Abstract
Objective
Insulin down-regulates hepatic production of sex hormone binding globulin (SHBG), which in turn influences sex hormone bioavailability. The effects of childhood-onset diabetes, and of insulin resistance in nondiabetics, on SHBG and testosterone in children and young adults are poorly understood.
Research Design and Methods
Individuals with diabetes diagnosed <18 years of age (n=48) and their siblings without diabetes (n=47) were recruited for the Chicago Childhood Diabetes Registry Family Study. SHBG and total and free testosterone were measured. Participants ranged in age from 10-32 years; 39% were Non-Hispanic white. The majority of individuals with diabetes were classical type 1 phenotype (75%), while the remainder exhibited features of type 2 or mixed diabetes; 96% were treated with insulin.
Results
SHBG and total testosterone were higher in males with diabetes compared to male siblings. Elevated SHBG was associated with the absence of endogenous insulin independent of gender; elevated total testosterone was similarly associated with the absence of C-peptide but only for males. Diabetes type and treatment were unrelated. In those without diabetes, greater insulin resistance had a small, nonsignificant association with lower SHBG and higher free testosterone.
Conclusions
SHBG and total testosterone appear to be higher in male children and young adults with diabetes compared to nondiabetic male siblings, apparently related to the absence of endogenous insulin. This may have implications for sex hormone-dependent processes across the lifespan in males diagnosed with diabetes as children.
Keywords: sex hormone binding globulin, testosterone, C-peptide, insulin resistance
Previous research has demonstrated that hepatic production of sex hormone binding globulin (SHBG) is down-regulated by insulin (1). SHBG in turn influences the relative balance and bioavailability of testosterone and estradiol (2). Conditions associated with altered systemic insulin levels such as type 1 diabetes and insulin resistance (IR) in nondiabetics could therefore affect SHBG, sex hormones, and their dependent physiological processes. For example, changes in bioavailable estradiol and testosterone levels may partly explain such factors as the delayed age at menarche (3) demonstrated in girls with type 1 diabetes. Understanding these associations may therefore have clinical implications.
Previous research evaluating the association of type 1 diabetes with SHBG has produced conflicting results demonstrating lower (children), similar (children and premenopausal women), and higher (postmenopausal women and adult men) levels in those with type 1 diabetes compared to controls (4-8). Research on testosterone in males with type 1 diabetes has consistently found elevated levels compared to controls (6), whereas studies in females (children and adults) have been inconsistent, demonstrating comparable to higher testosterone (4,7,8). Results regarding the effect of glycemic control (4,5,8) and insulin treatment (5,6) on sex hormones in type 1 diabetes have also been equivocal. However, these studies used small samples (<100) without adjustment for potential confounders such as age and body composition, and all were limited to Non-Hispanic whites.
Among individuals without diabetes, fasting insulin and IR are negatively associated with SHBG in both adult men and women (9,10). The associations of fasting insulin and IR with testosterone are negative in nondiabetic men (10) but positive in nondiabetic women (11). There have been no similar studies in children and adolescents without diabetes.
Therefore, we sought to understand whether altered sex hormones occur in those with childhood diabetes across the spectrum of demographic characteristics after adjustment for important confounders, and whether insulin is associated with sex hormones in children without diabetes. Based on the insulin-SHBG relationship, we hypothesized that SHBG is higher in those with diabetes compared to sibling controls and is related to absent endogenous insulin production, irrespective of diabetes type or treatment; and IR is associated with lower SHBG in siblings without diabetes. Based on previous studies in type 1 diabetes, we hypothesized that testosterone levels are higher in those with childhood diabetes compared to controls.
