain regions and hippocampus; effects were much more varied in diencephalon and midbrain structures. The effect of nicotine exposure on α4β2* receptors in periadolescents was noticeably smaller. The average binding across 38 regions was 27% higher after nicotine treatment, and only 13 of 38 regions showed a statistically significant increase (none were significantly decreased). The increase was larger in adults than in periadolescents for 35 of the 38 regions (for the remaining three, the larger increase in periadolescents was non-significant). The treatment effects of nicotine on binding in adults and adolescents is presented graphically in Figure 5, showing binding in nicotine-treated animals as a percent of that in saline-treated animals. To obtain a more global sense of the effects of age and treatment on α4β2* binding, we performed GLM analysis on the means collapsed across all regions measured. There was no interaction between age and treatment (F=0.17; p=0.6861). The overall effects of age (F=11.45; p=0.0021) and nicotine treatment (F=11.37; p=0.0022) were both highly significant for binding to the α4β2* nAChR.
Table 2.
Binding (fmoles) of [125I]A-85380 in the presence of 100 nM αCtxMII (to α4β2* nAChRs)
| Adults |
Periadolescents |
Treatment effects |
Age effects |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Saline |
Nicotine |
Saline |
Nicotine |
% chg from saline |
% chg from adult |
|||
| Mean+/−sem | Adult | Periadol. | Saline | Nicotine | |||||
| 1 | Nu. accumbens, core | 3.10±0.35 | 5.16±0.43 | 5.48±0.62 | 7.05±0.51 | 66.4** | 28.5* | 76.8** | 36.5* |
| 2 | Nu. accumbens, shell | 2.72±0.33 | 5.08±0.46 | 5.22±0.64 | 7.16±0.49 | 86.7** | 37.2* | 91.8** | 40.9* |
| 3 | Endopiriform nu. | 3.91±0.33 | 7.48±0.30 | 5.92±0.68 | 8.32±0.22 | 91.1*** | 40.4** | 51.4** | 11.3 |
| 4 | Striatum, rostral | 3.94±0.39 | 5.97±0.41 | 6.09±0.59 | 7.87±0.30 | 51.4* | 29.2* | 54.8** | 31.8* |
| 5 | Striatum, caudal | 3.39±0.35 | 5.36±0.50 | 5.40±0.66 | 7.13±0.48 | 58.3** | 32.1** | 59.2** | 32.9 |
| 6 | Lateral septum | 3.65±0.37 | 5.20±0.56 | 6.00±0.63 | 7.25±0.47 | 42.6* | 20.8 | 64.6** | 39.5* |
| 7 | Medial habenula | 8.14±0.26 | 8.49±0.15 | 8.70±0.04 | 8.57±0.13 | 4.3 | −1.5 | 6.9 | 0.9 |
| 8 | Fasiculus retroflexus | 6.02±0.36 | 6.96±0.32 | 7.66±0.35 | 8.19±0.25 | 15.6 | 6.9 | 27.2** | 17.7* |
| 9 | Posterior hypothalamus | 1.29±0.18 | 4.38±0.65 | 2.06±0.32 | 5.41±0.71 | 239** | 162*** | 59.4 | 23.3 |
| 10 | Ventral posterior thal. nu. | 6.12±0.44 | 8.21±0.32 | 7.54±0.41 | 8.50±0.21 | 34.2** | 12.8 | 23.2* | 3.5 |
| 11 | Posterior thal. nu. group | 6.91±0.41 | 8.46±0.24 | 8.08±0.27 | 8.63±0.13 | 22.4** | 6.8 | 16.9** | 2.0 |
| 12 | Lateral post. thal. nu. | 7.14±0.37 | 8.32±0.27 | 8.14±0.26 | 8.59±0.14 | 16.6* | 5.6 | 14.1* | 3.3 |
| 13 | Internal capsule | 1.52±0.17 | 2.32±0.33 | 4.12±0.71 | 4.82±0.66 | 52.6 | 16.8 | 171* | 107 |
| 14 | Optic tract | 1.92±0.20 | 2.00±0.32 | 4.58±0.87 | 5.28±0.63 | 4.6 | 15.2 | 139** | 163* |
| 15 | Optic chiasm | 5.88±0.45 | 6.98±0.39 | 7.43±0.44 | 8.16±0.28 | 18.7 | 9.9 | 26.4* | 16.9** |
| 16 | Superior collic., super. grey | 6.11±0.54 | 7.29±0.30 | 7.56±0.42 | 8.09±0.32 | 19.5 | 7.0 | 23.7 | 10.8* |
