Abstract
These studies explore the consequences of activating the prolyl hydroxylase (PHD) O2-sensing pathway in spontaneously twitching neonatal cardiomyocytes. Full activation of the PHD pathway was achieved using the broad-spectrum PHD inhibitor (PHI) dimethyloxaloylglycine (DMOG). PHI treatment of cardiomyocytes caused an 85% decrease in O2 consumption and a 300% increase in lactic acid production under basal conditions. This indicates a ∼75% decrease in ATP turnover rate, inasmuch as the increased ATP generation by glycolysis is inadequate to compensate for the lower respiration. To determine the extent to which decreased ATP turnover underlies the suppressed O2 consumption, mitochondria were uncoupled with 2,4-dinitrophenol. We were surprised to find that 2,4-dinitrophenol failed to increase O2 consumption by PHI-treated cells, indicating that electron transport chain activity, rather than ATP turnover rate, limits respiration in PHI-treated cardiomyocytes. Silencing of hypoxia-inducible factor-1α (HIF-1α) expression restored the ability of uncoupled PHI-treated myocytes to increase O2 consumption; however, basal O2 uptake rates remained low because of the unabated suppression of cellular ATP consumption. Thus it appears that respiration is actively “clamped” through an HIF-dependent mechanism, whereas HIF-independent mechanisms are responsible for downregulation of ATP consumption. In addition, we find that PHD pathway activation enables mitochondria to utilize fumarate as a terminal electron acceptor when cytochrome c oxidase is inactive. The source of fumarate for this unusual respiration is derived from aspartate via the purine nucleotide cycle. In sum, these studies show that the O2-sensing pathway is sufficient to actively “clamp” O2 consumption and independently suppress cellular ATP consumption. The PHD pathway also enables the mitochondria to utilize fumarate for respiration.
Keywords: mitochondrial membrane potential, hibernation, cardioprotection
although oxygenation of lower eukaryotes is accomplished by simple diffusion, an extensive cardiovascular and respiratory system has evolved in higher animals to enable tissue and organ oxygenation. In turn, cells, and the tissue they comprise, closely monitor O2 levels and initiate a complex response to restore homeostasis when lower O2 concentrations are detected. These responses include release of vasodilator agents, expression of angiogenic factors, and upregulation of anaerobic glycolytic metabolism, among others. Many of the cellular responses to lower O2 occur at concentrations higher than those limiting aerobic mitochondrial ATP production (38), an observation that first suggested the presence of a cellular O2-sensing mechanism distinct from the electron transport chain (ETC) or ATP depletion. More recently, a cellular O2-sensing mechanism has been identified as a family of proline- and asparagine-hydroxylating domain-containing enzymes [prolyl hydroxylase (PHD)] (5, 10, 27). Three PHDs (PHD1, PHD2, and PHD3) and one asparagine hydroxylase [factor inhibiting HIF (FIH-1)] have been identified. Under normoxic conditions, the hydroxylation of specific proline residues by PHD2 promotes the interaction of hypoxia-inducible factor-1α (HIF-1α) with the von Hippel-Lindau (VHL) protein and constitutively targets HIF-1α for proteasomal degradation (21, 22). Although PHD1 and PHD3 can hydroxylate HIF-1α in vitro, HIF does not appear to be their physiological target in the cell (3). During hypoxia, the reduced levels of proline hydroxylation lead to a cessation of HIF-1α degradation, causing an increase in its levels. In addition, hypoxia also blocks FIH-1 hydroxylation of asparagine 803 in the trans-activating domain, leading to full activation of HIF-1α (15, 27). Thus posttranslational hydroxylation regulates the stability and the activity of HIF-1α. The importance of the PHD pathway as a master regulator is highlighted by recent results showing that the O2-sensing signal cascade directs cellular responses independently from HIF-1α accumulation and activation (7, 26).
The classical protective maneuvers of the cell in response to hypoxia are the upregulation of anaerobic glycolysis, the Embden-Meyerhoff pathway (EMP), and downregulation of ATP-consuming processes that are nonessential to cellular viability (16, 17). The induction of multiple enzymes comprising the glycolytic pathway in response to hypoxia (37) is largely attributed to transcriptional activation via HIF-1α. The downregulation of ATP-consuming reactions has been recognized for over 20 years as a fundamental cellular survival response to hypoxia. However, very little is understood about the signaling pathways or the mechanisms for attenuation of ATP consumption. In the present studies, we have found evidence that the PHD pathway directs the downregulation of cellular ATP consumption in myocytes.
Enhanced anaerobic glycolytic metabolism and dampened cellular ATP consumption work to maintain ATP levels when O2 levels fall. Although maintaining ATP levels is of obvious benefit, our recent work suggested that other additional mechanisms protect the cell from irreversible damage independent of these energy-sparing maneuvers (39). In a previous study, we found that prior activation of the PHD O2-sensing signal cascade offered robust protection from the blockade of oxidative phosphorylation via increased EMP flux that preserved ATP levels. We were more surprised to also observe that activation of the O2 sensor conferred protection even when ATP depletion was forced with a glycolytic inhibitor. This protection was associated with the ability of the mitochondria to maintain inner membrane polarization, despite blockade of cytochrome c oxidase and glycolysis with metabolic poisons. The present studies were designed to reveal the mechanisms that underlie these phenomena.
In the present studies, the PHD inhibitor (PHI) dimethyloxaloylglycine (DMOG) was used to activate the O2-sensing signal cascade, and the subsequent changes in cardiomyocyte phenotype are examined. Recent studies have validated the idea that DMOG induces a robust hypoxic stress response with a remarkable degree of fidelity to responses that were previously attributed to the O2 sensor (9). DMOG produces a robust hypoxic response, because it inhibits the multiple, distinct prolyl and asparaginyl hydroxylases, eliciting HIF-1α-dependent and -independent responses (9). In the present study, we focus particularly on the metabolic and mitochondrial functional changes in response to PHD pathway activation. The results indicate that the PHD pathway directs profound changes in cellular metabolism and compensatory responses that culminate in a cytoprotected phenotype. The mechanisms employed by eukaryotic cells to protect themselves from O2 scarcity are of fundamental and translational interest.
MATERIALS AND METHODS
Reagents.
DMOG was purchased from Frontier Scientific (Logan, UT), antibody directed against HIF-1α from R & D systems (Minneapolis, MN), and all other reagents from Sigma.
Myocyte cultures.
