Abstract
In two-dimensional (2-D) culture systems, we have previously shown that cleaved two-chain high-molecular-weight kininogen (HKa) or its domain 5 induced apoptosis by disrupting urokinase plasminogen activator (uPA) receptor (uPAR)-integrin signal complex formation. In the present study, we used a three-dimensional (3-D) collagen-fibrinogen culture system to monitor the effects of HKa on tube formation. In a 3-D system, HKa significantly inhibited tube and vacuole formation as low as 10 nM, which represents 1.5% of the physiological concentration of high-molecular-weigh kininogen (660 nM), without apparent apoptosis. However, HKa (300 nM) completely inhibited tube formation and increased apoptotic cells about 2-fold by 20–24 h of incubation. uPA-dependent ERK activation and uPAR internalization regulate cell survival and migration. In a 2-D system, we found that exogenous uPA-induced ERK phosphorylation and uPAR internalization were blocked by HKa. In a 3-D system, we found that not only uPA-uPAR association but also the activation of ERK were inhibited by HKa. HKa disrupts the uPA-uPAR complex, inhibiting the signaling pathways, and also inhibits uPAR internalization and regeneration to the cell surface, thereby interfering with uPAR-mediated cell migration, proliferation, and survival. Thus, our data suggest that the suppression of ERK activation and uPAR internalization by HKa contributes to the inhibition of tube formation. We conclude that in this 3-D collagen-fibrinogen gel, HKa modulates the multiple functions of uPAR in endothelial cell tube formation, a process that is closely related to in vivo angiogenesis.
Keywords: urokinase plasminogen activator, urokinase plasminogen activator receptor, angiogenesis, extracellular signal-regulated kinase
angiogenesis, the formation of new capillaries from existing blood vessels, is crucial for normal physiological processes and pathological conditions (20). There are several steps involved in this process, requiring the coordinated regulation of multiple signaling pathways between endothelial cells (ECs) and extracellular matrix (ECM) proteins (28, 36). Most of our knowledge of signal transduction has been acquired from two-dimensional (2-D) culture systems, generating limited information regarding the role of the ECM. In 2-D cell cultures, the major cellular activity of ECs is proliferation with minimal morphological changes. However, in three-dimensional (3-D) collagen-fibrinogen cultures, ECs develop intracellular vacuoles, which in the presence of angiogenic factors, grow in size and elongate (28). Eventually, more adjacent cells coalesce, forming tube-like structures with well-defined lumens, mimicking the major steps of in vivo vessel formation by the fusion of vacuoles and tube formation in zebrafish (29).
Binding of urokinase-type plasminogen activator (uPA) to its specific receptor (uPAR) regulates cell migration and invasion and activates diverse cell signaling pathways. In many cell types, uPA binds to uPAR, resulting in the activation of MAPK ERK (46, 47, 60). This signaling response may be of central importance because the level of activated ERK controls many pathophysiological process, including cell growth, differentiation, apoptosis, and migration, and, in cancer, invasion and metastasis (50). In aggressive cancer cells, endogenously produced uPA and the uPAR may form a self-contained autocrine pathway, which serves as a major determinant of the basal level of ERK activation, in the absence of exogenous stimulants (1, 2, 41). Gondi et al. (21) showed that downregulation of uPARs and uPA by inhibitory RNA induced the downregulation of the EGF receptor (EGFR) and VEGF and inhibited angiogenesis in both in vitro and in vivo angiogenic assays. In addition, invasion and migration were inhibited, as indicated by in vitro spheroid cell migration, Matrigel invasion, and spheroid invasion assays. Maspin, a serine protease inhibitor, specifically inhibits prostate cancer-associated uPA in vitro and decreases angiogenesis in nude mice (10). The microvessel density (MVD) assessed immunohistochemically was significantly higher in gastric cancer patients with expression of uPA, uPAR, or VEGF, and stepwise analysis identified uPA as an independently correlated factor with MVD (30). Thus, the uPA-uPAR system plays a critical role in angiogenesis.
After the conversion of pro-uPA to uPA on the cell surface uPAR, internalization is triggered by plasminogen activator inhibitor (PAI)-1, which forms a ternary inactive complex. The uPA-PAI-1-uPAR complex is internalized by low-density lipoprotein-related protein (LRP), a member of the lipoprotein-related protein family (13, 48), which is degraded in the endosome. Lysosomal proteases degrade the complex, and eventually free uPARs recycle to the cell surface. Thus, the dynamic recycling of components of the uPAR complex enables it to simultaneously participate in diverse reactions in the pericellular space of individual cells.
The 3-D structure of the uPA-uPAR complex, solved recently by X-ray diffraction, revealed that the uPAR binds uPA in a pocket composed of all three domains (4, 27). In this laboratory, we (12) have shown that two-chain high-molecular-weight kininogen (HKa) binds to domain II (DII) and domain III (DIII) of the uPAR on ECs. We postulated that the binding of HKa to uPARs might result in conformational changes of uPARs affecting the function and signaling of the uPA-uPAR complex. In this study, we first compared the inhibitory potency of HKa and an anti-uPAR antibody on tube formation in a 3-D collagen-fibrinogen gel since the uPAR is a major receptor of HKa. We then provided evidence that HKa could disrupt the uPA-uPAR complex. Finally, we showed that HKa inhibited exogenous uPA-dependent ERK phosphorylation and uPAR internalization. Therefore, HKa exerts its inhibitory effect on tube formation in a 3-D collagen-fibrinogen gel.
MATERIALS AND METHODS
Reagents and antibodies.
Cleaved HKa was purchased from Enzyme Research Laboratories (South Bend, IN) with >95% purity. On nonreduced SDS gels, purified HKa appeared as a major band of 110 kDa. On reduced gels, HKa possesses a heavy chain (Mr = 62 kDa) and a light chain (Mr = 45 kDa). Endotoxin in HKa was measured and removed as described in a previous publication (40). Only HKa was used in these experiments; no intact high-molecular kininogen (HK) or domain 5 (D5) was used in this study. Collagen solution (purified rat type I collagen) was purchased from Cohesion (Palo Alto, CA) and ICN Biochemicals (Aurora, OH). Fibrinogen and protease inhibitor cocktail were purchased from Calbiochem (San Diego, CA). Antibodies against ERK and its phosphorylated form (pERK) were from New England Biolabs (Beverly, CA). Rabbit polyclonal anti-uPAR antibody (DIIDIII) and mouse monoclonal anti-uPAR antibody (E180) were gifts kindly provided by Drs. Andrew Mazar and Graham Parry (Attenuon, San Diego, CA). These antibodies were made against uPAR DII and DIII; thus, they block ligand binding, including HKa. Single-chain uPA was a gift kindly provided by Dr. Douglas B. Cines (University of Pennsylvania School of Medicine, Philadelphia, PA). High-molecular-weight uPA, a murine antibody to uPA, and human PAI-1 were obtained from American Diagnostica (Greenwich, CT). Phosphatidylinositol-specific PLC (PI-PLC) and PMA were purchased from Sigma (St. Louis, MO). Trypsin solution, trypsin neutralizing solution, and VEGF stock solution were from Clonetics (Walkersville, MD). Basic FGF (bFGF) was obtained from Life Technologies (Grand Island, NY). Mammalian Protein Extraction Reagent (M-per) was purchased from Pierce (Rockford, IL).
Cell culture.
Human umbilical vein ECs (HUVECs) were purchased from Clonetics and cultured as previously described (24). Zn2+ (20 mol/μl) was added to the culture mix whenever HKa was involved, as Zn2+ is required for HKa binding to ECs.
Cell migration assay.