| 17 | Dorsal lateral geniculate | 7.91±0.27 | 8.78±0.04 | 8.47±0.15 | 8.65±0.12 | 11.0 | 2.2 | 7.1 | −1.4 |
| 18 | Ventral lateral geniculate | 5.67±0.60 | 6.32±0.30 | 6.70±0.59 | 7.71±0.46 | 11.5 | 15.1 | 18.2* | 22.2 |
| 19 | Medial geniculate | 6.24±0.45 | 8.07±0.22 | 7.71±0.39 | 8.39±0.23 | 29.3** | 8.9 | 23.4** | 4.0 |
| 20 | Zona incerta | 2.44±0.29 | 5.84±0.67 | 4.63±0.66 | 6.96±0.55 | 140** | 50.2* | 90.0** | 19.1 |
| 21 | Tectal nuclei | 5.44±0.56 | 6.02±0.30 | 6.72±0.52 | 7.46±0.47 | 10.7* | 11.1 | 23.6** | 24.1 |
| 22 | Substantia nigra, P.C. | 6.07±0.75 | 7.44±0.55 | 8.06±0.26 | 8.31±0.28 | 22.6 | 3.0 | 32.8 | 11.6 |
| 23 | Ventral tegmental area | 4.88±0.60 | 6.05±0.56 | 6.89±0.52 | 7.99±0.32 | 24.1* | 16.1 | 41.3* | 32.2 |
| 24 | Dentate gyrus, hippocampus | 2.35±0.31 | 5.00±0.61 | 4.27±0.62 | 7.09±0.44 | 112** | 66.0* | 81.5* | 41.8 |
| 25 | CA1, hippocampus | 1.21±0.13 | 3.29±0.48 | 2.29±0.37 | 5.06±0.68 | 171** | 120** | 89.1* | 53.7 |
| 26 | Subiculum | 3.61±0.42 | 4.93±0.29 | 5.79±0.62 | 6.52±0.56 | 36.5* | 12.7 | 60.2* | 32.3 |
| 27 | Postsubiculum | 7.23±0.37 | 8.25±0.16 | 8.37±0.18 | 8.60±0.14 | 14.1** | 2.8 | 15.7** | 4.3 |
| 28 | Pontine nu. | 2.40±0.47 | 3.98±0.44 | 4.11±0.77 | 5.99±0.67 | 66.1 | 45.6 | 71.7* | 50.5 |
| 29 | Entorhinal cortex | 3.01±0.37 | 6.50±0.24 | 5.15±0.70 | 7.33±0.50 | 116** | 42.3* | 71.1** | 12.8 |
| 30 | Frontal cortex, L4 | 4.08±0.40 | 6.59±0.50 | 6.04±0.59 | 7.83±0.38 | 61.6** | 29.7* | 48.1** | 18.9 |
| 31 | Frontal cortex, L5 | 3.10±0.32 | 6.70±0.38 | 5.20±0.66 | 7.58±0.44 | 116*** | 45.6* | 67.9** | 13.0 |
| 32 | Cingulate cortex, outer | 5.48±0.40 | 7.09±0.36 | 7.35±0.46 | 8.19±0.28 | 29.3** | 11.4 | 34.3** | 15.6 |
| 33 | Cingulate cortex, inner | 4.89±0.43 | 7.13±0.38 | 6.91±0.54 | 8.45±0.16 | 45.9** | 22.2 | 41.4** | 18.5 |
| 34 | Retrosplenial cortex, L1–2 | 6.79±0.41 | 8.04±0.18 | 8.07±0.33 | 8.57±0.14 | 18.4** | 6.2 | 18.8** | 6.6 |
| 35 | Retrosplenial cortex, L3–4 | 6.36±0.44 | 7.93±0.18 | 7.93±0.37 | 8.44±0.19 | 24.7** | 6.5 | 24.6** | 6.5 |
| 36 | Visual cortex, L4 | 4.95±0.50 | 7.54±0.31 | 6.70±0.61 | 8.11±0.32 | 52.4** | 21.0 | 35.5* | 7.6 |
| 37 | Visual cortex, L5 | 3.42±0.44 | 6.44±0.53 | 5.60±0.68 | 7.72±0.42 | 88.0*** | 37.9* | 63.4** | 19.8 |
| 38 | Visual cortex, L6 | 4.47±0.44 | 6.73±0.32 | 6.53±0.63 | 7.87±0.37 | 50.7** | 20.5 | 46.1** | 16.9 |
Means determined from 5–8 replicates. Means from all four groups compared by 2-way ANOVA with Holm-Sidak post-test
p<0.05
p<0.01
p<0.001.
Figure 4. Age effects: Binding to three nAChR subtypes compared in saline controls from adults and periadolescent rats.
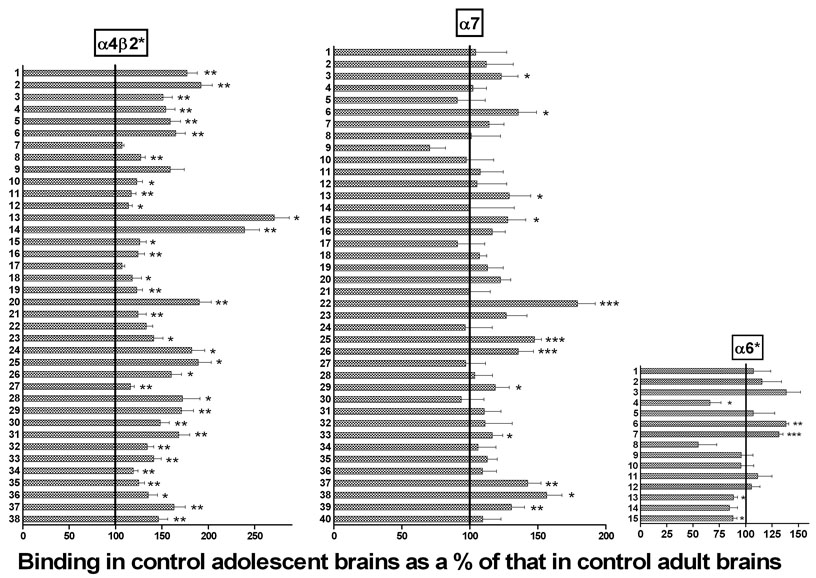
Binding in periadolescent brains is shown as a percent of that in the equivalent brain region in the adult animal; the100% line indicates where binding would be if it was equal in adults and periadolescents. Numbers refer to specific brain regions identified in Table 1 (for α4β2* nAChRs), Table 2 (for α7 nAChRs) or Table 3 (for α6* nAChRs). Different from binding in the equivalent brain region in adults: *p<0.05; **p<0.01; ***p<0.001; 2-way ANOVA with Holm-Sidak post-test.
Figure 5. Treatment effects: Binding to three nAChR subtypes in adult and periadolescent rat brains.