The animal procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee. Primary neonatal cardiomyocytes were isolated from 1- to 2-day-old Swiss Webster mouse pups by use of a previously described method (48) that produces cultures that exhibit spontaneous beating and >95% myocyte purity. Cells were plated on gelatin-coated plates at a density of 2 × 106 cells per well in a 6-well culture plate and 0.25 × 106 cells per well in 24-well plates in DMEM-Ham's F-12 medium supplemented with 10% fetal bovine serum. After 24 h, myocytes were washed and placed in maintenance medium consisting of DMEM-Ham's F-12 supplemented with insulin-transferrin-selenium solution (Cambrex, Baltimore, MD) and 1% fetal bovine serum. DMEM without glucose or pyruvate was used as the base solution for “metabolic inhibition” (MI) medium. All experiments were conducted 4–6 days after the isolation of myocytes. Protein separations were performed by standard procedures using SDS-PAGE Tris-glycine gels. Western blot analyses were performed and detected using ECL chemiluminescence detection reagents (Amersham Biosciences, Piscataway, NJ) and the manufacturer's protocols.
Determination of ATP equivalents.
ATP equivalents were calculated as described previously (6), with the assumption of a phosphorus-to-O2 ratio of 2.58 (4) and 1 mol of ATP per mole of lactic acid produced. Lactic acid was measured using a Sigma diagnostic kit according to the manufacturer's instructions.
Measurements of O2 consumption and extracellular acidification rates.
A Seahorse Bioscience XF24 instrument was used to measure the rate of change of dissolved O2 and pH in medium immediately surrounding cardiomyocytes cultured in 24-well plates. Measurements were performed using a cartridge where 24 optical fluorescent O2 and pH sensors are configured as individual well “plungers.” For measurements of rates, the plungers gently descended into the wells, forming a chamber that entraps the cells in ∼7 μl volume. O2 concentration and pH were measured over 1 min, a period of time that this is insufficient for >5–10% change in gross O2 levels. The rates of O2 consumption and extracellular acidification were obtained from the slopes of concentration changes vs. time. For preparation of the cell plate for assay with the XF24 instrument, 1 ml of Krebs-Henseleit buffer lacking bicarbonate (111 mM NaCl, 4.7 mM KCl, 2.0 mM MgSO4, 1.2 mM Na2HPO4, 0.24 mM MgCl2, 2.5 mM glucose, 0.5 mM carnitine, and 100 nM insulin) warmed to 37°C was added to each well containing 0.25 × 106 myocytes. Various inhibitors were introduced by automatic injectors followed by brief (15 s) mixing.
Measurement of cytotoxicity, NH3, ATP, and Δψmito.
The loss of cardiomyocyte viability was quantified by measurement of lactate dehydrogenase released from cells using a nonradioactive cytotoxicity assay kit (Cytotox 96, Promega, Madison, WI) according to the manufacturer's instructions. Intracellular ATP content was determined using an ATP bioluminescent assay kit (Sigma) according to the manufacturer's instructions. ATP content was calculated using a standard curve derived from known concentrations of ATP. Mitochondrial membrane potential (ψmito) was assessed using JC-1 (33) (Molecular Probes, Invitrogen, Carlsbad, CA). Control and PHI-treated myocytes were incubated in medium containing 5 μg/ml JC-1 during the final 15 min of the MI or recovery period. After the myocytes were stained, the cultures were washed twice with PBS (pH 7.4). When excited at 488 nm, the fluorescence emission of JC-1 was measured at wavelengths corresponding to its monomer (530 ± 15 nm) and J aggregate (>590 nm) forms. Fluorescence was measured in a fluorescent plate reader (Molecular Devices, Sunnyvale, CA) or via confocal microscopy. Ammonia was measured photometrically using Nessler's reagent (42). Statistical significance was determined using Student's t-test or ANOVA with Bonferroni's post hoc analysis. P ≤ 0.05 was taken as significant.
Measurements of carboxylic acids.
Analysis of carboxylic metabolites was performed according to previously published methods (25, 44). Briefly, 10 × 106 myocytes were extracted with a 10% TCA solution, neutralized, and derivatized with a 1:1 ratio of 100 mM 4-N,N-dimethylaminopyridine and 100 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide at 60°C for 120 min. Methylsuccinic acid was used an internal control. Sulfonated poly(styrenedivinylbenzene) polymer (Empore) disk cartridges were used to remove excess 4-N,N-dimethylaminopyridine, and samples were subjected to HPLC-mass spectrometric analysis as previously described (25, 44).
RESULTS
Activation of the O2-sensing pathway reduces ATP turnover rates and respiration.
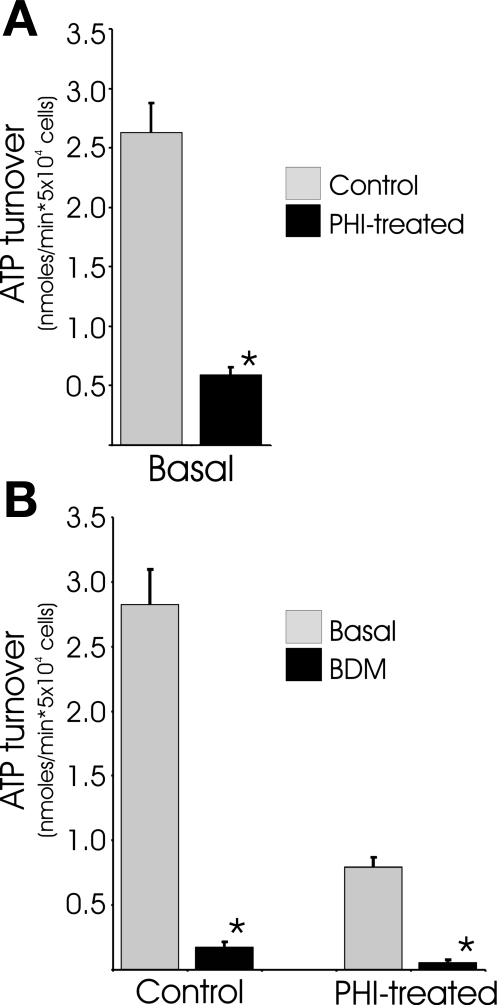
We previously reported that basal O2 consumption rates decrease by ∼85% in DMOG-treated cardiomyocytes (39). Here we report lactate production rates measured for these cells, which enables the calculation of ATP turnover rates (Fig. 1A). In basal conditions, control and DMOG-treated cardiomyocytes produce 24.3 ± 3.3 and 75.5 ± 10.1 pmol of lactate per minute per 5 × 104 myocytes, respectively. Even though lactic acid production is elevated threefold in the DMOG-treated cardiomyocytes, the EMP flux is far from adequate to compensate for the ∼85% reduction of oxidative phosphorylation (OxPhos) flux as measured from decreased O2 consumption. The decrease in OxPhos flux, even when offset with increased glycolytic flux, indicates a ∼75% decrease in ATP turnover in DMOG-treated cells. These results also demonstrate that the primary neonatal mouse cardiomyocytes derive ∼95% of their ATP from O2 respiration with little lactate production in basal conditions. In DMOG-treated cells, the percentage of ATP derived from OxPhos falls to 60% and EMP flux accounts for the remaining 40%. It should be noted here that steady-state levels of cellular ATP, mitochondrial morphology, and mitochondrial polarization of DMOG-treated cardiomyocytes are indistinguishable from those of control myocytes.
