Abstract
Recent studies have suggested that, in certain cases, necrosis, like apoptosis, may be programmed, involving the activation and inhibition of many signaling pathways. In this study, we examined whether necrosis induced by H2O2 is regulated by signaling pathways in primary hepatocytes. A detailed time course revealed that H2O2 treated to hepatocytes is consumed within minutes, but hepatocytes undergo necrosis several hours later. Thus, H2O2 treatment induces a “lag phase” where signaling changes occur, including PKC activation, Akt (PKB) downregulation, activation of JNK, and downregulation of AMP-activated kinase (AMPK). Investigation of various inhibitors demonstrated that PKC inhibitors were effective in reducing necrosis caused by H2O2 (∼80%). PKC inhibitor treatment decreased PKC activity but, surprisingly, also upregulated Akt and AMPK, suggesting that various PKC isoforms negatively regulate Akt and AMPK. Akt did not appear to play a significant role in H2O2-induced necrosis, since PKC inhibitor treatment protected hepatocytes from H2O2 even when Akt was inhibited. On the other hand, compound C, a selective AMPK inhibitor, abrogated the protective effect of PKC inhibitors against necrosis induced by H2O2. Furthermore, AMPK activators protected against H2O2-induced necrosis, suggesting that much of the protective effect of PKC inhibition was mediated through the upregulation of AMPK. Work with PKC inhibitors suggested that atypical PKC downregulates AMPK in response to H2O2. Knockdown of PKC-α using antisense oligonucleotides also slightly protected (∼22%) against H2O2. Taken together, our data demonstrate that the modulation of signaling pathways involving PKC and AMPK can alter H2O2-induced necrosis, suggesting that a signaling “program” is important in mediating H2O2-induced necrosis in primary hepatocytes.
Keywords: necrosis, protein kinase C, AMP-activated kinase, hydrogen peroxide, Akt
reactive oxygen species (ROS), generated during aerobic respiration and inflammation, have been implicated in mediating cell death in a wide range of disorders including atherosclerosis, Parkinson's disease, Alzheimer's disease, and drug-induced liver injury. ROS have been shown to induce both apoptosis and necrosis in cells (1, 5, 24, 58). Whether ROS induce apoptotic or necrotic cell death is dependent on the cell type and/or extent of exposure to ROS (1, 24). Higher ROS exposure generally leads to necrosis rather than apoptosis in many cell types due to extensive oxidation of proteins, including cysteines in caspases (cysteine proteases), which prevent caspase activity needed to mediate apoptosis (4, 22, 25). Cell death induced by ROS such as H2O2 has traditionally been attributed to oxidation and damage of cellular macromolecules (e.g., proteins, DNA, and lipids) leading to catastrophic damage that results in cell death. However, many recent studies have demonstrated that the activation of signal transduction pathways is important in mediating ROS-induced cell death. In various cell lines, ROS-induced apoptosis has been shown to be modulated by altered signaling pathways involving JNK, PKC, and Akt (PKB) (9, 11, 38, 55, 58). Therefore, understanding the signaling pathways involved in mediating ROS-induced cell death may have important implications in understanding many diseases associated with ROS-induced injury.
JNK, a member of the MAPK subfamily, has been shown to be activated by H2O2 and mediates ROS-induced apoptosis in a variety of cells (11, 41, 55, 62). Although JNK is important in the stress response, when JNK activation is sustained, JNK is believed to be proapoptotic (11, 41) and initiates apoptotic pathways including the induction of Bax translocation to mitochondria (56), promotion of second mitochondrial activator of caspases (SMAC) and cytochrome c release from mitochondria (7, 49), and inactivation of Bcl-xL (36). Similarly, Akt, a serine/threonine kinase, has been shown to be activated by H2O2 in some cell lines (48, 50). However, in contrast to JNK, Akt is believed to play a protective role against ROS-induced apoptosis, and pharmacological or genetic inhibition of Akt has been shown to sensitize cells to H2O2-induced apoptosis (17, 32). The activation of PKC has also been shown to occur in response to treatment with H2O2 or chemicals that generate ROS, such as menadione, to cells (13, 18, 57). In some cases, PKC activation plays a protective role (33): in RALA255 cells (a hepatocyte cell line), PKC inhibitors were found to sensitize cells to ROS-induced apoptosis (58). In other cases, PKC activation plays an injurious role (13): in a keratinocyte cell line, PKC activation was found to mediate apoptosis induced by ROS generated through UV light (16). Whether PKC activation protects against or promotes cell death caused by ROS may depend on the PKC isoform activated, which may be cell type and context specific. There are at least 11 isoforms of PKC, which are divided into 3 classes: the classical group (α, βI, βII, and γ), which is activated by diacylglycerol, Ca2+, and phorbol esters; the novel group (δ, ɛ, η, and θ), which is not activated by Ca2+; and the atypical group (ζ and λ/τ), which is insensitive to Ca2+, diacylglycerol, and phorbol esters (44).
Recent studies have shown that AMP-activated kinase (AMPK), an important energy sensor in cells, also plays an important role in cell survival/death (51). AMPK regulates energy-generating pathways (e.g., β-oxidation and glucose transport) and energy storage pathways (i.e., glycogen synthesis) in response to fluctuations in cellular energy levels (28, 42). Since cellular ATP levels are important in cell survival, AMPK may be an important regulator of cell death/survival in certain situations. AMPK has an important role in protecting the heart and liver from ischemia-reperfusion injury (45, 47). On the other hand, AMPK has also been shown to promote apoptosis or autophagy in some cell lines (35, 39). AMPK has been shown to be activated in response to H2O2 in some cells (8), but whether AMPK modulates ROS-induced cell death has not been extensively investigated.
While many signaling pathways involved in ROS-induced apoptosis have been well characterized, the signal transduction pathways that modulate ROS-induced necrosis have not been extensively explored. Traditionally, necrosis has been believed to be a passive process resulting from overwhelming cellular injury. However, recent studies have demonstrated that certain types of necrosis, like apoptosis, may be programmed and involve the activation and/or inhibition of signaling pathways important in cell death or survival (15, 46). In Jurkat cells, TNF-induced apoptosis was converted to programmed necrosis when Jurkat cells were treated with caspase inhibitor (zVAD) (15). The signaling pathway important in many types of programmed necrosis involves receptor-interacting protein kinase activity (RIP) (6, 30). In addition, we recently observed that JNK inhibition dramatically inhibited acetaminophen-induced liver injury, which primarily involves hepatocyte necrosis (21). Whether ROS can also induce programmed necrosis in cells and whether the signaling pathways involved in ROS-induced apoptosis (i.e., JNK, PKC, and Akt) mediate necrotic cell death have not been extensively explored.
ROS are believed to mediate liver injury in alcoholic liver disease, drug-induced liver injury, and during inflammation associated with various viral pathogens (34). In many of these pathophysiological states, such as inflammation, localized high concentrations of H2O2 may be an important component in promoting hepatocyte injury. Previously, we observed that treatment of primary cultured hepatocytes with H2O2 resulted in necrosis, with very little apoptosis (<2%) occurring at all doses of H2O2 examined (0–400 μM) (24). However, low nonlethal doses of H2O2 were observed to sensitize hepatocytes to TNF-induced apoptosis through the inhibition of NF-κB signaling, suggesting that H2O2 regulates important signal transduction pathways involved in survival and/or death in primary hepatocytes. In this study, we continued our investigation examining signaling pathways modulated by H2O2 in primary cultured hepatocytes. However, instead of investigating apoptotic signaling, we investigated signaling pathways involved in H2O2-induced necrosis. The question of whether altering key signaling pathways can modulate H2O2-induced necrosis was explored. Based on previous work, we focused on PKC and examined how PKC modulation affects H2O2-induced necrosis.
MATERIALS AND METHODS
Experimental protocols were approved by the University of Southern California Institutional Animal Care and Use Committee (protocol no. 10649).
Materials.
All inhibitors used in this study were purchased from Calibiochem (San Diego, CA). H2O2 was purchased from Sigma (St. Louis, MO).
Cell culture.
Hepatocytes were isolated as previously described from C57 BL/6 mice (26). Briefly, the liver was perfused with collagenase, and isolated hepatocytes (1.2 × 106, viability >90%) were plated in individual 60-mm-diameter LUX culture dishes coated with 0.03% rat tail collagen. After 3 h, the culture medium was changed to serum-free medium containing 100 U/ml penicillin and 0.1 mg/ml streptomycin. After 1 h in serum-free medium, hepatocytes were treated with various inhibitors, such as PKC inhibitors. H2O2 was added in 100-μM increments to hepatocytes following various treatments.
