Abstract
α4β1-Integrin plays a pivotal role in cell migration in vivo. This integrin has been shown to regulate the front-back polarity of migrating cells via localized inhibition of α4-integrin/paxillin binding by phosphorylation at the α4-integrin cytoplasmic tail. Here, we demonstrate that α4β1-integrin regulates directionally persistent cell migration via a more complex mechanism in which α4-integrin phosphorylation and paxillin binding act via both cooperative and independent pathways. We show that, in response to shear flow, α4β1-integrin binding to the CS-1 region of fibronectin was necessary and sufficient to promote directionally persistent cell migration when this integrin was ectopically expressed in CHO cells. Under shear flow, the α4β1-integrin-expressing cells formed a fan shape with broad lamellipodia at the front and retracted trailing edges at the back. This “fanning” activity was enhanced by disrupting paxillin binding alone and inhibited by disrupting phosphorylation alone or together with disrupting paxillin binding. Notably, the phosphorylation-disrupting mutation and the double mutation resulted in the formation of long trailing tails, suggesting that α4-integrin phosphorylation is required for trailing edge retraction/detachment independent of paxillin binding. Furthermore, the stable polarity and directional persistence of shear flow-stimulated cells were perturbed by the double mutation but not the single mutations alone, indicating that paxillin binding and α4-integrin phosphorylation can facilitate directionally persistent cell migration in an independent and compensatory manner. These findings provide a new insight into the mechanism by which integrins regulate directionally persistent cell migration.
Keywords: lamellipodia, fibronectin, shear stress
cell migration plays an important role in diverse biological processes, such as development, the immune response, and cancer metastasis. For a cell to migrate directionally, precise spatial and temporal coordination of membrane protrusion and retraction with cell adhesion and deadhesion are required. These events depend on polarization of the actin cytoskeleton, contractile activities, and adhesion complex assembly/disassembly, which are regulated by a Rho GTPase-mediated signaling network and other intracellular signaling modules (22, 35). Extrinsically, cell migration depends on soluble chemoattractants and the extracellular matrix (ECM). The ECM not only provides a substratum but also regulates the motile activities by sending chemical and mechanical signals to the cells. Both the anchoring and signaling functions of the ECM are predominantly mediated by the integrin family of cell adhesion receptors (10, 17). Integrins transduce signals across the plasma membrane bidirectionally via an allosteric mechanism by switching between active and inactive conformations (11). Activated integrins preferentially localize to the leading edge, where they bind to cytoskeletal and signaling proteins to form focal adhesions (19, 27). In motile cells, focal adhesions act as anchors for the cells to move forward and function as targeting and activation sites for Rac and other signaling proteins (5).
α4β1-Integrin belongs to a unique subgroup of integrins that not only mediate cell-ECM adhesion but also mediate heterophilic cell-cell adhesion by binding to VCAM-1, a member of the immunoglobulin superfamily. α4β1-Integrin-mediated cell-cell adhesion is essential for placental and cardiac development (38) and plays critical roles for leukocytes and pericytes to attach to vascular endothelial cells during extravasation (2) and tumor angiogenesis (9), respectively. α4β1-Integrin also binds to an alternatively spliced CS-1 region of fibronectin, the ECM ligand of α4β1-integrin. α4β1-Integrin-mediated cell-ECM adhesion is required for neural crest cell migration (18) and the migration of progenitor cells during cardiac development (30) and blood vessel remodeling (13). In culture, α4β1-integrin is required for primary embryonic pericytes to migrate in a directionally persistent manner in response to shear force (13). When ectopically expressed in CHO cells, α4β1-integrin promotes cell motility by facilitating the formation of lamellipodia in response to scratch wounding a cell monolayer (28). The mechanism by which α4β1-integrin regulates cell migration involves molecular interactions at the cytoplasmic tail of the α4-subunit (referred to as the α4-tail). The α4-tail is phosphorylated at Ser988 (14) and binds to paxillin, a signaling adaptor protein (24). Furthermore, α4-integrin and paxillin form a ternary complex with G protein-coupled receptor kinase interactor 1 (GIT1), an ADP-ribosylation factor-GTPase-activating protein, and this complex inhibits Rac activity (26). It has been proposed that the α4-integrin-paxillin-GIT1 complex polarizes Rac activities by inhibiting Rac activation at the sides and trailing edge of the cells (26).
Fluid shear stress associated with the flow of blood in the circulatory system plays an important regulatory role in angiogenesis (23), a process that involves the migration of vascular endothelial cells and pericytes. In α4 integrin-deficient mouse embryos, pericytes fail to cover angiogenic blood vessels, and the vessels are dilated (13). In culture, wild-type pericytes migrated in a directionally persistent manner in response to laminar shear flow, and this shear flow-induced motility was abolished by the α4-integrin-null mutation (13). Furthermore, an anti-α4-integrin antibody inhibited the functions of endothelial cells during tumor angiogenesis (9). As a critical physiological stimulus, shear flow has a profound effect on vascular endothelial cells and other cell types at the vessel wall and in the blood circulation. Studies on endothelial cells have revealed that shear flow modulates gene expression and promotes cytoskeletal alignment, cell polarization, and cell motility via the activation of intracellular signaling components, including Rac, FAK, and phosphatidylinositol 3-kinase (15, 16, 23, 33, 36, 39). α5β1-Integrin and αvβ3-integrin, which are the major components of focal adhesions, are required for some of these shear flow-induced signaling pathways (31).