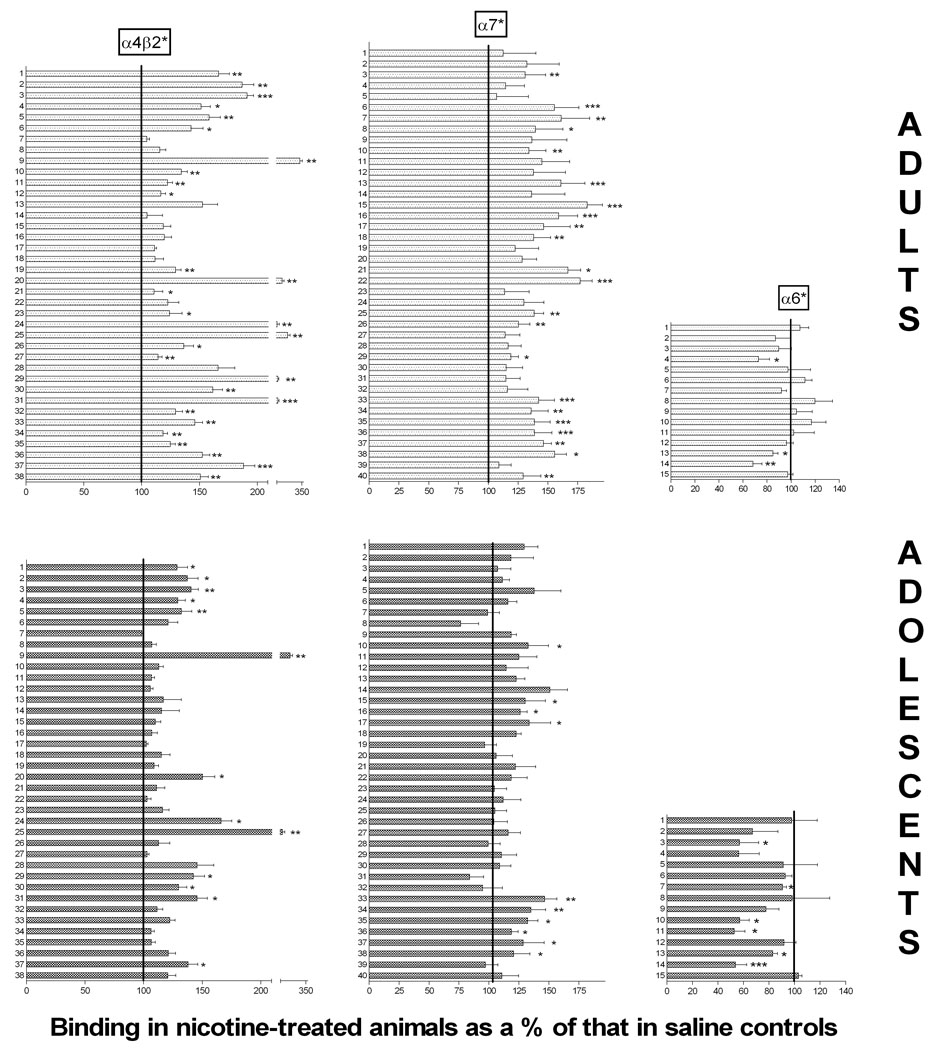
Binding in nicotine-treated animals is shown as a percent of that in the equivalent brain region in the saline-treated animals, for both adults (top three graphs) and periadolescents (bottom three graphs). The 100% line indicates where the binding would be if it was unaffected by nicotine treatment. Left two graphs show binding to α4β2* nAChRs; middle two graphs show binding to α7 nAChRs; right two graphs show binding to α3* nAChRs. Numbers on Y-axis refer to specific brain regions identified in Table 1 (for α4β2*), Table 2 (for α7) or Table 3 (for α6*). Different from binding in the equivalent brain region in saline controls: *p<0.05; **p<0.01; ***p<0.001; 2-way ANOVA with Holm-Sidak post-test.
Comparison of α7 binding in adult versus periadolescent saline-treated controls reveals similar but less dramatic age-related differences (Table 3; Figure 4). The mean binding across all 40 regions was 15% higher in saline-treated periadolescents compared to saline-treated adults, with 12 of 40 regions measured demonstrating significantly higher binding in the younger rats (none were significantly lower in periadolescents). For nicotine treated animals, there was overall no difference between older and younger rats: two regions were significantly higher in periadolescents, while three were significantly lower. As previously reported, chronic nicotine also causes up-regulation of α7 binding in adult rats (Rasmussen and Perry 2006) and mice (Pauly et al. 1991) although to a lesser degree and in fewer regions than occurs with α4β2* nAChRs. Across all 40 regions, there was a mean binding increase of 36% in adult rats, with 20 of 40 regions demonstrating significant increases; no region showed significantly lower binding after nicotine (Table 3; Figure 5). Up-regulation was most prominent in cerebral cortex, with several regions also up-regulated in hippocampus, hypothalamus and amygdala. As with α4β2*, the nicotine effect was much smaller in periadolescents. The mean increase was 16%, and only 10 of 40 regions showed significant increases (none showed significant decreases). To obtain a more global sense of the effects of age and treatment on α7* binding, we performed GLM analysis on the means collapsed across all regions measured. There was no interaction between age and treatment (F=2.32; p=0.1402). The overall effect of age was not significant (F=2.41; p=0.1334), but the overall effect of nicotine treatment was significant (F=9.62; p=0.0047).
Table 3.
Binding (fmoles) of [ 125I]α -bungarotoxin ( to α7 nAChRs)
| Adults |
Periadolescents |
Treatment effects |
Age effects |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Saline | Nicotine | Saline | Nicotine | %chg from saline | %chg from adult | |||
| Mean +/−sem | Adult | Periadol. | Saline | Nicotine | |||||
| 1 | Striatum | 1.51±0.46 | 1.70±0.43 | 1.58±0.24 | 2.04±0.17 | 12.6 | 29.4 | 4.5 | 20.0 |
| 2 | Nucleus accumbens | 1.78±0.48 | 2.35±0.64 | 1.99±0.27 | 2.36±0.55 | 32.5 | 18.3 | 12.1 | 0.1 |
| 3 | Septum | 6.03±1.04 | 7.90±1.34 | 7.43±0.63 | 7.97±1.07 | 31.0** | 7.2 | 23.2* | 0.8 |
| 4 | Nu. vertical limb diag. band | 5.65±0.93 | 6.47±0.99 | 5.79±0.19 | 6.43±0.52 | 14.6 | 11.2 | 2.5 | −0.6 |
| 5 | Ventral pallidum | 3.79±1.01 | 4.06±1.07 | 3.45±0.47 | 4.74±1.37 | 7.0 | 37.6 | −9.1 | 16.9 |
| 6 | Endopiriform nu. | 10.7±1.99 | 16.7±3.59 | 14.5±1.39 | 16.9±0.92 | 55.6*** | 15.9 | 35.7* | 1.1 |
| 7 | Bed nu., stria terminalis | 5.77±0.86 | 9.29±2.75 | 6.58±0.50 | 6.50±0.77 | 61.1** | −1.1 | 14.1 | −30.0* |
| 8 | Anterior hypothalamus | 9.67±2.52 | 13.5±2.86 | 9.79±1.61 | 7.47±1.01 | 39.5* | −23.7 | 1.3 | −45.0* |