RESEARCH DESIGN AND METHODS
Study and Sample
The Chicago Childhood Diabetes Registry Family Study is an ongoing study of the epidemiology of diabetes in an ethnically diverse sample. Individuals with diabetes were invited to participate if they were aged <18 years at diagnosis, their diabetes was not secondary to another medical condition, and they were currently living in the Chicago area. Recruitment occurred through diabetes clinics, health fairs, and mailings. All biological first and second degree relatives were also invited to participate. Currently 66 individuals with diabetes (probands) and their families have completed the examinations. The sub-sample for this analysis includes 48 probands and 47 full and half siblings without diabetes who were ≥10 years of age and therefore had sex hormone determinations. Probands and siblings from the same family, probands without siblings, and siblings without probands meeting these criteria, were all included in the analysis. None of the females reported current use of hormonal contraceptives. Examinations were conducted in the University of Chicago's 5 General Clinical Research Center or participants' homes. Study approval was obtained from the University of Chicago's Institutional Review Board. Participants ≥18 years of age, and parents of children <18 years of age, provided written informed consent; children 10-17 years old assented.
Data Collection and Variables
SHBG and Testosterone (dependent variables)
Plasma SHBG and total and free testosterone were determined from a fasting sample irrespective of menstrual phase as they vary little over the cycle. SHBG was measured with an assay standardized to the dialysis technique (12). Total and free testosterone were determined by a solid-phase 125I radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles, CA). SHBG and testosterone were measured by the University of Chicago's Endocrine Laboratory. The lower limits of detection for total and free testosterone were 10 ng/dL (0.3 nmol/L) and 2 pg/mL (6.9 pmol/L), respectively. Intra-assay coefficients of variation(CVs) were ≤10%.
Diabetes Characteristics (independent variables)
Diabetes status was the main predictor. Type of diabetes treatment (insulin, pills, both, or diet only), frequency of insulin injections, and disease duration were determined via questionnaire. Participants who reported using ≥3 insulin injections per day or an insulin pump were considered on intensive treatment. Participants were classified as type 1 (16 males, 20 females) if they 1) had no C-peptide, or 2) had C-peptide with <2 years diabetes duration, and were either positive for islet autoantibodies (GAD, IA2) or receiving intensive insulin monotherapy. Participants were classified as type 2 (4 males, 4 females) if they 1) had C-peptide and no antibodies, or 2) among those who were missing data on antibodies, 6 had C-peptide, and used oral antidiabetic medication, or received no treatment, or discontinued using insulin ≥2 years after diagnosis without severe complications, or had diabetes duration ≥2 years. Those classified as “mixed” phenotype (3 males, 1 female) had C-peptide and autoantibodies with ≥2 years diabetes duration.
Additional Blood Measurements (independent variables)
Fasting C-peptide, insulin and glucose were also determined. Individuals with diabetes with a fasting blood glucose <150 mg/dL (8.3 mmol/L) had a stimulated C-peptide measurement 90 minutes after ingestion of a 6 ml/kg standard nutrient solution (Boost, Novartis Nutrition Corporation, Minneapolis, MN). Plasma C-peptide was measured with a solid-phase, competitive chemiluminescent enzyme immunoassay (Immulite 2000, Diagnostic Products Corporation, Germany) in the University of Chicago's Diabetes Research and Training Center (DRTC) Lab. The lower limit of detection was 0.17 nmol/L and the intra-assay CV was 8%. Absent C-peptide was defined as a fasting and stimulated level below the detection limit. Serum insulin was measured with a solid-phase, two-site chemiluminescent immunometric assay (Immulite 1000, Siemens Medical Solutions Diagnostics, Los Angeles, CA) by the DRTC Lab. The intra-assay CVs were ≤8.0%. Fasting glucose was measured with a glucometer (One Touch Sure Step, Lifescan, Milpitas, CA). Using fasting insulin and glucose, IR was determined in nondiabetic siblings by the Homeostatic Model Assessment, version 2.0 (HOMA2) (13). IR was defined as a value ≥3.2 for those ≤18 years old (14) and ≥2.5 for those >18 years old (15).
HbA1c was measured in whole venous blood with a monoclonal antibody method (DCA 2000, Bayer Corporation, Elkhart, IN). The detection range was 2.5-14.0% and the intra-assay CVs were ≤5%.
