Abstract
Previous studies have shown that both murine and human anti-double-stranded DNA (anti-dsDNA) antibodies can develop from non-DNA–reactive B cells and suggest a crucial role for somatic mutation in dsDNA binding. However, since only a limited number of human anti-dsDNA antibodies have been analyzed previously, we could not exclude other mechanisms for the generation of anti-dsDNA antibodies in patients with systemic lupus erythematosus (SLE). Therefore, we isolated IgM anti-dsDNA antibodies from peripheral blood B cells of a patient with SLE. Three somatically mutated IgM anti-DNA antibodies with pathogenic potential (glomerular binding) were reverted to their germline configuration. Although all three IgM anti-dsDNA antibodies came from the same lupus patient, they displayed different profiles. Reversion to the germline sequence of autoantibodies A9 and B5 resulted in decreased dsDNA binding. In contrast, the germline form of G3-recognized dsDNA as well as the mutated counterpart. These results suggest that mutated IgM anti-dsDNA antibodies may develop from both DNA- and non-DNA–reactive B cells. The implications are that B cell activation occurs in response to self and non-self antigens, while selection after activation may be mediated by self antigen in SLE. Moreover, ineffective tolerance checkpoints may exist before and after antigen activation in SLE.
INTRODUCTION
Antibodies to a wide variety of autoantigens are a hallmark of systemic lupus erythematosus (SLE) (1). In particular, anti-double-stranded DNA (anti-dsDNA) antibodies are associated with lupus nephritis and disease activity (2–5). Two distinct models have been proposed to explain the origin of pathogenic antibodies in SLE. One model suggests that pathogenic anti-dsDNA autoantibodies arise from naïve autoreactive B cells through polyclonal B cell activation, which is antigen-independent; the alternative model proposes that anti-dsDNA antibodies acquire autoreactivity by somatic mutation and the anti-dsDNA response in SLE is antigen driven.
Analysis of various murine anti-dsDNA antibodies has demonstrated that somatic mutation, often with the introduction of basic amino acids, can result in dsDNA binding (6). Although this observation is consistent with antigen-dependent affinity maturation, the nature of the triggering and selecting antigens remains to be elucidated. Whether nucleosomes, DNA, or phospholipid antigens released by apoptotic cells, or alternatively, foreign antigens trigger the response is unclear. Back mutation of four human IgG anti-DNA antibodies derived from lupus patients demonstrates that in their germline configuration these antibodies may fail to bind DNA (7–9). This observation would suggest that polyclonal activation is not the mechanism for the generation of pathogenic autoantibodies and that self-DNA is a critical eliciting antigen. These antibodies are the only anti-dsDNA antibodies from lupus patients that have been analyzed for antigenic specificity in their germline configuration.
It has been reported recently that patients with SLE have a defect in early B cell tolerance checkpoints, leading to the accumulation of many autoreactive B cells in the mature naïve B cell compartment (10). This observation again raises the question whether pathogenic anti-dsDNA antibodies might originate from these naïve autoreactive B cells. Naïve B cells can become IgM memory B cells through antigen-dependent mechanisms and IgG-expressing cells through antigen-independent pathways (11). IgM memory B cells are diminished in lupus patients (12), probably due to increased Ig-class switching of IgM B cells which may be caused by elevated BAFF-levels, overexpression of costimulatory molecules, and certain cytokines, such as IL-10 and IL-21 (13–15). This subset, therefore, may be a major precursor population for pathogenic antibodies in SLE. We, therefore, chose to examine this subset to determine the origins of IgM anti-dsDNA antibodies and their antigenic cross-reactivity, because of the possibility that they might undergo class switch recombination even in the absence of cognate T-cell interaction.
So far, only a few mutated IgM anti-dsDNA antibodies have been isolated from lupus patients (16), and none have been back-mutated to assess germline-encoded antigenic specificity. Therefore, we isolated IgM anti-dsDNA antibodies from peripheral blood B cells of a patient with SLE. Three somatically mutated IgM anti-DNA antibodies with pathogenic potential, as reflected in their ability to bind to isolated glomeruli, were reverted to their germline configuration. Reactivity against dsDNA and other antigens was determined in both somatically mutated and reverted antibodies in order to understand the impact of somatic mutation on the generation of dsDNA-reactive IgM memory B cells in SLE, and to determine whether pathogenic human anti-DNA autoantibodies are derived from DNA- or non-DNA–reactive B cells.