| 9 | Supraoptic nu. hypothalamus | 26.1±3.67 | 35.6±14.5 | 18.5±1.43 | 21.9±0.36 | 36.7 | 18.4 | −29.2 | −38.6 |
| 10 | Posterior hypothalamus | 12.3±2.87 | 16.4±1.24 | 12.0±1.99 | 15.9±2.68 | 34.0** | 32.8* | −2.4 | −3.2 |
| 11 | Lateral hypothalamus | 4.85±1.35 | 7.03±1.44 | 5.23±0.33 | 6.54±1.43 | 44.9 | 24.9 | 7.8 | −7.1 |
| 12 | Ventromedial hypothalamus | 13.0±3.40 | 17.9±4.94 | 13.7±2.42 | 15.7±2.92 | 37.9 | 14.4 | 5.4 | −12.6 |
| 13 | Zona incerta | 3.84±1.16 | 6.18±0.87 | 4.95±0.23 | 6.08±0.58 | 60.9*** | 22.7 | 29.0* | −1.6 |
| 14 | Subthalamic nu. | 20.4±10.39 | 27.8±3.05 | 20.5±2.70 | 30.9±4.75 | 36.5 | 50.9 | 0.5 | 11.0 |
| 15 | Lateral amygdaloid nuclei | 5.23±0.58 | 9.56±1.33 | 6.68±1.01 | 8.70±1.59 | 82.9*** | 30.2* | 27.8* | −9.0 |
| 16 | Basal amygdaloid nuclei | 17.7±2.74 | 28.2±4.61 | 20.7±0.90 | 26.1±1.88 | 59.1*** | 26.0* | 16.6 | −7.6 |
| 17 | Posteromed. cort. amygdala | 19.3±4.70 | 28.2±5.93 | 17.5±2.65 | 23.4±4.65 | 46.4** | 33.6* | −9.0 | −17.0 |
| 18 | Amygdalohippocampal area | 18.1±1.11 | 24.9±5.08 | 19.4±0.88 | 23.8±0.92 | 38.0** | 22.6 | 7.2 | −4.7 |
| 19 | Stratum oriens, hippocampus | 7.58±1.23 | 9.28±2.11 | 8.57±0.64 | 8.26±0.98 | 22.5 | −3.5 | 13.0 | −11.0 |
| 20 | S. lac.-molec., hippocampus | 5.55±0.44 | 7.12±1.11 | 6.79±0.51 | 7.20±1.40 | 28.4 | 6.0 | 22.5 | 1.1 |
| 21 | Dentage gyrus, hippocampus | 11.3±1.46 | 18.9±1.77 | 11.4±1.82 | 13.9±2.47 | 66.8* | 22.1 | 0.6 | −26.0* |
| 22 | Ventral CA1, hippocampus | 6.18±0.80 | 10.9±0.93 | 11.1±1.43 | 13.1±1.80 | 77.2*** | 18.4 | 79.1*** | 19.6 |
| 23 | Posterior dentate gyrus | 58.8±12.38 | 66.9±13.6 | 74.5±8.03 | 77.8±7.45 | 13.8 | 4.5 | 26.7 | 16.4 |
| 24 | Parasubiculum | 15.1±2.89 | 19.6±2.96 | 14.6±2.96 | 16.3±1.65 | 29.8 | 11.8 | −3.1 | −16.6 |
| 25 | Tectal nuclei | 8.41±0.22 | 11.7±1.40 | 12.4±0.82 | 13.0±1.69 | 38.5** | 4.9 | 47.6*** | 11.8 |
| 26 | Ventral lateral geniculate | 11.1±1.57 | 13.9±0.89 | 15.1±1.34 | 15.7±2.13 | 25.2** | 4.0 | 35.7*** | 12.7 |
| 27 | Superior collic.,super. grey | 19.7±2.83 | 22.4±2.34 | 19.1±2.70 | 22.2±1.46 | 14.2 | 16.1 | −2.7 | −1.1 |
| 28 | Superior collic., optic L | 11.4±1.81 | 13.4±0.90 | 11.9±1.19 | 11.8±1.19 | 16.8 | −0.7 | 3.8 | −11.7 |
| 29 | Mammillary nu. | 19.5±1.23 | 23.2±1.56 | 23.1±3.15 | 25.5±3.05 | 18.9* | 10.3 | 18.7* | 10.1 |
| 30 | Dorsal raphe nu. | 11.7±2.78 | 13.5±0.73 | 11.0±1.08 | 12.0±1.06 | 15.0 | 8.9 | −6.3 | −11.3 |
| 31 | Parabigeminal nu. | 15.4±2.46 | 17.7±1.49 | 17.0±1.50 | 14.3±2.16 | 14.8 | −16.1 | 10.7 | −19.1 |
| 32 | Microcellular tegmental nu. | 12.3±2.43 | 14.2±2.10 | 13.6±2.73 | 12.9±1.68 | 16.1 | −5.3 | 11.2 | −9.3 |
| 33 | Frontal cortex, inner laminae | 5.76±0.49 | 8.20±1.31 | 6.72±0.46 | 9.84±1.24 | 42.4*** | 46.4** | 16.6* | 19.9* |
| 34 | Frontal cortex outer laminae | 3.73±0.59 | 5.08±0.66 | 3.96±0.41 | 5.35±0.72 | 36.0** | 35.1** | 6.2 | 5.4 |
| 35 | Cingulate cortex, lateral | 6.02±0.75 | 8.34±1.16 | 6.79±0.20 | 8.99±1.11 | 38.7*** | 32.4* | 12.9 | 7.8 |
| 36 | Cingulate cortex, medial | 4.79±0.82 | 6.64±0.87 | 5.24±0.20 | 6.23±0.43 | 38.7*** | 18.8* | 9.4 | −6.2 |
| 37 | Visual cortex, inner laminae | 3.15±0.15 | 4.61±0.37 | 4.50±0.59 | 5.77±1.21 | 46.3** | 28.5* | 42.6** | 25.2* |
| 38 | Visual cortex, outer laminae | 3.92±0.42 | 6.11±0.58 | 6.14±0.70 | 7.41±1.13 | 55.7* | 20.6* | 56.5* | 21.3 |
| 39 | Retrosplenial cortex | 5.18±0.67 | 5.64±0.43 | 6.76±0.48 | 6.56±0.90 | 9.0 | −3.0 | 30.6** | 16.2 |
| 40 | Entorhinal cortex | 10.4±1.41 | 13.5±2.14 | 11.4±1.52 | 12.7±1.83 | 29.3** | 10.8 | 9.5 | −6.1 |
Means determined from 5–8 replicates. Means from all four groups compared by 2-way ANOVA with Holm-Sidak post-test
p<0.05
p<0.01
p<0.001.