Adjustment Variables
Height was measured without shoes using a stadiometer rod, and weight and percent body fat were measured barefoot with a bio-electrical impedance analyzer scale (Tanita TBF-300A, Arlington Heights, IL). BMI was transformed into Z-scores using the Center for Disease Control Growth Chart (≤20 years old) (16) and NHANES III (>20 years old) (17) age and sex-matched reference data. Waist circumference was measured twice at the umbilical level and averaged. Other potential confounders included age at exam, sex, and race/ethnicity. Race/ethnicity was defined as that reported for ≥3 grandparents; if <3 grandparents shared the same race/ethnicity, race was mixed. When race/ethnicity was available on <3 grandparents, race/ethnicity was based on parental data. If parental data were missing, self-reported race/ethnicity was used. Pubertal stage was self-assessed with pubic hair Tanner diagrams. For 18 participants, pubertal stage was imputed using age, sex, and race-specific estimates (18). Females self-reported age at menarche.
Statistical Analyses
Probands and siblings were first compared using unadjusted mixed linear and logistic regression models, with family entered as a random effect to incorporate correlation due to clustering within families. Effects of diabetes status on SHBG, and on total and free testosterone, were then evaluated using multivariable mixed linear regression, again with family entered as a random effect. All multivariable models adjusted for pubertal stage and race/ethnicity. Potential confounders explored were body composition (BMI Z-score, percent body fat, or waist circumference), age at exam, diabetes treatment, and diabetes type; a covariate was retained in the model if it produced at least 10% change in the diabetes status regression parameter. If more than one body composition variable met the criterion for 8 confounding, the variable that produced the largest change was included. Interactions of diabetes status by gender were tested, and stratified models were fit when significant.
Analyses were performed for subgroups to examine effects of covariates applicable only to that subgroup (probands: disease duration, age at diagnosis, diabetes type, treatment, number of insulin injections per day, intensive insulin therapy; siblings: HOMA2, fasting insulin) or whose effects were expected to differ by diabetes status (fasting C-peptide, HbA1c, glucose). Models limited to probands included only one individual per family, and thus linear rather than mixed linear regression models were fit. Interactions with gender were tested for each covariate.
Sensitivity analyses were conducted by limiting the final models to those with type 1 diabetes or those receiving insulin treatment. Models excluding the 18 individuals with imputed puberty data were also analyzed. Results did not change substantially and therefore are presented using the entire sample.
Statistical tests were considered significant at p<0.05. Linear and mixed linear regression analyses were performed in SAS, version 8.0 (SAS Institute, Cary, NC) and mixed logistic regression in Stata/SE 9.2 (StataCorp LP, College Station, TX).
RESULTS
Age of participants ranged from 10 to 32 years; 39% were Non-Hispanic white (Table 1). Among females, 77% had begun menstruating at an average age of 12.6 years. Characteristics of probands and siblings were similar except that probands had significantly higher percent body fat, HbA1c and fasting glucose, and lower fasting C-peptide. Among probands, mean disease duration was 7.7 (range: 0.4 to 28.5) years and current HbA1c was 8.6%. All males were treated with only insulin; two females were treated with insulin plus 9 pills and two females were treated with pills only. Of the insulin users, 59% were on an intensive insulin regimen. All of the siblings without diabetes fell within the normal range for HbA1c (<6%) and only two met the criteria for IR.
Table 1.
