Abstract
Vascular endothelial cells (ECs) play a major role in regulating vascular tone and in revascularization. There is increasing evidence showing endothelial dysfunction in diabetes, although little is known about the contribution of connexins (Cxs) to vascular complications in the diabetic heart. This study was designed to investigate the role of Cxs in coronary endothelial dysfunction in diabetic mice. Coronary ECs isolated from diabetic mice exhibit lowered protein levels of Cx37 and Cx40 (but not Cx43) and a loss of gap junction intercellular communication (GJIC). Vasodilatation induced by the assumed contribution of EC-dependent hyperpolarization was significantly reduced in the diabetic coronary artery (CA). Cx40-specific inhibitory peptide 40GAP27 strongly attenuated endothelium-dependent relaxation in diabetic CA at the concentration that does not affect the relaxation in control CA, suggesting that the total amount of Cx40 is lower in diabetic CA than in control CA. In diabetic mice, coronary capillary density was significantly decreased in vivo. In vitro, GJIC inhibitor attenuated the ability of EC capillary network formation. High-glucose treatment caused a decrease in Cx40 protein expression in ECs and impaired endothelial capillary network formation, which was restored by Cx40 overexpression. Furthermore, we found that the hyperglycemia-induced decrease in Cx40 was associated with inhibited protein expression of Sp1, a transcriptional factor that regulates Cx40 expression. These data suggest that downregulation of Cx40 protein expression and resultant inhibition of GJIC contribute to coronary vascular dysfunction in diabetes.
Keywords: gap junction, endothelium-dependent relaxation, microvascular rarefaction, coronary vascular complications, Sp1
more than 20 million people in the United States have diabetes, a serious and life-long condition. Coronary artery disease is the leading cause of death among people with diabetes. Coronary blood flow and myocardial oxygen consumption are closely matched in the normal heart through changes in coronary vascular resistance in response to metabolic demands of the myocardium (3). Vascular endothelial cells (ECs) play a major role in regulating vascular tone and in revascularization, both of which contribute to determining vascular resistance. Endothelial dysfunction is considered a major risk factor of cardiovascular complications of Type 1 and Type 2 diabetes (19, 44).
The endothelium regulates vascular tone by producing and releasing nitric oxide (NO) and prostacyclin and by hyperpolarizing smooth muscle cell (SMC). ECs can evoke SMC hyperpolarization by releasing endothelium-derived hyperpolarizing factor (EDHF) and directly by intercellular communication through gap junctions. Gap junctions are cell membrane channels made of connexin (Cx) proteins, and 20 Cx genes have been identified in the mouse genome (37). Cx37, Cx40, and Cx43 (and Cx45 in some case) are described in the vascular wall, and Cx40 is highly expressed in ECs (45, 50). The Cx40-knockout mice are hypertensive (11), whereas polymorphisms in the promoter of Cx40 gene have been shown to associate with increased risk of hypertension in patients (21). Furthermore, in hypertensive rats, Cx40 protein expression is decreased in ECs (28, 41) [but not in the kidney (24) and the cardiomyocyte (2)]. These data suggest that Cx40 in ECs may play an important role in the development of hypertension; however, the role of Cx40 in coronary endothelial dysfunction in diabetes is unexplored.
Insufficient growth and rarefaction of capillaries, followed by endothelial dysfunction, may represent one of the most critical mechanisms involved in heart damage. It has been reported that capillary density in the heart is progressively decreased in Type 1 and Type 2 diabetic animal models, as well as in human diabetic patients (5, 23, 25, 47, 55). Decreased density of capillaries is due to loss of existing capillary network and attenuated regeneration of new capillaries. Increasing capillary density in the heart, which improves the capacity to transport oxygen to cardiac tissues with a high tissue perfusion and a short diffusion distance for oxygen, is therefore another potential therapeutic strategy for coronary vascular disease.
The present study was designed to investigate the role of Cx40 on vascular complication in the diabetic heart. To that end, we determined the protein expression level of Cxs in coronary endothelial cells and gap junction intercellular communication (GJIC) contribution on endothelial function in diabetes.