Comparison of [125I]α-CtxMII in adult versus periadolescent saline-treated controls did not show system-wide age-related differences (Table 4; Figure 4): the mean binding across 15 regions in periadolescents was 2% higher than in adults. Two regions (optic chiasm, optic tract) were significantly greater in periadolescents, while three (caudal striatum, dorsal lateral geniculate, superior colliculus) were significantly lower. The comparison across age in nicotine-treated animals showed that six regions were significantly lower in periadolescents, while one was higher (optic tract). In contrast to α4β2* and α7 nAChRs, autoradiographic studies have found that chronic nicotine causes either no change or a decrease in binding to α6* nAChRs (Perry et al. 2007;Mugnaini et al. 2006;Lai et al. 2005). In the current studies we also found a trend towards decreased binding with chronic nicotine exposure: a 5% average decrease across 15 regions; binding was significantly decreased in three regions, increased in none (Table 4; Figure 5). In contrast to the other two nAChR subtypes, the effect of chronic nicotine was more pronounced in periadolescents than adults: across all 15 regions, there was a mean decrease of 21%, and six of 15 regions in periadolescents showed a significant decrease with nicotine exposure. To obtain a more global sense of the effects of age and treatment on α6* binding, we performed GLM analysis on the means collapsed across all regions measured. There was no significant interaction between age and treatment (F=3.68; p=0.0653). The overall effect of age was not significant (F=0.23; p=0.6352), but the overall effect of nicotine treatment was significant (F=3.68; p=0.0044).
Table 4.
Binding (fmoles) of [125I]α-conotoxin MII (to α6* nAChRs).
| Adults |
Periadolescents |
Treatment effects |
Age effects |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain region | Saline |
Nicotine |
Saline |
Nicotinev |
%chg from saline |
%chg from adults |
|||
| Mean +/−sem | Adult | Periadol. | Saline | Nicotine | |||||
| 1 | Nu. ccumbens, shell | 0.11±0.01 | 0.11±0.01 | 0.12±0.03 | 0.11±0.02 | +7.3 | −2.2 | +7.3 | +0.1 |
| 2 | Nu. accumbens, core | 0.19±0.03 | 0.16±0.02 | 0.21±0.05 | 0.14±0.02 | −13.0 | −33.0 | +15.3 | −11.1 |
| 3 | Striatum, rostral | 0.21±0.02 | 0.19±0.02 | 0.29±0.05 | 0.17±0.02 | −10.3 | −43.1 | +38.7 | −11.9 |
| 4 | Striatum, caudal | 0.31±0.02 | 0.22±0.03 | 0.20±0.03 | 0.11±0.02 | −27.2* | −43.8* | −33.3* | −48.9* |
| 5 | Olfactory tubercle | 0.14±0.03 | 0.14±0.02 | 0.15±0.03 | 0.14±0.05 | −2.7 | −8.9 | +7.0 | +0.2 |
| 6 | Optic chiasm | 1.64±0.04 | 1.84±0.15 | 2.27±0.06 | 2.11±0.17 | +11.6 | −7.3 | +38.2** | +14.7 |
| 7 | Optic tract | 1.84±0.07 | 1.69±0.08 | 2.42±0.09 | 2.19±0.06 | −8.1 | −9.5* | +31.6*** | +29.7*** |
| 8 | Medial habenula | 0.26±0.03 | 0.31±0.05 | 0.14±0.04 | 0.14±0.04 | +19.9 | −1.8 | −45.1 | −55.1* |
| 9 | Fasiculus retrflexus | 0.40±0.05 | 0.42±0.06 | 0.38±0.03 | 0.30±0.03 | +4.3 | −22.3 | −4.1 | −28.5 |
| 10 | Ventral tegmental area | 0.28±0.04 | 0.32±0.03 | 0.26±0.02 | 0.15±0.01 | +16.9 | −43.0* | −4.4 | −53.4*** |
| 11 | Substantia nigra, PC | 0.28±0.05 | 0.29±0.05 | 0.32±0.03 | 0.17±0.01 | +2.1 | −47.4* | +11.4 | −42.6** |
| 12 | Tectal nuclei | 1.82±0.13 | 1.75±0.07 | 1.92±0.17 | 1.76±0.17 | −3.9 | −8.3 | +5.5 | +0.7 |
| 13 | Dorsal lateral geniculate | 1.95±0.07 | 1.66±0.08 | 1.73±0.07 | 1.43±0.05 | −15.1* | −17.1** | −11.6* | −13.7 |
| 14 | Ventral lateral geniculate | 1.84±0.12 | 1.26±0.11 | 1.56±0.13 | 0.84±0.08 | −31.8** | −46.4*** | −15.4 | −33.5* |
| 15 | Superior collic. sup. grey | 3.27±0.14 | 3.18±0.14 | 2.88±0.10 | 2.97±0.06 | −2.9 | +2.8 | −12.1* | −6.6 |
Means determined from 5–8 replicates. Means from all four groups compared by 2-way ANOVA with Holm-Sidak post-test
p<0.05
p<0.01
p<0.001.
3. DISCUSSION
These results demonstrate the first comprehensive characterization of the three major nAChR subtypes in adolescent and adult rat brain, and the first direct comparison of their differential response to chronic nicotine treatment in multiple brain regions. These data demonstrate that adolescent and adult rats exhibit distinct differences in the numbers of α4β2* nAChRs, and to a lesser extent α7 nAChRs, throughout the brain; however, α6* nAChRs are largely similar at both ages. Furthermore, the response of these receptors to chronic nicotine also differs by age: up-regulation of α4β2* and α7 receptors was greater in adults, but down-regulation of α6* receptors was greater in adolescents.
Developmental differences in nAChR expression have been reported in a few previous studies. Leslie and colleagues reported differences between PN20 and adult rats in expression of midbrain nAChR subunit mRNA (Azam et al. 2007). They found a gradual decline of midbrain [3H]nicotine binding after birth (although no significant differences were detected between adults and adolescents); midbrain [125I]α-Btx binding remained constant throughout development (Azam et al. 2007). [3H]cytisine binding was reported to be higher in cerebral cortex and hippocampus but not midbrain of PN45 rats compared to PN60 or PN75 rats (Trauth et al. 1999). A recent autoradiographic study in mice found that [3H]cytisine and [3H]epibatidine binding tended to peak developmentally at PN21 before declining to adult levels, while [125I]α-Btx binding peaked earlier at PN10, before also declining to adult levels (Yu et al. 2007). Our results are generally consistent with these studies, and extend them by directly comparing the three major nAChR subtypes at both ages.