Cell migration was assessed in 48-well Boyden chambers. The underside of the membrane of the upper chamber was coated with a collagen-fibrinogen mixture (10 μg/ml collagen and 30 μg/ml fibrinogen, ICN Biochemicals and Calbiochem) and 8 × 103 cells in endothelial basal medium (EBM; which does not contain serum) were seeded on the upper chamber and allowed to migrate for 6 h (32). After 6 h, cells that remained in the upper chamber were removed using a cotton swab. Cells that migrated to the other side of the membrane of the upper chamber were fixed with 4% paraformaldehyde and stained with 1% toluidine blue. We counted cells in six fields/well (×100 magnification), which essentially covered 80% of the well surface. The average number of cells from each of the triplicates represents the average number of cells that migrated in that treatment group. Each experiment had triplicate wells for every treatment group, and we repeated each experiment three times. The mean of all results from controls was considered as 100%, and all values were adjusted accordingly (including individual control results).
Preparation of the collagen-fibrinogen gel matrix and 3-D cell culture.
The collagen gel matrix was generated according to methods described previously (40). Briefly, HUVECs were resuspended in EBM and mixed with collagen solutions (rat type I collagen) to make a final collagen concentration of 1.7 mg/ml. Fibrinogen was added to a final concentration of 3 mg/ml, and the cell concentration adjusted to 1 million cells/ml; 200 μl of collagen-fibrinogen and cell mixture were added to each well (48-well plates). The matrix was allowed to form by incubation at 37°C. After gelation, EBM containing angiogenic factors [40 ng/ml bFGF (Life Technologies), 30 ng/ml VEGF (Clonetics) and 50 nmol/l PMA (Sigma)] was added on top of the collagen-fibrinogen gel matrix. Cells embedded in the gel were then placed in a humidified incubator with 5% CO2 and 95% air for the time periods stated in each individual experiment.
Microscopic analyses of cell morphogenesis in 3-D collagen-fibrinogen gel matrixes.
EC tube length was quantified following the protocol of Yang et al. (66). Briefly, 5 digital images/well were taken at ×100 magnification (∼70% of the total well surface). Pictures were taken from areas showing more abundant tube structures from each well. Images were analyzed using Image Pro-Plus 4.1 software. An observer blinded to the experimental protocol measured the total length of each tube (defined as structures exceeding 100 μm in length) that was in clear focus in the image field. In those instances where several tube-like structures merged together or branched, the total length of the tube was calculated as the sum of the length of the individual branches. To assess if our treatments had any effect on vacuole formation, we measured the surface area occupied by the vacuoles; a vacuole was defined as an open space within one cell or between two cells only. Results were standardized as percentages where controls were set at 100%.
Analysis of apoptosis in HUVECs cultured in 3-D collagen-fibrinogen gels.
Apoptotic cell death was determined by the combination of cell morphology changes (nuclear chromatin clumping and/or cell body disintegration observed through an inverted Leica microscope) and the detection of early apoptotic changes by TUNEL assay at the time endpoints indicated using a commercially available kit (DeadEnd Fluorometric TUNEL System, Promega). We counted the numbers of TUNEL-positive EC nuclei in 12 randomly chosen high-power fields/group (×400 magnification). The number of TUNEL-positive nuclei was divided by the number of total cells counted. Preimmune serum (IgG1) was used as the negative control for the anti-uPAR group. All experiments were run in triplicate.
Cell lysate preparation, immunoprecipitation, and immunoblot analysis.
Protein extraction from gels, SDS-PAGE separation of proteins, and Western blot analysis were performed as previously described (39). HUVECs in collagen-fibrinogen gel were washed with ice-cold PBS containing 0.7 mM CaCl2, 0.5 mM MgCl2, and 1 mM Na3VO4 before being harvested in extraction buffer A [composed of 1% Triton X-100, 60 mM octyl glucoside, 10 mM Tris·HCl (pH 7.6), 50 mM NaCl, 30 mM Na4P2O7, 50 mM NaF, 1 mM Na3VO4, and 2 mM CaCl2] plus mammalian protease inhibitor mixture (Sigma). After solubilization on ice for 15 min with intermittent vortexing, the extract was microcentrifuged for 10 min at 13,000 g, and the supernatant (cell lysate) was recovered.The complex formation of uPARs with other signaling molecules was determined by immunoprecipitation according to methods described by Chapman et al. (8) with some modifications. The cell lysate was incubated with an antibody to uPA followed by an incubation with protein A/G beads. Immunoprecipitates were subjected to SDS-PAGE under nonreduced conditions, and immunoblot analysis was performed as described below.
Separately, the immunoprecipitated complex or the cell lysate containing equal amounts of protein (10–20 μg) were solubilized in Laemmli's sample buffer and subjected to SDS-PAGE. Separated proteins were then transferred onto nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 and then probed with antibodies as indicated. Immunoblots were visualized by an enhanced chemiluminescence kit (Amersham Pharmacia Biotech) and analyzed by densitometry. pERK and uPAR were normalized to their corresponding total levels. pERK and uPAR in controls were set at 100%. Experiments were run in triplicate.
Internalization of uPARs by HUVECs and cellular distribution of uPARs by cytofluorimetric analysis.
To evaluate uPAR distribution after stimulation, we performed flow cytometry experiments as follows: subconfluent HUVECs (40–60%) were incubated with or without HKa (300 nM) in EBM for 1 h. Individually treated groups of HUVECs were further incubated with stimuli as indicated, after which cells were harvested and sequentially incubated with primary and secondary antibodies for 30 min on ice and washed with ice-cold Dulbecco's PBS. Finally, cells were fixed and aliquoted. We used a murine anti-uPAR antibody (directed against uPAR DIIDIII) in combination with FITC-conjugated secondary antibody (Santa Cruz Biotechnology), which allowed us to detect uPARs expressed on EC membranes. Samples were analyzed by fluorescence-activated cell sorting (FACSort, Becton Dickinson). Experiments were run in triplicate.
Statistical analysis.
Statistical analyses were performed with one-way ANOVA and all pairwise multiple-comparison procedures (Student-Newman-Keuls method). Results were considered significant when P ≤ 0.05. All experiments were run at least in triplicate, and results are presented as means ± SE.
RESULTS
HKa and anti-uPAR antibody inhibit HUVEC capillary tube formation in a collagen-fibrinogen gel matrix.