MATERIALS AND METHODS
Production of Human Anti-dsDNA Monoclonal Antibodies from Peripheral Blood of Lupus Patients
G3, A9, and B5 are human monoclonal antibodies derived from peripheral blood B cells of a lupus patient, M55, who met the revised ACR criteria for SLE (17). Patient M55 was 37 years old and presented no signs of active disease at the time of the blood draw. The patient exhibited elevated serum titers of anti-dsDNA antibodies and was being treated with hydroxychloroquine and low dose prednisone. In brief, individual B cells, identified by reactivity with a fluorochrome-tagged peptide mimetope of dsDNA (18), were sorted into 96-well PCR plates and IgH (μ only) and IgL chain gene rearrangements were amplified in two rounds of PCR (50 cycles each) before being cloned into human Ig γ1 and κ expression vectors (gift of MC Nussenzweig, Rockefeller University, New York, NY, USA). Human embryonic kidney fibroblast 293T cells were cotransfected with IgH- and IgL-encoding plasmid DNA by calcium phosphate precipitation as described previously (10,19). Supernatants were collected after 5 d of culture.
Reversion of Somatic Hypermutations into Germline Sequences
The plasmids with G3, A9, and B5 Ig gene segments were used as templates for the QuikChange Mutagenesis Kit (Stratagene, La Jolla, CA, USA). Mutation and junction analyses were conducted using the JOINSOLVER and IMGT/V-QUEST programs. Mutated IgH and IgL chain genes were reverted to their germline sequences and were sequenced to confirm the reversions before being expressed in vitro as described above.
Immunofluorescence Assay (IFA) and Glomerular Binding Assay
IFAs were performed following the manufacturer’s instructions using antibodies at 7–45 μg/mL for 1 h, followed by FITC anti-human IgG (Bion Enterprises Ltd., Des Plaines, IL, USA).
Murine glomeruli were isolated and attached to glass slides as described previously (20). Monoclonal antibodies were applied at 7 to 30 μg/mL for 1 h at room temperature and visualized with FITC anti-human IgG (Inova Diagnostics Inc, San Diego, CA, USA).
Crithidia assays were performed according to the manufacturer’s instructions. (IMMCO Diagnostics Inc, Buffalo, NY, USA)
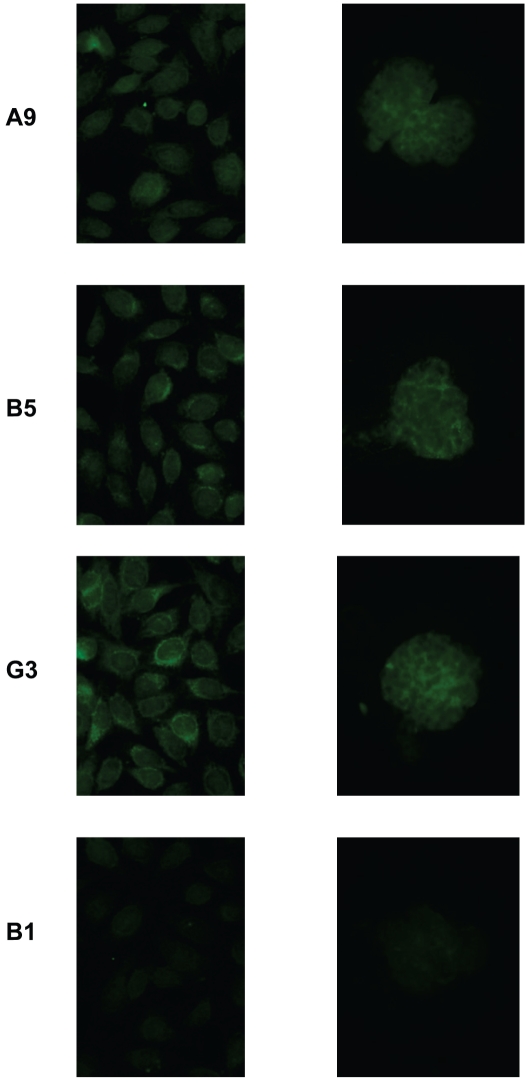
Monoclonal antibody B1, which does not recognize dsDNA by Enzyme-Linked ImmunoSorbent Assay (ELISA), was chosen as a negative control (45 μg/mL for IFA and 30 μg/mL for glomeruli and crithidia assays).