MATERIALS AND METHODS
Biological materials and reagents.
Antibodies and reagents used were as follows: anti-Cx37, anti-Cx40, medium 199 (M199), antibiotics reagents (Invitrogen, Carlsbad, CA), Cx43 (Chemicon International, Temecula, CA), anti-Sp1, anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD31, ECGS, Matrigel (BD Biosciences, San Jose, CA), streptozotocin (ALEXIS Biochemicals, San Diego, CA), collagenase II (Worthington Biochemical, Lakewood, NJ), and dispase II (Rhoche Diagnostics North America, Indianapolis, IN). Cx-mimetic peptides 40GAP27 (SRPTEKNVFIV) and control scrambled peptide (SRGGEKNVFIV) were from SynBioSci (Livemore, CA). All other chemicals were from Sigma-Aldrich (St. Louis, MO).
Preparation of diabetic mice.
Six-week-old male NIH Swiss mice were purchased from Harlan Sprague Dawley (Indianapolis, IN), and mice in the diabetic group received a single injection of streptozotocin (160 mg/kg iv, dissolved in citrate buffer). All data were obtained from mice 10–12 wk after injection. Plasma glucose levels were 7.9 ± 0.4 mM in control mice and 36.5 ± 1.6 mM in diabetic mice. This study was conducted in accordance with the guidelines established by Committee on Animal Research at the University of California, San Diego.
Isolation of coronary vascular endothelial cells.
Mouse coronary ECs were isolated using a modification of previously described methods (56). Briefly, dissected heart tissues were minced and incubated with M199 containing 1 mg/ml collagenase II and 0.6 U/ml dispase II, for 1 h at 37°C. The digested material was filtered through sterile 40-μm nylon mesh and washed in 2% fetal calf serum-M199. Subsequently, the cells were incubated with Dynabeads (Invitrogen), which were prepared as follows: beads coated with sheep anti-rat IgG were incubated with purified rat anti-mouse CD31 monoclonal antibody (1 μg/ml) at 4°C overnight and then washed with PBS containing 0.1% BSA and 2 mM EDTA. Cell suspension was incubated with beads for 1 h at 4°C, and then beads attached to endothelial cells were captured by Dynal magnet (Invitrogen). Purity of the EC population in cells isolated from hearts was tested by DiI-acLDL uptake and Bandeiraea Simplicifolia lectin-FITC (BS-l, Sigma Aldrich) staining in mouse coronary ECs. Cultured human coronary smooth muscle cells (HCSMC) were used as negative control. Mouse coronary ECs were seeded on a cover glass in a four-well chamber. After 3 days of incubation, cells were stained with Dil-acLDL (10 μg/ml, Invitrogen) and incubated for 4 h at 37°C. At the last 30 min, Hoechst (1 μg/ml, Invitrogen) was added to the cells to stain nuclei. Cells were washed with PBS, fixed with 4% paraformaldehyde, and incubated with BS-l for 30 min. The percentage of positive cells that exhibit both acLDL and BS-l signal was over 91% in mouse coronary EC cultures (the experiments were reproduced five times).
Western blot analysis.
Isolated ECs were lysed and centrifuged at 16,000 g for 10 min at 4°C. Supernatants were used as a sample protein. Samples were separated through a SDS-polyacrylamide gel and transferred to nitrocellular membranes. Blots were incubated with a primary antibody, anti-Cx37, Cx40, Cx43 antibodies (1:1,000), anti-Sp1 antibody (1:2,000), or anti-actin antibody (1:4,000), and followed by secondary antibody application. The immunoblots were detected with the ECL Western blotting detection reagents (Perkin-Elmer, Norton, OH). Band intensity was normalized to actin controls and is expressed in arbitrary units.
Detection of GJIC.
