Abstract
Acidosis (low pH) is the oldest putative agent of muscular fatigue, but the molecular mechanism underlying its depressive effect on muscular performance remains unresolved. Therefore, the effect of low pH on the molecular mechanics and kinetics of chicken skeletal muscle myosin was studied using in vitro motility (IVM) and single molecule laser trap assays. Decreasing pH from 7.4 to 6.4 at saturating ATP slowed actin filament velocity (Vactin) in the IVM by 36%. Single molecule experiments, at 1 μM ATP, decreased the average unitary step size of myosin (d) from 10 ± 2 nm (pH 7.4) to 2 ± 1 nm (pH 6.4). Individual binding events at low pH were consistent with the presence of a population of both productive (average d = 10 nm) and nonproductive (average d = 0 nm) actomyosin interactions. Raising the ATP concentration from 1 μM to 1 mM at pH 6.4 restored d (9 ± 3 nm), suggesting that the lifetime of the nonproductive interactions is solely dependent on the [ATP]. Vactin, however, was not restored by raising the [ATP] (1–10 mM) in the IVM assay, suggesting that low pH also prolongs actin strong binding (ton). Measurement of ton as a function of the [ATP] in the single molecule assay suggested that acidosis prolongs ton by slowing the rate of ADP release. Thus, in a detachment limited model of motility (i.e., Vactin ∼ d/ton), a slowed rate of ADP release and the presence of nonproductive actomyosin interactions could account for the acidosis-induced decrease in Vactin, suggesting a molecular explanation for this component of muscular fatigue.
Keywords: acidosis, fatigue, velocity, laser trap
after several minutes of intense contractile activity, the ability of skeletal muscle to generate force and power declines precipitously (45). Despite intensive investigation for more than 100 years, the etiology of this phenomenon, known as fatigue, remains elusive. With the increased rate of ATP utilization during such intense activity, the onset of fatigue is believed to result, in part, from the rapid accumulation of ATP hydrolysis products (ADP, Pi, and H+) (8). Evidence from skinned single muscle fibers demonstrates that elevated Pi significantly reduces force (28). Recent evidence suggests that acidosis (i.e., low pH) has only a small effect on force production (31) but significantly decreases both the shortening velocity (Vmax) and power-generating capacity of muscle (20). The reduction in maximum unloaded Vmax due to intracellular acidosis in muscle fibers has also been confirmed at the molecular level in the in vitro motility assay, where actin filament movement generated by isolated myosin was slowed at low pH (22). The mechanism of this depressed velocity may be due to acidosis slowing the ATPase rate of myosin, which has been demonstrated in skinned single muscle fibers (5). Whereas this finding remains equivocal (33), it is likely that the acidosis-induced reduction in Vmax results from an effect on one or more steps of the mechanochemical cycle of actomyosin. In fact, based on findings from solution (2, 4) and skinned single muscle fiber studies, Kentish (19) postulated that ATP hydrolysis and ADP release may be influenced by acidosis. To address this at the single molecule level, the three-bead laser trap assay (7) affords the ability to directly determine the molecular mechanism by which acidosis affects the mechanics and/or the kinetics of an individual cross-bridge.
In the present study, actin filament velocity (Vactin) was slowed at low pH. Based on a detachment limited model (15), Vactin is dependent on the step size of myosin (d) and the duration of the strongly bound state following the powerstroke (ton), i.e., Vactin ∼ d/ton. By characterizing the molecular mechanics of skeletal muscle myosin in the laser trap assay, lowering pH from 7.4 to 6.4 does not appear to affect myosin's inherent step size. However, low pH does alter the kinetics of the cross-bridge cycle by prolonging ton. This prolongation of ton appears to result from a threefold slower rate of ADP release from myosin. Additionally, low pH may increase the probability of a rigor-like, nonproductive interaction between actin and myosin that may act as an internal load to motion generation in an ATP-dependent manner, serving to further slow Vactin at low pH.
METHODS
Proteins
The myosin was prepared as previously described (43) from chicken pectoralis muscle, which expresses predominantly a fast myosin heavy chain isoform (11). Actin was isolated from the acetone powder of the pectoralis muscle preparation (30). Isolated actin was fluorescently labeled with an overnight incubation in 50% tetramethylrhodamine isothiocyanate-labeled phalloidin (Sigma-Aldrich) and 50% biotin-labeled phallodin. The myosin was further purified to remove inactive myosin molecules before each experiment by subjecting a small aliquot (200 μg/ml) to centrifugation (95 K) in the presence of an equimolar amount of filamentous actin in the presence of ATP (1 mM) in myosin buffer (see Solutions). Glass microspheres (1.0 μm silica, Duke Scientific) used in the laser trap assay were incubated overnight with N-ethylmaleimide (NEM)-modified skeletal muscle myosin, prepared as previously described (43), and with neutravidin (NAV).