Determination of apoptosis and necrosis.
After 16 h of various treatments, cells were double stained with 8 μg/ml Hoechst 33258 and 1 μM Sytox green. Hepatocytes were incubated with Hoechst 33258 for 15 min. Sytox was added just before analysis. After cells had been stained, culture dishes were observed under an OLYMPUS fluorescent microscope. Quantitation of total and apoptotic cells was performed as previously described by counting >1,000 cells in 10 different fields (24). Necrotic cells (Sytox green positive) were determined by counting the same field.
HPLC measurements for GSH and GSSG.
GSH and GSSG were detected using reverse-phase HPLC and a Coulochem II electrochemical detector (ESA Laboratories, Chelmsford, MA) as previously described (29). At collection time points, hepatocytes were washed with cold PBS and then treated with 5% metaphosphoric acid to prevent GSH autoxidation (24). Samples were centrifuged (12,000 g for 5 min), and the supernatant was injected into the HPLC.
Separation of membranes and the cytoplasm.
Following various treatments, hepatocytes were scraped in lysis buffer (20 mM Tris·HCl, 10 mM EGTA, 2 mM EDTA, 50 mM β-mercaptoethanol, 1 mM PMSF, and protease and phosphatase inhibitor cocktails from Sigma; pH 7.5). The lysate was sonicated and centrifuged for 1 h at 100,000 g at 4°C (31). The supernatant, which contained cytoplasmic proteins, was removed and frozen at −80°C. The pellet was treated with lysis buffer containing 0.2% Triton X-100, mixed, and incubated in ice for 1 h. The redissolved pellet was subsequently spun at 100,000 g for 1 h. The resulting supernant, which contained membrane proteins, was removed and stored at −80°C.
Immunoblot analysis.
Following various treatments, hepatocytes were scraped in lysis buffer. Cells were subsequently sonicated (3 × 15 s). Cell lysates were resolved by SDS-PAGE, and proteins were transferred to membranes and visualized by immunoblot analysis using antisera for Akt, phospho-Akt (Ser473), AMPK-α, phospho-AMPK, JNK, phospho-JNK, poly(ADP-ribose) polymerase (PARP), caspase 3 (Asp175), and PARP-1 (Asp214) (obtained from Cell Signaling Technologies, Danvers, MD). All gels shown are representative samples from three experiments. Densitometry was performed using the ImageJ program from the National Institutes of Health.
Measurement of H2O2 in culture media.
At the indicated times, media were removed, and H2O2 consumption was determined using an oxygen electrode following catalase treatment as previously described (2).
Antisense experiments.
Antisense oligonucleotides (ASO) targeting mouse PKC-α and PKC-ɛ (Isis Pharmaceuticals, Carlsbad, CA) and a chemical control oligonucleotide (Isis 141923) were synthesized as 20-nt uniform phosphorothioate chimeric oligonucleotides and purified as previously described (21). Oligonucleotides used in these experiments were chimeric oligonucleotides containing five nuclease-resistant 2′-O-methoxyethylribose-modified phosphorothioate residues on the 5′- and 3′-ends, flanking a 2′-deoxyribonucleotide/phosphorothioate region that supports RNase H-based cleavage of the targeted mRNA. To knockdown PKC-α and PKC-ɛ, mice were injected intraperitoneally with ASO seven times (50 mg every other day).
Statistical analysis.
When appropriate, means ± SD are presented in the text. For comparison between the two groups, paired t-tests were performed. Comparisons between multiple groups were performed with one-way ANOVA followed by Fisher's test. P < 0.05 was defined as statistically significant.
RESULTS
H2O2 induces necrosis in primary cultured hepatocytes.
H2O2 (300 μM) treatment of primary hepatocytes caused morphological alterations that included rounding up of hepatocytes (Fig. 1, a and b). However, cell death induced by H2O2 appeared to be primarily necrotic (∼82% with 300 μM H2O2), since Sytox green was readily taken up due to the disruption of the plasma membrane. Little nuclear fragmentation, characteristic of apoptosis, was observed with either Sytox green (Fig. 1b) or Hoechst staining of nucleus (<2% as determined by Hoechst dye examining chromatin condensation; data not shown), suggesting that apoptosis was not involved in H2O2-induced hepatocyte death. The lack of caspase activation following H2O2 treatment further confirmed that necrosis rather than apoptosis was the predominant form of hepatocyte death induced by H2O2. Fig. 1b shows that H2O2 (300 μM) failed to cause caspase 3 cleavage, whereas actinomycin D plus TNF treatment, which strongly initiates apoptosis, triggered caspase 3 cleavage in primary hepatocytes. The general caspase inhibitor zVAD failed to protect hepatocytes from H2O2, further confirming the lack of caspase activation in H2O2-induced hepatocyte death (Table 1). We also examined whether PARP-1, an important mediator of ROS-induced death in many cell lines (54), was activated by H2O2 treatment. Figure 1b shows that H2O2 did not induce the PARP cleavage necessary for activation. Neither caspase 3 nor PARP cleavage was observed at any time point or dose of H2O2 examined (data not shown), suggesting that H2O2-induced necrosis was not dependent on caspase or PARP activation.
Fig. 1.
H2O2 treatment induces necrosis in primary cultured hepatocytes that is caspase and poly(ADP-ribose) polymerase (PARP) independent. Cultured primary hepatocytes were treated with H2O2 (300 μM) for 18 h. A: microscopic images of primary hepatocytes stained with Sytox green following H2O2 treatment (8 h) in the absence or presence of Ro-31-8425 (Ro; 10 μM, 1-h pretreatment). In a and b, hepatocytes were treated with H2O2 [light microscopy image (a) and fluorescent microscopy image (b)]. In c and d, hepatocytes treated with H2O2 plus Ro [light microscopy image (c) and fluorescent microscopy image (d)]. B: caspase 3 and PARP cleavage are not associated with H2O2-induced necrosis. Caspase 3 and PARP cleavage were assessed in hepatocytes by immunoblot analysis 6 h following H2O2 (300 μM) treatment or in untreated controls (Cont.). Actinomycin D (Act D) plus TNF treatment, which induces apoptosis (>95%) in hepatocytes, was used as a positive control.
Table 1.
Effect of various inhibitors against H2O2-induced necrosis in primary cultured hepatocytes
| Inhibitor | Principal Function | Necrosis Caused by H2O2, % |
|---|---|---|
| Untreated (H2O2 alone) | 82±15 | |
| Z-VAD-DMK | Caspase inhibitor | 89±14 |
| α-Tocopherol | Antioxidant | 35±14* |
| BHT | Antioxidant | 40±13* |
| α-Tocopherol + BHT | Antioxidants | 29±15* |
| Ro-31-8425 | PKC inhibitor (α, βII, γ, and ɛ) | 14±7.6* |
| Bisindolymaleimide I (Go-6850) | PKC inhibitor (α, βI, γ, and ɛ) | 11±4.5* |
| Go-6983 | PKC inhibitor (α, β, γ, δ, and ζ) | 14±6.1* |
| PD-98059 | MEK inhibitor | 83±12.4 |
| 3-(2-Aminoethyl)-5-[(4-ethoxyphenyl)methylene]-2,4-thiazolidinedione | ERK inhibitor | 74±12 |
| Wortmannin | Phosphatidylinositol 3-kinase inhibitor | 86±18 |
| Okadaic acid | Protein phosphatase 2A inhibitor | 90±13 |
| Cyclosporin A | Mitochondrial permeability transition inhibitor | 98±5.7 |
Values are means ± SD. Hepatocytes were treated with H2O2 (300 μM) and various inhibitors [10 μM for all inhibitors except for butylated hydroxytoluene (BHT), cyclosporin A, and Z-VAD-FMK, which were used at doses of 20 μM]. Inhibitors were added 1 h prior to H2O2 treatment. Necrosis was determined 16 h after H2O2 treatment using Sytox green.
P < 0.05 compared with hepatocytes treated with H2O2 alone (untreated).
PKC inhibitors protect against necrosis induced by H2O2 in primary cultured hepatocytes.