ELISA
Antibody concentrations in supernatants were determined by using human IgG1 kappa as a standard. The capture antibody and detection antibody were unlabeled goat anti-human IgG and alkaline phosphatase conjugated goat anti-human kappa, respectively (Southern Biotechnology, Birmingham, AL, USA).
Ninety six-well plates (Corning Life Science, Pittsburgh, PA, USA) were coated with 100 μg/mL of calf thymus dsDNA (Sigma-Aldrich, St. Louis, MO, USA), single-stranded DNA (ssDNA),10 μg/mL of lipopolysaccharide (LPS), or 5 μg/mL of recombinant human insulin (Sigma-Aldrich). Antibody binding to phosphatidylserine (10 μg/mL) or cardiolipin (50 μg/mL) with β2-GPI was measured as described previously (21). In addition, a commercial anti-nucleosome assay was performed (IBL-America, Minneapolis, MN, USA). All ELISAs were developed with alkaline phosphatase-conjugated goat anti-human IgG (Southern Biotechnology) and OD405 was measured using a Victor microplate reader (Perkin Elmer, Waltham, MA, USA). Clone 53 (gift of MC Nussenzweig) was used as a negative control (10,19).
RESULTS
Characterization of Mutated Antibodies
Antibodies were obtained by single-cell PCR from peripheral blood IgM-producing B cells of a lupus patient and expressed as IgG1 antibodies (18). After screening all expressed antibodies for dsDNA binding, we identified eight somatically mutated IgM anti-dsDNA antibodies. None of eight mutated antibodies were clonally related. Since we were interested in determining the antigenic specificity of the germline-encoded antibodies, and it often is impossible to determine the germline sequence of complementarity determining region 3 (CDR3) of the heavy chains, all sequences were analyzed by IMGT junction analysis tool to distinguish somatic mutations from junctional diversity in CDR3. We chose three antibodies (A9, B5, and G3) for reversion study, as the IMGT analysis showed they had unambiguous germline sequences in CDR3 and different mutation profiles. Furthermore, all of these had more than three mutations making it unlikely that the mutations were introduced by PCR error. In their mutated configuration, these antibodies displayed anti-nuclear reactivity on HEp-2 cells. In addition, they bound to isolated mouse glomeruli (Figure 1). This tissue binding suggested a pathogenic potential of these antibodies once they undergo heavy chain class switching to IgG. Furthermore, DNase treatment did not diminish glomerular binding (data not shown), demonstrating cross-reactivity to glomerular antigens. It is important to note that glomerular binding is a surrogate for pathogenicity, but it is not clear that all glomerular-binding antibodies, in fact, trigger an inflammatory process.
Figure 1.
Potential pathogenicity of IgM anti-DNA antibodies. Three mutated antibodies were tested for binding to HEp-2 cells (left panel) and to isolated mouse glomeruli (right panel). Monoclonal antibody B1 was used as a negative control.
The amino acid sequences of these antibodies are shown in Figure 2. The heavy chain of antibody A9 was encoded by the IGHV1-69, D6-13, and J4; the light chain by IGKV3-20 and J2. A high ratio of replacement to silent mutations (R/S) (5:1 in the heavy chain, and 4:0 in the light chain) and a clustered distribution of replacement mutations in the CDRs of the heavy chain gene were additional features of A9, suggestive of antigen selection. Three basic amino acids (Arg, Lys, and His) were acquired through somatic mutation at amino acid residues 50, 58, and 64, respectively.
Figure 2.
Sequences of IgM anti-DNA antibodies. The sequences of the variable genes of three mutated antibodies were compared with their germline counterparts. The position of each mutated amino acid residue is indicated, with the germline encoded amino acid shown above the sequence. R/S represents the ratio of replacement to silent mutations. Underlined amino acids are within CDRs.