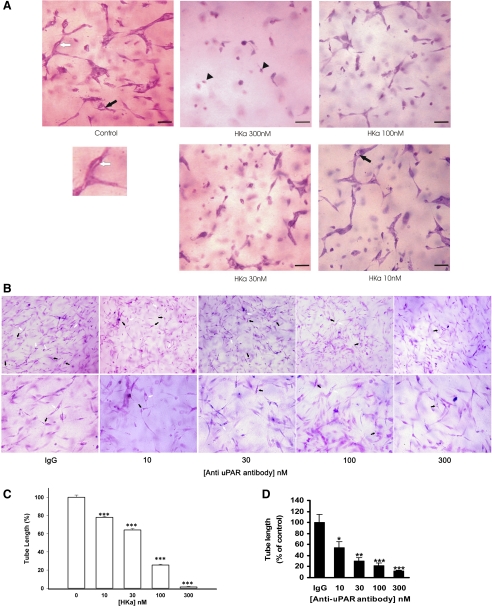
In previous studies, we found that the HKa functional domain, D5, inhibited angiogenesis on the chicken chorioallantoic membrane (11); we also identified that the uPAR is a receptor for HKa (12). We demonstrated that the binding of 125I-labeled HKa to HUVECs is inhibited by vitronectin, anti-uPAR DII and DIII antibodies, and recombinant soluble uPAR. However, Zhang et al. (67) showed that antitropomyosin monoclonal antibody TM-311 blocked the inhibition of bFGF and VEGF-induced EC proliferation in a 2-D system by HKa and D5 in a concentration-dependent manner, whereas antibodies that block the binding of HKa to the urokinase receptor did not (12). To clarify this apparent contradiction, we used a collagen-fibrinogen gel to compare the effect of HKa with the anti-uPAR antibody on tube and vacuole formation. The effects of HKa in a concentration range from 10 to 300 nM were tested, as shown in Fig. 1A. In Fig. 1, the control (angiogenic factors only) shows capillary tube structures; few vacuoles are present. HKa (10 nM) treatment decreased the formation of the more complex structures seen in the control. Intracellular vacuoles and tube formation were still present. HKa (30 nM) treatment showed an increased inhibition of vacuole and tube formation with scattered single cells and rare apoptotic cells. HKa (100 nM) treatment showed scattered apoptotic cells, elongated ECs, and very few tubes and vacuoles. HKa (300 nM) treatment showed sparse, elongated ECs with virtually no intracellular vacuole or tube formation and apoptotic cells. The addition of HKa at 300, 100, 30, and 10 nM to 3-D collagen-fibrinogen HUVEC cultures inhibited capillary tube formation (22-h incubation) by 98.6 ± 0.6%, 74.9 ± 0.9%, 36.7 ± 2.0%, and 28.0 ± 6.2%, respectively (Fig. 1C). The surface area of intracellular vacuoles decreased accordingly by 99.7 ± 0.1% (300 nM HKa), 65.4 ± 9.6 (100 nM HKa), 50.3 ± 9.5% (30 nM HKa), and 38.9 ± 7.9% (10 nM HKa). These data show that the inhibitory effect of HKa is concentration dependent. HKa could decrease the tube and vacuole formation at a concentration as low as 10 nM without an apparent apoptotic effect. The IC50 of the inhibitory effect of HKa on tube and vacuole formation was ∼30 nM, which represents 5% cleavage of HK in the plasma.
Fig. 1.
Effect of two-chain high-molecular-weight kininogen (HKa) and anti-urokinase plasminogen activator (uPA) receptor (uPAR) antibody on human umbilical vein endothelial cell (HUVEC) morphogenesis in three-dimensional (3-D) collagen-fibrinogen gel (22-h incubation). A: inhibition of capillary tube formation by HKa at different concentrations. HUVECs were cultured in 3-D collagen-fibrinogen gel matrixes with or without HKa for 22 h at 37°C. HUVECs were then fixed with 4% paraformaldehyde and stained with 1% toluidine blue. Black arrows point to vacuoles. A vacuole is an open space within a cell or between 2 cells only. Black rrowheads point to apoptotic cells. The lumen to the white arrow pointed to is magnified below. A lumen is a space formed by 3 or more cells. Bars = 100 μm. Magnification: ×200. B: inhibition of tube formation by mouse monoclonal anti-uPAR antibody at different concentrations. Concentrations of the monoclonal antibodies (in nM) were calculated by measuring the protein concentration of the antibodies and dividing them by molecular weight (150 kDa). HUVECs were cultured in 3-D collagen-fibrinogen gel matrixes with or without anti-uPAR antibody for 22 h at 37°C. HUVECs were then fixed with 4% paraformaldehyde and stained with 1% toluidine blue. Black arrows point to intracellular vacuoles. White arrows point to the lumen. Magnification: ×100 (top) and ×200 (bottom). C and D: quantification of tube length formation after an incubation with HKa (C) or anti-uPAR antibody (D). A tube is a cellular structure that measures >100 μm in length and is visibly formed by the body of 3 cells or more. Tube length was measured in μm/mm2. Results were normalized and reported as percentages of the tube length (means ± SE). All treatments were compared with the control. *P < 0.05; **P < 0.01; ***P < 0.005.
The anti-uPAR antibody compared with HKa exerted a similar effect on tube formation, as shown in Fig. 1B, which shows dose-dependent inhibited tube formation. The addition of anti-uPAR antibody at 300, 100, 30, and 10 nM to 3-D collagen-fibrinogen HUVEC cultures inhibited capillary tube formation (22-h incubation) by 87.9 ± 1.5%, 78.0 ± 4.6%, 69.3 ± 5.4%, and 45.8 ± 11.1%, respectively (Fig. 1, C and D). The anti-uPAR antibody mimicked the effect of HKa on tube formation, indicating that HKa inhibition of tube formation targets uPAR.
HKa disrupts the uPA-uPAR complex without an apparently inhibitory effect on endogenous uPA expression.
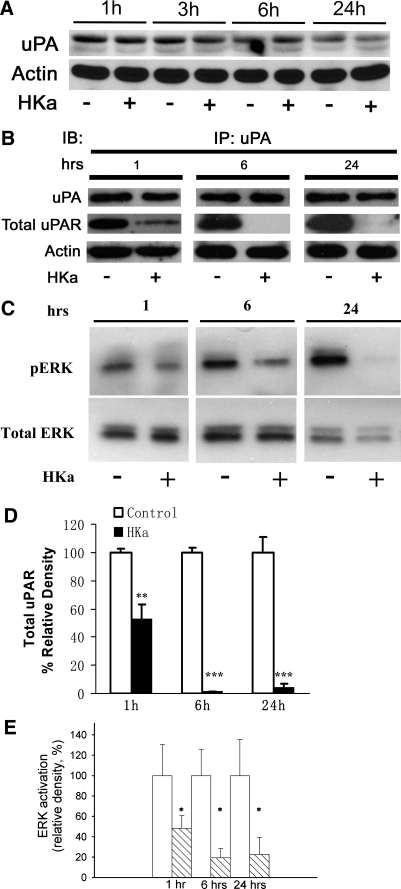
The angiogenic response of ECs initiated by different growth factors is accompanied by the assembly of cell surface-bound proteolytic machinery as a prerequisite for focal invasion. Pro-uPA binds to its receptor, uPAR, allowing the conversion of pro-uPA to active uPA, which is an initial step of invasion. Since HKa can bind to DII and DIII of the uPAR (12), we therefore hypothesized that HKa could block the uPA-uPAR complex by targeting uPAR. As shown in Fig. 2A, ECs in a 3-D gel constitutively expressed endogenous uPA from 1 to 24 h, as assessed by densitometry. HKa did not significantly inhibit endogenous uPA expression. Quantification did not show inhibition (data not shown).
Fig. 2.
Effect of HKa on uPA expression, the uPA-uPAR complex, and ERK phosphorylation in 3-D collagen-fibrinogen gels. A: uPA expression was determined by Western blot analysis. HUVECs were incubated in a 3-D gel with (+) or without (−) 300 nM HKa and harvested at the times indicated. Cell lysates were subjected to Western blot analysis as described in materials and methods. uPA expression was probed by a murine antibody to uPA. Data are representative of 4 independent experiments. B: disruption of the uPA-uPAR complex by HKa. HUVECs in a 3-D gel were incubated with or without 300 nM HKa and harvested at the times indicated by the addition of extraction buffer. Immunoprecipitation (IP) procedures were performed as described in materials and methods using an antibody to uPA. A comparison with β-actin showed equal protein loading. IB, immunoblot analysis. C: inhibitory effect of HKa on ERK phosphorylation. HUVECs in a 3-D gel were incubated with or without 300 nM HKa and harvested at the times indicated. Cell lysates were subjected to Western blot analysis as described in materials and methods. Activated ERK and total ERK were probed by antibodies to phosphorylated ERK (pERK) and nonphosphorylated ERK. D: relative density of total uPAR to uPA quantified by densitometry. Open bars, control; solid bars: HKa treatment. Data are means ± SE. **P ≤ 0.01; ***P ≤ 0.001. E: quantification of ERK activation at 1, 6, and 24 h with and without HKa. ERK activation was expressed as a percentage of the relative density. Open bars, control; hatched bars, HKa treatment. Data are means ± SE. *P ≤ 0.05. Experiments were performed in triplicate.
