Abstract
Membrane phosphatidylinositol-4,5-bisphosphate (PIP2) is critical for the function of many transient receptor potential (TRP) ion channels. The role of PIP2 in TRPA1 function is not well known. The effect of PIP2 on TRPA1 was investigated by direct application of PIP2 and by using polylysine and PIP2 antibody that sequester PIP2. In inside-out patches from HeLa cells expressing mouse TRPA1, polytriphosphate (PPPi) was added to the bath solution to keep TRPA1 sensitive to allyl isothiocyanate (AITC; mustard oil). Direct application of PIP2 (10 μM) to inside-out patches did not activate TRPA1, but AITC and Δ9-tetrahydrocannabinol (THC) produced strong activation. In inside-out patches in which TRPA1 was first activated with AITC (in the presence of PPPi), further addition of PIP2 produced a concentration-dependent inhibition of TRPA1 [agonist concentration producing half-maximal activity (K1/2), 2.8 μM]. Consistent with the inhibition of TRPA1 by PIP2, AITC activated a large whole cell current when polylysine or PIP2 antibody was added to the pipette but a markedly diminished current when PIP2 was added to the pipette. In inside-out patches with PPPi in the bath solution, application of PIP2 antibody or polylysine caused activation of TRPA1, and this was blocked by PIP2. However, TRPA1 was not activated by polylysine and PIP2 antibody under whole cell conditions, suggesting a more complex regulation of TRPA1 by PIP2 in intact cells. These results show that PIP2 inhibits TRPA1 and reduces the sensitivity of TRPA1 to AITC.
Keywords: allyl isothiocynanate, tetrahydrocannabinol, neomycin, magnesium
transient receptor potential (TRP) A1 (TRPA1) is highly expressed in a subset of sensory neurons and is activated by pungent and reactive chemicals such as allylisothiocyanate (from mustard), allicin (from garlic), cinnamaldehyde (from cinnamon), formaldehyde, N-methylmaleimide, and α,β-unsaturated aldehydes (2, 3, 14, 37). These chemicals are believed to act directly on the cytoplasmic regions of TRPA1 and cause local covalent modification of the protein, leading to a conformational change and opening of the channel (13, 25–27). TRPA1 may also serve as a sensor of cold temperature and mechanical deformation; however, these roles are currently controversial (17, 18, 28, 30, 35, 37, 39). In addition to pungent chemicals found in nature, endogenously generated molecules such as bradykinin, reactive oxygen species, and 4-hydroxynonenal that are produced during inflammation and oxidative stress, respectively, can activate TRPA1 (2, 4, 39). Thus TRPA1 detects and transduces signals produced by nociceptive chemicals, chemical-induced damage, and inflammation.
Recent studies show that activation, desensitization, and recovery from desensitization of several TRP ion channels in the TRPM (melastatin)and TRPV (vanilloid) families are dependent or regulated by membrane phosphatidylinositol-4,5-bisphosphate (PIP2) (5, 10, 21, 23, 33, 40). For example, activation of TRPM8 by menthol requires the presence of PIP2, and factors that reduce membrane PIP2 concentration such as PIP2 antibody, polylysine, and receptor agonists that activate phospholipase C (PLC), reduce activation by menthol (20, 32). The ability of PIP2 to regulate the channel function has also been reported for TRPM4, TRMP5, and TRPM7 (22, 29, 34). For TRPV1, initial studies reported that reduction of PIP2 enhanced the activation by capsaicin and that bradykinin and nerve growth factor-sensitized TRPV1 by hydrolysis of PIP2 (6). Subsequent studies showed, however, that PIP2 directly applied to the patch membrane activated TRPV1 (23, 36), and that sensitization of TRPV1 by PIP2 may depend on the concentration of the stimulus (23). TRPV5 is activated by PIP2 via relief of inhibition caused by intracellular Mg2+ (19). A recent study shows that TRPC4 is strongly inhibited by PIP2, but interestingly this inhibition required intact cytoskeletal network, as treatment of cells with cytochalasin D abolished the inhibition. This finding indicates that the PIP2 effect on this channel is not a simple one and requires a multiprotein complex (31). Thus PIP2 is a critical component of signaling that regulates the activity of a number of TRP ion channels. The precise regions of interaction between PIP2 and TRP ion channels, however, have not been clearly identified for a majority of TRP channels.