Isolated ECs were cultured in M199 supplemented with 20% FCS, 100 μg/ml endothelial cell growth supplement (ECGS), 100 IU/ml penicillin, 100 μg/ml streptomycin, 50 mg/l d-valine, and 16 U/ml of heparin. Cells were plated on the coverslips coated with 5% gelatin, cultured for 3–5 days, and used to determine GJIC activity. Cells on 25-mm coverslips were placed in a recording cell chamber on the stage of an inverted Nikon microscope (Eclipse/TE 200) with the TE-FM epifluorescence attachment. Excitation (428 nm) was provided by a mercury lamp. Fluorescence emission (536 nm) was collected using a ×40 Nikon Plan Fluor objective (0.75 numerical aperture) and an intensifier. The fluorescence images based on the fluorescence signals emitted from the cells were acquired at 1 Hz using an Image Intensifier Tube/Philips 1381 system (Stanford Photonics, Palo Alto, CA) and stored on an IBM-compatible computer for later analysis. Dye transfer through GJIC was studied using an electrode in the whole cell mode to deliver a known concentration (2 mM) of Lucifer Yellow (LY, Invitrogen) (49). GJIC activity was assessed by counting the number of surrounding cells showing LY fluorescence 30 min after loading LY into a cell in the middle of a group of cells.
Isometric tension measurement of coronary arterial ring.
The heart was isolated and placed in Krebs-Henseleit solution for dissection. Second- or third-order small coronary arteries (CAs) were cleaned of any adherent connective tissue and cardiomyocytes and cut into 1- to 1.5-mm segments. Rings were placed with tissue hooks onto force-displacement transducer, set at a resting tension of 0.1 g, and allowed to equilibrate for 60 min with intermittent washes every 20 min. After equilibration, each CA ring was contracted by treatment with PGF2α (1–5 μM). Functional vessels were confirmed by demonstrating the ring's ability to relax to 100 μM sodium nitroprusside (SNP). CAs in which 100% relaxation of the PGF2α response occurred were regarded as intact and functional vessels.
Electrophysiological measurements.
Isolated coronary ECs from control and diabetic heart were used for electrophysiological measurement. Whole cell K+ currents (IK) were recorded from ECs with an Axopatch 1D amplifier and a DigiData 1200 interface (Axon Instruments, Sunnyvale, CA) using conventional patch-clamp techniques. Briefly, a coverslip plated with cells was mounted on a Plexiglas perfusion chamber on a Nikon inverted microscope, and cells on the coverslip were bathed in Ca2+-free physiological salt solution (PSS) containing (in mM) 141 NaCl, 4.7 KCl, 3 MgCl2, 10 HEPES, 1 EGTA, and 10 glucose (pH 7.4). The pipette (intracellular) solution contained (in mM) 135 KCl, 4 MgCl2, 10 HEPES, 10 EGTA, and 5 Na2ATP (pH 7.2). All experiments were performed at room temperature (22–24°C). Patch pipettes (2–3 MΩ) were fabricated on an electrode puller (Sutter Instrument, Novato, CA) using borosilicate glass tubes and fire polished on a microforge (Narishige Scientific Instruments, Tokyo, Japan). Command voltage protocols and data acquisition were performed using pCLAMP-8 software (Axon Instruments). With the use of the 2- to 3-MΩ pipettes, the series resistance was at a range of 4–9 MΩ when the whole cell configuration was formed. Series resistance compensation was performed in most of the whole cell experiments. Leak and capacitative currents were subtracted using the P/4 protocol in pCLAMP software.
Analysis of capillary densities in left ventricular myocardium.