However, immunoprecipitation revealed that HKa disrupted the uPA-uPAR complex (Fig. 2B). HKa disrupted the uPA-uPAR complex at 1, 6, and 24 h by ∼47.14 ± 10.00%, 98.80 ± 0.34%, and 96.11 ± 2.83%, respectively (Fig. 2D). One downstream effect of uPA is to activate ERK. We tested whether HKa can inhibit ERK phosphorylation since HKa blocked the binding of uPA to uPAR. As shown in Fig. 2C, ERK was constitutively phosphorylated, reflecting activated endogenous uPA. However, the endogenous ERK phosphorylation was diminished by HKa. The HKa inhibition of ERK phosphorylation at 1, 6, and 24 h was ∼51.6 ± 12.4%, 80.5 ± 9.05, and 77.3 ± 16.9, respectively (Fig. 2E).
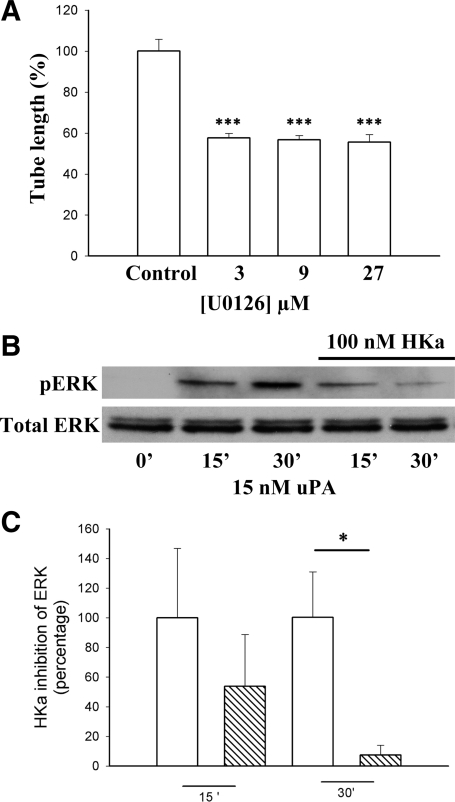
ERK inhibition decreases tube formation.
We have previously demonstrated that pERK is necessary for tube formation (65). In 3-D cultures, an ERK inhibitor (U-0126) at 3, 9, and 27 μM significantly inhibited tube formation by 42.3 ± 2.2%, 43.3 ± 2.1%, and 44.3 ± 3.6%, respectively (Fig. 3A). These results confirm the participation of ERK in EC tube formation.
Fig. 3.
HKa inhibition of ERK activation and tube formation. A: ERK activity was needed for tube formation. HUVECs in a 3-D gel were treated with the ERK inhibitor U-0126 at 3, 9, and 27μM. Tube length was quantified as in Fig. 1C. Comparisons were made with the control. ***P ≤ 0.005. B: HKa inhibition of ERK activation was time dependent in two-dimensional (2-D) cultures. HUVECs in monolayers were starved for 4 h and preincubated with or without HKa (100 nM) for 1 additional hour. Cells were incubated with uPA (15 nM) for the times indicated. Cells were lysed and subjected to Western blot analysis. ERK activation was probed by an antibody to pERK. C: quantification of pERK present after an incubation with HKa in 2-D cultures. HKa (100 nM) significantly decreased ERK activation at 30 min (*P ≤ 0.05). ERK activation was quantified by densitometry (n = 3). Data are means ± SE.
HKa inhibits ERK phosphorylation in a time-dependent manner in a 2-D culture system.
To determine whether the HKa inhibition of ERK phosphorylation is uPA dependent, we pretreated cells with 100 nM HKa and challenged cells with 15 nM uPA (Fig. 3B). Densitometry revealed that the inhibitory effect of HKa on ERK phosphorylation at 30 min was significant: HKa inhibited pERK by 54.9 ± 9.5% at 15 min of incubation and by 94.9 ± 3.9% at 30 min of incubation (P < 0.05; Fig. 3C). These data indicate that HKa prevented exogenous uPA-induced ERK phosphorylation.
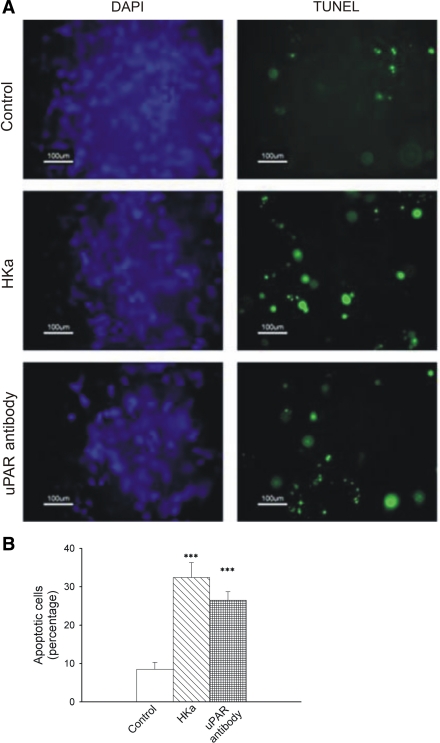
HKa induces apoptosis in HUVECs grown in a collagen-fibrinogen 3-D gel matrix.
A number of studies have demonstrated that uPA-activated ERK inhibits apoptosis (7, 23, 64). To test the effects of the endogenous uPA-uPAR system on apoptosis, we treated ECs with an antibody that blocks uPA binding to uPARs or with HKa. Cells treated with HKa showed 30% apoptotic cells as detected by the TUNEL assay, which is greater than a threefold increase compared with the control (8%). Anti-uPAR antibody-treated HUVECs showed 26% apoptotic cells (Fig. 4B). These differences from the control were highly significant (P < 0.005). The differences between the percentages of HKa- and anti-uPAR antibody-induced apoptosis were not statistically significant. Apoptosis was not seen in cells treated with HKa (10 or 30 nM, 22 h of incubation) or in cells treated with HKa (300 nM) at 6 h of incubation.
Fig. 4.
Effect of HKa and uPAR antibody on HUVEC apoptosis in 3-D collagen-fibrinogen gels at 22 h. A: apoptotic assays in 3-D gels with different treatments. HUVECs in a 3-D gel were treated with or without either HKa (300 nM) or an anti-uPAR antibody (100 μg/ml). After 22 h, cells were fixed, and the TUNEL assay was performed according to the manufacturer's instructions. Left, cells stained with 4′,6-diamidino-2-phenylindole (DAPI; blue); right, TUNEL-positive cells (green). B: quantification of apoptotic cells. Apoptotic and total cells were counted as described in materials and methods. The percentage of apoptotic cells over total cells was calculated in each experimental group. Comparisons were made with the control. ***P ≤ 0.005.
HKa inhibits uPA-uPAR complex internalization.