In the present study, we show that α4β1-integrin is required and sufficient for shear flow-induced directionally persistent cell migration when ectopically expressed in CHO cells. This model system allowed us to analyze the effects of mutations that disrupt phosphorylation and/or paxillin binding at the α4-tail on shear flow-induced cell motility. These experiments provide new insight into the α4β1-integrin-mediated signaling pathway that regulates shear flow-induced directionally persistent cell migration.
MATERIALS AND METHODS
Construction of plasmids, transfection, and cell culture.
pQN4S988AG and pQN4DMG, which carries the S988A single mutation and the Y991A/S988A double mutation in the α4-tail, respectively, were constructed using the same strategy as that for constructing pQN4Y991AG (28), which carries the Y991A single mutation, except that pQN4Y991AG was used as the PCR template for generating the double mutation in pQN4DMG.
Transfection was performed using FuGene6 transfection reagent (Roche Molecular Biochemical) following the manufacturer's instructions. The selection of stable transfectants and culturing of the cell lines have been described by Pinco et al. (28).
Integrin ligands.
Rat plasma fibronectin was purchased from Calbiochem (La Jolla, CA). Plasmids pGH-F12-V120-15 (expressing FNIII12-V120-15, which contains CS-1) and pGH-F7-11 (expressing FNIII7-11,which contains RGD and the synergy region), which were derived from pGH.PL (3), were provided by Richard Hynes (Massachusetts Institute of Technology, Cambridge, MA). CS-1- and RGD-containing fragments were purified from pGH-F12-V120-15- or pGH-F7-11-expressing Escherichia coli using glutathione-Sepharose beads (Pierce).
Flow cytometry, immunoprecipitation, and immunoblot analysis.
Flow cytometry, immunoprecipitation, and immunoblot analysis were performed as described by Pinco et al. (28). For flow cytometry, an anti-α4-integrin antibody (clone P1H4 from Millipore-Chemicon) and a goat anti-mouse allophycocyanin-conjugated secondary antibody (Santa Cruz Biotechnology) were used. For immunoprecipitation and immunoblot analysis, the following primary antibodies were used: mouse anti-α4-integrin (clone P1H4), mouse phosphorylation (phospho)-specific anti-α4-integrin (Invitrogen-Biosource), mouse anti-paxillin (BD Transduction Laboratories), and rabbit anti-green fluorescent protein (GFP; for GFP-tagged α4-integrin, Molecular Probes).
Shear flow migration assay.
Cells were plated on 35-mm suspension culture dishes precoated with 5 μg/ml fibronectin or other substrates for 2 h at 37°C. After cells had been allowed to adhere for 2 h at 37°C, the dish was assembled onto a perfusion chamber. The flow channel (1.3 × 0.5 × 0.0178 cm) was formed by a cutout in a silicone-based gasket, which was sealed to the transparent polycarbonate surface of the perfusion chamber. The flow chamber assembly was mounted onto the stage of an inverted microscope (Nikon TE300 or Zeiss Axiovert 200M) equipped with phase-contrast optics and enclosed in a 37°C incubator. Cells were subjected to shear stress by continuously perfusing Leibovitz's L-15 CO2-buffered medium (Invitrogen) supplemented with 10% FBS through the chamber for 4 or 6 h. Shear stress was set to 4 dyn/cm2, which is in the range of stresses encountered in the venous circulation in vivo. Digital images of a single ×10 field of view were acquired every 30 s. For each experiment, the time-lapse images were compiled into a movie at 30 frames/s. Three independent assays were performed on each cell line.
Immunofluorescence and confocal microscopy.
Cells were plated on 5 μg/ml fibronectin, stimulated with shear flow at various time periods, and fixed in 3% paraformaldehyde in PBS for 10 min at room temperature. Immunofluorescence staining was performed as described by Pinco et al. (28). The following primary antibodies were used: mouse anti-paxillin (1:50), rabbit anti-GFP (1:1,000), mouse anti-GFP (Zymed, 1:100), and rabbit anti-phospho-α4-integrin [generated and prepared as described by Han et al. (14), 1:50]. Confocal images were taken using a Zeiss LSM510 confocal microscope.
Detachment assay.
Suspension culture dishes (35 mm) were coated with 0.2 μg/ml of the FNIII12-V120-15 fragment of fibronectin for 1 h at 37°C, blocked with 2 mg/ml heat-inactivated BSA overnight at 4°C, assembled to the flow chamber, and mounted onto the microscope stage. Cells were injected into the chamber and allowed to settle for 1 min. Cells were then subjected to a shear stress of 4 dyn/cm2 for 1 min. Thereafter, the wall shear stress was increased stepwise to 32, 64, and 96 dyn/cm2 at 1-min intervals. At the end of each shear stress increment, cells were photographed, and the percentage of cells detached was determined. Means ± SE were calculated from the data of three independent assays for each cell line. Statistical analysis of the data was performed using ANOVA (Microsoft Excel).
Analysis of cell migration speed and directional persistence time.
The centroid position (x,y) of each cell migrating under shear was calculated at each time step (τ; 4.5 min) for the entire duration of the experiment (6 h) using ImageJ software (National Institutes of Health). Cells whose migration was not contained within the image field for the duration of the experimental run, as well as the cells that divided during the course of the flow assay, were excluded from this calculation. The distance that the centroid of the cell moved over each τ value in two-dimensional space was then used to calculate the cell migration speed during this time period. Migration speed data for each cell at all time points were then compiled to obtain the root mean square (RMS) cell migration speed (S) for the entire duration of the shear flow experiment using the following equation:
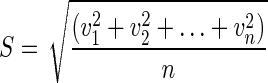 |
where v is velocity and n is the number of cells. Centroid coordinate data were also used to calculate individual time-averaged mean square displacement (MSD) in two-dimensional space using the following equation (7, 20, 32):
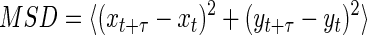 |
where t is the elapsed time.