We next examined a series of inhibitors and antioxidants to determine their potential to modulate H2O2-induced necrosis in primary hepatocytes. Not surprisingly, antioxidants [butylated hydroxytoluene (BHT) and/or α-tocopherol] decreased H2O2-induced necrosis by 40–64% (Table 1). While antioxidants such as BHT and α-tocopherol cannot detoxify H2O2, they can inhibit lipid peroxidation and other free radical reactions initiated by H2O2 that can injure cells (3). However, surprisingly, broad-spectrum PKC inhibitors Ro-31-8425 and bisindoylmaleimide I (Go-6850) [previously shown to potentiate ROS-induced apoptosis in RALA cells (58)] were found to significantly protect hepatocytes from H2O2-induced necrosis. PKC inhibitor treatment not only inhibited Sytox green uptake but also inhibited rounding up of hepatocytes induced by H2O2 in the majority of hepatocytes (Fig. 1, c and d). Similarly, Go-6983, another commonly used broad-spectrum PKC inhibitor, protected hepatocytes from necrosis caused by H2O2 treatment, confirming that PKC activation may be playing an important role in H2O2-induced necrosis. Inhibitors of other cell signaling pathways [ERK, MEK, phosphatidylinositol 3-kinase (PI3K), and protein phosphatase 2A] failed to protect hepatocytes from H2O2-induced necrosis. Overall, PKC inhibition was more effective than antioxidants in protecting hepatocytes against H2O2-induced necrosis. This suggests that cell signaling pathways involving PKC (blocked by PKC inhibitors) play a central role in mediating necrotic death induced by H2O2 treatment.
A dose-response curve of H2O2 was examined in the presence and absence of the PKC inhibitor Ro-31-8425 to further characterize the protective effects of PKC inhibition against H2O2 (Fig. 2A). Ro-31-8425 protected hepatocytes against up to 400 μM H2O2. Ro-31-8245 pretreatment also protected hepatocytes against necrosis caused by steady levels of H2O2 generated using glucose oxidase (Fig. 2B). Glucose oxidase delivers a more physiological steady-state level of H2O2 than bolus H2O2 treatment (1). Measurement of hepatic GSH levels confirmed Sytox green measurements that demonstrated the protective effects of Ro-31-8425 against H2O2 18 h following H2O2 treatment (Fig. 2C). H2O2 treatment alone caused an extensive loss of GSH at 18 h after H2O2 treatment, suggesting extensive hepatocyte death. With PKC inhibitor pretreatment, on the other hand, hepatocytes maintained GSH levels, suggesting that hepatocytes remained viable even after H2O2 treatment. These findings show the effectiveness of PKC inhibition in protecting primary hepatocytes against either bolus or steady-state levels of H2O2.
Fig. 2.
PKC inhibitor protects against necrosis induced by bolus or steady levels of H2O2. Primary cultured hepatocytes were treated with bolus additions of H2O2 or steady levels of H2O2 generated by various concentrations of glucose oxidase for 1 h in the presence of absence of Ro (10 μM, 1-h pretreatment). ⧫, H2O2 treatment alone; ▪, Ro + H2O2. A: dose-response curve of necrosis induced by bolus additions of H2O2. B: dose-response curve of necrosis induced by steady-state generation of H2O2 using glucose oxidase. Glucose oxidase at a dose of 1 μg is estimated to generate 0.52 nmol H2O2/min in culture medium. Necrotic cells were determined using Sytox green 16 h after treatment. C: cellular GSH levels as an assessment of hepatocyte viability. Hepatocytes were treated with various doses of bolus H2O2. Eighteen hours following H2O2 treatment, hepatocytes were washed with PBS and scraped in 5% metaphosphoric acid, and GSH levels measured using HPLC with electrochemical detection. Means ± SD are shown. *P < 0.05 compared with H2O2 treatment alone.
The protective effect of PKC inhibitor was not dependent on the synthesis of new proteins, since Ro-31-8425 pretreatment still protected against H2O2-induced necrosis in the presence of actinomycin D, a RNA synthesis inhibitor (data not shown). Similarly, actinomycin D did not protect hepatocytes against H2O2, suggesting that this H2O2-induced necrosis does not require synthesis of new genes (data not shown). These findings emphasize that H2O2-induced necrosis in hepatocytes depends only on the alteration of signal transduction pathways and not on gene transcription. Taken together, our findings suggest that cell signaling involving PKC plays a central role in H2O2-induced necrosis in primary hepatocytes.
Time course of H2O2 and its effect on hepatic GSH levels in primary hepatocytes.
To better understand the early signaling pathways involving PKC that mediate H2O2-induced necrosis, a detailed time course of cellular changes induced by H2O2 was performed. First, we examined the lifetime of H2O2 in media in the presence and absence of primary hepatocytes (Fig. 3A). The half-life of H2O2 in media alone (F-12-DMEM) was observed to be ∼10 min. When H2O2 was added to media containing primary hepatocytes, the majority of H2O2 was consumed within 1 min. This demonstrates that primary hepatocytes rapidly consume H2O2 and suggests that oxidative damage directly initiated by H2O2 occurs within minutes of treatment. The consumption of H2O2 by primary hepatocytes was not affected by PKC inhibitor pretreatment of hepatocytes (Fig. 3A), suggesting that PKC does not affect the antioxidant capacity of hepatocytes.
Fig. 3.
Effect of H2O2 and PKC inhibitor treatment on GSH/GSSG levels and H2O2 consumption in primary hepatocytes. A: H2O2 (300 μM) consumption by hepatocytes in the absence and presence of Ro (10 μM, 1-h pretreatment). ◊, H2O2 in medium without hepatocytes; ⧫, H2O2 in medium containing hepatocytes; ▪, H2O2 in medium containing hepatocytes pretreated with Ro. At the indicated times, media were removed, and H2O2 consumption was determined using an oxygen electrode following catalase treatment. B: effect of H2O2 (300 μM) on the GSH-to-GSSG ratio (GSH/GSSG ratio). ⧫, H2O2 treatment alone; ▪, Ro (10 μM) + H2O2. Primary cultured hepatocytes were treated with various doses of H2O2 with or without Ro. GSH and GSSG levels were analyzed in the supernatant using HPLC with electrochemical detection as described in materials and methods.
The rapid consumption of H2O2 in media by hepatocytes corresponded with a transient drop in GSH (from 99 ± 15 to 57 ± 22 nmol/106 cells at 5 min following H2O2 treatment), an increase in GSSG (from 0.9 ± 0.5 to 13 ± 8.3 nmol/106 cells at 5 min following H2O2 treatment), and a drop in the GSH-to-GSSG ratio in hepatocytes (Fig. 3B). H2O2 is primarily detoxified in the cytoplasm and mitochondria by GSH peroxidase, which utilizes the reducing power of GSH to reduced H2O2 to form GSSG and H2O (23). The addition of H2O2 to hepatocytes likely caused a transient decrease in the GSH-to-GSSG ratio due to the action of GSH peroxidase. The presence of PKC inhibitor (Ro-31-8425) had no significant effect on the transient drop in GSH (from 99 ± 15 to 49 ± 18 nmol/106 cells at 5 min following H2O2 treatment), increase in GSSG levels (from 0.9 ± 0.5 to 18 ± 7.5 nmol/106 cells at 5 min following H2O2 treatment), and GSH/GSSG ratio following H2O2 treatment (Fig. 3B). Overall, PKC inhibitor treatment had little effect on the GSH-to-GSSG redox ratio and H2O2 detoxification, suggesting that PKC inhibition does not affect the antioxidant capacity of hepatocytes.
Time course of H2O2-induced necrosis and PKC signaling in primary hepatocytes.
Although the majority of H2O2 was consumed within 5 min, hepatocyte necrosis was observed to ensue several hours following H2O2 treatment (Fig. 4A). Hepatocytes treated with 300 μM H2O2 began to undergo necrosis at 2 h (peaking at ∼4 h), whereas hepatocytes treated with 400 μM H2O2 began to undergo necrosis at 1 h (peaking at ∼2- 4 h) following H2O2 treatment. Hoechst staining demonstrated that H2O2 treatment, at all doses and times examined, induced very little nuclear fragmentation (<2%), confirming our previous observations that H2O2 does not induce apoptosis in primary hepatocytes unless TNF is present (24). These results suggest that necrosis following H2O2 treatment is not an immediate event but rather a process that develops with time. H2O2-induced necrosis is therefore associated with a “lag phase” during which cell survival or death may be determined by the activation or inhibition of various signaling pathways that involve PKC.
Fig. 4.