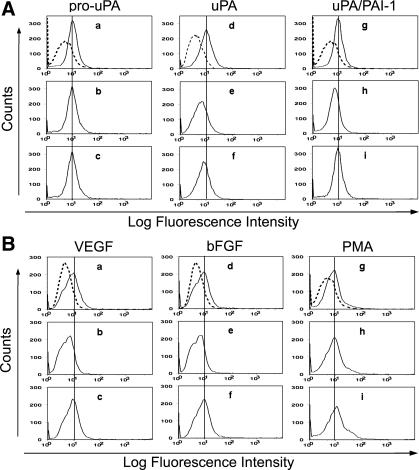
The uPAR binds to the internalization receptor (LRP) through DII and DIII regions (34), the same binding region that HKa binds to uPAR, indicating that HKa might modulate the uPAR internalization process. To quantify the level of uPARs on the cell surface and assess uPAR internalization, immunocytofluorimetric analysis was performed as described in Fig. 5. ECs synthesize and secrete PAI-1 (68). The active enzyme uPA has been shown to stimulate uPAR internalization (52). When HUVECs were incubated with pro-uPA (20 nM), uPA (20 nM), and uPA/PAI-1 (20:40 nM), uPA and uPA/PAI-1 decreased cell surface uPARs, as shown in Fig. 5A. As shown in Table 1, we found that uPA decreased cell surface uPARs from 9.86 ± 0.14 to 6.01 ± 0.06 (P < 0.001); uPA/PAI-1 decreased cell surface uPARs from 9.06 ± 0.03 to 6.37 ± 0.05 (P < 0.001), respectively. As expected, HKa (300 nM) reversed these effects to 8.15 ± 0.14 (P < 0.001) and 8.66 ± 0.08 (P < 0.001), respectively. In contrast, pro-uPA (20 nM) slightly increased cell surface uPARs from 9.07 ± 0.06 to 9.54 ± 0.09 (P < 0.05), indicating that exogenous pro-uPA might capture some uPARs, which are normally cycling at the basal level. HKa had no effect. In a 3-D gel, we used the combination of VEGF, bFGF, and PMA as angiogenic factors to stimulate tube formation. To determine which factor responds to uPAR internalization, subconfluent HUVECs were treated with VEGF (40 ng/ml), bFGF (40 ng/ml), and PMA (50 nM) separately. Cell surface uPARs were monitored by flow cytometry, as shown in Fig. 5B. VEGF and bFGF decreased cell surface uPARs, whereas PMA slightly increased cell surface uPARs, as shown in Fig. 5B. As shown in Table 1, VEGF decreased cell surface uPARs from 8.88 ± 0.04 to 5.12 ± 0.04 (P < 0.001) and bFGF decreased cell surface uPARs from 7.24 ± 0.06 to 5.18 ± 0.01 (P < 0.001), respectively. As shown in Table 1, HKa (300 nM) reversed these effects to 8.58 ± 0.03 (P < 0.001) and 8.06 ± 0.18 (P < 0.001), respectively. In contrast, HKa further elevated PMA-increased cell surface uPAR levels to 10.41 ± 0.23 (P < 0.001).
Fig. 5.
Immunocytofluorimetric analysis of HKa effects on uPAR internalization. uPAR expressed on the surface of HUVECs was measured by immunocytofluorimetric analysis as described in materials and methods. In A and B, a, d, and g are unstimulated cells. Dashed lines represent the secondary antibody only. Solid lines represent uPAR levels on the cell surface as determined by 3 independent experiments. Quantification of immunocytofluorimetric data was as described in Table 1. A: representative immunocytofluorimetric histograms of cell surface uPARs in HUVECs after stimulation with pro-uPA, uPA, and the uPA/plasminogen activator inhibitor-1 (PAI-1) complex. In b, e, and h, cells were challenged for 30 min with pro-uPA (20 nM), uPA (20 nM), and the uPA/PAI-1 complex (20:40 nM), respectively. In c, f, and i, in the presence of HKa (300 nM), cells were challenged for 30 min with pro-uPA (20 nM), uPA (20 nM), and the uPA/PAI-1 complex (20:40 nM), respectively. Cells were then harvested and subjected to the immunocytofluorimetric analysis procedure described in materials and methods. uPA and PAI-1 were added together at ratio of 1:2 and incubated for 1 h at room temperature to form the complex before the complex was added to the medium. B: representative immunocytofluorimetric histograms of cell surface uPARs in HUVECs after stimulation with VEGF, basic FGF (bFGF), and PMA. In b, e, and h, cells were challenged for 2 h with VEGF (40 ng/ml), bFGF (40 ng/ml), and PMA (50 nM), respectively. In c, f, and i, in the presence of HKa (300 nM), cells were challenged for 2 h with VEGF (40 ng/ml), bFGF (40 ng/ml), and PMA (50 nM), respectively. Cells were then harvested and subjected to the immunocytofluorimetric analysis procedure described in materials and methods.
Table 1.
Effect of stimulation and HKa on expression of uPARs
| pro-uPA | uPA | uPA/PAI-1 | VEGF | bFGF | PMA | |
|---|---|---|---|---|---|---|
| Cell surface uPAR concentrations | ||||||
| Unstimulated cells | 9.07±0.06 | 9.86±0.14 | 9.06±0.03 | 8.88±0.04 | 7.24±0.06 | 7.58±0.03 |
| Stimulated cells | 9.54±0.09 | 6.01±0.06 | 6.37±0.05 | 5.12±0.04 | 5.18±0.01 | 8.3±0.01 |
| Stimulated cells + HKa | 8.88±0.22 | 8.15±0.14 | 8.66±0.08 | 8.58±0.03 | 8.06±0.18 | 10.41±0.23 |
| Probability values for differences | ||||||
| Unstimulated cells vs. stimulated cells | 0.011 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Stimulated cells vs. stimulated cells + HKa | 0.050 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Unstimulated cells vs. stimulated cells + HKa | 0.229 | 0.001 | 0.008 | 0.002 | 0.012 | <0.001 |
Values for cell surface urokinase plasminogen activator (uPA) receptor (uPAR) concentrations are means ± SE (in arbitrary fluorescence units). Cell surface uPARs were measured by immunocytofluorimetric analysis as described in materials and methods. PAI-1, plasminogen activator inhibitor-1; bFGF, basic FGF. For cell surface uPAR concentrations, unstimulated cells represent cells without treatment as the control, stimulated cells represent cells treated with agonists, and stimulated cells + two-chain high-molecular-weight kininogen (HKa) represent cells preincubated with HKa (300 nM) before the addition of corresponding agonists. Immunocytofluorimetric data were collected from 3 independent experiments and quantified by FlowJo 7.2.2 software. For probability values for differences, comparisons of one group to the other group and statistical analysis were performed as described in materials and methods. Results were considered significant when P < 0.05.
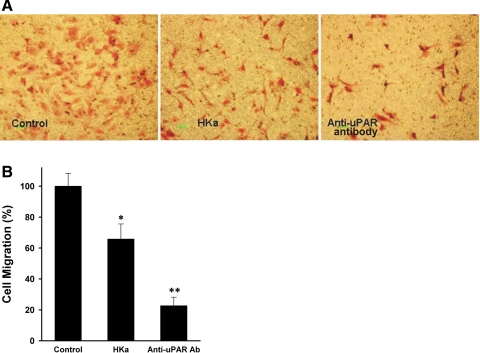
HKa and anti-uPAR antibody inhibit HUVEC migration.
Previous studies have shown that the inhibition of uPAR internalization dramatically reduces EC migration (9, 37, 52) and therefore reduces tube formation, which requires migration. We tested EC migration toward a collagen-fibrinogen mixture-coated membrane of the upper chamber of Transwell plates (a 2-D system). HKa inhibited HUVEC migration to the collagen-fibrinogen matrix to 40% of the control. Cells were not apoptotic after 6 h of incubation. Under the same conditions, anti-uPAR antibodies inhibited migration even more dramatically (>80% of the control; Fig. 6, A and B). These results indicate that both HKa and anti-uPAR antibody inhibit cell migration by preventing the binding of uPA to uPAR.
Fig. 6.
