Abstract
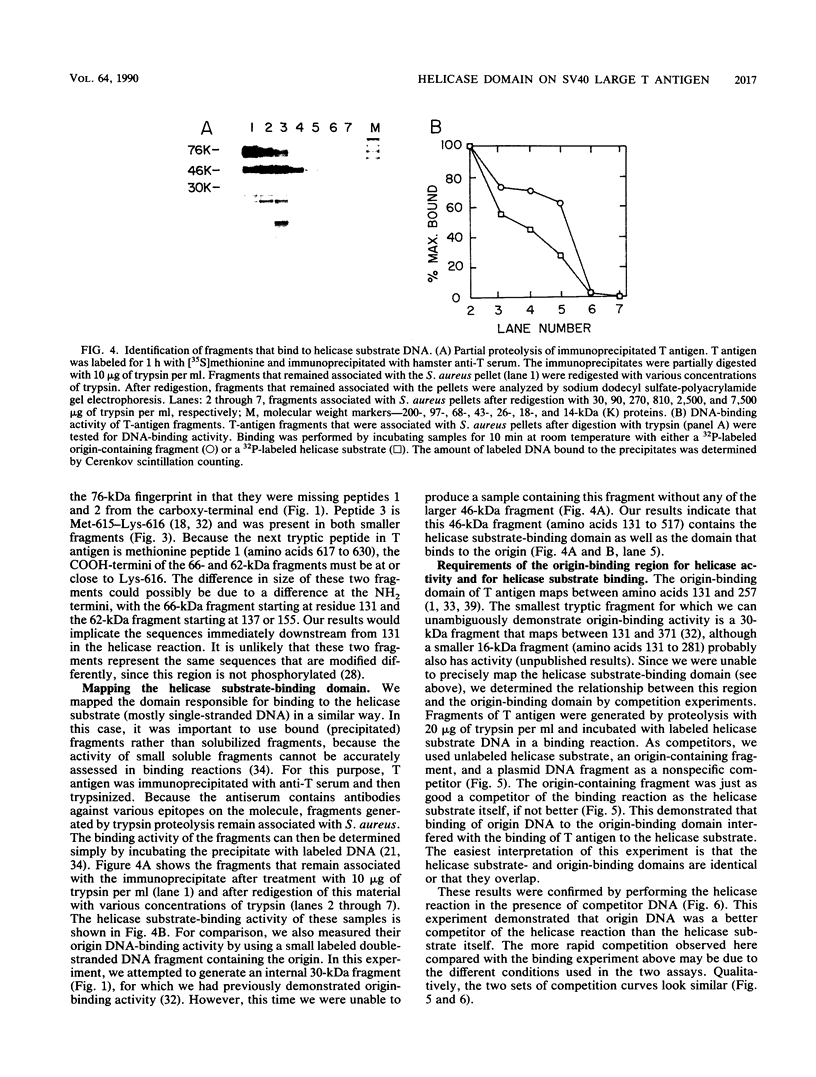
We generated fragments of simian virus 40 large tumor antigen (T antigen) by tryptic digestion and assayed them for helicase activity and helicase substrate (mostly single-stranded DNA)-binding activity in order to map the domain sites on the protein. The N-terminal 130 amino acids were not required for either activity, since a 76-kilodalton (kDa) fragment (amino acids 131 to 708) was just as active as intact T antigen. To map the helicase domain further, smaller tryptic fragments were generated. A 66-kDa fragment (131 to about 616) retained some activity, whereas a slightly smaller 62-kDa fragment (137 or 155 to 616) had none. This suggests that the minimal helicase domain maps from residue 131 to approximately residue 616. To map the helicase substrate-binding domain, we tested various fragments in a substrate-binding assay. The smallest fragment for which we could clearly demonstrate activity was a 46-kDa fragment (131 to 517). To determine the relationship between the helicase substrate domain and the origin-binding domain (131 to 257, minimal core region; 131 to 371, optimal region), we performed binding experiments with competitor DNAs present. We found that origin-containing double-stranded DNA was an excellent competitor of the binding of the helicase substrate to T antigen, suggesting that the two domains overlap. Therefore, full helicase activity requires at least a partial origin-binding domain as well as an active ATPase domain. Additionally, we found that the helicase substrate was a poor competitor of origin-binding activity, indicating that T antigen has a much higher affinity to origin sequences than to the helicase substrate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur A. K., Höss A., Fanning E. Expression of simian virus 40 T antigen in Escherichia coli: localization of T-antigen origin DNA-binding domain to within 129 amino acids. J Virol. 1988 Jun;62(6):1999–2006. doi: 10.1128/jvi.62.6.1999-2006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auborn K. J., Markowitz R. B., Wang E., Yu Y. T., Prives C. Simian virus 40 (SV40) T antigen binds specifically to double-stranded DNA but not to single-stranded DNA or DNA/RNA hybrids containing the SV40 regulatory sequences. J Virol. 1988 Jun;62(6):2204–2208. doi: 10.1128/jvi.62.6.2204-2208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. K., Smith T. F., Lathrop R. H., Livingston D. M., Webster T. A. Consensus topography in the ATP binding site of the simian virus 40 and polyomavirus large tumor antigens. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4026–4030. doi: 10.1073/pnas.84.12.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Stacy T. P. Biological properties of simian virus 40 host range mutants lacking the COOH-terminus of large T antigen. Virology. 1987 Nov;161(1):170–180. doi: 10.1016/0042-6822(87)90183-8. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Tornow J., Clark R., Tjian R. Properties of the simian virus 40 (SV40) large T antigens encoded by SV40 mutants with deletions in gene A. J Virol. 1986 Feb;57(2):539–546. doi: 10.1128/jvi.57.2.539-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Borowiec J. A., Ishimi Y., Deb S., Tegtmeyer P., Hurwitz J. Simian virus 40 large tumor antigen requires three core replication origin domains for DNA unwinding and replication in vitro. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8267–8271. doi: 10.1073/pnas.84.23.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Bullock P., Murakami Y., Wobbe C. R., Weissbach L., Hurwitz J. Simian virus 40 (SV40) DNA replication: SV40 large T antigen unwinds DNA containing the SV40 origin of replication. Proc Natl Acad Sci U S A. 1987 Jan;84(1):16–20. doi: 10.1073/pnas.84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M., Dean F. B., Bullock P., Echols H., Hurwitz J. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science. 1987 Nov 13;238(4829):964–967. doi: 10.1126/science.2823389. [DOI] [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz G. S., Dean F. B., Hurwitz J., Matson S. W. The unwinding of duplex regions in DNA by the simian virus 40 large tumor antigen-associated DNA helicase activity. J Biol Chem. 1988 Jan 5;263(1):383–392. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Brady J., Pipas J. M., Spence S. L., Sadofsky M., Khoury G. Carboxyl-terminal mutants of the large tumor antigen of simian virus 40: a role for the early protein late in the lytic cycle. Proc Natl Acad Sci U S A. 1988 Jan;85(2):354–358. doi: 10.1073/pnas.85.