Time course of H2O2-induced necrosis, PKC translocation, and PKC activation in primary cultured hepatocytes. A: time course of H2O2-induced necrosis in primary hepatocytes. ⧫, Cont; ▪, 200 μM H2O2; ▴, 300 μM H2O2; •, 400 μM H2O2. At the times indicated, the number of necrotic cells was determined using Sytox green. B: PKC translocation to membranes following H2O2 and PKC inhibitor treatment. Primary hepatocytes were treated with H2O2 (300 μM) with or without PKC inhibitors [Ro and bisindolymaleimide I (Bis; 10 μM), 1 h prior to H2O2 treatment]. Ten minutes following H2O2 treatment, hepatocytes were washed and scraped with lysis buffer. The cytoplasm and membrane were separated by high-speed centrifugation (100,000 g) as described in materials and methods. PKC levels in the cytoplasm and membrane were assessed by Western blot analysis. C: time course of PKC activation following H2O2 treatment. Primary hepatocytes were treated with H2O2 (300 μM) with or without PKC inhibitors or PMA (2 μM for 1 h). PKC activity in hepatocyte lysate was assessed by Western blot analysis using an antibody that recognizes proteins phosphorylated by PKC (PKC recognition motif: serine residues surrounded by arginine or lysine at the −2 and +2 positions and a hydrophobic residue at the +1 position).
The protective effect of PKC inhibitor treatment against H2O2-induced necrosis suggests that H2O2 treatment induces substantial PKC activation in primary hepatocytes. PKC activation generally entails phosphorylation and translocation to the membrane (44). To identify the PKC isoforms involved in H2O2-induced necrosis, PKC translocation to the membrane was analyzed by Western blot analysis. H2O2 treatment was not observed to cause PKC translocation to the membrane with any of the PKC isoforms reported to be expressed in the liver (α, βII, ɛ, δ, and ζ) (10, 31) or times (10, 30, and 60 min) examined (Fig. 4B; data not shown). H2O2 treatment, particularly in the presence of PKC inhibitors, appeared to cause some decrease of PKC-δ in both the cytoplasm and membrane, but this findings was not significant (data not shown). PKC inhibitor treatment alone caused the translocation of PKC-α, PKC-βII, and, to some extent, PKC-ɛ to membranes following Ro-31-8425 or bisindolymaleimide I treatment. The translocation of various PKC isoforms induced by PKC inhibitor treatment is in agreement with recently published results (52, 53). It is believed that the binding of PKC inhibitors to the ATP site in PKC, which inhibits its activity, causes a conformational change that promotes its translocation to the membrane. PKC-ζ was the only PKC isoform that was not affected by PKC inhibitor and, like other PKC isoforms, was not affected by H2O2 treatment of hepatocytes.
Although we were unable to unequivocally observe PKC activation, the fact that PKC inhibitors strongly protected against H2O2-induced necrosis suggests that PKC activation is an important signal in inducing necrosis, probably in the lag phase following H2O2 treatment. It is possible that the different PKC isoforms very transiently translocated to membranes and were missed in our experiments. In addition, in some cases, PKC activation has been reported not to involve membrane translocation (12, 19). Consequently, we next examined the time course of PKC activation following H2O2 treatment in hepatocyte lysates using an antibody that recognizes proteins phosphorylated by PKC (PKC recognition motif: serine residues surrounded by arginine or lysine at the −2 and +2 positions and a hydrophobic residue at the +1 position) (37). Figure 4C shows that PKC activity increased rapidly in hepatocytes following H2O2 treatment, peaking at ∼30 min. Control sample also had some bands, suggesting that there was some basal PKC activity in untreated hepatocytes. As expected, PKC inhibitor (Ro-31-8425 and bisindoylmaleimide I) pretreatment inhibited the phosphorylation of proteins by PKC. PMA, an activator of classical and novel PKC isoforms (10), was used as a positive control and caused a dramatic increase in proteins phosphorylated by PKC, similar to H2O2 treatment. These findings support the notion that H2O2 treatment causes rapid and sustained PKC activation in primary cultured hepatocytes.
In the next series of experiments, we examined the various signaling pathways in the lag phase that may be mediated by PKC and that may be important in modulating H2O2-induced necrosis. These experiments used PKC inhibitors to unmask signaling pathways that are important in protecting hepatocytes from H2O2-induced necrosis.
Protective effect of PKC inhibitors against H2O2-induced necrosis is not mediated through modulation of JNK signaling in primary cultured hepatocytes.
JNK is important in mediating apoptosis as well as programmed necrosis in many cell lines (11, 46). Recently, we observed that JNK inhibition dramatically protected the liver from acetaminophen, which primarily induces necrosis in hepatocytes (21). Consequently, the role of JNK in mediating H2O2-induced necrosis in primary hepatocytes was investigated. Figure 5A shows that treatment of hepatocytes with H2O2 caused JNK activation within 15 min, and JNK activation was sustained for up to 2 h. PMA treatment did not induce JNK phosphorylation, and PKC inhibitor treatment did not affect JNK activation (Fig. 5B), indicating that PKC does not modulate JNK activity under these conditions. JNK inhibitor (SP-600125; 20 μM) caused a slight protection (∼22%) against H2O2-induced necrosis in hepatocytes, suggesting that JNK activation may play some role in H2O2-induced necrosis (Fig. 5C). Based on our data, although JNK may play some role in H2O2-induced necrosis, the protective effect of PKC inhibitors does not involve the modulation of JNK.
Fig. 5.
Role of JNK activation in H2O2-induced necrosis in primary hepatocytes. A: time course of JNK activation by 300 μM H2O2 in primary hepatocytes. B: PKC inhibitor did not affect JNK activation induced by H2O2. Ro (10 μM) was administered to hepatocytes 1 h prior to H2O2 (300 μM) treatment. Western blot analysis was performed using antisera against phosphorylated (p-)JNK and JNK. C: effect of JNK inhibitor against H2O2-induced necrosis. ⧫, H2O2 treatment alone; ▴, JNK inhibitor + H2O2. Primary cultured hepatocytes were treated with various doses of H2O2 with or without JNK inhibitor [SP-600125 (20 μM), 1 h prior to H2O2]. Necrotic cells were determined 16 h after treatment using Sytox green. Means ± SD are shown. *P < 0.05 compared with H2O2 treatment alone.
H2O2 and PKC regulate the phosphorylation of Akt (Ser473).
Akt has been previously characterized in certain cell types (keratinocytes, A459 cells, and HEK-293 cells) to be negatively regulated by PKC (38, 59). In these experiments, PKC inhibitors were demonstrated to increase the phosphorylation of Akt (Ser473) and, consequently, Akt activity. Since Akt activity promotes cell survival, we investigated whether PKC inhibitors were modulating Akt, thereby protecting hepatocytes from H2O2-induced necrosis. Figure 6A shows that H2O2 treatment, in a dose-dependent manner, inhibited Akt phosphorylation (1 h following H2O2 treatment). H2O2 treatment rapidly inactivated Akt, with decreases in Akt phosphorylation being observed 5 min after H2O2 treatment (Fig. 6B), a time point that corresponded with a dramatic change in the GSH/GSSG redox status (Fig. 3, B–D). PMA, an activator of classical and novel PKC isoforms, also downregulated Akt (Fig. 6A), confirming that PKC is involved in the negative regulation of Akt phosphorylation, in agreement with previous results. Pretreatment of hepatocytes with PKC inhibitors alone (Ro-31-8425 or bisindolymaleimide I) resulted in increased Akt phosphorylation over control hepatocytes (Fig. 6C), suggesting that there is basal PKC activity in untreated hepatocytes that suppresses Akt phosphorylation. PKC inhibitors also prevented the dramatic decline in Akt phosphorylation caused by H2O2 treatment, allowing maximal Akt activation.
Fig. 6.
Inactivation of Akt by H2O2 in primary hepatocytes: regulation by PKC. A: dose response of H2O2 on Akt phosphorylation in primary hepatocytes. Hepatocytes were treated with various doses of H2O2 or PMA (0.5 μM). At 1 h following treatment, total cell extracts of hepatocytes were harvested, and Western blot analysis was performed using antisera against p-Akt (Ser473) and Akt. B: time course of Akt inactivation by H2O2. C: regulation of Akt by PKC. Ro (10 μM) and Bis (10 μM) were administered to hepatocytes 1 h prior to H2O2 (300 μM) treatment. At 1 h following H2O2 treatment, total cell extracts of hepatocytes were harvested, and Western blot analysis was performed.
Role of Akt in H2O2-induced necrosis.