Solutions
Isolated myosin was diluted in myosin buffer (0.3 M KCl, 25 mM imidazole, 1 mM EGTA, 4 mM MgCl2, and 10 mM dithiothreitol, pH 7.4). The final laser trap buffer contained 25 mM KCl, 25 mM imidazole, 1 mM EGTA, 4 mM MgCl2, 10 mM dithiothreitol, and oxygen scavengers (0.1 mg/ml glucose oxidase, 0.018 mg/ml catalase, and 2.3 mg/ml glucose) and was adjusted to either pH 7.4 or 6.4 (with the addition of HCl). The final motility buffer had the same composition as the final trapping buffer but also contained methylcellulose to help keep the actin filaments in contact with the myosin-coated surface. The MgATP concentration in the trapping and motility buffers was varied from 0.5 μM to 10 mM. To maintain a constant ionic strength and a 3 mM free Mg2+ concentration, the KCl and MgCl2 concentrations were adjusted according to the constants contained within the MaxChelator (Version 2.50) software program (32).
In Vitro Motility and Single Molecule Laser Trap Assays
The in vitro motility assay was performed at 30°C, and data were analyzed as previously described (43). The three-bead laser trap assay was performed as described by Guilford et al. (12) using the instrumentation detailed in Kad et al. (17). Briefly, manipulating the microscope stage allowed two 1-μm silica beads to be captured in separate laser traps, and a single actin filament was then attached to the NEM-myosin/NAV-coated beads (the combination of NEM-myosin/NAV coating allowed for a strong bead-actin-bead assembly at mM ATP concentrations). Pretension was then imposed on the bead-actin-bead assembly (∼4 pN), and the stiffness (∼0.02 pN/nm) of the combined assembly was determined using the equipartition method (12). The bead-actin-bead assembly was then lowered onto a third microsphere (3-μm diameter) sparsely coated with myosin. The laser trap experiments were performed at room temperature (20°C).
Analysis of Single Molecule Data Records
The raw displacement of the actin-attached bead was obtained from the output of a quadrant photodiode in the laser trap assay and was acquired and processed as previously described (12). Each recording consisted of roughly 2 min of data, containing hundreds of events. Myosin's unitary step size (d) and duration of strong actin binding (ton) were determined using mean-variance analysis as previously described (12). A Student's t-test for independent samples was used to determine pH-induced differences in d with significance set at P < 0.05. An ANOVA was used to analyze ton values as a function of [ATP] in the single molecule data with a Tukey-HSD post hoc test used to locate significant differences (P < 0.05).
RESULTS
Effect of Low pH on Vactin
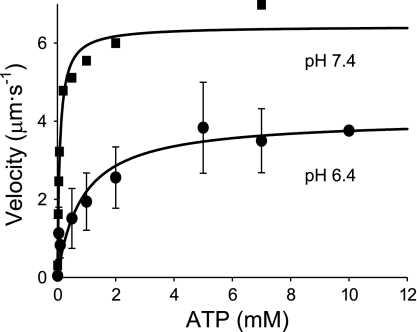
Decreasing the pH from 7.4 to 6.4 in the motility assay severely depressed the ability of myosin to translocate actin at all ATP concentrations between 0.001 and 10 mM (Fig. 1). This depressive effect of low pH was readily reversible with Vactin fully restored following the reinfusion of a pH 7.4 buffer (data not shown). When fit to a Michaelis-Menten relationship, the extrapolated maximum Vactin at pH 6.4 (4.1 μm/s) was reduced 36% compared with that at pH 7.4 (6.4 μm/s), as previously observed (13). In addition, low pH induced a more than 10-fold increase in the Km, from 83 μM at pH 7.4 to 850 μM at pH 6.4 (Fig. 1), indicating that the depressive effect of low pH on Vactin is more pronounced at subsaturating ATP concentrations.
Fig. 1.
Actin filament velocities (Vactin) versus ATP. Vactin from in vitro motility assays as a function of [ATP]. Squares and circles represent data for pH 7.4 and pH 6.4, respectively. Values displayed are means ± SD at pH 6.4 from several myosin preparations. At pH 7.4, each point represents the average of 20 filament velocities from one myosin preparation. Each data set was fit to the Michaelis-Menten equation yielding values for maximal velocity (Vmax, 6.4 and 4.1 μm/s at pH 7.4 and 6.4, respectively) and Km (83 and 850 μM at pH 7.4 and 6.4, respectively). The goodness-of-fit for each data set exceeded an R2 value of 0.85.
Effect of Low pH on Myosin Molecular Mechanics
To determine the underlying molecular mechanisms of the depressive effects of pH on Vactin, we characterized the mechanics (d) and kinetics (ton) of single skeletal muscle myosin molecules in the laser trap assay. These experiments were initially performed at low ATP concentrations (≤10 μM) to enhance the detection of myosin binding to actin by prolonging the attached lifetime following the powerstroke.