Values of S and time-averaged MSD calculated for each cell were then used to determine the directional persistence time (PT), which is the characteristic time in which cell movement persists in the same direction, using the following equation (7, 20, 32):
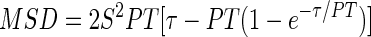 |
The value of PT for each cell at each time point was determined using the Solver function of Excel. The average PT for each cell over the entire duration of cell migration under shear flow (6 h) was then calculated. Moreover, ensemble-averaged PT (referred to as mean PT in the text) was calculated for each cell type.
Statistics.
Data are expressed as means ± SE. Statistical significance of differences between means was determined by Student's t-test or one-way ANOVA where appropriate. If means were shown to be significantly different, multiple comparisons by pairs were performed by the Holm test.
Online data supplements.
The online version of this article contains time-lapse movies that accompany Figs. 1 and 3 and additional supplemental movies. Online supplemental material is available at the American Journal of Physiology-Cell Physiology website.
Fig. 1.
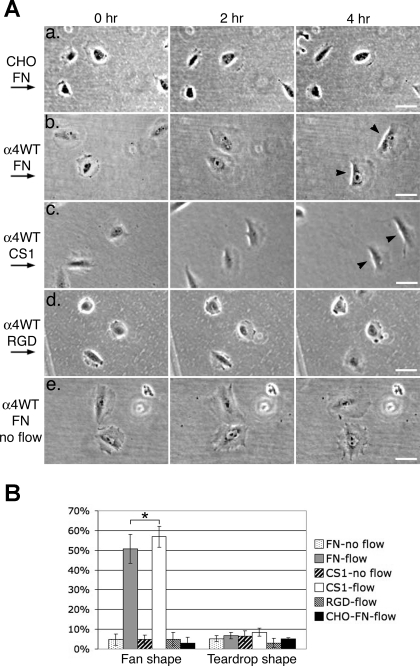
Shear flow-induced cell migration. A: time-lapse images of cells migrating under shear flow. CHO (a) or CHO-α4WT [wild type (WT); b–e] cells were plated on fibronectin (FN; a, b, and e), the FNIII12-V120-15 fragment of FN that contains the CS-1 region (c), or the FNIII7-11 fragment of FN that contains the RGD motif (d). Cells were imaged under shear flow (a–d) or without shear flow (e) at 30-s intervals for at least 4 h by time-lapse microscopy (see movies 1–6 in the online data supplements). Arrows on the left show the direction of the shear flow. For each time-lapse movie, 3 frames at designated time points from an area of a typical movie sequence are presented. Arrowheads point to cells with a fan shape (with a bright phase halo at its flattened and often concave trailing edge). Bars = 50 μm. B: percentages of cells under shear flow that formed fan or teardrop shape (with a pointed or elongated tail). In each experiment, at least 20 cells were analyzed (except for one of the experiments of CHO cells, where 17 cells were analyzed). Data represent means ± SE from 3 independent experiments. Note that FN and CS-1 had similar effects on the shear flow-induced fan shape formation of CHO-α4WT cells (*P > 0.5).
Fig. 3.
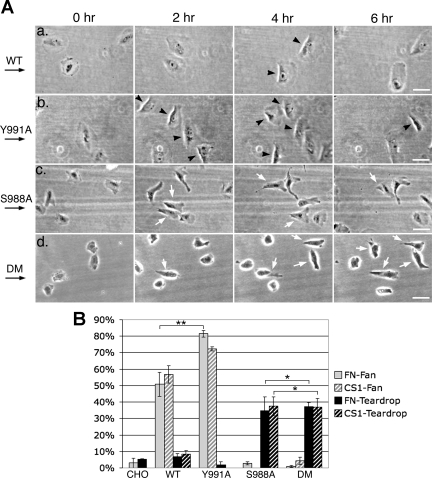
Effects of disruption of α4-integrin phosphorylation or paxillin binding on shear flow-induced motile activities. A: time-lapse images of cells migrating under shear flow. CHO-α4WT (a), CHO-α4Y991A (b), CHO-α4S988A (c), and CHO-α4DM (d) migrating on fibronectin under shear flow were imaged at 30-s intervals for 6 h by time-lapse microscopy (see movies 2, 7, 9, and 11 in the online data supplements). Arrows on the left show the direction of the shear flow. For each cell type, 4 frames at designated time points from an area of a typical movie sequence are presented. Black arrowheads point to fan-shaped cells; white arrows point to teardrop-shaped cells. Note that, in d, teardrop-shaped CHO-α4DM cells had less stable polarity, and many did not orient in the direction of the shear flow. Bars = 50 μm. B: percentages of cells under shear flow that formed fan or teardrop shapes. In each experiment, at least 20 cells were analyzed. Data represent means ± SE from 3 independent experiments. Note that CHO-α4Y991A cells had a higher percentage of fan-shaped cells than CHO-α4WT cells (**P < 0.05); CHO-α4S988A and CHO-α4DM cells formed a teardrop shape with a similar percentage (*P > 0.5).
RESULTS
α4β1-Integrin promotes leading edge lamellipodia protrusion and trailing edge detachment/retraction in response to shear flow stimulation.