HKa and the uPAR antibody inhibit endothelial cell migration. A: inhibition of HUVEC migration. The medium in the lower chamber was endothelial basal medium (EBM) with or without VEGF (10 ng/ml). The individual groups compared were HUVECs treated with only VEGF (control; left), VEGF and HKa (100 nM; middle), or VEGF and anti-uPAR antibody (66 nM; right). Magnification: ×100. Green bars = 100 μm. B: quantification of HUVEC migration. Cells were counted and standardized as percentages. Controls were set to 100%. Cell migration was inhibited by 40% with HKa and 80% with uPAR antibody. Comparisons were made with the control. *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
The uPAR functions in concert with coreceptors that include integrins (β1–β5) (50), EGFR (38), and FPR-like receptor-1/lipoxin A4 receptor (53). The uPAR has been implicated in several molecular events such as coordinating cell adhesion, migration, invasion, and proliferation and promoting cell survival (6). Since the uPAR is a major receptor for HKa, we started by comparing the effects of anti-uPAR antibody and HKa on tube formation of ECs in a novel 3-D culture system that includes fibrinogen (found in provisional ECM). We found that both molecules inhibited tube and vacuole formation to a similar extent. Our next step was to test if this effect was due to disruption of the uPA-uPAR complex. Our results showed that in the 3-D system, HKa mitigated the association of the uPA-uPAR complex without an apparent inhibition of uPA expression. We further demonstrated that HKa inhibited ERK phosphorylation in both 2-D and 3-D systems as well as uPAR internalization, each of which represented a novel finding in this study.
HK is a 120-kDa α-globulin with a plasma concentration of 80 μg/ml (0.67 μmol/l) and an isoelectric point of 4.3 (54, 55). In a healthy female, only 7.2% of HK is cleaved compared with a rheumatoid arthritis patient, in which 11.5% of HK is cleaved (56). In an extreme situation, such as the shock caused by a lethal dose (1.2 mg/kg) of pseudomonal elastase, the consumption of HK can range to up to 85% (33). As shown in Fig. 1C, HKa can significantly inhibit tube and vacuoles as low as 10 nM, which represents only 1.5% of the normal HK concentration. The IC50 of the inhibitory effect of HKa on tube and vacuole formation is ∼30 nM, which represents 5% cleavage of HK, similar to the cleavage observed in normal plasma. The concentration of HKa of 100 nM, which can be found in rheumatoid arthritis, represents 15% cleavage. A concentration of HK of 300 nM has been demonstrated only in septic shock.
HK is a proangiogenic molecule, whereas HKa is an antiangiogenic molecule. Together, they regulate the balance of pathophysiological angiogenesis. HKa (30 nM) inhibited ∼50% of tube formation, which can be found in physiological processes such as embryonic development, wound healing, and the female reproductive cycle. HKa (300 nM) almost completely inhibited tube and vacuole formation, which can be found in pathologic processes such as cancer and inflammation. Thus, HKa dose dependently inhibited tube formation, reflecting that HKa can play an important role in pathophysiological angiogenesis.
In a previous study (25), we found that cells treated with D5 in the presence of bFGF showed typical morphological features of apoptosis. We also showed that HKa inhibited cell adhesion to vitronectin by 90% and gelatin by 40%; in contrast, HKa had no apparent effect on cell adhesion to fibronectin. HKa induced apoptosis of ECs grown on vitronectin and gelatin but not cells grown on fibronectin, which closely parallels its antiadhesive potency, indicating that the apoptotic activity of HKa and D5 is highly regulated by their interactions with different ECM proteins (24). However, in 3-D gels, additional mechanisms might exist to explain the apoptotic effect of HKa. Recently, there have been several reports of apoptosis induced by the inhibition of ERK activity (22, 44). The ability of uPA to activate the Ras-ERK signaling pathway by binding to uPARs is well documented (1, 31, 34, 45, 46, 59). This process may depend on the integrity of a signaling receptor complex that includes uPARs and plasma membrane adaptor proteins, such as integrins and caveolin (5, 8). Ma et al. (41) showed that a uPAR-specific antibody, uPA mRNA-specific antisense oligodeoxynucleotides, or the inhibitor PD-098059 promoted apoptosis, as determined by caspase-3 activity. As shown Fig. 2C, 300 nM HKa can inhibit ERK phosphorylation from 1 to 24 h. As shown in Fig. 3B, Western blot analysis demonstrated that exogenous uPA-induced ERK phosphorylation was also prevented by 100 nM HKa. These observations suggest that the apoptotic effect of HKa at least partly mediated through the ERK pathway.
uPA is synthesized and secreted as a one-chain, 55,000-Mr, zymogen (pro-uPA). Limited proteolysis by plasmin can convert this pro-uPA into the two-chain, 55,000-Mr, active enzyme (uPA) composed of a 158-amino acid A chain and a 253-amino acid B chain linked by a disulfide bond (62). Active uPA forms complexes with its inhibitor, PAI-1, which binds to uPARs in a process involving clathrin-coated vesicle formation (26). Thereafter, uPAR itself can recycle back from the endocytotic compartment to preformed focal adhesions of the cell surface at the leading edge (48, 52). The pro-uPA/uPAR complex also can be internalized, which is mediated by a uPAR-associated protein (uPARAP/Endo 180) in the presence of collagen V (57). Internalization of pro-uPA/uPAR or uPA/uPAR/PAI-1 is cell type specific. For instance, when active uPA or pro-uPA are bound to uPAR, they are not internalized and remain on the cell surface in monocyte-like cell line U937 or in a epidermoid carcinoma cell line (15, 58, 62). Only active uPA is complexed to specific PAI-1, serpin, or protease nexin (PN)-1; the complex is internalized and degraded in the lysosomes of cell line U937 or in a human choriocarcinoma cell line (14, 16, 18, 48). Kounnas et al. (35) also demonstrated that pro-urokinase and two-chain urokinase bind directly to purified LRP and that LRP mediates their internalization and degradation in Hep G2 cells. As shown in Fig. 5A, the addition of pro-uPA did not decrease cell surface uPARs, whereas uPA or the uPA/PAI-1 complex decreased cell surface uPARs, suggesting that the internalization of the uPAR is mediated by the uPA-PAI-1-LRP complex but not by the pro-uPA-uPAR- uPARAP complex. It is generally agreed that the expression of uPARAP/Endo 180 in HUVECs is lower than in fibroblast-like cells. Most of uPARAP/Endo180 in HUVECs is localized throughout the cytoplasm in intracellular vesicles, and only a small portion of uPARAP/Endo 180 is localized on the cell surface in a fine punctuate distribution (57).
The conversion of pro-uPA to uPA in ECs is responsible for local fibrinolytic activity and is probably one of the initial steps in matrix degradation during the angiogenic process. Prager et al. (51) showed that VEGF induces rapid pro-uPA activation on the surface of ECs. The generation of active uPA, in the presence of PAI-1, induces the internalization of uPARs via a LDL receptor-like molecule (52). This concept is in agreement with our data, as shown in Fig. 5B. Furthermore, we showed that bFGF can decrease cell surface uPARs. Flaumenhaft et al. (19) showed that bFGF increase uPA activity by sixfold in ECs. Furthermore, VEGF-induced in vitro angiogenesis and uPA activity are dependent on endogenous bFGF (43). Thus, both VEGF and bFGF can convert pro-uPA to uPA and promote uPAR internalization. As shown in Table 1, our data also revealed that PMA increased cell surface uPARs, which coordinated with PMA to upregulate uPAR expression (49). The addition of HKa further increased cell surface uPARs, indicating that HKa might capture more uPARs when uPARs recycle to the cell surface. In the 3-D gel, PMA upregulated uPAR expression on the EC surface, whereas VEGF and bFGF induced the conversion of pro-uPA to uPA. The binding of active uPA to PAI-1/uPAR resulted in the internalization of uPARs via α2-macroglobulin receptor-LRP. Our flow cytometry data suggested that the targeting of HKa to uPARs halted this process and therefore prevented tube formation. The binding site of uPA to the LDL receptor is located within DII and DIII of the uPAR (17), which are the same uPAR domains with which HKa interacts (12). The binding of HKa should directly inhibit the association of uPARs and LDL receptors. Interference with the uPA-PAI-1-uPAR complex formation would decrease cell surface uPAR expression and cell motility (9, 37, 52). As shown in Fig. 6, either HKa or anti-uPAR antibody decreased cell motility.