Antibody G3 was encoded by IGHV3-30, D3-3, and J6, and IGKV1-5 and J1. In contrast to A9, the heavy chain of antibody G3 had only one replacement mutation, A40G, located in framework region 2 (FR2), and four silent mutations. Thus, this antibody displays no strong evidence of antigen selection.
The heavy and light chain variable regions of antibody B5 were encoded by IGHV3-07, D3-10, and J4, and IGKV1-NL1 and J1, respectively. Two replacement mutations and two silent mutations were present in the heavy chain variable region; one introduced the basic amino acid Lys at position 76.
The Effects of Somatic Mutation on Self- and Non-Self Reactivity
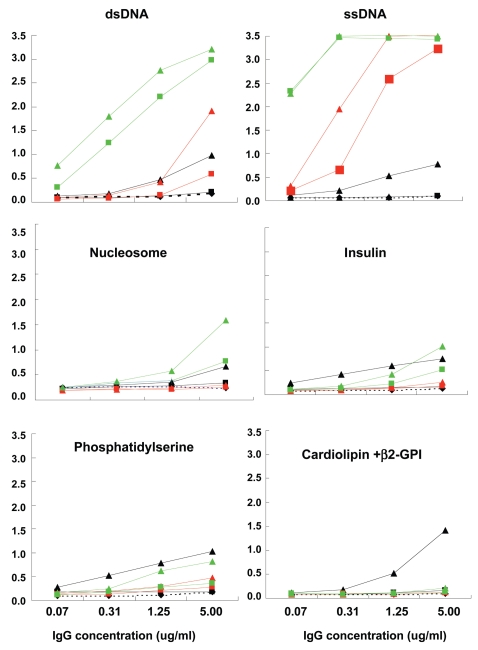
To evaluate the role of somatic mutation in the generation of dsDNA binding, we reverted the mutations of the above three anti-dsDNA antibodies to their germline sequence and tested the variants for self-reactivity (Figure 3). The three germline counterparts of the mutated antibodies differed with respect to antigenic specificity. While the germline form of G3 still recognized dsDNA, the germline forms of the other two antibodies displayed minimal dsDNA binding (Figure 3, top panel); thus, somatic mutation was essential for dsDNA binding of A9 and B5, but not G3.
Figure 3.
Antigenic reactivity of mutated antibodies in comparison to their unmutated counterparts. The reactivity of three mutated antibodies and their germline counterparts to different antigens was tested by ELISA at 5 μg/mL and three subsequent 1:4 dilutions. Clone 53 from MC Nussenzweig’s laboratory was used as a negative control (dotted lines). Mutated antibodies and unmutated counterparts are shown in the same color (A9: black; B5: red and G3: green). Triangles and squares represent mutated antibodies and their unmutated counterparts, respectively.
We also tested all antibodies for binding to dsDNA in the crithidia assay which has been shown to be specific for high affinity antibodies. None displayed reactivity in this assay (data not shown) suggesting that these antibodies bind dsDNA with relatively low affinity.
Previous studies have reported the presence of a large number of auto- and polyreactive antibodies in the transitional B cell repertoire of non-autoimmune individuals. A defect in B cell tolerance during B cell maturation in lupus patients results in an increased frequency of auto and polyreactive B cells in the naïve B cell repertoire. To ascertain the polyreactivity of the antibodies we isolated, we assayed the germline and mutated antibodies for binding to a set of antigens previously used to characterize polyreactivity (10,19).
Both mutated and germline-encoded G3 and B5 exhibited strong ssDNA binding. While the mutated A9 antibody bound to ssDNA, the unmutated counterpart did not. Thus, G3 bound both ssDNA and dsDNA prior to the acquisition of mutation; B5 bound ssDNA in the germline configuration but bound dsDNA only after mutation; A9 failed to bind ssDNA or dsDNA in the germline configuration but displayed both ssDNA and dsDNA binding after mutation. It has been reported in models of SLE that anti-chromatin antibodies arise before antibodies to isolated DNA. G3 bound nucleosomes, exhibiting higher binding in the mutated than the germline configuration. Mutated A9 displayed a low level of nucleosome binding. Thus, these antibodies did not appear to begin as anti-nucleosome antibodies, and acquire binding to naked dsDNA only as a consequence of somatic mutation. Rather, it appeared that even nucleosome binding was acquired or enhanced by somatic mutation. In addition, G3 displayed weak binding to human recombinant insulin in both the germline and mutated configuration; mutated A9 bound insulin weakly and B5 did not bind insulin either in the germline or the mutated configuration (see Figure 3). No binding to LPS was observed by any of the three antibodies, either in the germline or mutated configuration (data not shown). Thus, only G3 can be considered to have arisen from a polyreactive precursor.