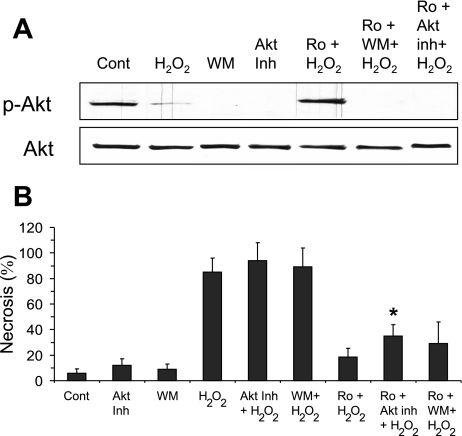
The fact that H2O2 treatment downregulates Akt and PKC inhibitor treatment upregulates Akt suggested that Akt may be an important determinant of H2O2-induced necrosis. To determine if PKC inhibitors act through Akt to protect hepatocytes against H2O2-induced necrosis, Akt activity was modulated using various inhibitors. Akt phosphorylation is regulated by PI3K-dependent serine/threonine kinase-1, a pathway inhibitable by the PI3K inhibitor wortmannin (38). Recently, several specific Akt inhibitors, such as Akt inhibitor VIII [1,3-dihydro-1-(1-{[4- (6-phenyl-1H-imidazo[4,5-g]quinoxalin-7-yl)phenyl]methyl}-4-piperidinyl)-2H-benzimidazol-2-one], have also been developed (40). Figure 7A shows that pretreatment of hepatocytes with wortmannin or Akt inhibitor VIII (30 min prior to PKC inhibitor treatment) inhibited Akt phosphorylation even in the presence of PKC inhibitors (Ro-31-8245 and bisindolymaleimide I). The loss of Akt phosphorylation by inhibitor treatments only minimally reversed the protection by Ro-31-8425 treatment (Fig. 7B) or slightly increased H2O2-induced necrosis. Akt inhibitor VIII caused a slight but significant decrease in viability (∼18% compared with Ro-31-8425 plus H2O2-treated cells), whereas wortmannin treatment only slightly negated the protective effects of PKC inhibitor (∼10%). The difference in effect between wortmannin and Akt inhibitor VIII may be due to specificity, since wortmannin may affect other downstream targets of PIK, whereas while Akt inhibitor VIII is believed to be a more selective inhibitor of Akt. Overall, these results suggest that the inhibition of Akt does not play a major role in H2O2-induced necrosis.
Fig. 7.
Role of Akt in H2O2-induced necrosis in primary hepatocytes. A: regulation of Akt phosphorylation by wortmannin (WM) and Akt inhibitor VIII (Akt Inh). Primary hepatocytes were treated with WM (250 nM) or Akt Inh (10 μM) 30 min prior to Ro (10 μM) and 90 min prior to H2O2. At 1 h following H2O2 treatment, total cell extracts of hepatocytes were harvested, and Western blot analysis was performed using antisera against p-Akt (Ser473) and Akt. B: effect of WM or Akt Inh, inhibitors of Akt phosphorylation, on the protective effects of PKC inhibitor against H2O2-induced necrosis. Means ± SD are shown. *P < 0.05 compared with Ro + H2O2 treatment.
Modulation of AMPK by PKC in primary hepatocytes.
A previous study (8) demonstrated that AMPK is upregulated by H2O2 treatment. The phosphorylation of a threonine residue (Thr172) within the activation domain of the α-subunit is required for AMPK activation (28). Consequently, AMPK phosphorylation (Thr172) was investigated following H2O2 treatment in primary cultured hepatocytes. In contrast to previous results, H2O2 (300 μM) caused a decrease in AMPK phosphorylation starting at 5 min, similar to Akt, when the GSH-to-GSSG ratio was at the lowest (Fig. 8, A and B). AMPK phosphorylation, like Akt, was restored with time, corresponding with a recovery of the GSH-to-GSSG ratio. Unlike Akt, AMPK was not affected by PMA treatment at any of the time points observed (Fig. 8, B and C). This suggests that AMPK is not regulated by classical or novel PKC isoforms, which are activated by PMA, as observed with Akt.
Fig. 8.
Modulation of AMP-activated kinase (AMPK) phosphorylation by H2O2: regulation by PKC. A: time course of AMPK phosphorylation (Thr172) in response to H2O2 treatment (300 μM). B: densitometry of AMPK phosphorylation in response to H2O2 and PMA (2 μM for 1 h). C: time course of the effect of PMA on AMPK phosphorylation. D: regulation of AMPK phosphorylation by PKC. Ro (10 μM) and Bis (10 μM) were administered to hepatocytes 1 h prior to H2O2 treatment. Western blot analysis was performed using antisera against p-AMPK and AMPK. Densitometry was determined using National Institutes of Health program Image J. Means ± SD are shown. *P < 0.05 compared with control hepatocytes.
PKC inhibitor (Ro-31-8425 and bisindolymaleimide I) treatment alone induced greater AMPK phosphorylation (Fig. 8D), suggesting that basal PKC activity was inhibiting AMPK, as observed with Akt. PKC inhibitor treatment prevented the decline in AMPK phosphorylation caused by H2O2 treatment and maximally activated AMPK, as observed with Akt. Taken together, these findings suggest that AMPK is inhibited by PKC (most likely an atypical PKC isoform since there was a lack of inhibition by PMA).
AMPK plays a protective role against H2O2-induced necrosis in hepatocytes.
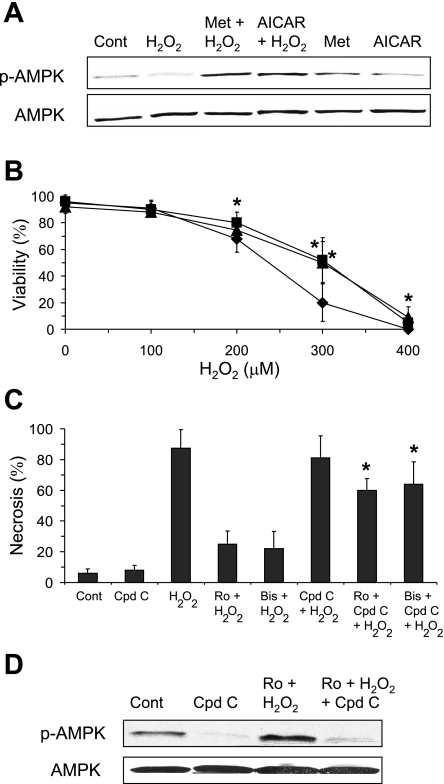
To determine whether the protective effect of PKC inhibitor was mediated through the upregulation of AMPK, various AMPK activators and inhibitors were examined. Metformin and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) are pharmaceutical agents that have been shown to activate AMPK (27). Pretreatment of hepatocytes with AICAR or metformin caused a modest increase in AMPK phosphorylation (Fig. 9A). Surprisingly, hepatocytes treated with both AMPK activators and H2O2 experienced a greater upregulation of AMPK than hepatocytes treated with AMPK activators alone, demonstrating a synergistic action of AMPK activators and H2O2 in upregulating AMPK. The upregulation of AMPK by AMPK activators was associated with increased resistance to H2O2-induced necrosis (∼32% at 300 μM H2O2) (Fig. 9B). Thus, upregulation of AMPK was associated with partial protection against H2O2-induced necrosis. Conversely, AMPK inhibition was associated with a dramatic decrease in the protective effects of PKC inhibitor against H2O2-induced necrosis. Treatment of hepatocytes with compound C, an inhibitor of AMPK (27), significantly reduced the protective effect of PKC inhibitors (Fig. 9C). Compound C has been shown to be a selective inhibitor of AMPK (61), and compound C treatment did not affect mitochondrial respiration in primary hepatocytes (data not shown), which is different from observations in mouse embryonic fibroblasts (14). Western blot analysis confirmed that compound C inhibited AMPK phosphorylation in parallel with abrogation of the protective effects of PKC inhibitors (Fig. 9D). These findings demonstrate that AMPK plays an important protective role against H2O2-induced necrosis and suggest that a major part of the protective effect of PKC inhibitors against H2O2-induced necrosis is mediated through the upregulation of AMPK.
Fig. 9.
AMPK activators and inhibitor modulate H2O2-induced necrosis and the protective effects of PKC inhibitors. A and B: effect of AMPK activators on H2O2-induced necrosis. Primary cultured hepatocytes were treated with H2O2 with or without metformin (Met; 500 μM) or 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; 100 μM) 2.5 h prior to H2O2 treatment. A: AMPK activators increased AMPK phosphorylation, particularly in conjunction with H2O2 (300 μM). B: AMPK activators protected primary hepatocytes from H2O2-induced necrosis. ⧫, H2O2 treatment alone; ▪, Met + H2O2; ▴, AICAR + H2O2. C: compound C (Cpd C; 40μM), an AMPK inhibitor, counteracted the protective effect of PKC inhibitors against H2O2-induced necrosis. Hepatocytes were treated with Cpd C 1 h prior to PKC inhibitor treatment and 2 h prior to H2O2 (300 μM) treatment. D: Cpd C treatment inhibited AMPK activation induced by PKC inhibitor. One hour following H2O2 treatment, total cell extracts of hepatocytes were harvested, and Western blot analysis was performed using antisera against p-AMPK and AMPK. Means ± SD are shown. *P < 0.05 compared with H2O2 treatment alone.