Fig. 1.
Activation of the prolyl hydroxylase (PHD) O2 sensor reduces ATP turnover by downregulating contractility. A: O2 consumption and lactic acid rates were used to estimate ATP turnover in twitching neonatal mouse myocytes after treatment with PHD inhibitor (PHI) dimethyloxaloylglycine (DMOG, 250 μM) for 22 h. B: 1 mM butanedione monoxime (BDM) was added 15 min before O2 consumption and lactic acid measurements in control cells and myocytes pretreated with DMOG for 22 h. Values are means ± SE (n = 6–9). *Significantly different from corresponding control (P < 0.05, by t-test).
In actively contracting cardiomyocytes, the great majority of ATP is used to support contractile activity, with only a small fraction used for cell maintenance processes such as protein synthesis. Neonatal mouse cardiomyocytes exhibit spontaneous twitch contractions in culture, and it was considered probable that the ∼75% reduction in ATP turnover could be attributed to dampened excitation contractions. To confirm that contractile-related activity accounts for the majority of ATP expenditures in the neonatal cultures, 2,3-butanedione monoxime (BDM) was used to block contractile activity. Treatment of cardiomyocytes with BDM resulted in a 90% reduction of calculated ATP turnover in control and DMOG-treated cultures (Fig. 1B). These results clearly indicate that a large majority of the metabolism in the cultured neonatal cardiomyocytes is utilized to support isometric twitch contractions and that contractile activity is the only sink of ATP consumption large enough to accommodate the ∼75% decline in ATP turnover in DMOG-treated cells. Although lower contractile activity clearly underlies the reduced ATP turnover rates, reductions in other ATP-consuming activities in the DMOG-treated cells cannot be ruled out. Taken together, these studies provide evidence that activation of the PHD O2-sensing pathway is sufficient to direct a profound repression of cardiomyocyte ATP consumption.
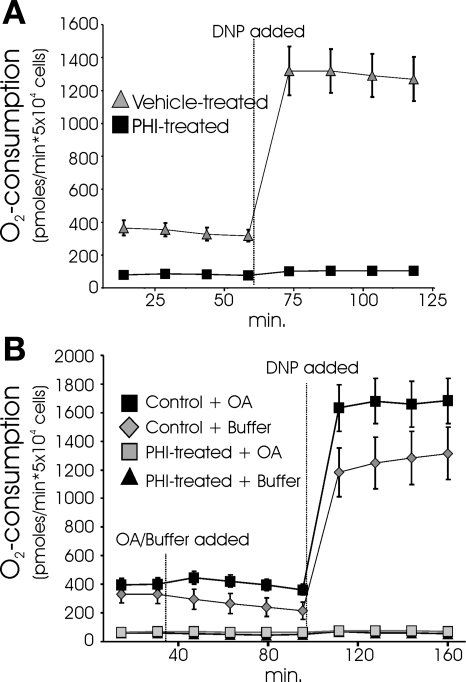
The 85% reduction in the O2 consumption rate in DMOG-treated cardiomyocytes could wholly be a consequence of reduced twitch activity. If diminished contractile ATP consumption underlies the depressed respiration, one would predict that uncoupled respiration would be roughly equivalent in control and DMOG-treated cells. Treatment of control cultures with the proton ionophore 2,4-dinitrophenol (DNP) caused a ∼350% increase in O2 consumption rates. However, DNP failed to elicit an increase in O2 uptake in the DMOG-treated cultures (Fig. 2A). The surprising finding that uncoupling causes no discernable increase in O2 consumption indicates that respiration is actively suppressed at the level of the mitochondrion and is not a simple consequence of reduced ATP turnover. Active suppression of respiration in hypoxia through an HIF-1α-dependent mechanism has recently been reported in studies using cancer cell lines (24, 32). The suppressed O2 uptake was ascribed to HIF-1α-mediated induction of pyruvate dehydrogenase kinase (PDK), which inhibits pyruvate dehydrogenase (PDH) (32). Inhibition of PDH shunts glucose metabolism from the citric acid cycle to the EMP. To examine whether the suppression of respiration observed in the DMOG-treated cells is solely due to PDH inhibition, octanoic acid (OA) was provided as a metabolic substrate in addition to glucose. OA is a short-chain fatty acid that freely diffuses into the mitochondria and has been demonstrated to be avidly metabolized by cardiomyocytes (2, 12). Provision of OA to control cells caused a small increase in basal O2 consumption and an increase in the uncoupled O2 consumption rate, which demonstrates that the cardiomyocytes can freely utilize OA. However, OA did not alleviate the DMOG-induced suppression of coupled or uncoupled respiration (Fig. 2B). The observation that OA is unable to rescue the repressed O2 consumption rate indicates that PHD-regulated mechanisms unrelated to elevated PDK are responsible for the blocked respiration. Although reduced PDH activity cannot fully account for the large reduction of respiration observed in our studies, the observation that HIF-1α actively suppresses mitochondrial respiration is generally consistent with our findings.
Fig. 2.
Active suppression of respiration by the PHD O2 sensor. A: neonatal cardiomyocytes were pretreated with DMSO (vehicle) or the PHI DMOG (250 μM) for 22 h before O2 consumption measurements. 2,4-Dinitrophenol (DNP, 1 μM) was injected to measure uncoupled respiration. B: octanoic acid (OA) was added to standard glucose-containing buffer 35 min before injection of DNP. Values are means ± SE (n = 9). Every PHI-treated respiration value was significantly lower than corresponding vehicle control values (P < 0.05, by ANOVA followed by Bonferroni's post hoc analysis).