Characteristics by diabetes status
| Characteristic | Probands with Diabetes |
Siblings without Diabetes |
||
|---|---|---|---|---|
| n | 48 | 47 | ||
| Demographics | ||||
| Age at Exam (years) | 17.1 (5.7) | 15.4 (3.7) | ||
| Tanner Stage (public hair) (%) | ||||
| 1 | 4.2 | 14.9 | ||
| 2 | 8.3 | 6.4 | ||
| 3 | 18.7 | 8.5 | ||
| 4 | 31.3 | 29.8 | ||
| 5 | 37.5 | 40.4 | ||
| Gender (%) | ||||
| Female | 52.1 | 40.4 | ||
| Male | 47.9 | 59.6 | ||
| Race/Ethnicity (%) | ||||
| Non-Hispanic White | 33.3 | 44.7 | ||
| Non-Hispanic Black | 39.5 | 23.4 | ||
| Other Race | 6.3 | 10.6 | ||
| Mixed Race | 14.6 | 14.9 | ||
| Mexican Hispanic | 6.3 | 6.4 | ||
| Anthropometrics | ||||
| BMI Z-score⊥ | 0.8 (0.9) | 0.4 (1.2) | ||
| Percent Bodyfat | 27.5 (10.0)* | 22.3 (11.7) | ||
| Waist Circumference (cm) | 78.7 (13.7) | 74.0 (13.4) | ||
| Blood Measurements | ||||
| Fasting Glucose (mg/dL) | 199.8 (101.2)** | 90.1 (6.9) | ||
| HbA1c (%) | 8.6 (2.0)** | 5.1 (0.3) | ||
| Fasting C-peptide (nmol/L) | 0.3 (0.4)** | 0.7 (0.4) | ||
| Absent C-peptide (%) | 66.7 | |||
| Diabetes | ||||
| Diabetes Duration (years) | 7.7 (6.1) | |||
| Age at Diagnosis (years) | 9.4 (3.7) | |||
| Diabetes Treatment (%) | ||||
| Insulin only | 91.6 | |||
| Insulin and Pills | 4.2 | |||
| Pills only | 4.2 | |||
| Insulin Therapy (%)I | ||||
| 1-2 Injections/day | 41.3 | |||
| 3-4 Injections/day | 37.0 | |||
| Pump | 21.7 | |||
| Diabetes Type (%)§ | ||||
| Type 1 | 75.0 | |||
| Type 2 | 16.7 | |||
| Mixed | 8.3 | |||
| Insulin Resistance | ||||
| Fasting Insulin (μU/mL) | 9.5 (10.1) | |||
| HOMA2 (using fasting insulin) | 1.2 (1.2) | |||
| Insulin Resistant (%)¶ | 4.3 | |||
HOMA2=Homeostatic Model Assessment 2 Numbers are means (standard deviations) and percentages To convert mg/dL to mmol/L for glucose, multiply by 0.055; to convert μU/mL to pmol/L for insulin, multiply by 6.0
p<0.05;
p<0.0001
BMI was transformed into Z-scores using CDC Growth Chart (≤20 years old) and NHANES III (>20 years old) age and sex-matched reference data
Subgroup using insulin, n=46
Diabetes type based on C-peptide levels, presence of diabetes antibodies, diabetes treatment, symptoms off insulin, and duration
Insulin resistant criterion based on fasting insulin HOMA2: if age≤18 years, insulin resistant=HOMA2 ≥3.2 if age>18 years, insulin resistant=HOMA2 ≥2.5
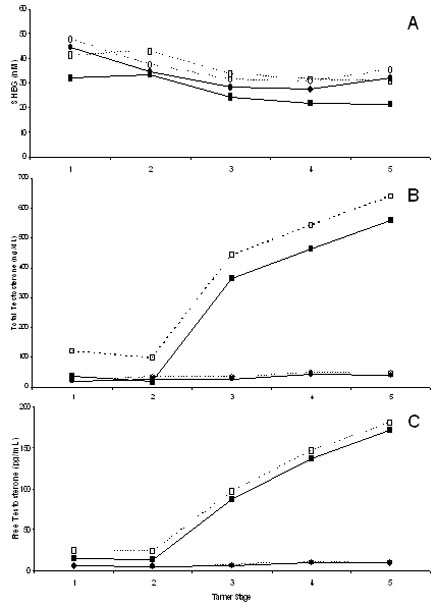
The effect of diabetes status on SHBG differed between males and females (p=0.008; Figure 1A). Among males, SHBG was significantly higher in probands than in siblings across pubertal stage (mean difference [Δ]=9.5 nM, 95% CI: 2.8, 16.1), adjusting for body composition and race. Higher levels were demonstrated in males both with type 1 (Δ=10.9 nM, 95% CI: 3.2, 18.6) and type 2 diabetes (Δ=10.3 nM, 95% CI: -2.4, 23.0). This association was not observed in females (Δ=3.0 nM, 95% CI: -5.2, 11.3). Among siblings, females had higher SHBG than males, while SHBG did not differ by gender among probands. SHBG was highest in Tanner stages 1-2 with lower levels during later puberty. SHBG did not differ by race/ethnicity.