As bradykinin activates TRPA1 via PLC (2), it seems likely that PIP2 may also be involved in the regulation of TRPA1. Indeed, a recent study reported that PAR2, a G protein-coupled receptor, sensitized TRPA1 to mustard oil via depletion of PIP2 (7), whereas other PLC-generated second messengers such as PKC, diacylglycerol, and Ca2+ had no effect on sensitization of TRPA1. This would suggest that membrane TRPA1 is normally in the inhibited state, and that the agonist-induced depletion of PIP2 results in a relief from inhibition, causing TRPA1 to open. However, another study reported that the pharmacological desensitization of TRPA1 involves PIP2 depletion, suggesting that PIP2 supports TRPA1 activity (1). Therefore, the role of PIP2 on the activation and sensitization of TRPA1 needs further clarification. Whether application of PIP2 directly to inside-out patches activates or inhibits TRPA1 has not yet been reported, although this protocol would provide more direct evidence for the role of PIP2, as has been done with other ion channels. Therefore, to clarify the role of PIP2 in the regulation of TRPA1, we assessed the effect of PIP2 directly applied to membrane patches, as well as the effects of polylysine and PIP2 antibody that sequesters PIP2 from the membrane. We also studied the effect of PIP2 and polylysine under whole cell conditions in response to allyl-isothiocyanate (AITC). The results show that PIP2 is a potent inhibitor of TRPA1 and reduces the sensitivity of the channel to the pungent chemicals AITC. Depletion of PIP2 by polylysine and PIP2 antibody activated TRPA1 in inside-out patches in the absence of AITC, suggesting that the basal membrane PIP2 keeps TRPA1 closed. However, polylysine did not activate the basal whole cell current, suggesting that the modulation of TRPA1 by PIP2 in intact cells is more complex possibly involving other regulatory molecules.
MATERIALS AND METHODS
Transfection in cultured cells.
Mouse TRPA1 was a gift from Dr. Artem Patapoutian (Scripps Research Institute, La Jolla, CA). Rat TRPV1 was a gift from Dr. Matoko Tominaga (Okazaki Institute for Integrative Bioscience, Okazaki, Japan). HeLa cells were seeded at a density of 2×105 cells per 35-mm dish 24 h before transfection in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. Cells were cotransfected with plasmids containing a channel and green fluorescent protein (GFP) DNA sequences in pcDNA3.1 using LipofectAMINE2000 and OPTI-MEM I Reduced Serum Medium (Life Technologies). Green fluorescence from cells expressing GFP was detected with the aid of a Nikon microscope equipped with a mercury lamp light source and a GFP filter (emission wavelength, 510 nm). Cells were used 1–2 days after transfection.
Electrophysiological studies.
Gigaseal was formed with pipettes with desired resistance (2–5 MΩ). Current was recorded with an Axopatch 200 patch-clamp amplifier, low-pass filtered at 3 kHz using an 8-pole Bessel filter (902-LPF), digitized (Digidata 1322A, Axon Instruments), and stored on computer disk. The sampling rate was 10 kHz. Digitized data were analyzed (pClamp 9.0) to obtain channel activity (NPo; where N is the number of channels in the patch and Po is the open probability), and amplitude histograms to obtain single channel conductance. Duration histograms were obtained from patches showing mainly one level of opening after setting the minimum duration at 0.2 ms and binning the data into log intervals. The histograms were fitted with two exponential probability functions using the maximum likelihood method (Clampfit software). Current tracings shown in figures have been filtered at 50–100 Hz, except for expanded tracings (1 kHz). For whole cell, cell-attached, outside-out and inside-out patches, pipette and bath solutions contained (in mM) 126 NaCl, 4 KCl, 2 EGTA, 1 MgCl2, 10 HEPES, and 5 glucose (pH 7.3). Student's t-test was used to test for significance (P < 0.05). Hill equation was used to fit the data points to obtain agonist concentration producing half-maximal activity (K1/2) and Hill coefficient using Origene programs. All experiments were done at room and bath temperatures of 23 ± 1°C.
Materials.
AITC, Δ9-tetrahydrocannabinol (THC), capsaicin, menthol, PIP2, wortmannin, and polytriphosphate (PPi) were purchased from Sigma Chemical and all other chemicals and enzymes were also purchased from Sigma Chemical. Monoclonal PIP2 antibody (stock 1 mg/ml) was purchased from Assay Designs (Ann Arbor, MI). AITC and capsaicin were dissolved in DMSO and used at the final DMSO concentration of 0.1% or less. DMSO (0.1%) had no effect on TRPA1 activation by AITC. THC was dissolved in ethanol at a stock concentration of 88 mM and diluted in recording solution as desired.
RESULTS
PIP2 does not activate TRPA1 in inside-out patches.