Some anti-dsDNA antibodies cross-react with phospholipids, which have been suggested to be triggering antigens in SLE. We, therefore, tested the binding of the mutated antibodies and their germline variants to phospholipid antigens (see Figure 3). Mutated A9 exhibited detectable binding to both phosphatidylserine and cardiolipin, while mutated G3 bound to phosphatidylserine only. All germline variants lacked detectable binding to phospholipids, suggesting that phospholipids are not eliciting antigens, although they might be selecting antigens after somatic mutation.
It is important to note that we tested all antibodies as IgG1, but they were present in the patient as IgM antibodies. In solution, IgM might be pentameric and display increased avidity for antigen. On the membrane of the B cell, however, the avidity for antigen would depend on the density of the BCR on the cell membrane.
Interestingly, while A9 required mutation for dsDNA binding and the R/S ratio suggests antigen selection, this antibody does not display the greatest dsDNA binding. Perhaps DNA was not the selecting antigen or there was a high density of antibody of the B cell membrane providing a high avidity for antigen.
Basic Amino Acids Contribute to dsDNA Binding
To understand which mutations were crucial for the acquisition of dsDNA binding, we produced a set of variants of A9 and B5, each with different mutations. As shown in Figure 4 (lower left panel), the A9 mutated heavy chain with an unmutated light chain (variant 5) exhibited diminished, but still detectable, binding to dsDNA. No difference was observed in dsDNA binding between variants 4 and 5, suggesting the mutation P46F does not affect dsDNA reactivity. However, when we introduced mutations S31G and S66N to generate variant 1, the dsDNA reactivity was similar to the wild-type A9. Thus, light chain mutations contribute to an increase in dsDNA binding.
Figure 4.
The effects of individual point mutations on the binding of antibodies to dsDNA. (A) Back-mutated variants. Both germline and mutated amino acid sequences are shown. An underline means the mutated residue was reverted to the germline sequence. # denotes the original mutated antibody and † denotes the unmutated counterpart. (B) A9 and its variants were tested by ELISA for reactivity with dsDNA at 5 μg/mL and three subsequent 1:4 dilutions. Clone 53 from MC Nussenzweig’s laboratory was used as a negative control (dotted line).
We then analyzed the effects of mutations in the heavy chain of A9. As shown in Figure 4 (lower right panel), variant 12, which possessed the unmutated heavy and mutated light chain, completely lost binding to dsDNA. The mutations G55N and Q64H had no effect on dsDNA binding. In contrast, the mutations N58K, G50R, and Y101S significantly enhanced dsDNA binding (variants 8, 9, and 10). The back mutation of any of these three mutations to their germline sequences (variants 6, 7, and 8) resulted in significantly decreased dsDNA binding.
Lys at position 76 is essential for dsDNA reactivity of B5, as the variant containing a K76N reversion significantly lost dsDNA binding (data not shown).
All variants derived from G3 showed identical dsDNA binding (data not shown).
DISCUSSION
Sequence analysis of IgG anti-dsDNA antibodies derived from autoimmune mouse models has revealed extensive somatic mutation in the CDRs and a role for basic amino acids, generally acquired by mutation, in DNA-binding. The germline variants of these antibodies often fail to bind dsDNA, although they may bind ssDNA, suggesting a crucial role for somatic mutation in dsDNA binding.
Only four human IgG anti-dsDNA antibodies from patients with SLE have been analyzed previously and all of these also fail to bind DNA in the germline configuration (7–9). Since the number of sequenced dsDNA-binding antibodies derived from lupus patients is still very limited compared with those isolated from different autoimmune mouse models, it remains a question whether conclusions drawn from murine models can be applied fully to the human dsDNA-antibody repertoire.