The endogenous uPA-uPAR system plays an essential role in determining the steady-state level of activated ERK. Recently, X-ray diffraction revealed that uPA binds to all three domains of a uPAR crystal (4, 27). Tarui et al. (61) showed that uPA binds to αvβ3-integrin by its kringle domain. We suggested a model in which uPA binds to domain I of the uPAR by its growth factor domain and binds to αvβ3-integrin by its kringle domain. αvβ3-Integrin binds to DII and DIII of the uPAR, which form a ternary complex.
As shown in Fig. 2C, HKa can disrupt this complex by targeting uPAR DII and DIII. Therefore, HKa inhibited uPA-dependent ERK phosphorylation and uPAR internalization. The Kd of uPA binding to uPARs is ∼1 nM, whereas the Kd of HKa binding to ECs is 2.5 nM (67). However, the Kd of HKa binding to uPARs is unknown at this time. The transition from HK to HKa involves major conformational changes (63) and leads to a greater exposure of the D5 region. Compared with HK, HKa has an increased antiadhesive effect that is due to domain rearrangement (3), which indicates that HKa might have a higher binding affinity to uPARs than HK. uPA is more effective in blocking native HK binding to scuPARs with increasing concentrations from 0.01 to 3,000 nM (42). We have shown that uPA at up to 500 nM cannot block the binding of HKa to ECs. We explain this observation by suggesting that 300 nM HKa can disrupt the uPA-uPAR complex but uPA can still associate with αvβ3-integrin on the surface of cells. Thus, HKa cannot prevent the binding of uPA to ECs.
In conclusion, we have shown that HKa dose dependently inhibited tube and vacuole formation, which correlated with pathophysiological angiogenesis processes. The antibody against the uPAR mimicked the inhibitory effect of HKa on apoptosis (HKa: 30% and uPAR antibody: 26%) and tube formation, indicating that HKa is as versatile as an inhibitor of the angiogenic process as is the uPAR as a stimulator. In tumor angiogenesis, cancer cells release angiogenic stimulators, such as VEGF and bFGF, thus stimulating the transformation of endogenous pro-uPA to uPA. The proteolytic enzyme uPA then binds to its receptor (uPAR) in a complex with its inhibitor PAI-1, which results in the internalization of this complex (52), and activates ERK. Recycling of the uPAR regulates the migration of ECs. ERK activation stimulates migration and proliferation and suppresses apoptosis of ECs. HKa disrupted the uPA-uPAR complex, inhibited ERK activation, and blocked the internalization of uPARs, eventually resulting in cell death and cell motility arrest. Both are critical steps in angiogenesis.
GRANTS
This work was supported by National Institutes of Health Grants R01-CA-83121, R01-AR-051713, and T32-HL-007777 (to R. W. Colman).
Acknowledgments
Present address of D. J. Cao: Div. of Cardiology, Dept. of Internal Medicine, The Univ. of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390 (e-mail: Dian.Cao@utsouthwestern.edu).
Present address of Y.-L. Guo: Dept. of Biological Sciences, Univ. of Southern Mississippi, Hattiesburg, MI 39406 (e-mail: Yanlin.Guo@usm.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aguirre Ghiso JA, Alonso DF, Farias EF, Gomez DE, de Kier Joffè EB. Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur J Biochem 263: 295–304, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol 147: 89–104, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asakura S, Hurley RW, Skorstengaard K, Ohkubo I, Mosher DF. Inhibition of cell adhesion by high molecular weight kininogen. J Cell Biol 116: 465–476, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barinka C, Parry G, Callahan J, Shaw DE, Kuo A, Bdeir K, Cines DB, Mazar A, Lubkowski J. Structural basis of interaction between urokinase-type plasminogen activator and its receptor. J Mol Biol 363: 482–495, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasi F Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the u-PA-u-PAR-PAI-1 system. Thromb Haemost 82: 298–304, 1999. [PubMed] [Google Scholar]
- 6.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 3: 932–943, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Chapman HA, Wei Y, Simon DI, Waltz DA. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemost 82: 291–297, 1999. [PubMed] [Google Scholar]
- 9.Chazaud B, Bonavaud S, Plonquet A, Pouchelet M, Gherardi RK, Barlovatz-Meimon G. Involvement of the [uPAR:uPA:PAI-1:LRP] complex in human myogenic cell motility. Exp Cell Res 258: 237–244, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Cher ML, Biliran HR Jr, Bhagat S, Meng Y, Che M, Lockett J, Abrams J, Fridman R, Zachareas M, Sheng S. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci USA 100: 7847–7852, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down- regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood 95: 543–550, 2000. [PubMed] [Google Scholar]
- 12.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2+3 of the urokinase receptor. J Clin Invest 100: 1481–1487, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. α-2 Macroglobulin receptor/Ldl receptor-related protein (Lrp)-dependent internalization of the urokinase receptor. J Cell Biol 131: 1609–1622, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conese M, Olson D, Blasi F. Protease nexin-1-urokinase complexes are internalized and degraded through a mechanism that requires both urokinase receptor and alpha 2-macroglobulin receptor. J Biol Chem 269: 17886–17892, 1994. [PubMed] [Google Scholar]
- 15.Cubellis MV, Nolli ML, Cassani G, Blasi F. Binding of single-chain prourokinase to the urokinase receptor of human U937 cells. J Biol Chem 261: 15819–15822, 1986. [PubMed] [Google Scholar]
- 16.Cubellis MV, Wun TC, Blasi F. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J 9: 1079–1085, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol Biol Cell 12: 1467–1479, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estreicher A, Muhlhauser J, Carpentier JL, Orci L, Vassalli JD. The receptor for urokinase-type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol 111: 783–792, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaumenhaft R, Abe M, Mignatti P, Rifkin DB. Basic fibroblast growth factor-induced activation of latent transforming growth factor beta in endothelial cells: regulation of plasminogen activator activity. J Cell Biol 118: 901–909, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folkman J Angiogenesis in female reproductive organs. In: Steroid Hormones and Uterine Bleeding, edited by Alexander NH, d'Arcangus C. Washington, DC: AAAS, 1992, p. 143.