After 10 wk of STZ injection, the mice were used for an analysis of myocardial capillary vasculature similar to previously described methods (56) with modifications. The ventricle was dissected, embedded in OCT compound (Sakura Finetek, Torrance, CA), frozen in 2-methylbutane precooled with liquid nitrogen, and then kept at −80°C until sectioned. Sections (6 μm) were fixed in 4% formaldehyde for 5 min, blocked with 5% BSA for 30 min, and incubated with Bandeiraea Simplicifolia lectin-FITC (BS-l) for 30 min. BS-l is used to probe the terminal α-galactosyl saccharides associated with endothelial cells surface of arterioles and venules as well as capillaries. Subepicardial regions of the left ventricular (LV) free wall on the section were photographed in sequence by a CCD camera connected to a fluorescence microscope with a ×20 objective lens. For every experimental condition, at least two sections from each sample were examined, and at least eight microscopic fields were investigated. Capillary count was analyzed with ImageJ 1.33 (National Institutes of Health, Bethesda, MD). The average capillary numerical density NA/mm2 (number of capillaries per mm2) was calculated for each heart.
Determination of Cxs localization in a CA.
Vertical section of the CA (6 μm) was fixed with ice-cold acetone and stained with BS-1 and α-smooth muscle action (SMA), makers for ECs and SMCs, respectively. The same heart but a different section was fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton-X, and stained with Cx37 or Cx40 (1:200). FITC-labeled secondary antibody (1:2,000) was applied to the section for visualization.
Assay for in vitro capillary network formation.
Four-well Permanox cell culture slides were coated with Matrigel (150 μl/well) and allowed to solidify for 1 h at 37°C. Rat vascular ECs (4 × 104 cells/well, Cell Applications, San Diego, CA) were seeded on the Matrigel. For high-glucose (HG) treatment, 30 mM glucose was added to the medium (the final glucose concentration was 35 mM). In a control group of cells, equimolar mannitol was added to exclude the potential effect of changes in osmolarity (NG: glucose concentration, 5 mM). After 24 h, 10 microscopic fields selected at random were photographed. Each cell was outlined manually, and the total tube lengths (L, mm/mm2) and the density of capillary network D/mm2 (D = number of cross section of cells and grid; grid width = 10 pixels in 640 × 480 pixel image) were analyzed by MATLAB software (The Math Works, Natick, MA).
Trasfection of Cx40 into human coronary ECs for capillary network formation assay.
Human coronary ECs were transfected with an empty adenovirus (Control-Adv) or adenovirus containing the mouse Cx40 gene (Cx40-Adv) (200 pfu/cell). Three days after infection, cells (104 cells/well) were seeded on four-well Permanox slides coated with Matrigel, and capillary network formation assay was performed as described above.
Statistical analysis.
Values are expressed as means ± SE. Responses were evaluated with one-way ANOVA, with a Dunn multiple-range test. Statistical comparison between dose-response curves was made by two-way ANOVA with the Bonferroni correction performed post hoc to correct for multiple comparisons. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Decreased Cx37 and Cx40 protein expression in coronary ECs from diabetic mice.
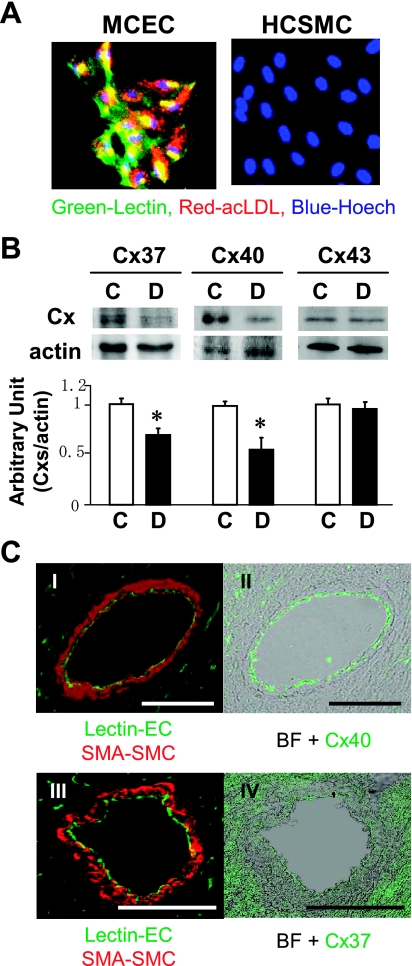
ECs isolated from hearts by magnetic beads exhibited a high purity of the EC population determined by acLDL uptake and BS-l staining (Fig. 1A). The cells were lysed, and Cxs protein levels were measured by Western blot analysis. Figure 1B shows that Cx37 and Cx40, but not Cx43, protein levels in coronary ECs were significantly lower in diabetic than the levels in control mice [protein expression levels; Cx37: 70.3% (P < 0.05), Cx40: 55.4% (P < 0.05), Cx43: 96.5% (P = 0.33), vs. control]. Immunohistological data shown in Fig. 1C demonstrate that Cx40 is dominantly expressed in ECs in the CA, whereas Cx37 is expressed in ECs and SMCs (low expression) as well as in cardiomyocytes (relatively high expression), suggesting that Cx40 might play an important role in the GJIC between ECs in diabetic heart.