In humans, a major checkpoint of peripheral B cell tolerance has been identified between the naïve and the IgM memory B cell compartments (22). It is well established that IgM antibodies are present in kidneys although their contribution to tissue damage is not defined clearly (23,24). However, in lupus patients, only four somatically mutated IgG anti-dsDNA antibodies have been isolated and characterized, and the effect of somatic mutations on human IgM anti-dsDNA antibodies has not been studied.
IgM producing cells displaying evidence of somatic mutation are considered to be IgM memory cells. These cells are diminished in lupus patients (12), most likely reflecting increased Ig-class switching of these B cells in a pro-inflammatory environment. We were interested in the characterization of these IgM antibodies as potential precursors of class-switched anti-dsDNA antibodies in lupus patients. We were particularly interested in determining whether these antibodies are derived from autoreactive naïve B cells that mature to immunocompetence due to an ineffective tolerance checkpoint, or whether they acquired autoreactivity through somatic mutation and reflect ineffectiveness of a later checkpoint, acting after antigen activation.
Although the three IgM anti-dsDNA antibodies in this report came from the same lupus patient, they displayed different profiles. They all differed with respect to R/S ratio and, therefore, differed with respect to evidence for antigen selection. Reversion of mutations in autoantibodies A9 and B5 resulted in decreased dsDNA binding. In contrast, the germline form of G3 recognized dsDNA. These results suggest that mutated IgM anti-dsDNA antibodies with pathogenic glomerulotropic potential may develop from both DNA- and non-DNA–reactive naïve B cells.
It is of interest to speculate that clinical disease may become apparent as these antibodies actually switch to IgG, and, then, can activate Fc-receptor-bearing cells and transport DNA to toll-like receptor 9 to lead to dendritic cell activation, especially in the pro-inflammation milieu in lupus patients which promotes class-switch recombination even outside of germinal center.
It has been reported that there is an accumulation of arginine, lysine, and asparagine (Arg, Lys, and Asn) residues at contact sites of anti-dsDNA antibodies. Arg and Lys are basic amino acids with positive charges, and might, therefore, increase the affinity of an antibody to negatively charged DNA by electrostatic interactions and hydrogen bonds. Asn is uncharged, but may interact with DNA either by donating or by accepting hydrogen bonds (16). Consistent with this paradigm, antibody A9 and B5 acquired several basic amino acids through somatic mutations. The Arg and Lys at position 50 and 58 of the heavy chain was crucial for the binding of A9 to dsDNA, and Lys at position 76 was essential for anti-dsDNA binding of B5. As Lys 76 was located in FR3, it is clear that not only CDRs but also FRs can contribute to the dsDNA binding. In addition, Asn at position 66 of the light chain was important for dsDNA binding of A9, whereas the acquisition of other Asn residues, such as G55N and S55N in the heavy chains of A9 and B5, and S93N and D17N in the light chains of B5 and G3, had no significant effect on dsDNA binding. Therefore, it is not possible to predict the effects of particular amino acid residues on reactivity to DNA without crystal structure. The data, however, are consistent with self antigen functioning in positive selection after B cell activation.
While some studies of murine anti-DNA antibodies have suggested a major contribution of the heavy chain to DNA reactivity, others have reported that the light chain also can play a critical role in DNA binding in some antibodies (6,25,26). Moreover, a recent analysis of two human IgG anti-dsDNA antibodies shows that reverting somatic mutations of either heavy or light chains independently results in a loss of dsDNA binding (9). This also was seen with antibody A9, as mutations in both heavy and light chains play an important role for dsDNA binding. Decoding the relative contribution of each chain will require the analysis of many more antibodies. Such an analysis also may reveal if there are particular heavy or light chains that are highly likely to confer DNA binding independent of their partner.
It has been reported that self-reactive antibodies rarely are found in the IgM memory B cell compartment in healthy humans, and that the rare self-reactive IgM memory B cells that were present are most likely not derived from naïve autoreactive B cells (22). DNA-binding IgM-producing B cells can exist in SLE because of a failure at an early tolerance checkpoint, as seen in antibody G3. Alternatively, their presence may reflect a failure in tolerance of B cells that acquire autoreactivity by somatic mutation, such as antibodies B5 and A9. The analysis of these three antibodies suggests that both these checkpoints might be defective in a single patient with SLE.