Go-6976, a selective inhibitor of PKC-α and PKC-β, fails to activate AMPK and is less effective in protecting hepatocytes from H2O2-induced necrosis.
The fact that PMA did not activate AMPK suggests that classical and novel PKC isoforms are not involved in regulating AMPK. To further test this notion, hepatocytes were treated with Go-6976, an inhibitor believed to be specific for classical PKC isoforms. Go-6976 has been reported to only inhibit PKC-α and PKC-βI (43), whereas bisindolymaleimide I and Ro-31-8425 have been shown to inhibit all PKC isoforms tested, including PKC-α, PKC-βI, PKC-δ, PKC-ɛ, and PKC-ζ (43, 60). Treatment of primary hepatocytes with Go-6976 promoted Akt phosphorylation but did not cause AMPK phosphorylation in either the absence or presence of H2O2 (Fig. 10A). These observations confirm that classical PKC isoforms (α, βI, βII, and γ) are important in suppressing Akt but are not important in regulating AMPK. The fact that PKC-α and PKC-βII are the only classical PKC isoforms that have been reported in the liver suggests that these PKC isoforms may be important in the negative regulation of Akt (10, 31). The lack of AMPK activation by Go-6976 was associated with less protection against H2O2-induced necrosis than Ro-31-8425 (Fig. 10B). Go-6976 protected hepatocytes less effectively than Ro-31-8425 (∼20–25% for 300 and 400 μM). These observations confirm that part of the protective effect of Ro-31-8425 treatment was mediated through the activation of AMPK, probably by the inhibition of an atypical PKC isoform.
Fig. 10.
Go-6976, a selective inhibitor of PKC-α and PKC-β, fails to activate AMPK and is less effective in protecting hepatocytes from H2O2-induced necrosis. Primary cultured hepatocytes were treated with H2O2 in the presence or absence of Go-6976 (10 μM, 1-h pretreatment). A: Go-6976, a PKC inhibitor believed to be specific for PKC-α and PKC-β, activated Akt but failed to activate AMPK. Samples were treated with H2O2 (300 μM) where noted. B: Go-6976 was less effective than Ro in protecting hepatocytes from necrosis induced by H2O2. ⧫, H2O2 treatment alone; ▪, Ro + H2O2; ▴, Go-6976 + H2O2. Means ± SD are shown. *P < 0.05 compared with hepatocytes treated with Ro; #P < 0.05 compared with hepatocytes treated with H2O2 alone.
Effect of knockdown of PKC-α and PKC-ɛ against H2O2-induced necrosis.
Since Go-6976, a more restricted PKC inhibitor than Ro-31-8425 and bisindolymaleimide I, also protected hepatocytes from H2O2-induced necrosis, although at more modest levels, suggests that classical PKC isoforms may be contributing to H2O2-induced necrosis. In an attempt to further elucidate which PKC isoform(s) may be mediating H2O2-induced necrosis, PKC-α and PKC-ɛ were knocked down in mice using ASO, and hepatocytes were isolated to assess their vulnerability to H2O2-induced necrosis. The knockdown of PKC-α slightly decreased H2O2-induced necrosis compared with control ASO (∼22%), wherease knockdown of PKC-ɛ had no effect (Fig. 11A). The fact that knockdown of PKC-α was only slightly protective suggests that the somewhat greater protective effect of Go-6976 was occurring through additional mechanisms besides PKC-α inhibition (e.g., PKC-βII inhibition, inhibition of PKC-like protein, PKC-βII translocation to the membrane, etc.). Assessment of the effect of ASO treatment confirmed that PKC-α and PKC-ɛ were knocked down (∼90%; Fig. 11A, inset). PKC-α treatment also caused a decline in PKC-ɛ protein levels without affecting the other PKC isoforms (βII, δ, and ζ; data not shown), suggesting that PKC-α may regulate PKC-ɛ. Taken together, these data suggest that PKC-ɛ is not important in H2O2-induced necrosis, whereas PKC-α, alone or in conjunction with PKC-ɛ, plays a small role in H2O2-induced necrosis in primary hepatocytes.
Fig. 11.
Effect of knockdown of PKC-α and PKC-ɛ on H2O2-induced necrosis in hepatocytes. A: effect of knockdown of PKC-α or PKC-ɛ on H2O2-induced necrosis in the absence and presence of PKC inhibitor. PKC-α or PKC-ɛ in mice were silenced using antisense oligonucleotide (ASO) injection (7 injections every other day). Cont mice were injected with scrambled ASO. Hepatocytes were isolated from ASO-treated mice, and their sensitivity to H2O2 (300 μM) was assessed in the presence and absence of Ro (10 μM, 1-h pretreatment). Western blot analysis demonstrated the effect of ASO on PKC-α and PKC-ɛ protein expression in hepatocytes (inset). B: Ro treatment of hepatocytes with suppressed PKC-α still induced AMPK and Akt phosphorylation. Fifteen minutes following H2O2 treatment, total cell extracts of hepatocytes were harvested, and Western blot analysis was performed using antisera against p-AMPK, AMPK, p-Akt, and Akt. NT, Cont from mice without ASO treatment. Means ± SD are shown. #P < 0.05 compared with Cont ASO sample; *P < 0.05 compared with PKC-α ASO without PKC inhibitor; **P < 0.05 compared with PKC-ɛ ASO without PKC inhibitor.
Ro-31-8425 treatment of hepatocytes from mice with PKC-α and PKC-ɛ knockdown still markedly protected from H2O2-induced necrosis (Fig. 11A). The protective effects of Ro-31-8425 treatment corresponded with an upregulation of AMPK even when PKC-α (Fig. 11B) and PKC-ɛ (data not shown) were knocked down. These findings confirm that PKC-α and PKC-ɛ are not important in AMPK inhibition and support the notion that most of the protective mechanism of Ro-31-8425 is mediated through the upregulation of AMPK. Ro-31-8425 treatment was also observed to increase Akt phosphorylation even when PKC-α (Fig. 11B) and PKC-ɛ (data not shown) were knocked down. Previous experiments with Go-6976 have suggested that classical PKC isoforms, likely PKC-α or PKC-βII, were important in the negative regulation of Akt. The fact that Ro-31-8425 treatment still upregulated Akt in hepatocytes with PKC-α knocked down suggests PKC-βII may be important in the negative regulation of Akt in primary hepatocytes.
DISCUSSION
Signal transduction pathways play an important role in H2O2-induced necrosis in cultured primary hepatocytes.
There is growing evidence in the literature suggesting that signaling pathways may modulate necrosis in certain situations (15, 46). Although necrosis has traditionally been characterized as a passive form of cell death due to catastrophic injury, recent studies have suggested that some forms of necrosis involve extensive programming. Similarly, H2O2-induced necrosis has been traditionally attributed to oxidative modification of proteins, lipids, and DNA that could be mainly inhibited by antioxidant treatment. However, our work demonstrates that the modulation of signaling pathways involving PKC and AMPK could alter H2O2-induced necrosis. Although H2O2-induced necrosis in hepatocytes did not extensively involve JNK (a common feature of programmed necrosis) (21), it is clear from our work that modulating signaling pathways involving PKC and AMPK were as or more effective than antioxidants (e.g., BHT and α-tocopherol) in protecting hepatocytes from H2O2-induced necrosis. This suggests that H2O2-induced necrosis involves two factors: 1) oxidative damage and an alteration in cellular redox status, which have been previously characterized to be prevented by antioxidants; and 2) activation of signaling pathways involving PKC activation that suppress signaling pathways such as AMPK and Akt. While oxidative damage and changes to the GSH redox status induced by H2O2 are likely to be important in activating or inhibiting signaling pathways that induce necrosis, our data suggest that even in the presence of extensive changes to the GSH redox status, altering PKC signaling pathways alone can protect hepatocytes from H2O2-induced necrosis. Consequently, our work suggests that necrosis induced by H2O2 involves a “program” that includes PKC activation and AMPK inhibition to promote hepatocyte death.