Age differences in nAChR function have also been reported. Nicotine-stimulated 86Rb efflux was found to be higher in four brain regions in PN35 rats compared to PN28 or PN63 rats (Britton et al. 2007). This method measures primarily presynaptic nAChRs, primarily α4β2* (Marks et al. 1999). These results are consistent with our findings that α4β2* binding was significantly higher in periadolescent rats (PN42) in most areas sampled from these four brain regions, suggesting that this adolescent “peak” response is the result of increased receptor expression. Nicotine-stimulated striatal dopamine release was found to be greater in PN30 compared to PN40 or adult rats (Azam et al. 2007). In vivo microdialysis studies showed that adult rats responded to an acute nicotine challenge with an increase in extracellular striatal dopamine, whereas adolescent rats did not exhibit significant increases to such a challenge (Badanich and Kirsteina 2004). Nicotine-stimulated dopamine release from in vitro striatal preparations is mediated both by α4β2* and α6* receptors located on dopamine terminals (Salminen et al. 2004;Perry et al. 2007), whereas in vivo, nicotine-stimulated dopamine release is largely mediated by α4β2* receptors (Champtiaux et al. 2003). Overall we found more α4β2* receptors in both dopamine cell body and terminal regions in adolescents compared to adults, but no net difference in α6* receptors. This difference in the balance between these two subtypes may contribute to an enhanced sensitivity to the rewarding effects of nicotine in adolescents.
Nicotine has long been known to cause up-regulation of its receptors following chronic administration. One previous study directly compared nicotine’s effects on nAChRs in adults and adolescents, using the same treatment protocol. Up-regulation of [3H]cytisine binding (largely to α4β2* nAChRs) was detected in midbrain, hippocampus and cerebral cortex of both adolescents and adults, but differences were seen in regional specificity and persistence (Trauth et al. 1999). Up-regulation varied by region in adults but was uniform in adolescents; in addition, adolescent up-regulation showed greater persistence (Trauth et al. 1999). Direct comparison with the present results is difficult given their use of homogenate binding, and the different time points employed. Similar to their findings, we saw greater increases in α4β2* binding in adult hippocampal regions compared to adolescents. We saw only modest and often non-significant increases in midbrain structures, in both age groups. As for the greater regional variability of response in adults, this is difficult to quantify; instead, we found an overall much greater responsiveness of adult α4β2* binding to up-regulation by nicotine.
Relatively less attention has been paid to the non-α4β2 nAChRs in adolescents, in part due to the paucity of selective tools. One study used homogenate binding to demonstrate modest up-regulation of [125I]α-Btx binding in the striatum and brainstem of periadolescent rats (Slotkin et al. 2004). They noted that previous studies failed to detect up-regulation in adults, and concluded that this represented a developmental difference. However, none of the studies cited used the dosing regimen employed in their study, which was the same as that used in the current study, and which we have previously shown does cause up-regulation of [125I]α-Btx binding in adult rats (Rasmussen and Perry 2006). Clearly this subtype is less prone to up-regulation compared to α4β2* (Pauly et al. 1991). This may be a consequence of the relatively lower affinity of α7 receptors to nicotine compared to the α4β2* subtype. A similar explanation has been advanced for the resistance of α3β4* nAChRs to nicotine-induced up-regulation (Nguyen et al. 2003;Dávila-García et al. 2003). However, affinity differences alone cannot explain all subtype differences in nicotine regulation: α6* nAChRs exhibit affinity for nicotine in the same nanomolar range as α4β2* nAChRs (Zoli et al. 2002), and this subtype is either not affected or is down-regulated by chronic nicotine exposure. The present results demonstrate that this subtype is unique in other ways as well. First, it does not demonstrate the same developmental arc as the α4β2* and α7 subtypes, but instead shows relatively constant levels from adolescence to adulthood. Second, its responsiveness to nicotine regulation shows a different age-dependence than these other two subtypes: α6* nAChRs are more prone to nicotine-induced changes in adolescents than in adults. One possible explanation for these differences would be if the subunit composition of α6* nAChRs differed in the adolescent. We have recently shown that α6* nAChRs co-expressing the β3 subunit are resistant to nicotine-induced down-regulation compared to α6* nAChRs lacking this subunit (Perry et al. 2007).
Because this treatment protocol relies upon initial body weight for dose calculations, and because adolescents grow at a faster rate during the two-week dosing period, it was not surprising to find that by the end of the period, the actual per-weight dose in adolescents, as well as the corresponding blood levels of both nicotine and cotinine, were approximately half that in adults; similar results were reported previously by Slotkin and colleagues (Trauth et al. 2000b). The finding that brain nicotine levels in adults were also roughly twice those in adolescents is novel. While this difference is unrelated to differences in receptor expression between age groups, we cannot rule out that it contributes to differences in responsiveness to chronic nicotine exposure. Note, however, that the brain concentrations were quite high; even the lower concentration in adolescent brain would yield >99% occupancy of both α4β2* and α6* nAChRs, which suggests that the differences in concentration achieved may not be relevant to differential responsiveness of at least these two high-affinity receptor subtypes.
Our studies do not differentiate between an increased number of receptors per neuron or an increase in the number of neurons expressing nAChRs. It is recognized that synaptic pruning occurs during adolescence (Spear 2004). The widespread difference in α4β2* and α7 receptors between periadolescent and adult brains that we detected could be a result of such pruning. The age differences in responsiveness to nAChR up-regulation are more difficult to explain, in part because the mechanism(s) for such regulation remain elusive. Evidence from in vitro expression systems suggests an effect of nicotine on subunit assembly (Kuryatov et al. 2005); it is unclear how such a process might differ in adolescent brain. A generalization of the current data might be that up-regulation is greater when initial receptor levels are lower; this suggests a possible “ceiling” effect on the process. Evidence for a ceiling effect on nAChR up-regulation has not been seen with in vitro models, which often demonstrate far greater increases than seen in vivo. Furthermore, the generalization of less up-regulation with higher initial expression is far from uniform across brain regions. We recently reported that co-expression of α5 with α4β2* nAChRs is associated with resistance to nicotine-induced up-regulation (Mao et al. 2008); as discussed above with the α6* nAChR, it is possible that adolescent α4β2* nAChRs demonstrate a different subunit composition than adults, which could affect sensitivity to up-regulation by nicotine.