It will be necessary to study a large number of patients in detail in a similar fashion to determine if multiple tolerance checkpoints, some preceding antigen-activation and some after antigen-activation, must fail for a lupus-like phenotype to develop, or if it is possible to display defects only in early or late tolerance checkpoints and still develop disease.
This study also suggests that the triggering antigen(s) in SLE may be more promiscuous than some studies suggest. In the context of multiple defects in generation and maintenance of B cell tolerance, both self and non-self antigens may activate or sustain autoreactivity.
ACKNOWLEDGMENTS
The study was supported by a grant from the National Institutes of Health (BD). AMJ is supported by the Irvington Institute Fellowship Program of the Cancer Research Institute.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Tan EM. Autoantibodies in pathology and cell biology. Cell. 1991;67:841–2. doi: 10.1016/0092-8674(91)90356-4. [DOI] [PubMed] [Google Scholar]
- 2.Renaudineau Y, et al. Association of alpha-actinin-binding anti-double-stranded DNA antibodies with lupus nephritis. Arthritis Rheum. 2006;54:2523–32. doi: 10.1002/art.22015. [DOI] [PubMed] [Google Scholar]
- 3.Vlahakos D, et al. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol. 1992;2:1345–54. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- 4.Vlahakos DV, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 5.Winfield JB, Faiferman I, Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977;59:90–6. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radic MZ, et al. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150:4966–77. [PubMed] [Google Scholar]
- 7.Lambrianides A, et al. Arginine mutation alters binding of a human monoclonal antibody to antigens linked to systemic lupus erythematosus and the antiphospholipid syndrome. Arthritis Rheum. 2007;56:2392–401. doi: 10.1002/art.22743. [DOI] [PubMed] [Google Scholar]
- 8.Rahman A, et al. The importance of somatic mutations in the V(lambda) gene 2a2 in human monoclonal anti-DNA antibodies. J Mol Biol. 2001;307:149–60. doi: 10.1006/jmbi.2000.4491. [DOI] [PubMed] [Google Scholar]
- 9.Wellmann U, et al. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–63. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 12.Wehr C, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113:161–71. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Briere F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757–62. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litinskiy MB, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Pene J, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–7. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 16.Rahman A, Giles I, Haley J, Isenberg D. Systematic analysis of sequences of anti-DNA antibodies—relevance to theories of origin and pathogenicity. Lupus. 2002;11:807–23. doi: 10.1191/0961203302lu302rr. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, et al. Identification of DNA-reactive B cells in patients with systemic lupus erythematosus [technical note] J Immunol Methods. 2008;338:79–84. doi: 10.1016/j.jim.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52:522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 21.Seal SN, Monestier M, Radic MZ. Diverse roles for the third complementarity determining region of the heavy chain (H3) in the binding of immunoglobulin Fv fragments to DNA, nucleosomes and cardiolipin. Eur J Immunol. 2000;30:3432–40. doi: 10.1002/1521-4141(2000012)30:12<3432::AID-IMMU3432>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Tsuiji M, et al. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankewycz OG, Migliorini P, Madaio MP. Polyreactive autoantibodies are nephritogenic in murine lupus nephritis. J Immunol. 1987;139:3287–94. [PubMed] [Google Scholar]
- 24.Xie C, Liang Z, Chang S, Mohan C. Use of a novel elution regimen reveals the dominance of polyreactive antinuclear autoantibodies in lupus kidneys. Arthritis Rheum. 2003;48:2343–52. doi: 10.1002/art.11092. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim SM, Weigert M, Basu C, Erikson J, Radic MZ. Light chain contribution to specificity in anti-DNA antibodies. J Immunol. 1995;155:3223–33. [PubMed] [Google Scholar]
- 26.Radic MZ, Mascelli MA, Erikson J, Shan H, Weigert M. Ig H and L chain contributions to autoimmune specificities. J Immunol. 1991;146:176–82. [PubMed] [Google Scholar]