Fig. 1.
Downregulated connexin (Cx) 37 and Cx40 protein expression in coronary endothelial cells (ECs) from diabetic mice. A: fluorescence images show that mouse coronary ECs (MCEC) isolated from hearts exhibits high purity of the EC population. Purity was tested by both acLDL uptake (red) and lectin staining (green) in MCEC and human smooth muscle cells (HCSMC) as control. Nuclear was stained by Hoechst. The percentage of positive cells that exhibit both colors was over 91% (5 times tested) in MCEC. B: Western blot analysis showing protein levels of Cx37, Cx40, and Cx43 in the coronary ECs from control (C) and diabetic (D) mice (top). Summarized data (bottom) showing protein expression levels of Cx37 (n = 5), Cx40 (n = 6), and Cx43 (n = 5), normalized by the protein expression level of actin are shown. Data are expressed as means ± SE. *P < 0.05 vs. control. C: localization of Cxs in the cornary artery (CA). Left (I and III): location of ECs (lectin, green) and SMCs (SMA, red). Right: localization of Cx40 (II, green) and Cx37 (IV, green). BF, bright field. Bar = 100 μm.
Inhibited GJIC in coronary ECs from diabetic mice.
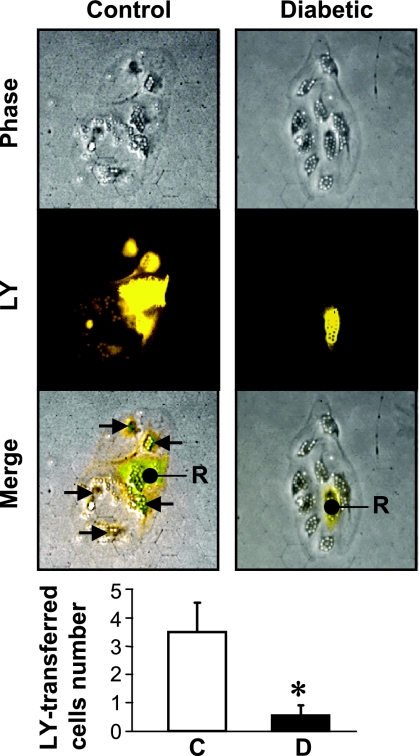
To investigate the GJIC activity between ECs in vitro, LY assay was performed with isolated ECs. Figure 2 demonstrates that the number of cells with dye, around the cell that was injected with dye (indicated by “R” in Fig. 2), was significantly lower in coronary ECs isolated from diabetic mice compared with cells from control mice. These results imply that attenuated GJIC might be due to the decreased expression and function of Cx40 in diabetic coronary ECs.
Fig. 2.
Inhibited gap junction intercellular communication (GJIC) in coronary ECs in diabetic mice. Representative phase contrast and fluorescence images showing the recipient cell (R) that was injected with Lucifer-Yellow (LY) and cells around R. Arrow indicates the cells to which LY is transported via gap junctions from R. Cells shown are coronary ECs from control (left) and diabetic (right) mice. Small particles on the cells are magnetic beads. Bottom shows averaged data from control (n = 6) and diabetic (n = 6) coronary ECs. Data are expressed as means ± SE. *P < 0.05 vs. control.