Differences between subtypes in their regulation by chronic nicotine mean that the overall balance of receptor subtypes will shift with continued exposure, and presumably the response pattern to subsequent nicotine exposure. It should be pointed out that these experiments do not assess receptor functionality; because nAChRs desensitize with continued agonist stimulation, it is possible that the rate or extent of desensitization, or recovery from desensitization, differs in adult and adolescent animals. We have previously shown that the increased numbers of α4β2-like receptors following identical chronic nicotine exposure in adult rats represent functional receptors, as assayed by 86Rb efflux (Nguyen et al. 2004), and that the decreased numbers of α6* receptors in striatum were accompanied by a like decrease in αCtxMII-sensitive nicotine stimulated dopamine release from striatal synaptosomes (Perry et al. 2007).
In conclusion, adolescent rats have a distinct pattern of nAChR expression, and respond differently to chronic nicotine exposure, compared to adult rats. A different pattern of CNS nicotinic receptor expression may play a role in the initiation of smoking among adolescents. Furthermore, the distinct pattern of responses of nAChR subtypes to nicotine during adolescence may contribute to the higher daily consumption and decreased probability of cessation observed in smokers who initiate tobacco use during adolescence.
4. EXPERIMENTAL PROCEDURES
Materials
[125I]α-conotoxin MII ([125I]α-CtxMII) was synthesized by the method of Whiteaker (Whiteaker et al. 2000), as adapted by us (Perry et al., 2007). Briefly, Tyr0-α-CtxMII (kind gift of J. Michael McIntosh) was reacted with 10 mCi Na125I (22 µl; PerkinElmer Life Sciences, Boston, MA) using the chloramine-T method. The reaction mixture was then purified by reversed phase HPLC and fractions collected. This protocol readily separates unreacted Tyr0-α-CtxMII from the mono-iodo and di-iodo forms (Whiteaker et al. 2000); only the mono-iodo form was utilized, and based on the purification was assumed to be maximally iodinated (2200 Ci/mmol). [125I]A-85380 and [125I]αBtx were purchased from Perkin-Elmer Life Sciences (Shelton, CT). All other chemicals not otherwise mentioned were obtained from Sigma-Aldrich (St. Louis, MO).
Animal Treatment
Osmotic minipumps (Alzet model 2002; Durect Corporation, Cupertino, CA) were filled with sterile saline or with nicotine hydrogen tartrate dissolved in saline, at concentrations calculated to achieve a dose of 6 mg/kg/day, calculated as nicotine free base (37 µmole/kg).
Minipumps were implanted into male Sprague-Dawley rats (Hilltop Lab Animals, Scottdale, PA) of two ages, at postnatal (PN) day 29 or 70–90; eight animals were used for each treatment group. The period from PN28–40 in rats is typically labeled periadolescence, that from PN40–52 middle adolescence, and PN52–60 late adolescence; “puberty” generally occurs during the last days of periadolescence (Spear 2004). Thus, our treatment was performed at the early, periadolescent stage. Rats were anesthetized with isoflurane and the minipumps inserted into a subcutaneous pocket via a small incision made over the shoulders. While under anesthesia, animals were administered buprenorphine (0.1 mg/kg, s.c.) for post-operative pain. The wound was closed with clips and the area swabbed with antiseptic. After recovery from anesthetic (10–30 min), animals were returned to individual cages. Fourteen days after minipump implantation (PN42 for adolescents PN83–103 for adults), animals were lightly anesthetized with isoflurane and sacrificed by decapitation.
Because the per weight dose changes as animals grow, we used a parallel set of animals to test for the blood and brain levels of nicotine and cotinine occurring in adolescent and adult rats treated with nicotine as described. Animals were weighed prior to sacrifice; trunk blood was collected and plasma prepared and frozen. Forebrains were then extracted as previously described (Ghosheh et al. 2001). Briefly, forebrains were removed, rinsed in saline and dried, then homogenized in 3 volumes of ice-cold 1.15% KCl. After centrifugation for 30 min at 3000×G at 4°C, the supernatant was treated with 1 ml of 2% w/v ZnSO4 for 1 hr at 34°C to precipitate proteins. This mixture was then centrifuged at 30,000×G for 60 min at 4°C, after which the supernatant was removed and frozen.
Concentrations of nicotine and cotinine were determined in the laboratory of Dr. Neal Benowitz (San Francisco General Hospital Clinical Pharmacology Laboratory) by gas chromatography with nitrogen-phosphorus detection (Jacob, III et al. 1981), modified for simultaneous extraction of nicotine and cotinine, and determination using capillary GC (Jacob, III et al. 1991). The internal standards, 5-methylnicotine and ortho-cotinine, were obtained from Dr. Peyton Jacob, III (Division of Clinical Pharmacology of the Department of Medicine, University of California, San Francisco).
Animal use and procedures were approved by the George Washington University Medical Center Institutional Animal Care and Use Committee.
Autoradiography
Following decapitation, brains were rapidly removed and frozen on dry ice. Frozen coronal brain sections (16 µm) were cut and mounted onto Superfrost Plus slides (Fisher Scientific, Newark, DE) and stored at −80°C until use. For each of the three autoradiographic experiments described below, sections from all four treatment groups were incubated together and in random order, to avoid artifacts from in vitro processing.
[125I]α-CtxMII autoradiography was adapted from Perry et al. (Perry et al. 2007). Sections were preincubated for 15 min in buffer 1 (20 mM HEPES, pH 7.5, 144 mM NaCl, 1.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM PMSF, 0.1 % BSA) at room temperature. This was followed by incubation for 60 min at room temperature in buffer 2 (= buffer 1 plus 5 mM EGTA, 5 mM EDTA, 10 µg/ml aprotinin, 10 µg/ml pepstatin A, 10 µg/ml leupeptin) containing 0.8 nM [125I]α-CtxMII (2200 Ci/mmol). Adjacent sections were incubated in the same buffer with 100 µM nicotine added to determine non-specific binding. Slides were then rinsed for 5 min at room temperature in buffer 1, followed by 10 min in buffer 1 on ice, then sequential dips in ice-cold 5 mM HEPES and H2O followed by rapid air-drying.