We have previously shown that α4β1-integrin is required for primary pericytes to respond to shear force and migrate in a directionally persistent manner (13). To elucidate how α4β1-integrin regulates cell motility, we used a CHO cell system. CHO cells do not express α4-integrin endogenously but do express α5β1-integrin. It has previously been shown that, when α4β1-integrin is stably transfected into CHO cells, this integrin promotes the formation of broad lamellipodia in response to scratch wounding a cell monolayer (12, 28). To study the role of α4β1-integrin in directionally persistent cell migration, we tested the motile activities of α4β1-integrin-expressing CHO cells (referred to as CHO-α4WT cells) when stimulating the cells with a unidirectional shear flow using a parallel plate flow chamber system (6, 21). When plated on fibronectin or a recombinant FNIII12-V120-15 fragment of fibronectin that contains the CS-1 region (referred to as CS-1), CHO-α4WT cells responded to the shear flow and migrated in the direction of the flow. Many migrating cells (51 ± 7.3% and 57 ± 5.3% on fibronectin and CS-1, respectively, P > 0.5) formed broad leading edge lamellipodia in a manner similar to that seen at the edges of scratch wounds (Fig. 1B) (28). These cells also had flattened and often concave trailing edges with a distinct bright phase-contrast halo along the edges, giving the cells a fan-like shape (Fig. 1A,b and c, and movies 2 and 3 in the online data supplements). These fan-shaped cells migrated in a directionally persistent manner, resembling migrating fish epidermal cells (8). In the absence of α4β1-integrin, on the other hand, parental CHO cells (referred to as CHO cells) plated on fibronectin had only a low basal level of the “fanning” activity under shear flow (Fig. 1A,a and movie 1 in the online data supplements), as did CHO-α4WT cells plated on a recombinant FNIII7-11 fragment of fibronectin that contained RGD and synergy regions (Fig. 1A,d and movie 4 in the online data supplements). The majority of these cells protruded membrane extensions transiently in random directions. The fanning activity of CHO-α4WT cells on fibronectin and CS-1 depended on the shear flow. In the absence of shear flow, most CHO-α4WT cells failed to form the fan shape, and their protrusive activities were similar to those of CHO cells (Fig. 1A,e and movies 5 and 6 in the online data supplements). These results indicate that binding between α4β1-integrin and CS-1 is necessary and sufficient for shear flow-induced fanning activities characterized by the formation of a broad leading edge lamellipodium and a flattened trailing edge.
Phosphorylation and paxillin binding at the α4-tail regulate shear flow-induced motile activities.
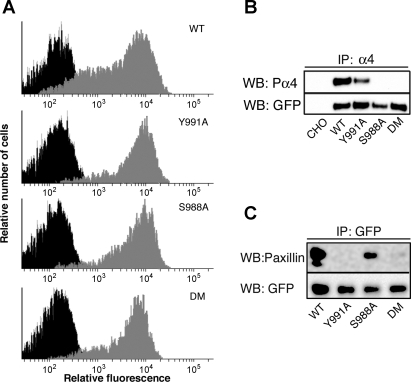
The α4-tail binds to paxillin and can be phosphorylated at Ser988. It has been previously shown that the lamellipodia-promoting activity of α4β1-integrin is negatively regulated by paxillin binding (28) and requires α4-subunit phosphorylation (12). These conclusions were derived from studies involving two point mutations in the α4-tail: Y991A, which markedly reduces paxillin binding by substituting Tyr991 to Ala, and S988A, which impairs phosphorylation by substituting Ser988 to Ala. Y991A and S988A enhance and inhibit the lamellipodia-promoting activities of α4β1-integrin, respectively (12, 28). To investigate the mechanisms by which α4β1-integrin regulates shear flow-induced cell migration, we examined the effects of these mutations using a shear flow assay. We compared CHO-α4WT cells with CHO cell lines that stably express α4-integrin mutants carrying Y991A, S988A, or a Y991A/S988A double mutation (referred to as CHO-α4Y991A, CHO-α4S988A, and CHO-α4DM, respectively). In all of these cell lines, α4-integrin was tagged with GFP, which has been shown not to interfere with the motile activities of α4β1-integrin (28). Flow cytometry analysis showed that the expression levels of α4-integrin were fairly equivalent among all cell lines (Fig. 2A). Furthermore, immunoblot analysis showed that S988A and the double mutation disrupted the phosphorylation site at Ser988 (Fig. 2B) and that Y991A and the double mutation markedly reduced the ability of α4-integrin to coimmunoprecipitate with paxillin (Fig. 2C).
Fig. 2.
Characterization of CHO cells expressing α4β1-integrin (WT) and α4-integrin mutants [Y991A, S998A, and the double mutation (DM)]. A: cells were analyzed by flow cytometry in the absence (in black, on the left) or presence (in gray, on the right) of an anti-α4 integrin antibody. B: lysates from CHO cells expressing α4β1-integrin or α4-integrin mutants were analyzed by immunoprecipitation (IP) with an anti-α4-integrin antibody followed by immunoblot analysis with an antibody against phospho-α4-integrin. The immunoblot was reprobed with an anti-green fluorescent protein (GFP) antibody that recognized GFP-tagged WT and mutant α4-integrin. C: cell lysates were immunoprecipitated with the anti-GFP antibody followed by immunoblot analysis with an antibody against paxillin or GFP. Note that the DM disrupted both the phosphorylation site and the paxillin-binding site in the α4-tail. WB, Western blot.
To determine the effect of S988A and Y991A mutations on shear flow-induced motile activities, mutant α4-integrin-expressing cells were compared with CHO-α4WT cells for their ability to form the fan shape when plated on fibronectin or CS-1 under shear flow. We found that shear flow-induced fanning activities were also observed among CHO-α4Y991A cells plated on fibronectin (Fig. 3A, b and movie 7 in the online data supplements) or CS-1 (movie 8 in the online data supplements). The percentage of CHO-α4Y991A cells with fanning activity (82 ± 1.8% and 72 ± 1.3% on fibronectin and CS-1, respectively) was significantly higher than that of CHO-α4WT cells (P < 0.05; Fig. 3B). On the other hand, CHO-α4S988A and CHO-α4DM had only low basal levels of fanning activy under shear flow (Fig. 3A,c and d, and B and movies 9–12 in the online data supplements). Thus, fanning activity is enhanced by Y991A but inhibited by S988A and the double mutation.