Endothelium-dependent CA relaxation is attenuated in diabetes due to Cx40 downregulation.
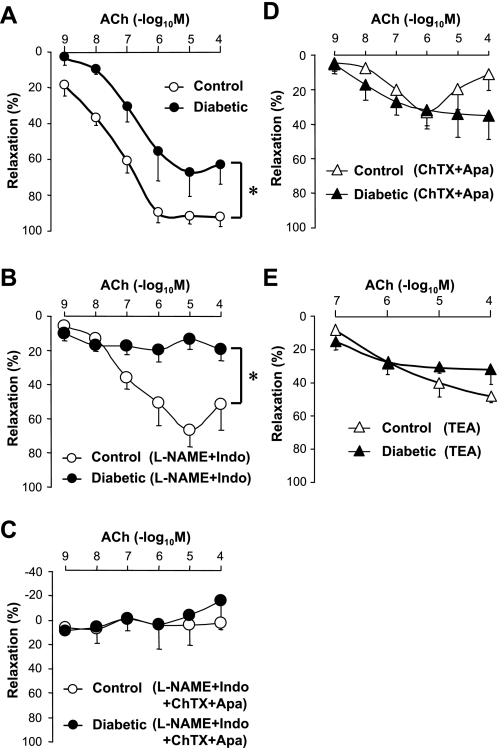
To investigate the role of GJIC in arteriole EC function, isometric tension was measured in isolated CA rings from control and diabetic mice. Acetylcholine (ACh)-induced vascular relaxation was normalized by maximum relaxation induced by 100 μM SNP. There was no significant difference in SNP-induced CA relaxation (as determined by percentage of PGF2α-induced contraction) between control (132.2 ± 6.6%) and diabetic (135.2 ± 5.9%, P = 0.20) mice. However, ACh-induced relaxation in CAs from diabetic mice was significantly attenuated compared with CAs from control mice (Fig. 3A).
Fig. 3.
Endothelium-dependent hyperpolarization (EDH)-induced vasorelaxation is attenuated in diabetic CA. A: dose-response curves of acetylcholine (ACh)-induced vasodilatation in CA rings isolated from control (open circles, n = 5) and diabetic (closed circles, n = 6) mice. Endothelium-dependent relaxation (EDR) was significantly decreased in diabetic CAs. *P < 0.05 vs. control. B: in the presence of N-nitro-l-arginine methyl ester (l-NAME, 100 μM) and indomethacin (Indo, 10 μM), EDR was almost abolished in diabetic CAs (closed circles, n = 4) but not in control CAs (open circles, n = 4). *P < 0.05 vs. control. C: EDR was abolished by the combinational treatment with l-NAME, Indo, apamin (Apa, 100 nM), and charybdotoxin (ChTX, 100 nM) in control (open circles, n = 4) and diabetic (closed circles, n = 4) CAs. D and E: EDR is inhibited by combinational treatment of Apa and ChTX (D, n = 4, respectively) or 10 mM tetraethylammonium (TEA) (E, control; n = 3, diabetic; n = 4) in both control (open triangles) and diabetic (closed triangles) CA rings. Data are expressed as means ± SE.
In the presence of N-nitro-l-arginine methyl ester (l-NAME, 100 μM), an endothelial NO synthase inhibitor, and indomethacin (Indo, 10 μM), a cyclooxygenase (COX) inhibitor, ACh-induced vasodilatation was significantly attenuated in diabetic CAs compared with control CAs (Fig. 3B). Combinational treatment with Indo, l-NAME, and apamin (Apa, 100 nM) plus charybdotoxin (ChTX, 100 nM), inhibitors of Ca2+-activated K+ channels, abolished ACh-induced vascular relaxation in both control and diabetic CAs (Fig. 3C). Pretreatment with Apa + ChTX or 10 mM tetraethylammonium (TEA, a nonselective K+ channel blocker) attenuated ACh-induced coronary vascular relaxation in both control and diabetic mice. Importantly, the magnitude of ACh-induced relaxation under either treatment (Apa + ChTX or TEA) was almost the same between control and diabetic rings (Fig. 3, D and E). Taken together, the data shown in Fig. 3 suggest that ACh-induced endothelium-dependent hyperpolarization (EDH) is attenuated in diabetic CA.