Autoradiography for [125I]-5-iodo-3-(2(S)-azetidinylmethoxy)pyridine ([125I]A-85380) was adapted from Perry et al. (Perry et al. 2007) Sections were first pre-incubated in buffer 1 for 15 min at room temperature, followed by incubation for 60 min at room temperature in buffer 1 with 0.6 nM [125I]A-85380 (2200 Ci/mmol) plus 100 nM unlabeled α-CtxMII. Adjacent sections were incubated in the same buffer with 100 µM nicotine added to determine non-specific binding. After incubation, sections were rinsed twice for five min in buffer 1, followed by a water dip and rapid air-drying.
[125I]α-bungarotoxin ([125I]αBtx) binding was adapted from Rasmussen and Perry (Rasmussen and Perry 2006). Sections were pre-incubated for 30 min at room temperature in 50 mM TrisHCl, pH 7.3, containing 0.1% BSA, then transferred to this same buffer containing 0.72 nM 125I-α-bungarotoxin (120 Ci/mmole). Adjacent sections were incubated in the same buffer with 100 µM nicotine added to determine non-specific binding. After 120 min incubation at room temperature, sections were then dipped in ice-cold Tris buffer, then rinsed three times for 10 min in ice-cold buffer, followed by a dip in distilled water and air-dried.
After overnight desiccation, the sections were apposed to film (Kodak BioMax MR) for 4 days (for [125I]α-CtxMII and [125I]A-85380) or 38 days (for [125I]α-bungarotoxin) along with 125I standards (GE Healthcare, Piscataway, NJ); film was developed in an automatic developer. Film images were digitized and quantitative densitometric analysis of binding was done using the Loats Inquiry digital densitometry system (Loats Associates, Winchester, MD). Quantification of binding was done by comparison with standard curves constructed from 125I standards; regions were identified by comparison with the rat brain atlas of Paxinos and Watson (Paxinos and Watson 2007). Non-specific binding in adjacent sections was subtracted from the total binding in the paired section to calculate specific binding. While eight animals were used for each treatment group, for some regions the number of replicates measured per treatment group was less (5–8) due to damaged sections or other technical considerations.
Log-transformed means of specific binding in individual brain regions for each radioligand were compared using two-way ANOVA (SigmaStat 3.5) followed by Holm-Sidak post-hoc comparison of the four treatment groups; differences were accepted at p<0.05. To determine the global effects of age and treatment on binding of each receptor subtype, we used the general linear model (GLM) procedure in SAS (version 8) to test main effects and interactions for the age and treatment variables. Due to non-normality of the dependent variables of the [125I]αCtxMII binding, even after a log transformation, the GLM procedure was done on the ranks of the variables.
ACKNOWLEDGEMENTS
This work was supported by NIH grant DA015767 (DCP). We thank Dr. Samuel Simmens and Jacqueline Milton for their gracious help with statistical analysis.
Non-standard abbreviations
- A-85380
5-iodo-3-(2(S)-azetidinylmethoxy)pyridine
- α-CtxMII
α-conotoxin MII
- nAChR
neuronal nicotinic acetylcholine receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Res. 2003;988:164–172. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Deroche-Gamonet V, Le MM, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184:382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Kirsteina CL. Nicotine administration significantly alters accumbal dopamine in the adult but not in the adolescent rat. Ann N Y Acad Sci. 2004;1021:410–417. doi: 10.1196/annals.1308.054. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Britton AF, Vann RE, Robinson SE. Perinatal nicotine exposure eliminates peak in nicotinic acetylcholine receptor response in adolescent rats. J Pharmacol Exp Ther. 2007;320:871–876. doi: 10.1124/jpet.106.112730. [DOI] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new α-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio JL, Kellar KJ. Chronic nicotine administration does not increase nicotinic receptors labeled by [125I]epibatidine in adrenal gland, superior cervical ganglia, pineal or retina. J Neurochem. 2003;85:1237–1246. doi: 10.1046/j.1471-4159.2003.01774.x. [DOI] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2'-(14)C]nicotine. Drug Metab Dispos. 2001;29:645–651. [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Middaugh LD. Periadolescent nicotine exposure reduces cocaine reward in adult mice. J Addict Dis. 1999;18:27–39. doi: 10.1300/J069v18n03_04. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom JM. Nicotine acts as a pharmacological chaperone to upregulate human {alpha}4{beta}2 AChRs. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment decreases striatal {alpha}6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor α6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. Two pharmacologically distinct components of nicotinic receptor- mediated rubidium efflux in mouse brain require the β2 subunit. J Pharmacol Exp Ther. 1999;289:1090–1103. [PubMed] [Google Scholar]
- McMillen BA, Davis BJ, Williams HL, Soderstrom K. Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. Eur J Pharmacol. 2005;509:161–164. doi: 10.1016/j.ejphar.2005.01.002. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [(125)I]Y(0)-alpha-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137:565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates {alpha}6- and {beta}3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Perry DC. An autoradiographic analysis of [(125)I]alpha-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci Lett. 2006;404:9–14. doi: 10.1016/j.neulet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Seidler FJ. Administration of nicotine to adolescent rats evokes regionally selective upregulation of CNS alpha 7 nicotinic acetylcholine receptors. Brain Res. 2004;1030:159–163. doi: 10.1016/j.brainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Piattoni-Kaplan M, Campbell JE, McKenna DG, Molinari E, Hettinger AM, Garvey DS, Wasicak JT, Holladay MW, Williams M, Arneric SP. A-85380 [3-(2(S)-azetidinylmethoxy) pyridine]: in vitro pharmacological properties of a novel, high affinity α4β2 nicotinic acetylcholine receptor ligand. Neuropharmacology. 1996;35:725–734. doi: 10.1016/0028-3908(96)84644-2. [DOI] [PubMed] [Google Scholar]
- Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: effects on cholinergic systems in rat brain regions. Brain Res. 2000a;873:18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000b;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-α-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000;57:913–925. [PubMed] [Google Scholar]
- Yu WF, Guan ZZ, Nordberg A. Postnatal upregulation of alpha4 and alpha3 nicotinic receptor subunits in the brain of alpha7 nicotinic receptor-deficient mice. Neuroscience. 2007;146:1618–1628. doi: 10.1016/j.neuroscience.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