Under shear flow, a significant proportion of CHO-α4S988A and CHO-α4DM cells had a polarized “teardrop” shape featured with a pointed or elongated trailing tail (35 ± 8.0% and 37 ± 5.8% on fibronectin and CS-1, respectively, for CHO-α4S988A; and 37 ± 2.6% and 37 ± 5.0% on fibronectin and CS-1, respectively, for CHO-α4DM, P > 0.5; Fig. 3A,c and d, and movies 9–12 in the online data supplements). This phenotype was rarely observed in the other cell types under the same conditions (Figs. 1B and 3B). The trailing tail phenotype of CHO-α4S988A and CHO-α4DM cells indicates that α4-integrin phosphorylation plays a critical role in retracting/detaching the trailing edge of shear flow-stimulated cells. This event is likely to be independent of α4-integrin/paxillin interactions because the trailing tail phenotype of the double mutation resembled that of the S988A but not Y991A mutation. Teardrop-shaped CHO-α4S988A and CHO-α4DM cells did form leading edge lamellipodia, but compared with the broad lamellipodia of fan-shaped CHO-α4WT and CHO-α4Y991A cells, the lamellipodia protruded by teardrop-shaped cells were narrower and often had irregular shapes. Therefore, α4-integrin phosphorylation is not essential for lamellipodia protrusion, but it does somehow contribute to this event, again in a manner dominant over α4-integrin/paxillin interactions.
α4β1-Integrin promotes directionally persistent cell migration under shear flow.
Although both CHO-α4S988A and CHO-α4DM cells had the same capacity to form a teardrop shape, they did not migrate in the same manner under shear flow. Teardrop-shaped CHO-α4S988A cells appeared to migrate in a directionally persistent manner similar to fan-shaped CHO-α4WT and CHO-α4Y991A cells, whereas teardrop-shaped CHO-α4DM cells had less stable front-back polarity (white arrows in Fig. 3A,d) and appeared to be less directionally persistent. To quantify directional persistence, we calculated the directional persistence time (PT), which was the characteristic time that a cell moved in the same direction. We calculated the mean PT for each cell type plated on fibronectin in the absence or presence of shear flow (Fig. 4A). In the absence of shear flow, mean PTs of all cell types were <6 min. Under shear flow, mean PTs for CHO (23 ± 3.5 min) and CHO-α4DM cells (25 ± 3.8 min) were similar (P > 0.5); both had an increased mean PT compared with the same cell types without shear flow, consistent with these cells having some directionally persistent movement under shear flow. However, these mean PT values were much lower than those for CHO-α4WT (99 ± 25 min), CHO-α4Y991A (183 ± 32 min), and CHO-α4S988A (75 ± 11 min) cells under shear flow. These data show that α4β1-integrin drastically enhances directionally persistent cell migration, and the ability of α4β1-integrin to promote directionally persistent migration is abolished by disrupting both α4-integrin phosphorylation and paxillin binding but not by the single mutations. Consistent with the enhancing effect of Y991A on fanning activities, under shear flow, the mean PT of CHO-α4Y991A cells was significantly higher than that of CHO-α4WT cells (P < 0.05). Surprisingly, no statistical differences were observed between mean PT values of CHO-α4WT and CHO-α4S988A cells under shear flow (P > 0.3), although the double mutation had a strong inhibitory effect (P < 0.005). These results show that 1) paxillin binding negatively regulates the shear flow-induced directional persistence (as shown by CHO-α4Y991A cells), 2) α4-integrin phosphorylation is dispensable for α4β1-integrin to promote shear flow-induced directional persistence when paxillin can bind to the α4-tail (as shown by CHO-α4S988A cells), and 3) α4-integrin phosphorylation is required for α4β1-integrin to promote shear flow-induced directional persistence when paxillin binding is inhibited (as shown by CHO-α4DM cells compared with cells with single mutations). These results suggest that both α4-integrin phosphorylation and paxillin binding/dissociation contribute to the ability of α4β1-integrin to promote directionally persistent cell migration in response to shear force, but via a complex mechanism where the two events act in a compensatory manner.
Fig. 4.
Quantitative analysis of directional persistence, speed, and adhesive strength of migrating cells under shear flow. A: mean directional persistence times (±SE) for CHO, CHO-α4WT, CHO-α4Y991A, CHO-α4S988A and CHO-α4DM cells with (n ≥ 99) or without (n ≥ 30) shear flow. *P > 0.3; **P < 0.05. B: mean directional persistence times (±SE) for fan- or teardrop-shaped cells as well as cells with neither shape (n > 50 for all cells except CHO-α4Y991A cells with neither shape, which had a sample size of n = 17). *P > 0.5; **P < 0.001. C: average root mean square (RMS) speed, which is the migration speed of cells along the path, was graphed (means ± SE) for each cell type that formed a fan or teardrop shape or neither shape (n > 50 for all cells except CHO-α4Y991A cells with neither shape, which had a sample size of n = 17). *P > 0.5; **P < 0.001. D: detachment assays performed under the designated shear stresses. In each experiment, at least 50 cells were initially attached to the CS-1-coated surface (except for one of the experiments for CHO-α4WT cells, which had 38 cells initially attached). Data represent means ± SE from 3 independent experiments for each cell type. Note that there were no significant differences among the cell types (P > 0.5).