Step size.
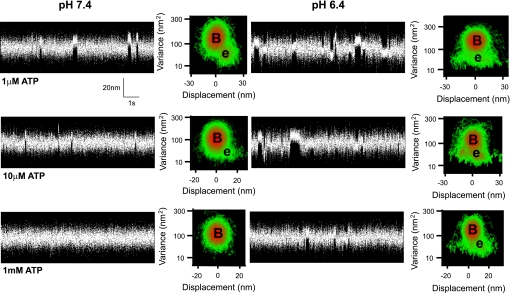
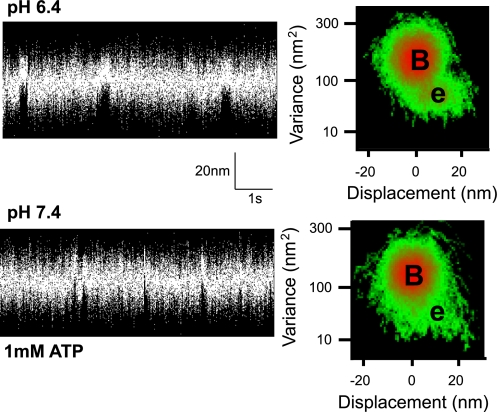
At pH 7.4 and low ATP (≤10 μM), binding events (Fig. 2) were characterized by an average step size of 10 nm (Table 1), as determined by mean-variance (MV) analysis, consistent with previous estimates from whole chicken skeletal muscle myosin (12). In contrast, at pH 6.4 and low ATP, binding events were broadly distributed and centered near 0 nm, which drastically altered the profile of the MV histogram (Fig. 2). The average d, at pH 6.4 and 10 μM ATP, was reduced by 40% to 6 ± 5 nm and by 80% at 0.5 and 1 μM ATP to 2 ± 1 nm (Table 1).
Fig. 2.
Sample single molecule data records. Short sections (∼10 s) of 1- to 3-min data records that typically contained 100–200 events are displayed. Data were obtained using a three bead laser-trap assay (12). The entire 1- to 3-min data were used to construct the adjacent three-dimensional mean-variance (MV) histogram (see methods). B, estimated peak of the baseline population; e, peak of the event population. Separate experiments were performed at pH 7.4 and pH 6.4 at each ATP concentration indicated. No significant event population was discernable at 1 mM ATP and pH 7.4, likely due the lifetime of strong attachment being more rapid than the resolution of the instrumentation (see discussion).
Table 1.
Mean single molecule parameters
| [ATP] |
d, nm |
ton, ms
|
||
|---|---|---|---|---|
| pH 7.4 | pH 6.4 | pH 7.4 | pH 6.4 | |
| 0.5 μM | N/A | 2±2 | N/A | 200±114 |
| 1 μM | 10±2 | 2±1* | 178±67 | 119±22 |
| 10 μM | 11±2 | 6±5 | 21±31 | 70±31* |
| 1 mM | 10† | 9±3 | 10† | 31±9 |
The values displayed, obtained by mean variance analysis, are the means ± SD from 4 to 10 single molecule data records, each 1–3 min in length, containing hundreds of events. d, unitary step size of myosin; ton, strong binding variable; N/A, not applicable.
Extrapolated or estimated value from Baker et al. (3).
Significantly different (P < 0.05) from the value at the corresponding [ATP] at pH 7.4. The data were analyzed using a two-way ANOVA (pH × ATP) with a Tukey-HSD post hoc test to locate specific differences. A subset of data using smooth muscle myosin at 1 mM ATP showed that d was unaffected by low pH (9 nm at pH 7.4 vs. 9 nm at pH 6.4), whereas ton was threefold longer (27 ms at pH 7.4 vs. 100 ms at pH 6.4), consistent with the observations in skeletal myosin.
To determine whether the apparent reduction in the myosin step size at low pH was ATP dependent, experiments were also performed at 1 mM ATP. At pH 6.4, binding events were no longer broadly distributed, with d being restored at 1 mM ATP to 9 ± 3 nm, statistically equivalent to that at pH 7.4 at both 1 and 10 μM ATP (Table 1). Although a comparison to d at pH 7.4 and 1 mM ATP would have been more appropriate, skeletal muscle myosin binding events under these conditions are extremely short lived (<10 ms; Ref. 3) and difficult to resolve in the laser trap assay (see Fig. 2, left). However, based on the two ATP concentrations (i.e., 1 and 10 μM) used in this study and other studies from our laboratory, using slower muscle myosin isoforms, there is no apparent effect of ATP concentration on inherent step size of myosin at pH 7.4 (3, 23, 29). Therefore, the dependence of d on ATP concentration at low pH suggests that lowering pH can affect processes that result in a productive powerstroke (see discussion).