- 21.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res 13: 4051–4060, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grethe S, Porn-Ares MI. p38 MAPK regulates phosphorylation of Bad via PP2A-dependent suppression of the MEK1/2-ERK1/2 survival pathway in TNF-alpha induced endothelial apoptosis. Cell Signal 18: 531–540, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Guillonneau X, Bryckaert M, Launay-Longo C, Courtois Y, Mascarelli F. Endogenous FGF1-induced activation and synthesis of extracellular signal-regulated kinase 2 reduce cell apoptosis in retinal-pigmented epithelial cells. J Biol Chem 273: 22367–22373, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Guo YL, Wang S, Cao DJ, Colman RW. Apoptotic effect of cleaved high molecular weight kininogen is regulated by extracellular matrix proteins. J Cell Biochem 89: 622–632, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Guo YL, Wang S, Colman RW. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler Thromb Vasc Biol 21: 1427–1433, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71: 411–421, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, Furie B, Furie BC, Cines DB, Huang M. Structure of human urokinase plasminogen activator in complex with its receptor. Science 311: 656–659, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Ingber DE Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91: 877–887, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442: 453–456, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci 94: 43–49, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanse SM, Benzakour O, Kanthou C, Kost C, Lijnen HR, Preissner KT. Induction of vascular SMC proliferation by urokinase indicates a novel mechanism of action in vasoproliferative disorders. Arterioscler Thromb Vasc Biol 17: 2848–2854, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Katkade V, Soyombo AA, Isordia-Salas I, Bradford HN, Gaughan JP, Colman RW, Panetti TS. Domain 5 of cleaved high molecular weight kininogen inhibits endothelial cell migration through Akt. Thromb Haemost 94: 606–614, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Khan MM, Yamamoto T, Araki H, Ijiri Y, Shibuya Y, Okamoto M, Kambara T. Pseudomonal elastase injection causes low vascular resistant shock in guinea pigs. Biochim Biophys Acta 1182: 83–93, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Konakova M, Hucho F, Schleuning WD. Downstream targets of urokinase-type plasminogen-activator-mediated signal transduction. Eur J Biochem 253: 421–429, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Kounnas MZ, Henkin J, Argraves WS, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates cellular uptake of pro-urokinase. J Biol Chem 268: 21862–21867, 1993. [PubMed] [Google Scholar]
- 36.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem 271: 24313–24316, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Knisely JM, Lu W, McCormick LM, Wang J, Henkin J, Schwartz AL, Bu G. Low density lipoprotein (LDL) receptor-related protein 1B impairs urokinase receptor regeneration on the cell surface and inhibits cell migration. J Biol Chem 277: 42366–42371, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1: 445–457, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Pelekanakis K, Woolkalis MJ. Thrombin and tumor necrosis factor alpha synergistically stimulate tissue factor expression in human endothelial cells: regulation through c-Fos and c-Jun. J Biol Chem 279: 36142–36147, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Sainz IM, Wu Y, Pixley R, Espinola RG, Hassan S, Khan MM, Colman RW. The inhibition of tube formation in a collagen-fibrinogen, three-dimensional gel by cleaved kininogen (HKa) and HK domain 5 (D5) is dependent on Src family kinases. Exp Cell Res 314: 774–788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci 114: 3387–3396, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Mahdi F, Shariat-Madar Z, Kuo A, Carinato M, Cines DB, Schmaier AH. Mapping the interaction between high molecular weight kininogen and the urokinase plasminogen activator receptor. J Biol Chem 279: 16621–16628, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Mandriota SJ, Pepper MS. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci 110: 2293–2302, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Michaelis M, Suhan T, Michaelis UR, Beek K, Rothweiler F, Tausch L, Werz O, Eikel D, Zörnig M, Nau H, Fleming I, Doerr HW, Cinatl J Jr. Valproic acid induces extracellular signal-regulated kinase 1/2 activation and inhibits apoptosis in endothelial cells. Cell Death Differ 13: 446–453, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MJ, Gonias SL. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol 146: 149–164, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen DH, Hussaini IM, Gonias SL. Binding of urokinase-type plasminogen activator to its receptor in MCF-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Biol Chem 273: 8502–8507, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen DH, Webb DJ, Catling AD, Song Q, Dhakephalkar A, Weber MJ, Ravichandran KS, Gonias SL. Urokinase-type plasminogen activator stimulates the Ras/Extracellular signal-regulated kinase (ERK) signaling pathway and MCF-7 cell migration by a mechanism that requires focal adhesion kinase, Src, and Shc. Rapid dissociation of GRB2/Sps-Shc complex is associated with the transient phosphorylation of ERK in urokinase-treated cells. J Biol Chem 275: 19382–19388, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J 16: 2610–2620, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nykjaer A, Moller B, Todd RF 3rd, Christensen T, Andreasen PA, Gliemann J, Petersen CM. Urokinase receptor. An activation antigen in human T lymphocytes. J Immunol 152: 505–516, 1994. [PubMed] [Google Scholar]
- 50.Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol 12: 613–620, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Prager GW, Breuss JM, Steurer S, Mihaly J, Binder BR. Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood 103: 955–962, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Prager GW, Breuss JM, Steurer S, Olcaydu D, Mihaly J, Brunner PM, Stockinger H, Binder BR. Vascular endothelial growth factor receptor-2-induced initial endothelial cell migration depends on the presence of the urokinase receptor. Circ Res 94: 1562–1570, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA 99: 1359–1364, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmaier AH, Bradford H, Silver LD, Farber A, Scott CF, Schutsky D, Colman RW. High molecular weight kininogen is an inhibitor of platelet calpain. J Clin Invest 77: 1565–1573, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmaier AH, Zuckerberg A, Silverman C, Kuchibhotla J, Tuszynski GP, Colman RW. High-molecular weight kininogen. A secreted platelet protein. J Clin Invest 71: 1477–1489, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma JN, Zeitlin IJ, Brooks PM, Buchanan WW, Dick WC. The action of aspirin on plasma kininogen and other plasma proteins in rheumatoid patients: relationship to disease activity. Clin Exp Pharmacol Physiol 7: 347–354, 1980. [DOI] [PubMed] [Google Scholar]
- 57.Sheikh H, Yarwood H, Ashworth A, Isacke CM. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J Cell Sci 113: 1021–1032, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Stoppelli MP, Tacchetti C, Cubellis MV, Corti A, Hearing VJ, Cassani G, Appella E, Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell 45: 675–684, 1986. [DOI] [PubMed] [Google Scholar]
- 59.Tang DG, Li L, Zhu Z, Joshi B. Apoptosis in the absence of cytochrome c accumulation in the cytosol. Biochem Biophys Res Commun 242: 380–384, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Tang H, Kerins DM, Hao Q, Inagami T, Vaughan DE. The urokinase-type plasminogen activator receptor mediates tyrosine phosphorylation of focal adhesion proteins and activation of mitogen-activated protein kinase in cultured endothelial cells. J Biol Chem 273: 18268–18272, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Tarui T, Akakura N, Majumdar M, Andronicos N, Takagi J, Mazar AP, Bdeir K, Kuo A, Yarovoi SV, Cines DB, Takada Y. Direct interaction of the kringle domain of urokinase-type plasminogen activator (uPA) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost 95: 524–534, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol 100: 86–92, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villanueva GB, Leung L, Bradford H, Colman RW. Conformation of high molecular weight kininogen: effects of kallikrein and factor XIa cleavage. Biochem Biophys Res Commun 158: 72–79, 1989. [DOI] [PubMed] [Google Scholar]
- 64.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Yang B, Cao DJ, Sainz I, Colman RW, Guo YL. Different roles of ERK and p38 MAP kinases during tube formation from endothelial cells cultured in 3-dimensional collagen matrices. J Cell Physiol 200: 360–369, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Yang S, Graham J, Kahn JW, Schwartz EA, Gerritsen ME. Functional roles for PECAM-1 (CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am J Pathol 155: 887–895, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JC, Donate F, Qi X, Ziats NP, Juarez JC, Mazar AP, Pang YP, McCrae KR. The antiangiogenic activity of cleaved high molecular weight kininogen is mediated through binding to endothelial cell tropomyosin. Proc Natl Acad Sci USA 99: 12224–12229, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao R, Shen GX. Involvement of heat shock factor-1 in glycated LDL-induced upregulation of plasminogen activator inhibitor-1 in vascular endothelial cells. Diabetes 56: 1436–1444, 2007. [DOI] [PubMed] [Google Scholar]