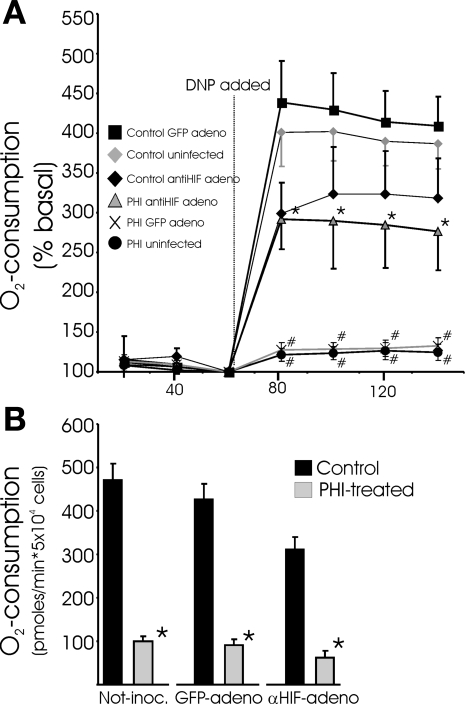
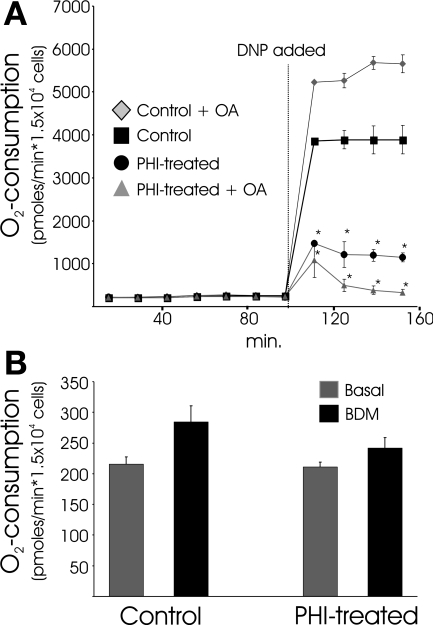
The HIF dependence of the PHD pathway-directed responses observed in the above-described experiments was examined using an adenovirus expressing a short hairpin RNA that we previously showed to selectively and effectively silence HIF-1α expression (49). Primary cardiomyocyte cultures are infected with very high efficiency by adenovirus, and HIF-1α induction in response to DMOG treatment was almost completely (>95%) prevented with prior application of the short hairpin RNA expression vector. With prior ablation of HIF-1α expression, DMOG is no longer effective at clamping respiration and preventing increased O2 uptake in response to DNP (Fig. 3A). This result is consistent with a role for HIF-1α in the PHD-directed suppression of respiration. Strikingly, however, DMOG treatment remained equally effective at lowering basal O2 consumption in cells in the absence of HIF-1α expression (Fig. 3B). Thus basal ATP turnover rates are suppressed by PHI, which leads to less O2 consumption, in an HIF-independent fashion. In addition, HIF-dependent mechanisms are involved in the direct clamping of mitochondrial respiration. We interpret the results shown in Fig. 3 to indicate that the direct suppression of OxPhos and the downregulation of ATP turnover (i.e., contractile activity) are separately directed by the PHD pathway through HIF- and non-HIF-dependent mechanisms, respectively. Following this reasoning, we predicted that, in a nonworking heart culture model, PHI would result in a phenotype where basal O2 uptake rates remain unchanged but the ability to respond to uncoupler with increased O2 consumption would remain depressed. For this experiment, we used feline adult cardiomyocytes that are stable in culture and do not display spontaneous contractile activity. Consistent with the predicted phenotype, PHI treatment of adult feline cardiomyocytes leads to greatly attenuated O2 consumption in response to DNP, whether or not OA is provided as substrate. In stark contrast to the spontaneously contracting neonatal cells, PHI or BDM does not depress basal O2 uptake rates in quiescent adult myocytes (Fig. 4). Taken together, the findings shown in Figs. 3 and 4 support the conclusion that the PHD pathway is responsible for directing the downregulation of ATP consumption associated with contractile activities through an HIF-independent branch in cardiomyocytes.
Fig. 3.
Active, but not passive, suppression of respiration is dependent on hypoxia-inducible factor-1α (HIF-1α). A: coupled and uncoupled O2 consumption measurements in neonatal myocytes in which HIF-1α expression was silenced. Myocytes were inoculated with adenovirus (adeno, 50 plaque-forming units/cell) that expresses short hairpin RNA targeting HIF-1α or control green fluorescent protein (GFP) 30 h before an additional 22 h of DMOG or vehicle treatment. O2 consumption is normalized to basal consumption rates (100%). Values are means ± SE (n = 14). *Significant difference between DNP- and PHI-treated cells in noninfected or GFP-infected groups; #significant difference between PHI-treated cells and respective controls (P < 0.05, by ANOVA followed by Bonferroni's post hoc analysis of individual comparisons). B: nonnormalized basal O2 uptake rates for myocytes treated as described in A. Values are means ± SE (n = 9). *Significantly different from corresponding control (P < 0.05, by t-test).
Fig. 4.
Activating the PHD pathway in quiescent adult feline myocytes suppresses uncoupled respiration but does not affect basal O2 consumption. A: O2 consumption rates in adult myocytes treated with DMOG (PHI) or vehicle (control) for 22 h. The uncoupler DNP was added where indicated. Some cultures where treated with 250 μM OA in addition to 1 mM glucose as metabolic substrate. Values are means ± SE (n = 8). *Significant difference between DNP and corresponding control (P < 0.05, by ANOVA followed by Bonferroni's post hoc analysis of individual comparisons). B: O2 consumption of DMOG (PHI) and untreated (control) myocytes 10 min before (basal) and 10 min after BDM treatments. No significant differences were found between treatment groups.
The above-described studies indicate that electron flow through the ETC is severely restricted by mechanisms activated by the PHD pathway. It is unclear whether this is achieved by limiting the reducing power that enters the ETC, lowering the capacity of the ETC to shuttle electrons, or a combination of the two. Whatever the cause, the finding that electron flow is not stimulated by DNP indicates that basal electron flow is maximal in the DMOG-treated cultures. This would seemingly create a situation where ψmito would be expected to be highly sensitive to any additional perturbations in electron flow. Contrary to this expectation, ψmito is found to be highly refractory, with PHI-treated cells exhibiting remarkable maintenance of mitochondrial polarization during cyanide and 2-deoxyglucose (2-DG) poisoning (39). The next series of studies explored how ψmito is maintained in PHI-treated cells in conditions (i.e., cyanide treatment) where it rapidly dissipates in control cultures.
PHD pathway directs changes that allow cardiomyocytes to use fumarate respiration.