Attached lifetime, ton.
Although the apparent reduction in d at ≤10 μM ATP, pH 6.4, may contribute to the slowed Vactin at these ATP concentrations, the inability of Vactin to recover at elevated ATP (see Fig. 1) even though d was restored to normal at 1 mM ATP, suggests that one or more biochemical transitions in the actomyosin ATPase cycle might be slowed by low pH. This is further emphasized by the large shift in Km (Fig. 1). Therefore, we examined the effects of low pH on myosin's attached lifetime (ton) since ton is determined by the time waiting for ADP release (1/k−ADP) and subsequent ATP binding [1/(k+ATP·[ATP])] to the active site {i.e., ton = (1/k−ADP)+[1/(k+ATP·[ATP])] (29), where k−ADP is the rate of ADP release and k+ATP is the second-order ATP binding rate}. By measuring ton as a function of ATP concentration, we can determine whether k−ADP and/or k+ATP are altered by lowering pH.
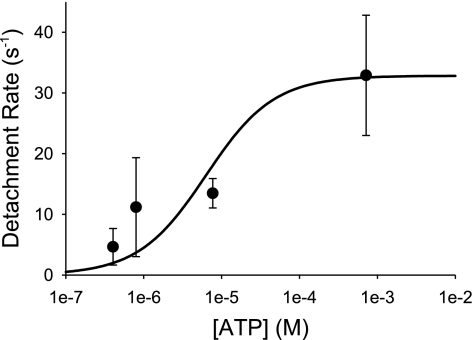
As expected, ton was ATP dependent at both high and low pH levels (Table 1). At 1 μM ATP, low pH did not significantly affect ton but at 10 μM ATP, ton was prolonged threefold at pH 6.4 (Table 1). Additionally at 1 mM ATP, under acidic conditions the observed ton value of 31 ± 9 ms (Table 1) is once again threefold longer than an extrapolated estimate of ton at saturating ATP and pH 7.4, based on our previous work (3). A comparison to an extrapolated estimate of ton was necessary since binding events at 1 mM ATP and pH 7.4 are typically below our detection limit (Fig. 2, bottom left). We fit the myosin detachment rate [1/ton (see above)] at pH 6.4 as a function of ATP concentration (Fig. 3) to the following equation to estimate k−ADP and k+ATP:
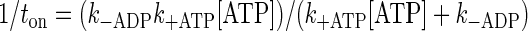 |
Fig. 3.
Detachment rate as a function of [ATP] at pH 6.4. Detachment rates on the y-axis were obtained by taking the reciprocal of the strongly bound lifetime (1/ton). Data points are means ± SD. The data were fit to a two-parameter hyperbola {detachment rate = (k−ADP·k+ATP·[ATP])/[(k+ATP·[ATP]] + k−ADP} using SigmaPlot 8.0 and had an R2 value of 0.75. The fit yielded mean estimates ± SE for k−ADP (33 ± 7.s−1) and k+ATP 5.2 ± 4.6 × 106 .M−1·s−1.
Based on the fit, k+ATP was estimated to be 5.2 ± 4.6 × 106 M−1·s−1(means ± SE), whereas k−ADP was 33 ± 7.s−1 (means ± SE).
DISCUSSION
Mechanism for Decreased Vactin at Low pH
The pH dependence of Vactin observed in the present investigation using chicken skeletal myosin confirms our previous experiments examining Vactin over a range of pH values (13) with similar results observed for mammalian skeletal (22) and cardiac muscle myosin (46). At the single molecule level, the observed decrease in Vactin with low pH could be due either to a decrease in d and/or an increase in ton (i.e., Vactin∼ d/ton) (15). In addition, at the ensemble level we have shown that Vactin is also governed by the mechanical interactions between myosin molecules that simultaneously interact with the actin filament (43). Here we propose that lowering pH at physiological ATP does not alter skeletal myosin's inherent motion-generating capacity, d, but rather the reduced Vactin results from slowed kinetics associated with ton and that the emergence of a population of nonproductive actomyosin interactions may act as an internal load in the motility assay.