Figure 1.
Mean sex hormone levels by childhood diabetes status for males and females across Tanner stages 1-5 (n=9, 7, 13, 29, 37, respectively). Levels adjusted for body composition and race. Solid lines with black symbols=siblings without diabetes (n=47); dashed lines with white symbols=probands with diabetes (n=48; 36 type1, 8 type 2, 4 mixed phenotype); squares=males (n=51); circles=females (n=44). A: Sex hormone binding globulin, significant interaction between diabetes status and gender; B: Total testosterone, significant interaction between diabetes status and gender (to convert ng/dL to nmol/L, multiply by 0.03467); C: Free testosterone (to convert pg/mL to pmol/L, multiply by 3.467).
In males, total testosterone differed significantly by diabetes status (Figure 1B). In contrast, total and free testosterone in females were uniformly low with virtually no difference across diabetes status (Figure 1B, 1C). Specifically, males with diabetes had significantly higher total testosterone compared to male siblings without diabetes (Δ=80.4 ng/dL [2.8 nmol/L], 95% CI: 0.1, 160.7), adjusting for body composition and race. Higher levels were found in males both with type 1 (Δ=106.4 ng/dL [3.7 nmol/L], 95% CI: 4.4, 208.4) and type 2 diabetes (Δ=75.7 ng/dL [2.6 nmol/L], 95% CI: -103.4, 254.7). A difference was not observed in females (Δ=5.1 ng/dL [0.2 nmol/L], 95% CI: -8.5, 18.8; interaction p=0.01). Testosterone did not differ by race/ethnicity.
In final models adjusted for sex, pubertal stage, body composition and race, higher SHBG in probands was associated with absent versus detetable C-peptide levels (Δ=7.6 nM, 95% CI: 2.1, 13.0), and also with longer diabetes duration (years) (Δ=0.7 nM, 95% CI: 0.2, 1.2). These associations did not differ by gender. In males, but not females, the lack of C-peptide was also significantly associated with higher total testosterone (males: Δ=152.6 ng/dL [5.3 nmol/L], 95% CI: 26.2, 279.0; females: Δ=24.7 ng/dL [0.9 nmol/L], 95% CI: - 106.7, 156.0). SHBG and total testosterone were not significantly associated with age at diagnosis, HbA1c, fasting glucose, diabetes type or treatment, or number of insulin injections per day or intensive insulin therapy. There were no significant associations with free testosterone.
In the combined group of male and female siblings without diabetes, adjusting for sex, pubertal stage, body composition, and race, SHBG and testosterone were not significantly associated with fasting C-peptide, HbA1c, glucose, or insulin. While SHBG and free testosterone levels were not significantly associated with HOMA2, weak trends were observed. A one unit increase in HOMA2 was associated with a -4.6 nM (95% CI: - 11.0, 1.9, p=0.15) decrease in SHBG and a 23.5 pg/mL [81.5 pmol/L] (95% CI: -6.2, 53.3, p=0.12) increase in free testosterone. HOMA2 was not significantly related to total testosterone (p=0.43). None of these associations differed by gender.