The enhanced directional persistence of CHO-α4Y991A correlated with enhanced fanning activity. To directly assess the correlation between the cell shape and directional persistence, we calculated the mean PT for cells that formed the fan or teardrop shape or for those with neither shape (Fig. 4B). For all cell types except CHO-α4DM cells, cells with polarized shapes (fan or teardrop shape) had similar mean PTs (P > 0.3) and much higher mean PTs than cells without polarized shapes (P < 0.001). Thus, there is a tight correlation between stably polarized shapes and directional persistence. We conclude that α4β1-integrin promotes directionally persistence migration by stabilizing front-back cell polarity.
α4β1-Integrin enhances cell migration speed but does not influence cell adhesion strength on fibronectin under shear flow.
It is possible that α4β1-integrin-promoted cell shape changes also influence the speed of cell migration. Using the coordinates of the centroid positions of the cells, we calculated, for each cell, RMS speed, defined as the square root of the averaged square of cell speeds at each time step value (Fig. 4C). This calculation reflects the migration speed of a cell along the migration path. As observed for the directional persistence, for all cell types except CHO-α4DM cells, cells with polarized shapes (fan or teardrop shape) had significantly higher migration speeds than cells without these shapes (P < 0.001). Furthermore, fan-shaped CHO-α4WT and teardrop-shaped CHO-α4S988A cells, which had similar directional persistence, had no significant differences in migration speeds (P > 0.5). Differing from directional PTs for specific cell shapes (Fig. 4B), fan-shaped CHO-α4Y991A cells migrated at a faster pace than fan-shaped CHO-α4WT cells (P < 0.001), indicating that cell shape and migration speed do not have a tight causal relationship.
Adhesive strength is known to play critical roles in regulating cell migration. The mutations at the α4-tail may confer changes in adhesion strength that could potentially affect the motile activities of the cells. To test this possibility, the adhesion strength of α4β1-integrin-expressing cells on CS-1 under shear flow was determined by assessing the percentage of cells detaching from CS-1-coated surfaces under increasing shear stresses up to 96 dyn/cm2, a force much stronger than the 4 dyn/cm2 force used in our shear flow assays (Fig. 4D). Among the wild-type and mutant α4-integrin-expressing cell types, no significant differences were observed in the adhesion strength on CS-1, even at 96 dyn/cm2 (P > 0.5). We conclude that in our CHO cell system, paxillin binding and α4-integrin phosphorylation do not play a major role in modulating the adhesive strength of the cells under shear flow.
Distribution of paxillin, α4-integrin, and phospho-α4-integrin under shear flow.
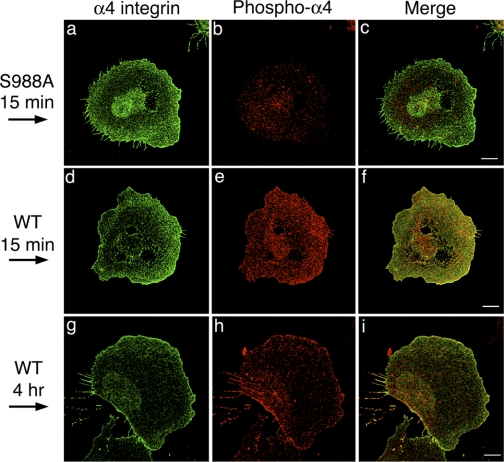
It has been reported that, in randomly migrating cells, paxillin and α4β1-integrin colocalized at the sides and trailing edge but not at the leading edge, whereas phospho-α4-integrin is localized preferentially at the leading edge (12). This observation is consistent with a model in which α4-integrin phosphorylation inhibits α4-integrin/paxillin binding at the leading edge, thus promoting the formation of leading edge lamellipodia (26). Our shear flow experiments suggested that α4-integrin phosphorylation may have additional functions. To understand how α4-integrin phosphorylation and paxillin binding regulate shear flow-induced motile activities, we examined the distribution of α4-integrin, phospho-α4-integrin, and paxillin in CHO-α4WT cells when cells were plated on fibronectin and subjected to shear flow. Cells began to polarize in shape after 15 min of shear flow stimulation, and, after 4 h, many cells formed the fan shape. At both time points, α4-integrin localized throughout the cell surface with higher intensity of staining at the leading and trailing edges and phospho-α4-integrin colocalized with α4-integrin (Fig. 5, d–i). The staining of phospho-α4-integrin was specific since there was little staining of phospho-α4-integrin on the plasma membrane of CHO-α4S988A cells (Fig. 5b). The distribution of phospho-α4-integrin in CHO-α4WT cells is consistent with α4-integrin phosphorylation acting at both the leading and trailing edges of the cells under shear flow.
Fig. 5.
Localization of α4-integrin and phosphorylated (phospho-)α4-integrin under shear flow. CHO-α4S988A cells (a–c) or CHO-α4WT (d–i) were plated on fibronectin, stimulated with shear flow for the designated times, and double stained with anti-GFP antibody (for GFP-tagged α4-integrin) and anti-phospho-α4-integrin antibody. Stained cells were imaged by confocal microscopy, and the slice closest to the coverslip is presented for each cell. Arrows on the left show the direction of the shear flow. Note that both α4-integrin and phospho-α4-integrin were localized at both the leading and trailing edges of the cells. Bars = 10 μm.