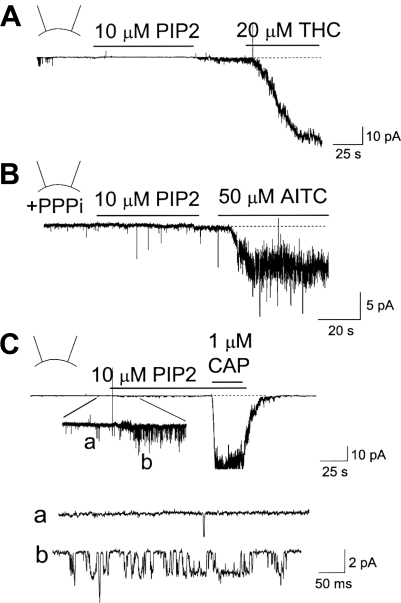
PIP2 has been shown to activate many different types of ion channels, including TRPV1 and TRPM8. To test whether PIP2 also affects TRPA1, inside-out patches were formed from HeLa cells expressing mouse TRPA1, and the purified bovine brain PIP2 preparation was applied to the cytoplasmic side of the membrane. In our recent study, we have characterized the kinetic properties of TRPA1 after expression in HeLa cells, and therefore, they are not repeated here in detail (16). Ca2+-free physiological solution was used in the pipette and bath solutions to prevent desensitization and inactivation. In all six patches tested, PIP2 (10 μM) failed to activate TRPA1 (Fig. 1A). In the same patch, the TRPA1 agonist THC (20 μM) caused a strong activation of the channels (Fig. 1A). In the current tracing shown in Fig. 1A, 16 TRPA1 channels were activated by THC. In cells expressing only GFP or in nontransfected cells, THC did not activate any channels (n = 6 each). In patches where THC activated only a few channels, the single channels were analyzed to determine their kinetic properties. The single channel conductance was 63 ± 4 pS (n = 3) at the membrane potential of −40 mV, and 82 ± 6 pS at +40 mV, producing a slightly outward-rectifying current-voltage relationship. These channel properties are similar to those previously reported for TRPA1 (16, 28).
Fig. 1.
Phosphatidylinositol-4,5-bisphosphate (PIP2) does not activate transient receptor potential A1 (TRPA1) in inside-out patches. HeLa cells were transfected with TRPA1 (A and B) or TRPV1 (C) and green fluorescent protein (GFP) DNAs. Unless indicated otherwise, the pipette potential was held at +40 mV to record inward current. A: application of 10 μM PIP2 to an inside-out patch containing TRPA1 did not activate TRPA1. After washout of PIP2, subsequent application of 20 μM Δ9-tetrahydrocannabinol (THC) activated TRPA1 (NPo; 7.2 ± 2.8; n = 5). B: an inside-out patch was formed in the presence of 5 mM PPPi. Addition of 10 μM PIP2 to the bath solution did not activate TRPA1. Subsequent application of allyl-isothiocyanate (AITC, 50 μM) caused activation of TRPA1 (NPo, 5.8 ± 1.9; n = 5). C: inside-out patch was formed from a HeLa cell expressing TRPV1. Application of 10 μM PIP2 caused a small activation of TRPV1 (NPo, 1.3 ± 0.4; n = 5; see tracings a and b). Subsequent application of capsaicin produced a much larger activation (NPo, 12.8 ± 3.7; n = 5).
Our recent study showed that TRPA1 switches from the native state that is sensitive to pungent chemicals to an insensitive state when the patch is removed from the cell (inside-out patch), possibly due to loss of a cytosolic factor that is required for channel activation (16). It was also found that the presence of a polyphosphate such as PPPi could keep TRPA1 in the AITC-sensitive state even in inside-out patches. To test whether TRPA1 needs to be in the native conformation to be activated by PIP2, inside-out patches were formed with 5 mM PPPi in the bath solution, and then PIP2 was applied to the patches. Still no activation was observed with PIP2, although AITC evoked a large activation in the same patches (Fig. 1B). Lower and higher concentrations of PIP2 (2 and 20 μM) also failed to activate TRPA1 (n = 3 each). In inside-out patches containing TRPV1, application of PIP2 produced a small but significant increase in channel activity, as shown in the inset of Fig. 1C. Capsaicin caused a much larger activation in the same patch (Fig. 1C). These results show that PIP2 does not activate TRPA1 when directly applied to inside-out patches.
PIP2 is an inhibitor of TRPA1.