Low pH Increases Nonproductive Actomyosin Interactions
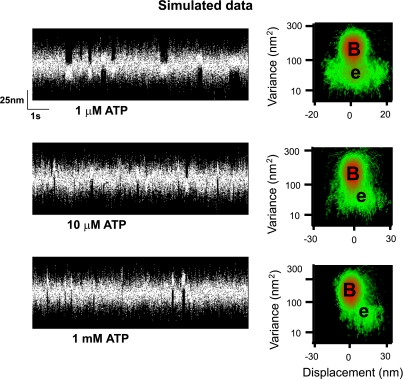
At limiting concentrations of ATP (<10 μM), the step size decreased as much as 80% at pH 6.4 (Table 1). The step size of myosin is due to a rotation of the light chain binding domain, acting as a lever arm to amplify small conformational changes in the myosin head (34, 44). Therefore, low pH could induce a structural alteration in myosin to reduce its lever arm rotation (21) and/or stiffness (37) to account for the diminished step size. We do not favor this explanation and propose that the inherent step size of myosin is unchanged by low pH and that the presence of a broadly distributed population of binding events centered at 0 nm displacement biases the estimate of d to lower values. In support of this, at pH 7.4 and 1 μM ATP, the step size for a given myosin molecule is clearly defined by a distinct 10-nm population (see Fig. 2). In contrast at pH 6.4, the event population becomes broadly distributed about a mean of 2 nm. Based on a model simulation (see Fig. 4 for details), the apparently shorter step size at pH 6.4 could arise from myosin generating a normal 10-nm displacement but more often binds to actin without generating a powerstroke. This nonproductive interaction would randomly capture the actin filament during its Brownian excursion in the laser trap, resulting in a broadly distributed event population near 0 nm (40). Additionally, these nonproductive binding events are seemingly ATP dependent given that at pH 6.4 and 1 mM ATP, step sizes are no longer broadly distributed and are restored to the well-defined 10-nm population.
Fig. 4.
Simulated single molecule data. Single molecule data were simulated with two kinds of binding events: one that binds actin productively and generates a full displacement (10 nm) and a second, rigor-like population, that binds actin but generates no net displacement (0 nm), having a very broad displacement distribution. The simulations were performed using custom software previously described (12) with the ratio of nonproductive to productive events set at 6 to 1. In the simulations the ADP release rate was set to 33.s−1 (Fig. 3), whereas the rigor state lifetime was varied to simulate increasing ATP concentrations based on a second-order ATP-dependent dissociation constant of 5 × 106 .M−1·s−1 (Fig. 3). The lifetime of the productive binding events was the sum of the ADP and rigor state lifetimes, whereas the nonproductive events were determined by only the rigor lifetime. At 1 μM ATP, the event population is broadly distributed (see MV histogram) giving a value for d (2.5 nm) equivalent to that observed at pH 6.4 (and 1 μM ATP) for real data (see Fig. 2). However, with increasing ATP concentrations, the lifetime of the nonproductive events become shorter and barely evident at 10 μM ATP. The step size for the 10 μM ATP condition is 6 nm, similar to the real data (see Table 1). At 1 mM ATP, the nonproductive events are below the detection limits of the MV analysis so that only the productive, 10-nm population remain, and thus restore the step size back to its 10-nm normal value.
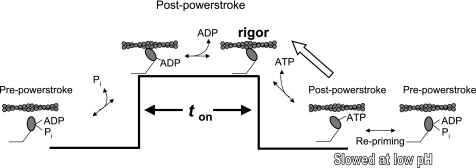
What is the origin of these nonproductive actomyosin interactions and why should they be ATP dependent? The cross-bridge cycle is illustrated in Fig. 5 in which myosin is either weakly or strongly bound to actin depending on the nucleotide present in the active site. Following the powerstroke, myosin remains strongly bound to actin (i.e., ton) until ADP is released and subsequent binding of ATP to the rigor state. Upon ATP-induced detachment, myosin-ATP may remain in a short-lived postpowerstroke state (42) before transitioning to a myosin-ATP state that is in rapid equilibrium between pre- and postpowerstroke states, as indicated by the position of the lever arm in crystallographic data (35). Upon hydrolysis, the lever arm is reprimed, completing the cross-bridge cycle (39). Given that low pH may slow hydrolysis, there is a finite probability that the postpowerstroke myosin-ATP state might exist and that rebinding of this state to actin would induce ATP release from myosin, which is supported by biochemical data (38). This scenario would effectively return myosin to the strongly bound, nucleotide-free rigor state. In the laser trap, this process would be mechanically expressed as a binding event that merely captures the actin filament during its Brownian motion and thus gives rise to the broad distribution of events as shown previously using slowly hydrolyzable analogs of ATP (39).
Fig. 5.
A model of the cross-bridge cycle at low pH. In a normal cross-bridge cycle, ATP binding causes dissociation from actin after which myosin goes through a series of transitions leading to the hydrolysis of ATP and repriming of the lever arm. Upon rebinding to actin, myosin releases Pi and generates a lever arm rotation as it goes from a pre- to postpowerstroke state. Myosin then releases ADP while attached to actin and remains bound until ATP again induces the release from actin. At low pH, repriming may be slowed, thus favoring the myosin-ATP state (38). Myosin will then rebind to actin in the myosin-ATP state, governed by the reverse rate constant, allowing actin to induce the release of ATP from myosin reforming the rigor state. This nonproductive binding will lead to a broadly distributed event population around 0 nm due to Brownian capture.