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky K., Chandrasekaran K., Mora P. T., Simmons D. T. Immunoaffinity chromatography of a cellular tumor antigen from mouse neuroblastoma cells. Int J Cancer. 1983 Nov 15;32(5):597–602. doi: 10.1002/ijc.2910320513. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke H. K., Hunter T., Walter G. Structural relationship between the 100,000- and 17,000- molecular-weight T antigens of simian virus 40 (SV40) as deduced by comparison with the SV40-specific proteins coded by the nondefective adenovirus type 2-SV40 hybrid viruses. J Virol. 1979 Jan;29(1):390–394. doi: 10.1128/jvi.29.1.390-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber G., Parsons R., Tegtmeyer P. The zinc finger region of simian virus 40 large T antigen. J Virol. 1989 Jan;63(1):94–100. doi: 10.1128/jvi.63.1.94-100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Genetic and biochemical analysis of transformation-competent, replication-defective simian virus 40 large T antigen mutants. J Virol. 1985 Jan;53(1):120–127. doi: 10.1128/jvi.53.1.120-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J Virol. 1985 May;54(2):569–575. doi: 10.1128/jvi.54.2.569-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. M. Simian virus 40 (SV40) large tumor antigen causes stepwise changes in SV40 origin structure during initiation of DNA replication. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3939–3943. doi: 10.1073/pnas.86.11.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Wessel R., Stahl H. Sequence independent duplex DNA opening reaction catalysed by SV40 large tumor antigen. Nucleic Acids Res. 1989 Jan 11;17(1):93–106. doi: 10.1093/nar/17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieg F. I., Simmons D. T. Characterization of the in vitro interaction between SV40 T antigen and p53: mapping the p53 binding site. Virology. 1988 May;164(1):132–140. doi: 10.1016/0042-6822(88)90628-9. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Chou W., Rodgers K. Phosphorylation downregulates the DNA-binding activity of simian virus 40 T antigen. J Virol. 1986 Dec;60(3):888–894. doi: 10.1128/jvi.60.3.888-894.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. DNA-binding region of the simian virus 40 tumor antigen. J Virol. 1986 Mar;57(3):776–785. doi: 10.1128/jvi.57.3.776-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Geometry of the simian virus 40 large tumor antigen-DNA complex as probed by protease digestion. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2086–2090. doi: 10.1073/pnas.85.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Loeber G., Tegtmeyer P. Four major sequence elements of simian virus 40 large T antigen coordinate its specific and nonspecific DNA binding. J Virol. 1990 May;64(5):1973–1983. doi: 10.1128/jvi.64.5.1973-1983.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Martin M. A., Mora P. T., Chang C. Relationship among Tau antigens isolated from various lines of simian virus 40-transformed cells. J Virol. 1980 Jun;34(3):650–657. doi: 10.1128/jvi.34.3.650-657.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Stepwise phosphorylation of the NH2-terminal region of the simian virus 40 large T antigen. J Biol Chem. 1984 Jul 10;259(13):8633–8640. [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Argani P., Mohr I. J., Gluzman Y. Studies on the origin-specific DNA-binding domain of simian virus 40 large T antigen. J Virol. 1987 Oct;61(10):3326–3330. doi: 10.1128/jvi.61.10.3326-3330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Pipas J. M., Kierstead T., Cole C. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3' end of SV40 gene A. Virology. 1988 Jan;162(1):76–89. doi: 10.1016/0042-6822(88)90396-0. [DOI] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Dröge P., Stahl H. Monoclonal antibodies as probes for a function of large T antigen during the elongation process of simian virus 40 DNA replication. J Virol. 1987 Feb;61(2):411–418. doi: 10.1128/jvi.61.2.411-418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiekowski M., Schwarz M. W., Stahl H. Simian virus 40 large T antigen DNA helicase. Characterization of the ATPase-dependent DNA unwinding activity and its substrate requirements. J Biol Chem. 1988 Jan 5;263(1):436–442. [PubMed] [Google Scholar]
- Wobbe C. R., Weissbach L., Borowiec J. A., Dean F. B., Murakami Y., Bullock P., Hurwitz J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1834–1838. doi: 10.1073/pnas.84.7.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]