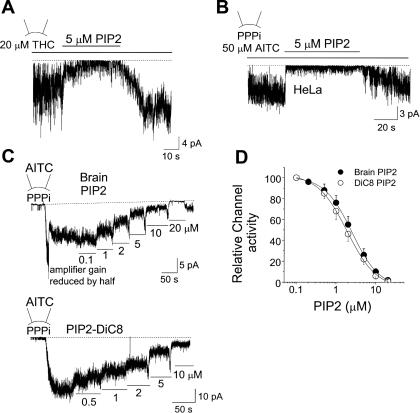
We tested the possibility that PIP2 is an inhibitor of TRPA1. Inside-out patches from HeLa cells expressing TRPA1 were formed, and THC or AITC was applied to the bath solution to activate TRPA1. PIP2 (5 μM) was then applied to the patch together with THC or AITC. This concentration of PIP2 produced a rapid and strong inhibition of TRPA1 activated by THC and AITC (Fig. 2, A and B). Channel activity recovered nearly completely following washout of PIP2. To determine the inhibitory potency of PIP2, TRPA1 was first activated with AITC added to the pipette in the presence of 5 mM PPPi in the bath solution, and a low concentration of PIP2 (0.1 μM) was applied to the inside-out patch. The concentration of PIP2 was then progressively increased to 20 μM (Fig. 2C, top). PIP2-DiC8, a synthetic PIP2 with shorter lipid side chains, produced a similar concentration-dependent inhibition of TRPA1 (Fig. 2C, bottom). Channel activity at each [PIP2] was determined, and the data were fitted to the Hill equation of the form: y = (An)/(K1/2 + An), where y is the fractional activation, A is the concentration of the agonist, K1/2 is the agonist concentration producing half-maximal activity, and n is the Hill coefficient. K1/2 values for natural and DiC8 forms of PIP2 were 2.8 and 2.1 μM, respectively, and n values were 1.5 and 1.5, respectively (Fig. 2D). PIP2-DiC8 (5 μM) caused a 82 ± 7% inhibition (n = 3) of TRPA1 in Mg2+-free solution, not significantly different from that observed with 1 mM Mg2+ (76 ± 6% inhibition). Because TRPA1 desensitized mildly with time after activation with THC, we were unable to obtain the concentration-dependent curve for inhibition of THC-activated TRPA1 by PIP2. Nevertheless, the inhibition of TRPA1 by PIP2 was evident in all patches tested.
Fig. 2.
PIP2 inhibits TRPA1 activated by AITC and THC. A: an inside-out patch was formed, and THC was applied to the bath solution to activate TRPA1 (NPo, 5.5 ± 1.7; n = 4). Further addition of PIP2 caused an inhibition of TRPA1 activity (NPo, 0.8 ± 0.3; n = 4). B: an inside-out patch was formed in the presence of 5 mM polytriphosphate (PPPi). Addition of AITC elicited a strong activation of TRPA1 (NPo, 6.8 ± 2.6; n = 5). Further addition of PIP2 caused a reversible inhibition of TRPA1 (NPo, 0.5 ± 0.2; n = 5). C: an inside-out patch was formed in the presence of 5 mM PPPi with AITC in the pipette. Either bovine brain PIP2 (natural) or PIP2-DiC8 was applied in increasing concentrations up to 20 μM. D: graph shows relative TRPA1 activity as a function of [PIP2] when AITC was used to activate TRPA1. Each point is the mean ± SD of 4 determinations. See text for details.
Figure 3 shows a current tracing in which TRPA1 was activated with AITC, and low concentrations of PIP2 were used to inhibit TRPA1 in an inside-out patch held at the membrane potential of −40 mV. Expanded current tracings are shown for different concentrations of PIP2 as indicated by lower-case letters (Fig. 3B). Comparison of the amplitude histograms shows that PIP2 does not significantly affect the unitary conductance at either −40 mV or +40 mV (Fig. 3E). Open time duration histograms were also obtained from patches that showed mainly one level of opening. The open duration histograms obtained from channel openings at −40 mV were well fitted with two exponential probability function (Fig. 3D). According to these analyses, TRPA1 has two open states with mean open times of 2.9 ± 0.8 ms (τ01) and 11.5 ± 2.9 (τ02) ms with approximately equal probability, as judged by the areas covered by the exponential curves. PIP2 caused a progressive shortening of the long openings with small effects on the short openings (Fig. 3F). At 2 μM PIP2, only 9% (area) of the total current was contributed by longer open state when compared with 55% without PIP2. At higher PIP2 concentrations (10–20 μM), the open time duration histograms could be fitted with only one exponential function, the mean open time decreased to <1 ms (0.7–0.9 ms), and the unitary conductance also decreased from 65 to ∼35 pS (at −40 mV; not shown). Therefore, the effect of PIP2 at concentrations above 10 μM is unlikely to be physiological, because TRPA1 channels with such properties are not present under normal conditions. These results show that PIP2 is a potent inhibitor of TRPA1 and reduces the effectiveness of AITC to activate TRPA1, mainly by destabilizing the long open state of the channel.
Fig. 3.