The fact that a broad event population is seen at low ATP (≤10 μM) and not at 1 mM ATP offers additional support that these binding events involve rigor-like actomyosin complexes that are sensitive to ATP. Based on a second-order ATP-binding rate of 5 × 106 M−1·s−1, at 1 mM ATP the nonproductive events would be extremely short-lived (<1 ms) and therefore not detected in the laser trap. In fact, additional simulations (as described above and presented in Fig. 4) demonstrate that the contribution of the broadly distributed nonproductive events to the overall event population observed at 1 μM ATP is reduced as the ATP concentration is increased to 1 mM. As the duration of nonproductive events is decreased with increasing ATP concentrations, the productive events once again predominate, restoring the step size to its normal 10-nm value. Therefore, we propose that lowering pH does not directly affect the inherent motion generation of myosin but rather its kinetics so that slowing of the transition out of the ATP-bound postpowerstroke state or hydrolysis itself (Fig. 5) would thus favor myosin rebinding to actin in a nonmotion generating strongly bound state.
The presence of nonproductive actomyosin interactions could serve as an internal load to slow Vactin at all ATP concentrations studied, even physiological ATP concentrations. We have shown previously in the motility assay that chemically modified myosins that trap myosin in a weak- (pPDM-modified) or strong-binding (NEM-modified) state impede the motion generated by normally cycling myosins (43). With the duration of these putative nonproductive binding events at pH 6.4 being sensitive to ATP, the effective load presented by these events would be greater at lower ATP concentrations. This internal load might explain the rightward shift in the Vactin/[ATP] relationship and therefore the apparent change in Km, supporting the hypothesis that acidosis slows Vmax in muscle fibers by imposing an increased resistive drag (36).
Low pH Prolongs ton
At 1 mM ATP, which is near physiological (18), Vactin at pH 6.4 was reduced 65% compared with that at pH 7.4 (Fig. 1). Although the resistive load created by nonproductive binding events may contribute to this slowing, the prolonged ton at 1 mM ATP is the most likely determinant of Vactin. To demonstrate this directly, we took advantage of the slower kinetics associated with smooth muscle myosin (23) so that the effect of low pH on ton at 1 mM ATP could be determined without relying on an assumed value of ton for skeletal myosin at pH 7.4 (3). At 1 mM ATP and pH 6.4, smooth muscle binding events were characterized by a step size of 9 nm, as at pH 7.4, but as predicted for skeletal myosin, ton was approximately threefold longer than at pH 7.4 (Fig. 6), providing direct support that low pH does prolong ton at normal physiological ATP concentrations.
Fig. 6.
Sample single molecule records for smooth muscle myosin. Displacement records and MV histograms from smooth muscle myosin at 1 mM ATP at pH 7.4 and 6.4. Chicken gizzard whole smooth muscle myosin was used under conditions identical to those for skeletal muscle myosin (see methods). One 90-s trace was used to determine d and ton at low pH, while at pH 7.4 d and ton were determined based on five 90- to 120-s data records. The mean d was unaffected by low pH (9 nm at pH 7.4 vs. 9 nm at pH 6.4), whereas the mean ton was threefold longer (27 ms at pH 7.4 vs. 100 ms at pH 6.4), consistent with the observations in skeletal myosin.
Based on the ATP dependence of ton at pH 6.4 (Fig. 3), low pH has little or no effect on the rate of ATP binding to myosin since the estimated k+ATP is within the range of values reported for solution (24) as well as laser trap studies (3). This is further confirmed by the observation that ton in the laser trap assay is unaffected at low ATP (1 μM) where the lifetime is dominated by rigor. However, low pH does appear to slow the rate of ADP release from myosin, since k−ADP is at least threefold slower than our previous estimate at pH 7.4 [100.s−1 (3)] providing support for the hypothesis that proton exchange occurs with the ADP-release step (19). In a detachment limited model of Vactin, this slowed ADP release rate would significantly contribute to the low pH-induced depression of Vactin in the motility assay (Fig. 1). The increased ADP lifetime would increase ton and thus might be expected to increase maximal isometric force, in contrast to evidence from single muscle fibers (5, 31). However, this could be reconciled if acidosis also slows the overall ATPase rate of myosin (5) to a greater extent than the attached lifetime. This scenario would effectively reduce the duty ratio of myosin, resulting in a lower average force per cross-bridge.