CONCLUSIONS
To our knowledge, this study is the first to show that, among males, SHBG and total testosterone are higher in children and young adults with diabetes compared to nondiabetic siblings, even after adjustment for relevant confounders. Among females, SHBG was slightly higher in those with diabetes compared to siblings, but the difference was not significant. Total testosterone in females also showed no association with diabetes status. Furthermore, we demonstrated that the absence of endogenous insulin as measured by C-peptide was significantly associated with elevated SHBG and, in males, with total testosterone, and that higher SHBG was positively related to disease duration.
Prior research in children and adolescents with type 1 diabetes (aged ~ 6-20 years) has been inconsistent, demonstrating lower, similar, and slightly elevated SHBG compared to controls (5,7), even though each study controlled for pubertal status. These contradictory findings may be due to the small and/or select clinical samples examined. The results of this study suggest that prior conflicting reports may also be a function of the lack of adjustment for body composition, a failure to account for gender differences, and/or heterogeneity in C-peptide status and diabetes duration. In contrast, results by diabetes status in adults have been more consistent. SHBG has been demonstrated to be similar in premenopausal women (aged ~20-55 years) with and without type 1 diabetes (8), while significantly higher SHBG has been found in middle-aged men (aged ~20-55 years) (6) and postmenopausal women (aged ~50-70 years) (4) with type 1 diabetes.
Prior research on testosterone by diabetes status has also produced divergent results by gender. In males, studies have consistently found elevated testosterone in individuals with type 1 diabetes compared to controls (6,7). Studies in females have demonstrated less coherence, with similar or elevated testosterone in both children (7) and adults (4,8) with type 1 diabetes compared to controls. These inconsistent results in females could again be due to small samples. However, one key reason for the contradictory results in females may be a lack of adjustment for body composition, as adiposity is strongly related to testosterone (19).
Within the context of the previous literature, this study demonstrates that males diagnosed with diabetes in childhood have significantly elevated SHBG and testosterone compared to males without diabetes. In contrast, among females with childhood diabetes, any alterations in sex hormones are small, suggesting gender differences in the effect of diabetes on sex hormone physiology.
The associations of higher SHBG and total testosterone with diabetes among males reported here are consistent with free testosterone levels that do not differ by diabetes status, and echo previous reports in adult males (6). This lack of association results from the high affinity with which SHBG binds to testosterone to influence its bioavailability (2). In addition to varying by diabetes status, SHBG and testosterone exhibited the predicted changes across pubertal stage, irrespective of diabetes. Also, consistent with known gender differences in nondiabetics, females had higher SHBG than males (2).
Elevated SHBG in males and females and total testosterone in males were strongly associated with the absence of C-peptide in those with diabetes, regardless of phenotype. This is consistent with the up-regulation of hepatic SHBG production in response to low insulin levels (1). Thus, higher SHBG in those with childhood diabetes may reflect hepatic hypoinsulinemia despite peripheral hyperinsulinemia from exogenous treatment (6). The relationship of absent endogenous insulin with elevated testosterone in males with childhood diabetes is likely more complicated, as insulin has been shown to stimulate gonadal testosterone production in individuals without diabetes (20). Perhaps peripheral hyperinsulinemia associated with insulin treatment (which increases with decreasing C-peptide) (6) has a greater effect on gonadal testosterone production than does portal insulin level. However, we did not find an association between testosterone and intensive insulin therapy. A more precise measure of exogenous insulin exposure may be needed to determine whether insulin treatment mediates the relationship between absent C-peptide and elevated testosterone. Unfortunately, those data were not available in this study. Disease duration was also positively associated with SHBG. However, neither SHBG nor testosterone was associated with HbA1c, fasting glucose, or type or intensity of treatment. These results indicate that sex hormones in those with childhood-onset diabetes may be more strongly affected by the long-term absence of portal insulin and the clinical course of diabetes, rather than by short-term, more modifiable, factors such as glycemia.