Effect of PIP2 on single TRPA1 channels. A: an inside-out patch was formed with 5 mM PPPi and 20 μM AITC in the pipette. Patches showing a low level of channel activity were used. Low concentrations of PIP2 were then applied to the bath solution. Channel openings at time points a through f are shown at expanded scale in B. C: amplitude histograms were obtained from channel recordings at different [PIP2], and typical histograms are shown. D: duration histograms were obtained from patches showing mainly one level of opening. The duration histograms were fitted with two exponential probability function. E: graph shows the effect of PIP2 on single channel conductance determined at −40 mV and +40 mV. Each bar is the mean ± SD of 3 determinations. No significant differences were present at the same membrane potentials (P > 0.05). F: graph shows the effect of PIP2 on two mean open times (τo1 and τo2) at each [PIP2]. PIP2 produced a progressive reduction in τo2. The percent values shown in the open bars indicate the relative contribution of the longer-open state to the total current.
Role of PIP2 in TRPA1 function.
To further test the role of PIP2 on TRPA1 function, polylysine and PIP2 antibody were used to reduce the level of PIP2 in the membrane. First, whole cell currents in response to AITC were recorded at −30 mV in HeLa cells expressing TRPA1 with pipette solution containing PIP2, polylysine, PIP2 antibody, or none of the three compounds (control). Under these conditions, the use of PPPi was not necessary, as cell cytoplasm presumably contains the factor that maintains TRPA1 in the functional state and dialysis of this factor is relatively slow (16). In Ca2+-free physiological solution, AITC produced a large ruthenium red-sensitive whole cell current in cells dialyzed with control solution for ∼4 min (Fig. 4A). With polylysine (10 μg/ml) or PIP2 antibody (20 μg/ml) in the pipette, the AITC-evoked currents were slightly larger than those observed in control cells. However, when PIP2-DiC8 (10 μM) was present in the pipette, the response to AITC (50 μM) was greatly diminished (Fig. 4A). The averaged results obtained at −30 mV are shown in Fig. 4B. In another set of cells expressing TRPA1, whole cells were held at −60 mV, and ramp pulses were applied from −60 to +60 mV (1-s duration) to record whole cell currents (Fig. 4A, right). The results from these experiments show that PIP2-DiC8 markedly inhibits activation of TRPA1 by AITC for both inward and outward current.
Fig. 4.
Effect of polylysine (PL) and PIP2 antibody on the activation of TRPA1 current by AITC. A, left: whole cell currents were recorded from HeLa cells held at −30 mV before and after application of AITC (50 μM). PL, PIP2 antibody, or PIP2-DiC8 was added to the pipette solution as indicated for ∼4 min before addition of AITC. Ruthenium red (RR; 10 μM) was applied in some experiments. Right, whole cell currents were recorded following a ramp pulse (−60 to +60 mV) applied to the cells. The pipette solution was same as the bath solution. Dotted lines indicate the zero current level. B: results of the whole cell current recordings are summarized in the graph. Each bar is the mean ± SD of 5 determinations; *significant difference from the control bar; **significant difference from all other bars (P < 0.05).
The strong inhibition of TRPA1 by PIP2 suggests that the basal level of membrane PIP2 might be keeping the channels in the inhibited state, as observed in cell-attached patches that normally show low, basal TRPA1 activity. If so, lowering [PIP2] may relieve TRPA1 from inhibition leading to activation. However, this was not obvious from the whole cell current recordings shown in Fig. 4. It was possible that the endogenous cytosolic factor that keeps TRPA1 in the AITC-sensitive state was slowly dialyzed out of the cell during whole cell recording and prevented activation of basal TRPA1 by polylysine. To keep TRPA1 in the functional state, PPPi (5 mM) was added to the pipette along with polylysine (10 μg/ml), and the whole cell current was recorded at −30 mV. Even in the presence of PPPi, we failed to observe an increase in basal current, although subsequent application of AITC caused further activation of TRPA1 in all six cells tested (not shown). The lack of activation of TRPA1 by polylysine was further investigated using inside-out patches.
Activation of TRPA1 by polylysine and PIP2 antibody in inside-out patches.