It is unclear how low pH affects the kinetics of the cross-bridge cycle at a structural level. However, one or more amino acids within the active site that are crucial to nucleotide-dependent transitions in the cross-bridge cycle may be protonated at low pH. This might alter the ability of myosin to cleave the gamma phosphate of ATP and increase its affinity for ADP both of which are suggested by the data presented. Histidine residues could play a role in these effects because they have a pKa (6.0) closest to the range of pH values used in the present investigation. Interestingly, the depressive effects of low pH have been shown to be dependent on the isoform of myosin heavy chain (MHC) expressed in a muscle fiber (26), thus a comparison of the sequences of the MHC could provide information regarding the residues crucial to low pH-induced decreases in Vactin.
Implications for Fatigue
Intracellular acidosis has been a primary suspect in muscular fatigue and reduced muscle force generation since the nineteenth century (10). Edman and Mattiazzi (6) were among the first to attribute the decline in Vmax to increased myoplasmic H+ in isolated muscle preparations. Whereas the depressive effect of acidosis on maximal isometric force appears to be minimal near physiological temperatures (20, 31), the effect on unloaded shortening appears to be temperature independent over the range between 15 and 30°C (20). The large acidosis-induced decrease in velocity, observed in single fibers, was also evident under loaded shortening and suggests that acidosis can reduce the peak power-generating capacity of muscle (9, 20).
The motility assay and single molecule experiments can provide insight into the molecular mechanism underlying pH-dependent muscular fatigue. Even at or slightly below normal physiologically ATP concentrations (18), the 10-fold increase in Km for ATP caused by low pH (Fig. 1) could lead to a significant reduction in Vmax in fibers. The reduction in ATP may be even more important than previously thought because there is evidence that reductions in intracellular ATP can be as great as 80% following short bouts of intense exercise (18). This effect is fiber-type dependent with a greater reduction in intracellular ATP in fast type II versus slow type I fibers (18). Thus combining this observation with an increased acid production and Km for ATP may help explain the greater rate of fatigue of fast type II versus slow type I muscle (8).
The present findings may not apply entirely to the situation in both skinned and intact muscle for several reasons. For example, Metzger and Moss (25) suggests that acidosis may decrease filament lattice spacing (1), which can slow unloaded Vmax in muscle fibers (41). Such an effect would of course be dependent on the highly ordered structure of muscle, which is not present in the assays employed in the present investigation. In addition, actin filaments used in the present experiments do not include the regulatory proteins tropomyosin and troponin, which are known to modulate actomyosin kinetics (14) and also mediate the pH effect on the force-calcium relationship in muscle fibers (27). Thus future studies that incorporate fully regulated thin filaments in the laser trap assay (16) could partition the effect of low pH between the direct effects on actomyosin performance from the effects mediated through regulatory proteins. Regardless, this study does provide direct evidence that low pH profoundly affects the ability of myosin to translocate actin, whereby Vactin is slowed by both a reduced rate of ADP release from myosin and the appearance of an increased proportion of nonproductive actomyosin interactions, both of which at the ensemble level would create an effective internal load to movement.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-059408 and HL-085489.
Acknowledgments
E. P. Debold is now affiliated with the Department of Kinesiology at the University of Massachusetts. E. P. Debold posthumously thanks Devra Hendelman for inspiring him to rigorously pursue the question of muscular fatigue. The authors also thank members of the Warshaw Laboratory for helpful comments and discussions during the development of this project. In addition, we thank Guy Kennedy of the Instrumentation and Modeling Facility at the University of Vermont, for expertise in design and maintenance of the laser trap.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.April EW, Brandt PW, Elliott GF. The myofilament lattice: studies on isolated fibers. II. The effects of osmotic strength, ionic concentration, and pH upon the unit-cell volume. J Cell Biol 53: 53–65, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw CR, Trentham DR. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J 141: 331–349, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker JE, Brosseau C, Joel PB, Warshaw DM. The biochemical kinetics underlying actin movement generated by one and many skeletal muscle myosin molecules. Biophys J 82: 2134–2147, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chock SP The mechanism of the skeletal muscle myosin ATPase. III Relationship of the H+ release and the protein absorbance change induced by ATP to the initial Pi burst. J Biol Chem 254: 3244–3248, 1979. [PubMed] [Google Scholar]
- 5.Cooke R, Franks K, Luciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol 395: 77–97, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edman KA, Mattiazzi AR. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil 2: 321–334, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368: 113–119, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Fitts RH Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Fitts RH The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104: 551–558, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Gaskell WH On the tonicity of the heart and blood vessels. J Physiol 3: 48–92, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauthier GF, Lowey S. Polymorphism of myosin among skeletal muscle fiber types. J Cell Biol 74: 760–779, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilford WH, Dupuis DE, Kennedy G, Wu J, Patlak JB, Warshaw DM. Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. Biophys J 72: 1006–1021, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris DE, Warshaw DM. Smooth and skeletal muscle actin are mechanically indistinguishable in the in vitro motility assay. Circ Res 72: 219–224, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Homsher E, Nili M, Chen IY, Tobacman LS. Regulatory proteins alter nucleotide binding to acto-myosin of sliding filaments in motility assays. Biophys J 85: 1046–1052, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huxley HE Sliding filaments and molecular motile systems. J Biol Chem 265: 8347–8350, 1990. [PubMed] [Google Scholar]