Compared to male nondiabetics, males with both type 1 and type 2 childhood diabetes had elevations in SHBG and testosterone of similar magnitudes; the comparisons with type 2 were not significant likely due to limited power. In contrast, previous research has shown that adult males with type 2 diabetes have lower SHBG and testosterone compared to controls (21). One explanation for these conflicting results could be that males categorized as having type 2 diabetes in our sample may actually have type 1. However, even though they were diagnosed before age 18 and used insulin, all were antibody negative and had detectable C-peptide after ≥5 years duration. Thus, type 2 diabetes appearing in childhood may well differ from adult type 2 diabetes in its association with sex hormones. For example, prospective research indicates that low SHBG and testosterone are risk factors for type 2 diabetes in older men (i.e. sex hormones affect diabetes risk) (21). In contrast, alterations in sex hormones by childhood type 2 diabetes may be more akin to childhood type 1 diabetes (i.e. diabetes characteristics such as endogenous insulin affect sex hormones). This is consistent with research demonstrating that diabetes in children represents a spectrum combining various levels of beta-cell dysfunction with insulin resistance, rather than two distinct phenotypes (22). Studies using larger samples of children with non-type 1 diabetes are needed to confirm these results.
The gender differences in absolute levels of sex hormones by diabetes status detected in our and other studies may be due to gender differences in biologic processes linking diabetes and sex hormone production. For example, gender differences may exist in the associations of SHBG and total testosterone with C-peptide and disease duration, even though our study had limited power to detect this. Research must continue to explore the potential reasons for gender differences in the relationship between childhood diabetes and sex hormone physiology.
In the siblings without diabetes, IR tended toward a negative association with SHBG and a positive association with free testosterone, although neither reached statistical significance. The magnitude of the decrease in SHBG for a one unit increase in HOMA2 was approximately one-half the difference attributable to diabetes status in males and therefore has clinical relevance. The trend with SHBG, although weak, is consistent with research in both male and female adults without diabetes, demonstrating IR to be negatively associated with SHBG (9,10). In comparison, previous research in nondiabetic adults has found testosterone to be lower in males, and higher in females, with IR (10,11). In our study, the lack of a statistically significant association between IR and SHBG, and interaction between IR and gender on testosterone, may be due to the low values and limited variability in IR in this group of relatively young, healthy siblings, as only two met criteria for IR. It may also be due to the imprecision of using a single fasting serum insulin level, and thus one HOMA value (1).
The current study was cross-sectional, which limits conclusions on how sex hormones change with age in individuals with childhood diabetes, though they appear to follow a pattern consistent with age differences in individuals without diabetes. This study was also underpowered to detect subtle differences in SHBG and testosterone by diabetes type, gender differences in the associations of C-peptide with SHBG and testosterone in those with diabetes, and associations of IR with SHBG and free testosterone in siblings without diabetes. Lastly, data on insulin dose was not available to fully explore whether insulin therapy mediated the association between C-peptide and testosterone. Yet, the research had a number of strengths, including the first ethnically diverse sample. Using sibling controls, and measuring and adjusting for potential confounders (especially body composition), also minimized genetic and lifestyle differences between those with and without diabetes to better estimate the effect of diabetes. This is the first study to our knowledge to examine the association of IR with sex hormones in children and young adults without diabetes.
Alterations in SHBG have important physiological relevance for sex hormones. High SHBG lowers the proportion of sex hormone available by binding estradiol and testosterone and influences the relative balance of estradiol to testosterone through bidirectional feedback (2). These alterations may then affect sex hormone-dependent processes. It is possible that the changes detected in the sex hormone milieu in children and young adults with diabetes are not modifiable and may only be averted by preventing the disease in the first place. However, sex hormone abnormalities in young people without diabetes may perhaps be avoided by preventing IR.
In summary, SHBG and total testosterone appear to be significantly higher in male children and young adults with diabetes, apparently a function of the absence of endogenous insulin. Whether IR and SHBG are associated in young people without diabetes calls for further research. Both associations may have implications for sex hormone-dependent processes across the lifespan.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health grants DK44752 and RR00055. Parts were presented at the 67th American Diabetes Association Meeting. We gratefully acknowledge the study participants and the staff of the Chicago Childhood Diabetes Registry Family Study, particularly Latrisha Hampton and Carmela Estrada.