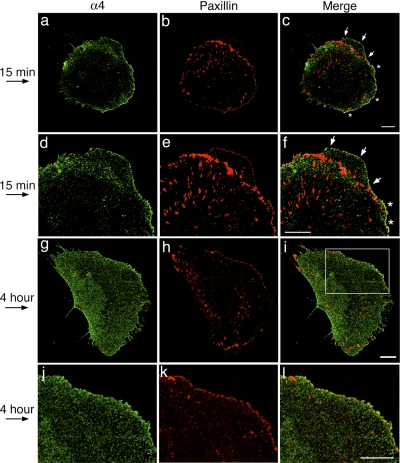
At the 15-min time point, some CHO-α4WT cells responded to the shear flow and began to protrude lamellipodia at the leading edge. As reported for CHO-α4WT cells stimulated with scratch wounding (12, 28), when the cells were plated on fibronectin and stimulated with shear flow, α4-integrin was localized in punctate structures at the leading edge of protruding lamellipodia, where most of the α4-integrin positive puncta had little overlap with paxillin-positive focal complexes and focal adhesions (Fig. 6, c and f, marked by arrows). In the areas at the leading edge flanking the protruding membrane, however, prominent colocalization of the two proteins was observed (Fig. 6, c and f, marked by asterisks). At the 4-h time point, α4-integrin and paxillin had little apparent colocalization at the leading edge (Fig. 6l). These results are consistent with α4-integrin/paxillin binding playing a negative regulatory role in the formation of lamellipodia. At both the 15-min and 4-h time points, little apparent colocalization of α4-integrin and paxillin was observed at the trailing edge, suggesting that these two proteins had little interactions in this area.
Fig. 6.
Localization of α4-integrin and paxillin under shear flow. Cells were plated on fibronectin, stimulated with shear flow for the designated times, and double stained with anti-GFP antibody (for GFP-tagged α4-integrin) and anti-paxillin antibody. Stained cells were imaged by confocal microscopy, and the slice closest to the coverslip is presented for each cell. Arrows on the left show the direction of the shear flow. In c and f, white arrows point to protruding lamellipodia. *Areas of the leading edge flanking the protruding membrane where α4-integrin and paxillin partially colocalize. The boxed area in I is enlarged in l, where there is little apparent colocalization of α4-integrin and paxillin. Bars = 10 μm.
DISCUSSION
Role of phosphorylation and paxillin binding at the α4-tail in regulating directionally persistent cell migration under shear flow.
In this study, we showed that α4β1-integrin is necessary and sufficient to promote directionally persistent cell migration in response shear flow when ectopically expressed in CHO cells. In addition to the reported role of α4β1-integrin in facilitating the formation of lamellipodia at the leading edge (12, 29), we found that α4β1-integrin also regulated trailing edge detachment/retraction. Notably, we found that disruption of serine phosphorylation alone or together with disruption of paxillin binding at the α4-tail led to the formation of long trailing tails, which is in contrast to the flattened and retracted trailing edges formed by cells expressing wild-type α4-integrin. This trailing tail phenotype is reminiscent of that caused by inhibiting RhoA and its downstream effectors (37) and by knockdown of nonmuscle myosin IIA (34). We propose that α4-integrin phosphorylation at the trailing edge transduces signals into cells via the Rho-mediated contractility pathway, which regulates trailing edge retraction and/or focal adhesion dynamics (4), and this function is likely to be independent of the binding between α4-integrin and paxillin.
In a recent model proposed by Ginsberg and colleagues (11), restriction of α4-integrin phosphorylation to the leading edge of a migrating cell limits α4-integrin-paxillin binding to the sides and trailing edge of the cell; the α4-integrin-paxillin complex inhibits Rac1 activities, thus confining lamellipodia formation to the leading edge (26). This model is based on studies of randomly migrating cells. In this study, we examined cells stimulated with shear flow and provided evidence for a more complex mechanism. Consistent with Ginsberg et al.'s model, we showed that inhibiting paxillin binding to the α4-tail enhances lamellipodia protrusion and that there is little colocalization of α4-integrin and paxillin at the edges of protruding membrane at the leading edge, whereas the two proteins colocalize in areas flanking the protruding membrane. These results support a role for paxillin binding/dissociation in regulating the protrusive activities at the leading edge. However, we also found that α4-integrin phosphorylation functions at the trailing edges of shear flow-stimulated cells. Furthermore, the double mutation, which disrupts both α4-integrin phosphorylation and paxillin binding, impaired the ability of α4β1-integrin to promote directionally persistent cell migration, whereas the single mutations had no inhibitory effects. Therefore, both α4-integrin phosphorylation and paxillin binding/dissociation contribute to stabilizing front-back polarity and directional persistence, but they are likely to act via independent pathways. Our results, nevertheless, do not rule out a possible role of α4-integrin phosphorylation in regulating paxillin binding to establish the polarity of migrating cells. Although we showed that α4-integrin phosphorylation can occur at both the leading and trailing edges, the possibility that there are subtle temporal and spatial differences in α4-integrin phosphorylation levels between the front and back of shear flow-stimulated cells still remains.
Role of α4β1-integrin in transducing signals in response to shear stress.