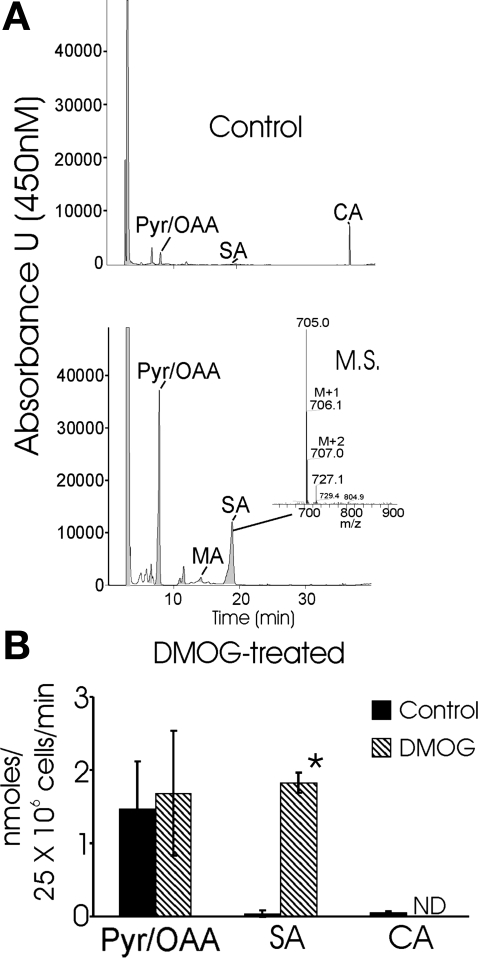
Previously, we suggested that PHD activation induces anaplerotic pathways that culminate in production of fumarate, which is used to accept electrons via reduction to succinate at complex II. In this scenario, complex II provides an outlet for electrons, enabling complex I to initiate electron flux from NADH oxidation and to pump protons in the absence of cytochrome c oxidase activity. Several metabolic pathways that consume aspartate and culminate in fumarate reduction to succinate by complex II have been proposed to explain the anaerobic production of succinate by, and the performance of, the musculature of diving mammals (18, 19, 30). Thus the next experimental series addressed the possibility that the ability of DMOG-treated cells to maintain ψmito during MI with cyanide and 2-DG is due to the use of fumarate as an electron acceptor. Cultures were provided with U-13C-labeled aspartate during 1 h of MI, and the concentrations of citric acid cycle intermediates, including succinate, were measured using an HPLC-mass spectrometric assay. In control cultures, succinate was barely detectable in cellular extracts treated under these conditions. In contrast, a much larger accumulation of succinate was measured in the DMOG-treated cultures (Fig. 5A). A significant portion, >55%, of the succinate produced was labeled with 13C, indicating that it originated from exogenous, as well as endogenous, aspartate. The finding of greatly increased succinate production in the PHI-preconditioned cultures provides direct evidence that the anaplerotic pathway proposed by Hochachka and colleagues (16) is potently upregulated by the PHD pathway. When coupled with our previous findings that the complex I inhibitor rotenone is required, in addition to cyanide, to collapse ψmito in DMOG-treated cells, these results strongly support the notion that the reduction of fumarate by complex II stabilizes ψmito during MI.
Fig. 5.
Activation of PHD O2-sensing pathway results in succinate accumulation during metabolic inhibition: evidence of fumarate respiration. A: representative HPLC of carboxylic acids extracted from mouse neonatal myocyte cultures pretreated with vehicle or DMOG for 24 h. Cultures were metabolically inhibited with 2 mM KCN and 10 mM 2-deoxyglucose (2-DG) in glucose-free medium that contained 13C isotopomer-labeled asparagine (1 mM) for 1 h before extraction and derivatization of carboxylic acids with 4-N,N-dimethylaminopyridine. Citric acid (CA) is also shown. Values are absorbance units at 450-nm wavelength. Inset: mass spectrum (MS) of the peak demonstrating isotopomer labeling of succinic acid (SA). B: levels of selected citric acid cycle intermediates extracted from untreated and DMOG-pretreated cultures after 1 h of metabolic inhibition. Values are means ± SE (n = 3). Values were calculated using 2-methylsuccinic acid as an internal standard. Pyruvic/oxaloacetic acid (Pyr/OAA) resolved as a single peak, whereas citric acid was not detected (ND) in DMOG-treated cultures under these conditions. *Significantly different from corresponding vehicle control (P < 0.05, by Student's t-test).
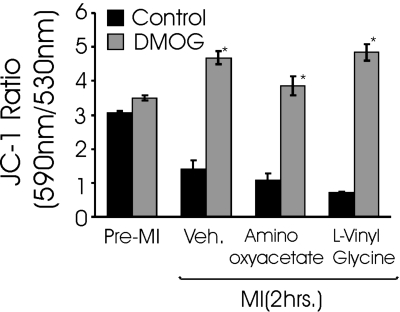
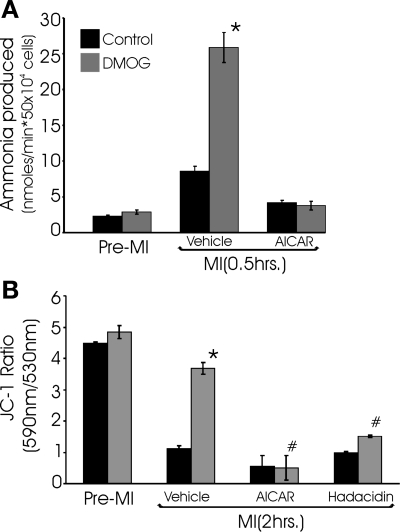
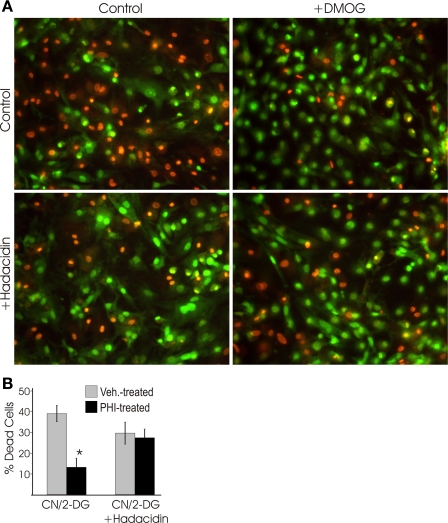
In the anaplerotic pathway proposed by Hochachka and co-workers (18, 30), aspartate and α-ketoglutarate are converted to glutamate and oxalacetate through the action of aspartate aminotransferase. Following the results shown in Fig. 5, we predicted that inhibition of aspartate aminotranferase activity would prevent the stabilization of ψmito that is mediated by the PHD pathway during MI. Contrary to these expectations, two potent aminotransferase inhibitors exhibited little effect on the ability of DMOG-treated cells to maintain mitochondrial polarization during cyanide poisoning (Fig. 6). These observations caused us to reevaluate the limited number of metabolic pathways that could account for aspartate-derived fumarate production. The purine nucleotide cycle (PNC) regenerates AMP from IMP, consumes aspartate, and produces fumarate and ammonia as by-products. Our observation that ammonia production is elevated threefold during MI in the DMOG-treated cultures compared with controls (Fig. 7A) is consistent with a PHI-induced increase in flux through the PNC. In contrast to the aminotransferase inhibitors, inhibitors of the PNC effectively abolished the PHD pathway-mediated stabilization of ψmito during cyanide treatment (Fig. 7B). These results support the proposed role for the PNC in providing the fumarate that allows the continued operation of complexes I and II in the presence of cyanide. It also provides an elegant mechanism to increase fumarate respiration when the cardiomyocyte experiences overt metabolic disturbances and AMP levels rise. Next, the importance of PNC activity in the cytoprotection conferred by PHI treatment was tested. As we previously reported, PHI pretreatment lessens the cell death in response to MI (Fig. 8). Here we find that inhibition of the PNC with hadacidin results in the loss of the cytoprotection afforded by PHI treatment, thus suggesting the necessity of PNC-derived fumarate in the resulting protection of anaerobic ETC activity. Taken together, these results indicate that DMOG-treated cells are capable of true hybrid respiration, utilizing O2 for respiration, but with the capacity to switch to fumarate when cytochrome c oxidase is inactive and glycolysis is insufficient to maintain ATP levels, a situation that most commonly arises with anoxia in cardiac tissue.