REFERENCES
- 1.Nestler JE. Sex hormone-binding globulin: a marker for hyperinsulinemia and/or insulin resistance? J Clin Endocrinol Metab. 1993;76:273–274. doi: 10.1210/jcem.76.2.8432767. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 3.Danielson KK, Palta M, Allen C, D'Alessio DJ. The association of increased total glycosylated hemoglobin levels with delayed age at menarche in young women with type 1 diabetes. J Clin Endocrinol Metab. 2005;90:6466–6471. doi: 10.1210/jc.2005-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyholm H, Djursing H, Hagen C, Agner T, Bennett P, Svenstrup B. Androgens and estrogens in postmenopausal insulin-treated diabetic women. J Clin Endocrinol Metab. 1989;69:946–949. doi: 10.1210/jcem-69-5-946. [DOI] [PubMed] [Google Scholar]
- 5.Holly JM, Dunger DB, al Othman SA, Savage MO, Wass JA. Sex hormone binding globulin levels in adolescent subjects with diabetes mellitus. Diabet Med. 1992;9:371–374. doi: 10.1111/j.1464-5491.1992.tb01799.x. [DOI] [PubMed] [Google Scholar]
- 6.Yki-Jarvinen H, Makimattila S, Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80:3227–3232. doi: 10.1210/jcem.80.11.7593430. [DOI] [PubMed] [Google Scholar]
- 7.Meyer K, Deutscher J, Anil M, Berthold A, Bartsch M, Kiess W. Serum androgen levels in adolescents with type 1 diabetes: relationship to pubertal stage and metabolic control. J Endocrinol Invest. 2000;23:362–368. doi: 10.1007/BF03343739. [DOI] [PubMed] [Google Scholar]
- 8.Salonia A, Lanzi R, Scavini M, Pontillo M, Gatti E, Petrella G, Licata G, Nappi RE, Bosi E, Briganti A, Rigatti P, Montorsi F. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care. 2006;29:312–316. doi: 10.2337/diacare.29.02.06.dc05-1067. [DOI] [PubMed] [Google Scholar]
- 9.Preziosi P, Barrett-Connor E, Papoz L, Roger M, Saint-Paul M, Nahoul K, Simon D. Interrelation between plasma sex hormone-binding globulin and plasma insulin in healthy adult women: the telecom study. J Clin Endocrinol Metab. 1993;76:283–287. doi: 10.1210/jcem.76.2.8432770. [DOI] [PubMed] [Google Scholar]
- 10.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 11.Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Amer J Epidemiol. 2004;160:540–8. doi: 10.1093/aje/kwh250. [DOI] [PubMed] [Google Scholar]
- 12.Moll G, Jr, Rosenfield R, Helke J. Estradiol-testosterone binding interactions and free plasma estradiol under physiological conditions. J Clin Endocrinol Metab. 1981;52:868–874. doi: 10.1210/jcem-52-5-868. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–e503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics 2000 CDC Growth Charts: United States. 2007 http://www.cdc.gov/growthcharts. [PubMed]
- 17.National Center for Health Statistics Anthropometric reference data, United States, 1988-1994. 2007 http://www.cdc.gov/nchs/about/major/nhanes/Anthropometric%20Measures.htm.
- 18.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM. Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: epidemiological and clinical correlation. Int J Obes Relat Metab Disord. 2000;24:S56–S58. doi: 10.1038/sj.ijo.0801279. [DOI] [PubMed] [Google Scholar]
- 20.Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev. 1987;8:132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- 21.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 22.Pinhas-Hamiel O, Zeitler P. Clinical presentation and treatment of type 2 diabetes in children. Pediatr Diabetes. 2007;8(Suppl 9):16–27. doi: 10.1111/j.1399-5448.2007.00330.x. [DOI] [PubMed] [Google Scholar]