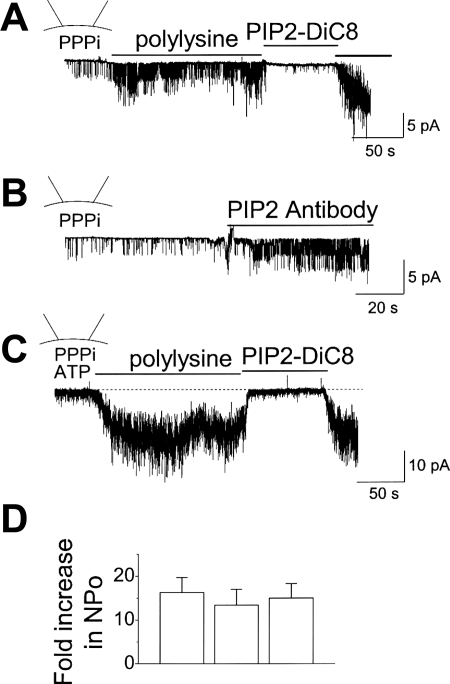
The whole cell studies above suggest that the reduction of PIP2 by polylysine and PIP2 antibody does not activate TRPA1 under basal conditions. To confirm these findings, the effect of PIP2 depletion on TRPA1 was further tested in inside-out patches. Inside-out patch was formed in the presence of 5 mM PPPi, and polylysine was added to the cytoplasmic side of the membrane. Interestingly, polylysine (10 μg/ml) produced a significant increase in TRPA1 activity above the basal level observed in the presence of PPPi alone (Fig. 5A). This activation of TRPA1 did not occur when the concentration of polylysine was 1 μg/ml (n = 4; not shown), suggesting that a small reduction of PIP2 may be insufficient to activate TRPA1. Similarly, adding PIP2 antibody (20 μg/ml) also evoked activation of TRPA1 in all patches tested when used at a concentration of 20 μg/ml but not at 2 μg/ml (Fig. 5B). Activation of TRPA1 by polylysine (or by PIP2 antibody; not shown) was strongly inhibited by application of 10 μM PIP2-DiC8 (Fig. 5). A similar activation of TRPA1 by polylysine was also observed when 2 mM ATP was present in the bath solution (Fig. 5C). The activation of TRPA1 by polylysine in the presence and absence of ATP was similar when normalized to the initial basal level (Fig. 5D). These results show that in inside-out patches, polylysine and PIP2 antibody lead to opening of TRPA1 and suggest that membrane PIP2 keeps TRPA1 in the low activity, basal state.
Fig. 5.
Activation of TRPA1 by polylysine and PIP2 antibody in inside-out patches. A and B: inside-out patch was formed in the presence of 5 mM PPPi in the bath solution. Addition of polylysine (10 μg/ml; A) or PIP2 antibody (20 μg/ml; B) caused activation of TRPA1. Subsequent addition of PIP2 (10 μM) quickly and reversibly inhibited the channels. C: inside-out patch was formed in the presence of 5 mM PPPi and 2 mM ATP in the bath solution. Addition of polylysine (10 μg/ml) caused activation of TRPA1 and further addition of PIP2 (10 μM) inhibited the channels. D: bar graph summarizes the data from A–C. Each bar is the mean ± SD of 5 determinations. No significant differences were present (P > 0.05).
We also tested whether neomycin, a positively charged aminoglycoside antibiotic known to bind PIP2, activates TRPA1 by reducing membrane [PIP2]. Neomycin (300 μM), however, failed to activate TRPA1 when applied to inside-out patches (n = 4). When neomycin (300 μM) was applied to patches in which TRPA1 was first activated with AITC (50 μM), the antibiotic caused a significant inhibition (42 ± 8%; n = 4) of TRPA1 activity. This result might suggest that shielding PIP2 by neomycin is associated with reduced TRPA1 activity and therefore that PIP2 is a positive modulator of TRPA1. However, the result that PIP2 inhibits TRPA1 in inside-out patches suggests that neomycin may have a direct inhibitory effect on TRPA1. Similarly, high concentrations of Mg2+ (10 mM) also caused inhibition (56 ± 9%; n = 4) of AITC-activated TRPA1, and no activation of TRPA1 by Mg2+ was ever observed under basal conditions in all six patches tested. Like neomycin, the inhibitory effect of high Mg2+ on TRPA1 could be partially due to shielding of PIP2. However, based on the strong inhibitory effect of PIP2 on TRPA1, it seems more likely that high Mg2+ has a direct inhibitory effect on TRPA1.
Inhibition of PIP2 synthesis reduces membrane [PIP2], and wortmannin has been used often to reduce membrane [PIP2] (38). Therefore, the effect of 20 μM wortmannin, an inhibitor of phosphatidylinositol-3-kinase and phosphatidylinositol-4-kinase at this high concentration, was studied on TRPA1 activity in cell-attached patches and compared with that observed in control patches incubated similarly without the drug for 1 h. The basal levels of activity in the two groups were low (NPo: 0.013 ± 0.005 vs. 0.016 ± 0.005; n = 5 each; P > 0.05) and were not significantly different. In wortmannin-treated cells, AITC produced a strong activation of TRPA1 in cell-attached condition, similar to those of untreated control cells. The concentration-dependent inhibition of AITC-activated TRPA1 by PIP2 was also similar to those observed in control cells (K1/2, 2.5 μM, n = 1.5; n = 4). The lack of effect of wortmannin could be that, in the presence of normal levels of intracellular ATP, it did not produce a large enough reduction of membrane PIP2 that is necessary to release TRPA1 from inhibition. This would be in keeping with the observation that activation of TRPA1 under basal conditions required relatively high concentrations of polylysine and PIP2 antibody (see discussion).