Fig. 6.
Aspartate aminotransferase inhibitors do not affect the ability of DMOG-treated cultures to maintain mitochondrial membrane potential (ΔΨmito) using fumarate respiration in metabolic inhibition (MI) conditions. Cultures were treated as described in Fig. 5 legend with cyanide and 2-DG for 2 h in the presence of 1 mM aminooxyacetate or 1 mM l-vinyl glycine. JC-1 was loaded during the final 15 min of MI. ΔΨmito is expressed as ratio of 590-nm to 530-nm fluorescence as quantified with a fluorescent plate reader after JC-1 staining (n = 6) under respective conditions. The mitochondrial uncoupler carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone was used a positive control (not shown). Values are means ± SE (n = 8). *Significantly different from corresponding control (P < 0.05, by t-test).
Fig. 7.
Activation of the PHD O2-sensing pathway enhances myocyte NH3 production, and purine nucleotide cycle (PNC) inhibitors reduce ability of DMOG-treated cultures to maintain ΔΨmito in MI conditions: evidence that enhanced PNC activity provides the fumarate necessary for anaerobic respiration. A: NH3 production in myocytes that were pretreated with vehicle or DMOG for 22 h and subjected to 0.5 h of MI. B: cultures were treated as described in A with DMOG and subjected to 2 h of MI. The PNC inhibitors 5-aminoimidazole-4-carboxamide-1β-d-ribofuranoside (AICAR) and hadacidin were included with MI conditions in the indicated cultures. Ability of cells to maintain mitochondrial polarization in these conditions was assessed with JC-1. Values are means ± SE (n = 6). *Significantly different from corresponding non-DMOG-treated control; #significantly lower than corresponding DMOG-treated control (P < 0.05, by t-test).
Fig. 8.
Inhibition of PNC activity with hadacidin abolishes cytoprotection conferred by DMOG treatment. Myocytes were pretreated with vehicle or DMOG for 22 h, subjected to 3 h of MI (with or without hadacidin), and stained with acridine orange (fluoresces green, indicating live cells) and ethidium bromide (fluoresces orange, indicating dead cells). A: representative fluorescent images of myocytes. B: quantification of data generated from cultures treated with DMOG and subjected to 3 h of MI. CN, cyanide; 2-DG, 2-deoxyglucose. Values are means ± SE of an average of 12 randomly selected fields of view in 3 independent samples. *Significantly different from corresponding non-DMOG-treated control (P < 0.05, by t-test).
DISCUSSION
These studies have explored the multifactorial series of compensatory changes that are directed by the PHD pathway and, especially, those that occur at the level of the mitochondria. It is well established that anaerobic carbohydrate metabolism is enhanced by HIF-1α through increased glucose uptake at the plasma membrane and the induction of multiple enzymes of the glycolytic pathway (36). More recently, it has been shown that the shunting of carbohydrate metabolism away from oxidation through the inhibition of PDH is accomplished by the HIF-1α-driven induction of PDK (32). These changes provide for continued ATP generation, albeit with reduced capacity, during hypoxia/anoxia.
Although it is relatively well understood how the cell upregulates anaerobic ATP generation in response to hypoxia, it is much less clear how cellular ATP utilization is regulated. Interestingly, the suppression of ATP consumption is seen in different cell types, and the phenomenon has variously been described as O2 conformance, metabolic suppression, hibernation, and specialized dedifferentiation (1, 14, 16, 17, 31, 35). Thus anaerobic ATP generation is reinforced by conservation of ATP use, which in working cardiomyocytes is mainly achieved via suppression of contractile activity. Our studies indicate that the PHD pathway directs the suppression of ATP utilization and, furthermore, that this effect is mediated independently of HIF-1α. The identification of the PHD pathway as responsible for O2 conformance is a key finding that will enable further mechanistic insights into how ATP use is suppressed in heart and other tissue types.
Although the downregulation of ATP consumption is protective from the cellular standpoint, in the context of the organism it must be considered dysfunction. Clearly, a global 85% reduction of respiration in heart, similar to the reduction we observe in cultured cardiomyocytes, would be incompatible with viability. On the other hand, the functional “hunkering down,” and subsequent survival, of smaller regions of cardiac tissue that, in parallel, produce angiogenic factors would be of eventual benefit, especially in heart, where regeneration of dead tissue is limited. Given that the poorly understood phenomenon of myocardial hibernation is characterized by increased glucose uptake and lower contractile activity in underperfused portions of the heart, it is reasonable to speculate that the PHD O2 sensor may play a large role in the noted dysfunction (14).