The present work revealed many important steps involved in H2O2-induced necrosis. First, we observed that H2O2 is rapidly consumed by hepatocytes, within minutes of H2O2 treatment. The rapid H2O2 consumption by hepatocytes was associated with a dramatic decrease in the GSH-to-GSSG ratio, which gradually recovered before the onset of necrosis. This suggests that most of the oxidative damage and changes in the GSH redox status important in H2O2-induced necrosis occur immediately following H2O2 treatment. However, hepatocytes died hours after H2O2 treatment (depending on the dose), indicating that there is a lag phase during which signaling changes that promote necrosis might be activated. Indeed, the dramatic drop in the GSH/GSSG redox status that occurred immediately following H2O2 treatment was associated with increased PKC activity [as shown by increased phosphorylation of proteins by PKC (Fig. 4C)]. Based on our work with inhibitors and ASO, classical PKC isoforms, which inhibit Akt, and atypical PKC isoforms, which inhibit AMPK, were activated in response to H2O2 treatment and/or changes in the GSH/GSSG redox status. The activation of atypical PKC isoforms and the consequent inhibition of AMPK were the key signaling changes induced by H2O2 treatment that promoted severe necrosis. Activation of classical PKC isoforms may also contribute to H2O2-induced necrosis, but probably not through its inhibitory effect on Akt.
PKC isoforms involved in H2O2-induced necrosis in primary hepatocytes.
Although all PKC inhibitors tested protected hepatocytes against H2O2-induced necrosis, identification of the PKC isoforms involved in H2O2-induced necrosis was complex. Part of the problem lies in the fact that PKC inhibitors not only inhibit PKC activity but also affect PKC translocation to membranes (52, 53). Based on our data, atypical PKC and classical PKC isoforms, which regulate AMPK and Akt, respectively, are activated in response to H2O2 treatment. The regulation of AMPK by atypical PKC isoforms is based on observations that AMPK is downregulated by H2O2 treatment of hepatocytes but upregulated by treatment with broad-spectrum PKC inhibitors (Ro-31-8425 and bisindolymaleimide I). In addition, PKC inhibitor (Ro-31-8425 and bisindolymaleimide I) treatment alone induced greater AMPK phosphorylation in the absence of H2O2 (Fig. 8D), suggesting that there was some basal PKC activity inhibiting AMPK. The selective PKC inhibitor Go-6976 (43), however, did not active AMPK, suggesting that classical PKC isoforms were not involved in AMPK regulation. In agreement with this finding, PMA, an activator of classic and novel PKC isoforms, did not affect AMPK phosphorylation. Since AMPK activation played a protective role against H2O2-induced necrosis, our data suggest that atypical PKC isoforms may be activated following H2O2 treatment to inhibit AMPK and promote H2O2-induced necrosis. However, based on our data, the possibility that PKC inhibitors were inhibiting a PKC-like protein that regulates AMPK signaling cannot be ruled out. Further experiments using ASO or short interfering RNA against atypical PKC isoforms will be needed to confirm that atypical PKC isoforms regulate AMPK activity in primary hepatocytes.
Although Go-6976 did not upregulate AMPK, it did partially prevent H2O2-induced necrosis (although not as strongly as Ro-31-8425), suggesting that the activation of classical PKC isoforms contributes to H2O2-induced necrosis. Akt phosphorylation was inhibited by H2O2 treatment but upregulated by the selective PKC inhibitor Go-6976, suggesting that classical PKC isoforms, likely PKC-βII, are involved in the negative regulation of Akt. This is in agreement with previous studies (38, 59) that showed that Akt is negatively regulated by classical PKC isoforms in some cell types (keratinocytes, A459 cells, and HEK-293 cells). Knockdown of PKC-ɛ using ASO showed that PKC-ɛ was not important in H2O2-induced necrosis, whereas knockdown of PKC-α partially protected hepatocytes against H2O2. However, the protection afforded by knockdown of PKC-α was smaller (∼22%) than Go-6976 treatment (∼45%), suggesting that Go-6976 may work through other pathways to protect hepatocytes from H2O2-induced necrosis. Part of the protective mechanism of PKC inhibitors may be mediated by PKC inhibitor treatment initiating translocation of these PKC isoforms to the membrane. Although PKC isoforms that translocate to membranes following PKC inhibitor treatment remain inactive, PKC translocation to the membrane may still affect signal transduction pathways (through protein-protein interactions, inhibiting the access of other proteins to membrane). Thus, it is possible that Go-6976 may be protecting hepatocytes through a combination of inhibition of PKC-α and induction of translocation of PKC-β to the membrane that alters other signaling pathways. At this point, it appears that atypical PKC isoforms and PKC-α play some role in H2O2-induced necrosis. However, other PKC isoforms or kinase targets of inhibitors may also play a contributory role, and this needs to be further elucidated.
Signal transduction pathways involved in H2O2-induced necrosis in primary hepatocytes.
Regardless of the mechanism, PKC inhibitors helped to unmask signaling pathways that are important in modulating H2O2-induced necrosis. Immediately following H2O2 treatment, Akt and AMPK were downregulated, whereas JNK was gradually activated. PKC inhibitors (Ro-31-8425 and bisindolymaleimide I) upregulated Akt and AMPK but did not affect JNK activation. AMPK was observed to be an important pathway in modulating H2O2-induced necrosis, whereas Akt and JNK were much less important in H2O2-induced necrosis. JNK activation has been shown to play a key role in apoptosis and programmed necrosis in many cell lines. In the liver, we observed that sustained JNK activation plays a central role in acetaminophen-induced liver injury (21), which is associated with hepatocyte necrosis and GSH depletion (20). JNK inhibitor and JNK ASO treatment significantly limited liver injury caused by acetaminophen without affecting GSH levels (21). Therefore, it is somewhat surprising that PKC inhibition could prevent H2O2-induced necrosis despite the fact that JNK activation remained sustained. JNK activation played some role in H2O2-induced necrosis, as seen by the partial protection by JNK inhibition. However, PKC inhibitor protected hepatocytes despite sustained JNK activation and alterations in the GSH redox status. This suggests that sustained JNK requires other important factors (activated during acetaminophen-induced liver injury) to promote hepatocyte necrosis. In contrast to JNK, Akt is believed to be prosurvival and has been shown to protect cells against ROS and other stresses (17, 32). Akt inhibition by Akt inhibitor VIII only slightly decreased the effectiveness of PKC inhibitor, suggesting that Akt plays a small role in mediating hepatocyte survival against H2O2-induced necrosis. Despite the prosurvival aspect of Akt, our data suggest that Akt activity does not play a major role in mediating the protective effects of PKC inhibitors.
Based on experiments with AMPK activators and inhibitors, the most important protective pathway affected by H2O2 and PKC inhibitor (Ro-31-8425 and bisindolymaleimide I) was AMPK. The upregulation of AMPK (phosphorylation) by PKC inhibitors or AMPK activators significantly reduced H2O2-induced necrosis (Fig. 12B). In addition, treatment of hepatocytes with compound C, an AMPK inhibitor, almost completely abrogated the protective effects of PKC inhibitor, suggesting that PKC inhibitors work primarily through the upregulation of AMPK to protect hepatocytes from H2O2-induced necrosis (Fig. 12C). Taken together, our data suggest that the upregulation of AMPK promotes hepatocyte survival and significantly reduces H2O2-induced necrosis. AMPK is an important energy sensor in cells and activates energy-generating pathways including increased β-oxidation, upregulation of glucose transporters, and inhibition of glycogen synthesis (28, 42). AMPK is also important in cell repair and a regulator of autophagy. Thus, a possible mechanism by which AMPK activation may be protecting hepatocytes is through modulating energy-generating pathways as well as cell repair through autophagy. H2O2 treatment is likely to drain energy levels in cells because ATP is required for repair of damaged proteins, lipid peroxidation, and DNA damage initiated by H2O2. Additional studies will be needed to determine if AMPK protects hepatocytes against H2O2-induced necrosis through the upregulation of energy-generating pathways.
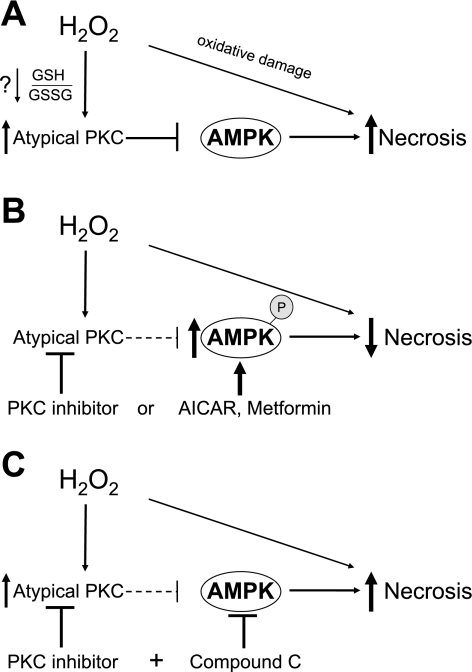
Fig. 12.