- 16.Kad NM, Kim S, Warshaw DM, VanBuren P, Baker JE. Single-myosin crossbridge interactions with actin filaments regulated by troponin-tropomyosin. Proc Natl Acad Sci USA 102: 16990–16995, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kad NM, Rovner AS, Fagnant PM, Joel PB, Kennedy GG, Patlak JB, Warshaw DM, Trybus KM. A mutant heterodimeric myosin with one inactive head generates maximal displacement. J Cell Biol 162: 481–488, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karatzaferi C, de Haan A, Ferguson RA, van Mechelen W, Sargeant AJ. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflügers Arch 442: 467–474, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kentish JC Combined inhibitory actions of acidosis and phosphate on maximum force production in rat skinned cardiac muscle. Pflügers Arch 419: 310–318, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J Physiol 575: 887–899, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler D, Ruff C, Meyhofer E, Bahler M. Different degrees of lever arm rotation control myosin step size. J Cell Biol 161: 237–241, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kron SJ, Spudich JA. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci USA 83: 6272–6276, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauzon AM, Tyska MJ, Rovner AS, Freyzon Y, Warshaw DM, Trybus KM. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil 19: 825–837, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Marston SB, Taylor EW. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol 139: 573–600, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Metzger JM, Fitts RH. Role of intracellular pH in muscle fatigue. J Appl Physiol 62: 1392–1397, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Metzger JM, Moss RL. Effects of tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J Physiol 428: 737–750, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger JM, Parmacek MS, Barr E, Pasyk K, Lin WI, Cochrane KL, Field LJ, Leiden JM. Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice. Proc Natl Acad Sci USA 90: 9036–9040, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nosek TM, Fender KY, Godt RE. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 236: 191–193, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Palmiter KA, Tyska MJ, Dupuis DE, Alpert NR, Warshaw DM. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J Physiol 519: 669–678, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol 85: 164–181, 1982. [DOI] [PubMed] [Google Scholar]
- 31.Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol 486: 689–694, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium 35: 427–431, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Potma EJ, van Graas IA, Stienen GJ. Influence of inorganic phosphate and pH on ATP utilization in fast and slow skeletal muscle fibers. Biophys J 69: 2580–2589, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science 261: 58–65, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261: 50–58, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Ricciardi L, Bottinelli R, Canepari M, Reggiani C. Effects of acidosis on maximum shortening velocity and force-velocity relation of skinned rat cardiac muscle. J Mol Cell Cardiol 26: 601–607, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Sherwood JJ, Waller GS, Warshaw DM, Lowey S. A point mutation in the regulatory light chain reduces the step size of skeletal muscle myosin. Proc Natl Acad Sci USA 101: 10973–10978, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleep JA, Hutton RL. Actin mediated release of ATP from a myosin-ATP complex. Biochemistry 17: 5423–5430, 1978. [DOI] [PubMed] [Google Scholar]
- 39.Steffen W, Sleep J. Repriming the actomyosin crossbridge cycle. Proc Natl Acad Sci USA 101: 12904–12909, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steffen W, Smith D, Simmons R, Sleep J. Mapping the actin filament with myosin. Proc Natl Acad Sci USA 98: 14949–14954, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umazume Y, Onodera S, Higuchi H. Width and lattice spacing in radially compressed frog skinned muscle fibres at various pH values, magnesium ion concentrations and ionic strengths. J Muscle Res Cell Motil 7: 251–258, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Volkmann N, Liu H, Hazelwood L, Krementsova EB, Lowey S, Trybus KM, Hanein D. The structural basis of myosin V processive movement as revealed by electron cryomicroscopy. Mol Cell 19: 595–605, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Warshaw DM, Desrosiers JM, Work SS, Trybus KM. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding velocity in vitro. J Cell Biol 111: 453–463, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warshaw DM, Guilford WH, Freyzon Y, Krementsova E, Palmiter KA, Tyska MJ, Baker JE, Trybus KM. The light chain binding domain of expressed smooth muscle heavy meromyosin acts as a mechanical lever. J Biol Chem 275: 37167–37172, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Westerblad H, Lee JA, Lannergren J, Allen DG. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol Cell Physiol 261: C195–C209, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita H, Sugiura S, Serizawa T, Sugimoto T, Iizuka M, Katayama E, Shimmen T. Sliding velocity of isolated rabbit cardiac myosin correlates with isozyme distribution. Am J Physiol Heart Circ Physiol 263: H464–H472, 1992. [DOI] [PubMed] [Google Scholar]