The key insight that the reduced activity of dioxygenase hydroxylases signals lower O2 levels provided the rationale for using broad-spectrum PHIs to activate the PHD pathway. PHI treatments have been reported to potently induce hypoxic responses such as angiogenesis and protective compensatory changes in culture and in vivo (23, 39, 43, 47). Because the broad-spectrum PHIs inhibit PHD1, PHD2, PHD3, and FIH-1, they produce an extremely robust, fully realized activation of the O2-sensing cascade. Elvidge et al. (9) recently compared the genomic expression profile of cells subjected to either treatment with DMOG and authentic hypoxia. They found a remarkable concordance between the direction, as well as degree, of gene expression changes between the two groups. In addition, a subset of gene expression changes occurred independently of HIF-1α. This report largely validates the use of DMOG to faithfully recapitulate the responses of the PHD pathway. It also emphasizes the salient point that HIF-1α represents but one component, albeit an important one, of the O2-sensing signal cascade. For instance, PHD1 was shown recently to regulate IKKβ and, thus, NF-κB activity directly through posttranslational hydroxylation (7). Our present studies indicate that suppression of ATP consumption is directed by an HIF-1α-independent mechanism, further pointing to an emerging role for non-HIF branches of the PHD pathway in modulating cellular and mitochondrial function.
The maneuvers discussed above work to protect ATP levels and maintain cellular functions necessary for the viability of the cell. However, we find no difference in the kinetics or degree of ATP decline between DMOG-treated and control cardiomyocytes exposed to cyanide and 2-DG, even though DMOG-treated cardiomyocytes sustain less irreversible damage. This protection is associated with the ability of DMOG-treated cells to persistently maintain mitochondrial membrane polarization, despite ATP depletion and in the absence of O2 respiration. Further studies showed that the complex I inhibitor rotenone was necessary, in addition to cyanide, to depolarize the mitochondria of DMOG-treated cells. These findings indicated that complex I remained active during the blockade of cytochrome c oxidase, which required evoking an alternative (to O2) electron acceptor (39). Precedence exists in nature for fumarate respiration where fumarate accepts electrons at complex II and is reduced to succinate. The parasite Ascarus suum changes subunits within complex II and utilizes fumarate respiration during the portion of its life cycle within the anoxic gut of the host. In the 1970s, Owen and Hochachka (30) proposed several metabolic pathways to explain the anaerobic production of succinate by, and the performance of, the musculature of diving mammals. Since the original proposal, these metabolic pathways have been reported to operate in anoxic heart (20, 28, 34, 40, 41, 46) and kidney (11, 13, 44, 45) tissue of rodents. Findings of miniscule flux, as assessed by succinate production (the terminal metabolite), through these metabolic pathways led investigators to conclude that they play no significant role in ischemic tolerance (20, 28, 46). However, these previous studies were conducted in naive cells or tissue without prior activation of the PHD pathway. We show evidence that activation of the PHD pathway leads to a large increase in the capacity of mammalian cardiomyocytes to employ fumarate respiration and stabilize ψmito.
The protection of mitochondrial polarization during cessation of O2-supported ETC flux can be envisioned to have several benefits in promoting cell survival. Previously, we showed that PHI treatments allow cardiomyocytes to maintain ATP levels during cyanide poisoning to a remarkable degree (39). The findings here concerning fumarate respiration and stabilization of ψmito may have further clarified those previous findings. The ATP synthase of depolarized mitochondria often begins to consume EMP-produced ATP (29). Thus it is likely that maintenance of ψmito through fumarate respiration could prevent the materialization of this large cellular sink of precious ATP. Another potential benefit of fumarate respiration is that, by providing an alternative outlet for electrons from the ETC, it is possible that radical formation may be dampened during ischemia or reperfusion. Finally, the mitochondrial permeability transition is voltage modulated (8), such that its occurrence may be prevented when mitochondrial polarization is maintained. The induction of fumarate respiration revealed by these studies, and the consequent stabilization of mitochondrial membrane polarization, represents a protective mechanism employed by cells to survive low-O2 conditions. In a similar vein, the active suppression of O2 consumption that is observed may also represent a protective adaptation to hypoxia. Our data indicate that downregulation of ATP turnover by the PHD pathway is sufficient to lower basal O2 consumption. Thus the following question arises: why are additional mechanisms put in place to prevent increased O2 consumption when mitochondria are uncoupled, the functional equivalent of high ADP levels? We can only speculate, but the clamping of respiration may moderate large tissue O2 gradients and the precipitous occurrence of total anoxia that can occur in portions of tissue as ADP-to-ATP concentration ratios rise as a result of metabolic insufficiency. The above-described possibilities are under investigation.
Previously, we showed that when cyanide and 2-DG are present, ATP levels decline with identical kinetics in control and DMOG-treated cultures. Thus the increased NH3 production from the PNC during MI is not likely to be due simply to greater AMP levels in the PHD-preconditioned cells but, instead, represents a PHD-mediated increase in PNC activity. The suggestion that the PHD pathway may upregulate the PNC is of potential importance beyond the obvious implications for enhanced fumarate respiration observed in these studies. During severe ATP depletion, AMP is either salvaged by the PNC or dephosphorylated by the 5′-nucleotidase to adenosine. Uncharged adenosine freely diffuses from cells and is lost on reperfusion. Thus, in addition to participating in the stabilization of ψmito, enhanced PNC activity may also act to conserve the pools of AMP nucleotide during metabolic stress.
In summary, these studies have explored the impact of the PHD O2-sensing signal cascade on cardiomyocyte metabolism with a focus on adaptive changes effected at the level of the mitochondria. Activation of the PHD O2 sensor causes respiration to be clamped at 15–20% of basal levels. Despite the reduction of ETC flux, the clamped mitochondria display a remarkable ability to maintain polarization during complex IV blockade and severe ATP depletion. Our data indicate that maintenance of ψmito is accomplished through the ability to switch to the utilization of fumarate as an alternative terminal electron acceptor. The source of this fumarate is suggested to be the PNC, a pathway that is operable when high AMP levels materialize. Finally, these studies indicate that cellular ATP consumption is suppressed through a PHD-dependent, but HIF-independent, mechanism. We speculate that the PHD pathway may trigger a dedifferentiation-like program that downregulates ATP-consuming processes that are nonessential to cellular survival. Although the differentiated function of the cardiomyocyte is contraction, lower ATP turnover in response to hypoxia is observed universally across many cell types, suggesting that the PHD pathway may broadly influence cellular differentiation across various cell types.
GRANTS
This work was supported by National Institutes of Health Grants RO1 HL-084302 (to G. L. Wright) and C06 RR-015455 (to the Medical University of South Carolina).