Regulation of H2O2-induced necrosis by PKC and AMPK in primary hepatocytes. A: role of AMPK and atypical PKC isoforms in H2O2-induced necrosis. H2O2 treatment of hepatocytes results in the activation of atypical PKC isoforms, which downregulates AMPK. The downregulation of AMPK leaves hepatocytes susceptible to H2O2-induced necrosis. Not shown is the H2O2-induced activation of classical PKC isoforms, activation of JNK, and downregulation of Akt, which also modulate, to some degree, H2O2-induced necrosis. B: upregulation of AMPK promotes hepatocyte survival. Inhibition of atypical PKC isoforms by PKC inhibitor treatment results in the upregulation of AMPK (phosphorylation), significantly preventing H2O2-induced necrosis. Similarly, upregulation of AMPK by activators (AICAR and Met) also reduces H2O2-induced necrosis. C: inhibition of AMPK by Cpd C negates the protective effect of PKC inhibitor. Cpd C, an AMPK inhibitor, negates the protective effects of PKC inhibitor against H2O2-induced necrosis, suggesting that PKC inhibitor works primarily through the upregulation of AMPK to protect hepatocytes from H2O2-induced necrosis.
Perspectives
When normal hepatocytes were treated with broad-spectrum PKC inhibitors, we observed activation of Akt and AMPK, suggesting that basal PKC activity exerts an inhibitory tone on these kinases. H2O2 further activated PKC, leading to greater inhibition of Akt and AMPK. PKC inhibitor fully restored the activation of these kinases, preventing the inhibition by H2O2. AMPK played a dominant protective role in the PKC inhibitor effect. Much of our data support the conclusion that H2O2-induced necrosis is mediated by atypical PKC isoform-induced suppression of AMPK, and PKC inhibition allowed maximal activation of this protective kinase. Other pathways activated by H2O2, such as JNK or classical/novel PKC isoform-dependent suppression of Akt, appeared to play a small role in the signal transduction events leading to necrosis induced by H2O2.
GRANTS
This work was supported by a grant from the Wright foundation (to D. Han), by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-067215 (to N. Kaplowitz), and by the Cell Culture Core of the National Institutes of Health Research Center for Liver Diseases (NIDDK Grant DK-48522). D. Han was also supported by a grant from the Veronica Budnick Chair.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H2O2: submicromolar levels of H2O2 induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med 30: 1008–1018, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 475: 121–126, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Antunes F, Salvador A, Marinho HS, Alves R, Pinto RE. Lipid peroxidation in mitochondrial inner membranes. I. An integrative kinetic model. Free Radic Biol Med 21: 917–943, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Baker A, Santos BD, Powis G. Redox control of caspase-3 activity by thioredoxin and other reduced proteins. Biochem Biophys Res Commun 268: 78–81, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273: 11401–11404, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278: 51613–51621, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem 278: 17593–17596, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H2O2. Biochem Biophys Res Commun 287: 92–97, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol 44: 918–929, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Croquet F, Brehier A, Gil S, Davy J, Feger J. Five isoenzymes of protein kinase C are expressed in normal and STZ-diabetic rat hepatocytes: effect of phorbol 12-myristate 13-acetate. Biochim Biophys Acta 1315: 163–168, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Czaja MJ Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis 27: 378–389, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Guerra MJ, Bosca L. Lack of translocation of protein kinase C from the cytosol to the membranes in vasopressin-stimulated hepatocytes. Biochem J 269: 163–168, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domenicotti C, Paola D, Vitali A, Nitti M, d'Abramo C, Cottalasso D, Maloberti G, Biasi F, Poli G, Chiarpotto E, Marinari UM, Pronzato MA. Glutathione depletion induces apoptosis of rat hepatocytes through activation of protein kinase C novel isoforms and dependent increase in AP-1 nuclear binding. Free Radic Biol Med 29: 1280–1290, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett 581: 5727–5731, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta 1757: 1371–1387, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga M, Oka M, Ichihashi M, Yamamoto T, Matsuzaki H, Kikkawa U. UV-induced tyrosine phosphorylation of PKC delta and promotion of apoptosis in the HaCaT cell line. Biochem Biophys Res Commun 289: 573–579, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2. Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem 276: 46379–46385, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med 28: 1349–1361, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Grabarek J, Ware JA. Protein kinase C activation without membrane contact in platelets stimulated by bryostatin. J Biol Chem 268: 5543–5549, 1993. [PubMed] [Google Scholar]
- 20.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67: 322–328, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 131: 165–178, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414: 552–556, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol 64: 1136–1144, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Han D, Hanawa N, Saberi B, Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med 41: 627–639, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Han D, Hanawa N, Saberi B, Kaplowitz N. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol 291: G1–G7, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid-induced necrosis of cultured mouse hepatocytes: inhibition of mitochondrial function and oxidative stress. Biochem Pharmacol 67: 439–451, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Hardie DG AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47: 185–210, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol 574: 7–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey PR, Ilson RG, Strasberg SM. The simultaneous determination of oxidized and reduced glutathiones in liver tissue by ion pairing reverse phase high performance liquid chromatography with a coulometric electrochemical detector. Clin Chim Acta 180: 203–212, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immun 1: 489–495, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Hsu C, Hsieh YC, Hsu HK, Jao SC, Yang RC. Alteration of protein kinase C isoforms in the liver of septic rat. Shock 17: 41–46, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Jeon BW, Kim KT, Chang SI, Kim HY. Phosphoinositide 3-OH kinase/protein kinase B inhibits apoptotic cell death induced by reactive oxygen species in Saccharomyces cerevisiae. J Biochem 131: 693–699, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Lopez JM, Cederbaum AI. Protein kinase C signaling as a survival pathway against CYP2E1-derived oxidative stress and toxicity in HepG2 cells. J Pharmacol Exp Ther 312: 998–1006, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kaplowitz N Biochemical and cellular mechanisms of toxic liver injury. Semin Liver Dis 22: 137–144, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Kefas BA, Heimberg H, Vaulont S, Meisse D, Hue L, Pipeleers D, Van de Casteele M. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 46: 250–254, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-xL in response to DNA damage. J Biol Chem 275: 322–327, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Kohn EA, Yoo CJ, Eastman A. The protein kinase C inhibitor Go6976 is a potent inhibitor of DNA damage-induced S and G2 cell cycle checkpoints. Cancer Res 63: 31–35, 2003. [PubMed] [Google Scholar]
- 38.Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem 281: 3237–3243, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9: 218–224, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett 15: 761–764, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Lo CR, Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35: 772–778, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116: 1776–1783, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993. [PubMed] [Google Scholar]
- 44.Newton AC Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101: 2353–2364, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpi E, Rosello-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology 34: 1164–1173, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res 283: 1–16, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Russell RR, 3rd Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salsman S, Felts N, Pye QN, Floyd RA, Hensley K. Induction of Akt phosphorylation in rat primary astrocytes by H2O2 occurs upstream of phosphatidylinositol 3-kinase: no evidence for oxidative inhibition of PTEN. Arch Biochem Biophys 386: 275–280, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem J 372: 359–369, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 336: 241–246, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 3329–3335, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stensman H, Raghunath A, Larsson C. Autophosphorylation suppresses whereas kinase inhibition augments the translocation of protein kinase Calpha in response to diacylglycerol. J Biol Chem 279: 40576–40583, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi H, Namiki H. Mechanism of membrane redistribution of protein kinase C by its ATP-competitive inhibitors. Biochem J 405: 331–340, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tapodi A, Debreceni B, Hanto K, Bognar Z, Wittmann I, Gallyas F Jr, Varbiro G, Sumegi B. Pivotal role of Akt activation in mitochondrial protection and cell survival by poly(ADP-ribose)polymerase-1 inhibition in oxidative stress. J Biol Chem 280: 35767–35775, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2: 222–228, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J 23: 1889–1899, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem 279: 27482–27493, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem 279: 31089–31097, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Wen HC, Huang WC, Ali A, Woodgett JR, Lin WW. Negative regulation of phosphatidylinositol 3-kinase and Akt signalling pathway by PKC. Cell Signal 15: 37–45, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J 294: 335–337, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem 104: 325–335, 2008. [DOI] [PubMed] [Google Scholar]