DISCUSSION
Membrane PIP2 regulates many ion transporters including TRP ion channels. The level of PIP2 in the membrane is thought to be a critical determinant of the basal level of activity and of the sensitivity of a channel to its modulators (10, 33, 40). Therefore, any changes in [PIP2] produced by altered synthesis and degradation elicited by altered activities of lipid kinase/phosphatase and PLC would have a significant impact on the channel activity and thus the cell excitability. It is generally believed that channels with low affinity for PIP2 are more sensitive to changes in PIP2, whereas channels with high affinity for PIP2 are less sensitive changes in PIP2 (10). Most ion channels show increased basal activity and sensitivity when membrane [PIP2] is elevated. Here, we studied the role of PIP2 in TRPA1 function by directly applying it to inside-out patches and by using PIP2 scavengers and found that PIP2 is a potent inhibitor of TRPA1. Thus TRPA1 can be added to the group of channels that are inhibited by PIP2. This group includes drosophila TRPL, TRPV1, TRPP2 (PKD2), and TRPC4 (6, 11, 23, 24, 31). It must be noted that the response to PIP2 in excised patches and in intact cells (in vivo) may be different. For example, TRPV1 may be activated by PIP2 in excised patches (23, 36), but it may be inhibited in intact cells (6, 23). Similarly, drosophila TRPL is inhibited by PIP2 in inside patches (9), but such inhibition was not apparent in vivo (12).
Our results, together with the earlier findings by Dai et al. (7), show that PIP2 inhibits TRPA1 in both excised patches and whole cell conditions. The finding that PIP2 inhibits TRPA1 suggests that membrane PIP2 could be keeping the basal level of channel activity low, as typically observed in cell-attached patches in the absence of any chemical activators. Our results that PIP2 antibody and polylysine applied to excised patches increase TRPA1 activity support such a role for PIP2, at least in inside-out patches. However, our findings were obtained in the presence of PPPi that is not likely to be a physiological modulator of TRPA1. The highly negatively charged character of polyphosphates may also affect the function of other proteins that might be associated with the channel protein. Therefore, until we identify the cytosolic factor that supports the function of TRPA1, the results need be interpreted with caution.
If polylysine were to remove TRPA1 from tonic inhibition, polylysine in the pipette would be expected to slowly increase the basal whole cell current in the absence of AITC. Surprisingly, we did not observe a significant increase in basal current with polylyine or PIP2 antibody in the pipette. Therefore, we were unable to reproduce our finding in excised patches at the whole cell level with respect to activation by PIP2 scavengers. What could explain this difference? In a recent study, TRPC4, a channel that is also inhibited by PIP2, was found to be associated with actin skeleton through interaction with PDZ protein Na/H exchanger regulatory factor and ezrin (31). For TRPC4, PIP2 depletion alone was not sufficient to activate the channel, as additional factors such as G protein and Ca2+ were involved (31). Therefore, it is possible that the basal TRPA1 activity in vivo is also dependent on the functional network of proteins, and simply reducing the level of PIP2 does not cause activation of TRPA1 in intact cells. Breakdown of the network that probably occurs in excised patches could remove such factors and may allow an easier activation by polylysine. In this regard, we were also unable to measure an increase in basal activity of TRPA1 when wortmannin was used to block generation of PIP2 in intact cells. It may also be that the activation of TRPA1 by AITC and other pungent chemicals is highly sensitive to [PIP2], but the opening of the channel in the absence of such chemicals (or receptor agonists) is less sensitive to [PIP2] and require much greater depletion of PIP2. This interpretation would be consistent with the observation that a low concentration of polylysine (1 μg/ml) or PIP2 antibody (2 μg/ml) did not activate TRPA1 in inside-out patches. Clearly, further studies are needed to better understand the regulation of TRPA1 by PIP2 in the absence of chemical agonists in the native system.