Acknowledgments
The authors acknowledge the excellent technical support of Dr. Venkat Ramshesh of the Medical University of South Carolina confocal facility, as well as helpful discussions concerning mitochondrial metabolism with Dr. John J. Lemasters.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arthur PG, Giles JJ, Wakeford CM. Protein synthesis during oxygen conformance and severe hypoxia in the mouse muscle cell line C2C12. Biochim Biophys Acta 1475: 83–89, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ben Cheikh R, Guendouz A, Moravec J. Control of oxidative metabolism in volume-overloaded rat hearts: effects of different lipid substrates. Am J Physiol Heart Circ Physiol 266: H2090–H2097, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22: 4082–4090, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand MD The stoichiometry of proton pumping and ATP synthesis in mitochondria. Biochemist 16: 20–24, 1994. [Google Scholar]
- 5.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Casey TM, Pakay JL, Guppy M, Arthur PG. Hypoxia causes downregulation of protein and RNA synthesis in noncontracting mammalian cardiomyocytes. Circ Res 90: 777–783, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci USA 103: 18154–18159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lisa F, Bernardi P. Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol Cell Biochem 184: 379–391, 1998. [PubMed] [Google Scholar]
- 9.Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J Biol Chem 281: 15215–15226, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Feldkamp T, Kribben A, Roeser NF, Senter RA, Kemner S, Venkatachalam MA, Nissim I, Weinberg JM. Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules. Am J Physiol Renal Physiol 286: F749–F759, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Fritz IB, Kaplan E, Yue KT. Specificity of carnitine action on fatty acid oxidation by heart muscle. Am J Physiol 202: 117–121, 1962. [DOI] [PubMed] [Google Scholar]
- 13.Gronow GH, Cohen JJ. Substrate support for renal functions during hypoxia in the perfused rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F618–F631, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Heusch G, Schulz R. Myocardial hibernation. Ital Heart J 3: 282–284, 2002. [PubMed] [Google Scholar]
- 15.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem 277: 26351–26355, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 93: 9493–9498, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochachka PW, Dunn JF. Metabolic arrest: the most effective means of protecting tissues against hypoxia. Prog Clin Biol Res 136: 297–309, 1983. [PubMed] [Google Scholar]
- 18.Hochachka PW, Owen TG, Allen JF, Whittow GC. Multiple end products of anaerobiosis in diving vertebrates. Comp Biochem Physiol B 50: 17–22, 1975. [DOI] [PubMed] [Google Scholar]
- 19.Hochachka PW, Storey KB. Metabolic consequences of diving in animals and man. Science 187: 613–621, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Hohl C, Oestreich R, Rosen P, Wiesner R, Grieshaber M. Evidence for succinate production by reduction of fumarate during hypoxia in isolated adult rat heart cells. Arch Biochem Biophys 259: 527–535, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Kasiganesan H, Sridharan V, Wright G. Prolyl hydroxylase inhibitor treatment confers whole-animal hypoxia tolerance. Acta Physiol (Oxf) 190: 163–169, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kubota K, Fukushima T, Yuji R, Miyano H, Hirayama K, Santa T, Imai K. Development of an HPLC-fluorescence determination method for carboxylic acids related to the tricarboxylic acid cycle as a metabolome tool. Biomed Chromatogr 19: 788–795, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kuznetsova AV, Meller J, Schnell PO, Nash JA, Ignacak ML, Sanchez Y, Conaway JW, Conaway RC, Czyzyk-Krzeska MF. von Hippel-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci USA 100: 2706–2711, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16: 1466–1471, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laplante A, Vincent G, Poirier M, Des RC. Effects and metabolism of fumarate in the perfused rat heart. A 13C mass isotopomer study. Am J Physiol Endocrinol Metab 272: E74–E82, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls DG The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem 50: 305–315, 1974. [DOI] [PubMed] [Google Scholar]
- 30.Owen TG, Hochachka PW. Purification and properties of dolphin muscle aspartate and alanine transaminases and their possible roles in the energy metabolism of diving mammals. Biochem J 143: 541–553, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantely GA, Arai AE, Grauer SE, Bristow JD. Metabolic aspects of hibernating myocardium. Z Kardiol 84 Suppl 4: 101–105, 1995. [PubMed] [Google Scholar]
- 32.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3: 187–197, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260: 406–417, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Sanborn T, Gavin W, Berkowitz S, Perille T, Lesch M. Augmented conversion of aspartate and glutamate to succinate during anoxia in rabbit heart. Am J Physiol Heart Circ Physiol 237: H535–H541, 1979. [DOI] [PubMed] [Google Scholar]
- 35.Schumacker PT, Chandel N, Agusti AG. Oxygen conformance of cellular respiration in hepatocytes. Am J Physiol Lung Cell Mol Physiol 265: L395–L402, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol 59: 47–53, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 88: 1474–1480, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 19: 176–182, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Sridharan V, Guichard J, Bailey RM, Kasiganesan H, Beeson C, Wright GL. The prolyl hydroxylase oxygen-sensing pathway is cytoprotective and allows maintenance of mitochondrial membrane potential during metabolic inhibition. Am J Physiol Cell Physiol 292: C719–C728, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Taegtmeyer H Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ Res 43: 808–815, 1978. [DOI] [PubMed] [Google Scholar]
- 41.Taegtmeyer H, Peterson MB, Ragavan VV, Ferguson AG, Lesch M. De novo alanine synthesis in isolated oxygen-deprived rabbit myocardium. J Biol Chem 252: 5010–5018, 1977. [PubMed] [Google Scholar]
- 42.Thompson JF, Morrison GR. Determination of organic nitrogen. Anal Chem 23: 1153–1157, 1951. [Google Scholar]
- 43.Warnecke C, Griethe W, Weidemann A, Jurgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt KU. Activation of the hypoxia-inducible factor pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J 17: 1186–1188, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97: 2826–2831, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol 279: F927–F943, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiesner RJ, Rosen P, Grieshaber MK. Pathways of succinate formation and their contribution to improvement of cardiac function in the hypoxic rat heart. Biochem Med Metab Biol 40: 19–34, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Wright G, Higgin JJ, Raines RT, Steenbergen C, Murphy E. Activation of the prolyl hydroxylase oxygen sensor results in induction of GLUT1, heme oxygenase-1, and nitric oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J Biol Chem 278: 20235–20239, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Wright G, Singh IS, Hasday JD, Farrance IK, Hall G, Cross AS, Rogers TB. Endotoxin stress response in cardiomyocytes: NF-κB activation and tumor necrosis factor-α expression. Am J Physiol Heart Circ Physiol 282: H872–H879, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Kon T, Wang H, Li F, Huang Q, Rabbani ZN, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, Li CY. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1α. Cancer Res 64: 8139–8142, 2004. [DOI] [PubMed] [Google Scholar]