The physiological role of the α4β1-integrin-dependent shear flow response has been implicated in blood vessel remodeling (13). This integrin also contributes to the functions of endothelial cells during tumor angiogenesis (9) and may mediate the shear flow-induced migration of these cells. In the present study, we demonstrated that the role of α4β1-integrin in shear flow-induced cell migration is recapitulated when α4β1-integrin is ectopically expressed in CHO cells. Analysis of this model system allowed us to elucidate the mechanism by which α4β1-integrin facilitates the shear flow response. One possible mechanism is that shear flow may regulate cell migration by modulating the adhesive activities of α4β1-integrin. Shear flow has been shown to activate α4β1-integrin in leukocytes to facilitate adhesion of these cells to endothelial cells (40), which is regulated by α4-integrin- paxillin binding (1, 25). However, under shear flow, CHO cell types expressing wild-type and mutant α4-integrin did not have any significant differences in their cell adhesion strengthening to the CS-1 fragment of fibronectin, the α4β1-integrin-specific ligand, yet the cells display different shear flow-induced motile activities. Therefore, the shear flow-induced motile activities we observed are not likely due to changes of adhesion strengthening. A similar observation has also been made in primary pericytes isolated from mouse embryos. Knockout of the α4-integrin gene impaired the ability of pericytes to migrate in a directionally persistent manner under shear flow but had no significant effect on the adhesion strength of the cells (13). Therefore, in CHO cells and pericytes, the mechanism by which α4β1-integrin responds to shear force is different from that in leukocytes. α4β1-Integrin may transduce forces when a directional shear flow exerts pulling and pushing forces on the trailing and leading edges of the cell, respectively. Alternatively, α4β1-integrin may act downstream of other mechanosensors or mechanotransducers to regulate directionally persistent cell migration.
In summary, we showed that α4β1-integrin is sufficient and required for promoting directionally persistent migration of CHO cells in response to shear flow by facilitating leading edge protrusions and trailing edge detachment/retraction. Furthermore, we found a novel role of α4-integrin phosphorylation in trailing edge retraction/detachment that is likely to be independent of paxillin binding and a requirement for α4-integrin phosphorylation and paxillin binding in stabilizing the front-back polarity of shear flow-induced cells. These results provide new insight into the role of integrins in directed cell migration.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-GM-063710 and R01-DK-063096, American Heart Association (AHA) Grant-In-Aid 0755432U, NIH Ruth L. Kirschstein National Research Service Award F31-GM067581 (a fellowship provided to L. A. Rivera Rosado), and a predoctoral fellowship from the AHA (to C. S. Alves).
Supplementary Material
Acknowledgments
We thank Susan Napier and Bill Hanley for the help in setting up the shear flow assay and Lita Braiterman for time-lapse microscopy. We are grateful to Richard Hynes for providing pGH plasmids, and Mark Ginsberg for providing the anti-phospho-α4-integrin antibody. We are also grateful to Douglas Robinson and Lew Romer for suggestions and critical comments on this article.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alon R, Feigelson SW, Manevich E, Rose DM, Schmitz J, Overby DR, Winter E, Grabovsky V, Shinder V, Matthews BD, Sokolovsky-Eisenberg M, Ingber DE, Benoit M, Ginsberg MH. Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain. J Cell Biol 171: 1073–1084, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell 85: 997–1008, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Bloom L, Ingham KC, Hynes RO. Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13. Mol Biol Cell 10: 1521–1536, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133: 1403–1415, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol 4: 232–239, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science 243: 1483–1485, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson R, Tranquillo R. Optimal estimation of cell movement indices from the statistical analysis of cell tracking data. AIChE J 39: 1995–2010, 1993. [Google Scholar]
- 8.Euteneuer U, Schliwa M. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature 310: 58–61, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest 115: 1542–1551, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 19: 173–206, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol 17: 509–516, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of α4 integrin phosphorylation regulates lamellipodial stability and α4β1-dependent cell migration. J Cell Biol 162: 731–741, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grazioli A, Alves CS, Konstantopoulos K, Yang JT. Defective blood vessel development and pericyte/pvSMC distribution in alpha 4 integrin-deficient mouse embryos. Dev Biol 293: 165–177, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Liu S, Rose DM, Schlaepfer DD, McDonald H, Ginsberg MH. Phosphorylation of the integrin alpha 4 cytoplasmic domain regulates paxillin binding. J Biol Chem 276: 40903–40909, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, Konstantopoulos K. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci USA 102: 14010–14015, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu YL, Li S, Miao H, Tsou TC, del Pozo MA, Chien S. Roles of microtubule dynamics and small GTPase Rac in endothelial cell migration and lamellipodium formation under flow. J Vasc Res 39: 465–476, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hynes RO Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev Biol 202: 29–42, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Kiosses WB, Shattil SJ, Pampori N, Schwartz MA. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol 3: 316–320, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kole TP, Tseng Y, Wirtz D. Intracellular microrheology as a tool for the measurement of the local mechanical properties of live cells. Methods Cell Biol 78: 45–64, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Delivery Res 33: 141–164, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA 99: 3546–3551, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature 402: 676–681, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Manevich E, Grabovsky V, Feigelson SW, Alon R. Talin 1 and paxillin facilitate distinct steps in rapid VLA-4-mediated adhesion strengthening to vascular cell adhesion molecule 1. J Biol Chem 282: 25338–25348, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol 7: 343–352, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nishizaka T, Shi Q, Sheetz MP. Position-dependent linkages of fibronectin- integrin-cytoskeleton. Proc Natl Acad Sci USA 97: 692–697, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinco KA, He W, Yang JT. alpha4beta1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol Biol Cell 13: 3203–3217, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of α4β1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol 157: 873–882, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91: 769–775, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Tseng Y, Kole TP, Wirtz D. Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophys J 83: 3162–3176, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 21: 6791–6800, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol 176: 573–580, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells–over and over and over again. Nat Cell Biol 4: E97–E100, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol 161: 429–439, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol 154: 147–160, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 121: 549–560, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Zaidel-Bar R, Kam Z, Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J Cell Sci 118: 3997–4007, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Zwartz GJ, Chigaev A, Dwyer DC, Foutz TD, Edwards BS, Sklar LA. Real-time analysis of very late antigen-4 affinity modulation by shear. J Biol Chem 279: 38277–38286, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.