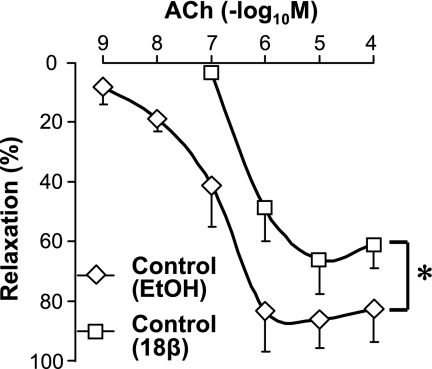
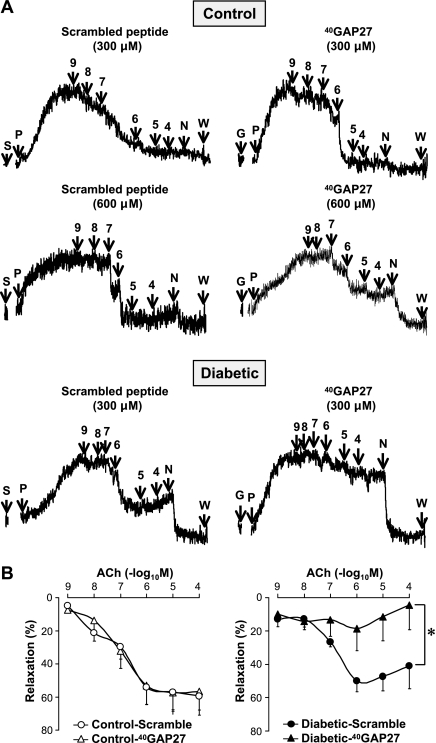
In addition, 20-min treatment of the CA rings isolated from control mice with 18β-glycyrrhetinic acid (18β-GA, 50 μM), a nonspecific gap junction inhibitor (1, 46), markedly attenuated ACh-induced vasodilatation (Fig. 4). Pretreatment of CA rings for 1 h with 300 μM 40GAP27, a selective inhibitor of Cx40, had no effect on ACh-induced vasorelaxation in control CA but strongly inhibited the relaxation in diabetic CA (Fig. 5). Only after the concentration of 40GAP27 was increased to 600 μM, it finally exhibited the inhibitory effect on the relaxation in control CA, indicating that diabetic CA forms much less gap junction with Cx40. These data suggest that GJIC is required for endothelium-dependent coronary vasodilatation, and attenuated vasodilatation observed in diabetic CA results in reduced GJIC function by a decrease in Cx40 expression in coronary ECs. An alternative explanation for the change in sensitivity of ACh-mediated EDR in diabetic CAs to 40GAP27 is that the gap junction-mediated EDR is altered in diabetes to be more dependent on Cx40.
Fig. 4.
Endothelium-dependent coronary vasodilatation is significantly attenuated by 18β-glycyrrhetinic acid (18β-GA). Dose-response curves of ACh-induced vasodilatation in control CA rings treated with 50 μM 18β-GA (open squares, n = 4) or vehicle (0.25% EtOH, open diamonds, n = 4). *P < 0.05 vs. vehicle. Data are expressed as means ± SE.
Fig. 5.
Cx40-specific inhibitor abolishes EDR in diabetic CAs but does not affect EDR in control CAs at the same concentration. A: representative record of vascular relaxation in CAs after application of a scramble peptide (left, indicated by S) or 40GAP27 (right, indicated by G). Each symbol indicates as follows: P, PGF2α; 9, 10−9 M ACh (same manner for 4∼8); N, sodium nitroprusside (SNP); W, wash. The concentration of 300 μM 40GAP27 exhibits no inhibitory effect on EDR, whereas 600 μM 40GAP27 starts to affect EDR in control CAs. However, 300 μM 40GAP27 is sufficient to inhibit EDR in diabetic CAs. B: dose-response curves of ACh-induced vasodilatation in control (n = 5) and diabetic (n = 5) CA rings treated with 300 μM scramble peptide and 300 μM 40GAP27. Data are expressed as means ± SE. *P < 0.05 vs. scramble peptide.