The finding that PIP2 inhibits TRPA1 suggests that activation of TRPA1 by agonists may occur via PLC-induced degradation of PIP2. The recent work of Dai et al. (7) shows that PAR2 (a G protein-coupled receptor agonist) expressed in dorsal root ganglion neurons sensitizes the response of TRPA1 to AITC. In this study, application of 10 μM PIP2 to the pipette solution almost completely inhibited the sensitization produced by PAR2, and PIP2 antibody fully recovered the sensitization. From these results, the authors concluded that the agonist-mediated potentiation of TRPA1 activity occurs by PIP2 depletion. Our results that PIP2 strongly inhibits AITC- or THC-activated TRPA1 support the observations by Dai et al. (7) that PIP2 inhibits sensitization. Our findings also support the general conclusion that depletion of PIP2 is involved in sensitization of TRPA1 to AITC. Thus membrane PIP2 is a critical modulator of TRPA1 function, and any intervention that alters the level of PIP2 would be expected to produce the associated change in TRPA1 sensitivity to other activators such as AITC. It is interesting that PAR2 itself did not activate TRPA1 in HEK cells expressing PAR2 receptor and TRPA1 (7), suggesting that either the reduction of [PIP2] by PLC was not sufficient or that factors other than depletion of PIP2 are involved, as demonstrated for TRPC4 (31). This could explain the lack of activation of TRPA1 by polylysine under whole cell conditions in the absence of a chemical activator such as AITC.
A recent study reported that one mechanism that leads to pharmacological desensitization of TRPA1 involves PIP2 depletion (1). Our study as well as that of Dai et al. (7) are not consistent with this idea, as PIP2 inhibits TRPA1. However, because TRPV1 was coexpressed with TRPA1 and several mechanisms may be involved in desensitization under these conditions, it is possible that the role of PIP2 in regulating TRPA1 is different when TRPV1 is coexpressed.
In our study, we had to use PPPi to activate TRPA1 with AITC in inside-out patches, because TRPA1 could not be activated by AITC without PPPi (16). However, in other studies, AITC has been reported to activate TRPA1 in inside-out patches without adding any foreign molecules (8, 41). We do not have a satisfactory answer for these differences but can offer a plausible explanation. We propose that a cytosolic factor that can be functionally mimicked by PPPi keeps TRPA1 in the AITC-sensitive conformation. Upon formation of inside-out patch, the cytosolic factor is lost and TRPA1 shifts to a conformation that is insensitive to AITC. It is possible that in patches that contain many TRPA1 channels, one or two channels may remain sensitive to AITC because of the presence of the cytosolic factor associated with the membrane until it is gradually washed away. In some patches from HeLa cells containing many channels, we have observed activation of one or two channels by AITC that eventually closed with time, but this was a rare event in most patches. Further studies are clearly needed to identify the cytosolic factor and its mechanism of action and to resolve the issue of polyphosphate requirement for activation.
While our paper was under review, Karashima et al. (15) reported that PIP2 delays desensitization of TRPA1 and that PIP2 and Mg-ATP induces recovery of TRPA1 in inside-out patches. It was therefore concluded that PIP2 has a positive modulatory effect on TRPA1 (15). These findings are in contradiction to our results that PIP2 inhibits TRPA1 and the results of Dai et al. (7). Clearly, further studies are needed to address the complex nature of TRPA1 regulation under different conditions.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-55363 (to D. Kim) and in part by a grant from Rosalind Franklin University to (D. Kim).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 583: 175–193, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248–12252, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118: 1899–1910, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, Rosenmann E, Gonzalez-Nilo F, Latorre R. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA 104: 10246–10251, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 117: 1979–1987, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 282: 13180–13189, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Estacion M, Sinkins WG, Schilling WP. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J Physiol 530: 1–19, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci 8: 921–934, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hardie RC TRP channels in Drosophila photoreceptors: the lipid connection. Cell Calcium 33: 385–393, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron 30: 149–159, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103: 19564–19568, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch, 2008. May 7 [Epub ahead of print]. [DOI] [PubMed]
- 16.Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci 27: 6500–6509, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci 10: 568–577, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Cha SK, Sun TJ, Huang CL. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J Gen Physiol 126: 439–451, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 1674–1681, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 4835–4843, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100: 15160–15165, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci 27: 7070–7080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 451–455, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci 27: 11412–11415, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104: 13525–13530, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata K, Duggan A, Kumar G, and Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25: 467–478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 115: 2393–2401, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. Isoform-specific Inhibition of TRPC4 Channel by Phosphatidylinositol 4,5-Bisphosphate. J Biol Chem 283: 10026–10036, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8: 626–634, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflügers Arch 455: 157–168, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 4: 329–336, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res 1160: 39–46, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128: 509–522, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35: 507–520, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104: 13519–13524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voets T, Nilius B. Modulation of TRPs by PIPs. J Physiol 582: 939–944, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca(2+). Nat Neurosci 10: 277–279, 2007. [DOI] [PubMed] [Google Scholar]