Whole cell K+ currents are comparable in coronary ECs between control and diabetic mice.
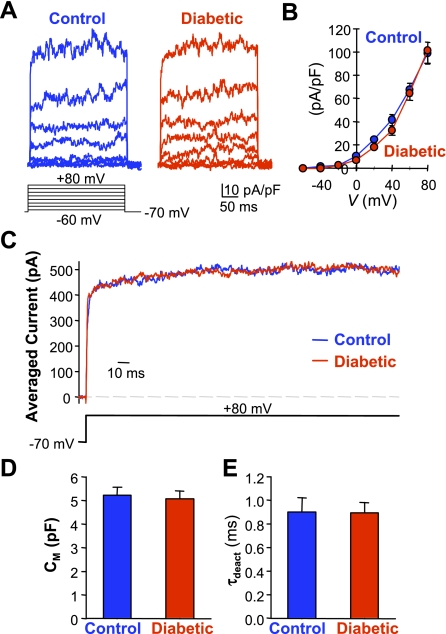
To test whether attenuated EDH-induced vasodilatation in diabetic CAs results from impaired K+ channel activity in diabetic ECs, we examined and compared the amplitude and kinetics of whole cell K+ currents in ECs isolated from heart of control and diabetic mice. As shown in Fig. 6, the amplitude of the currents and the current-voltage relationship (I-V) curves were comparable in control and diabetic ECs (Fig. 6, A and B). The averaged current at +80 mV had similar activation kinetics between control and diabetic ECs (Fig. 6C). Furthermore, cell capacitance (Cm, Fig. 6D) and the time constant for current deactivation (τdeact, Fig. 6E) were also comparable between control and diabetic ECs. These data suggest that attenuated EDH-mediated vascular relaxation in diabetes is not due to changes in K+ channel activity.
Fig. 6.
Comparison of whole cell K+ currents (IK) in control and diabetic ECs. A: representative currents, elicited by 300-ms step depolarizations at potentials ranging between −60 and +80 mV from a holding potential of −70 mV. B: summarized current-voltage relationship (I-V) curves constructed from control (n = 5) and diabetic (n = 5) cells. C: averaged currents at +80 mV showing that the activation kinetics is comparable between control and diabetic cells. D and E: summarized data (means ± SE) showing cell capacitance (Cm, D) and the time constant for current deactivation (τdeact, E) in control and diabetic ECs.
Reduced capillary density in LV myocardium in diabetic mice.
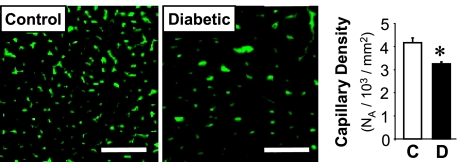
To determine the capillary density, heart sections of subepicardial regions in LV were photographed (Fig. 7, left), and at least two sections from each sample with eight microscopic fields were examined to calculate the average of capillary numerical density (see materials and methods). As shown in Fig. 7, the capillary density in LV myocardium was significantly decreased in diabetic mice (3,259 ± 60 /mm2, P < 0.05) compared with control mice (4,176 ± 202 /mm2).
Fig. 7.
Chronic hyperglycemia reduces capillary density in left ventricular (LV) myocardium. Representative photographs showing capillary images in LV of control and diabetic mice (left). Bar = 50 μm. Averaged data showing the capillary density in control (n = 5) and diabetic (n = 5) LV (right). Data are expressed as means ± SE. *P < 0.05 vs. control.
HG treatment decreases Cx40 protein level and attenuates capillary network formation in ECs.
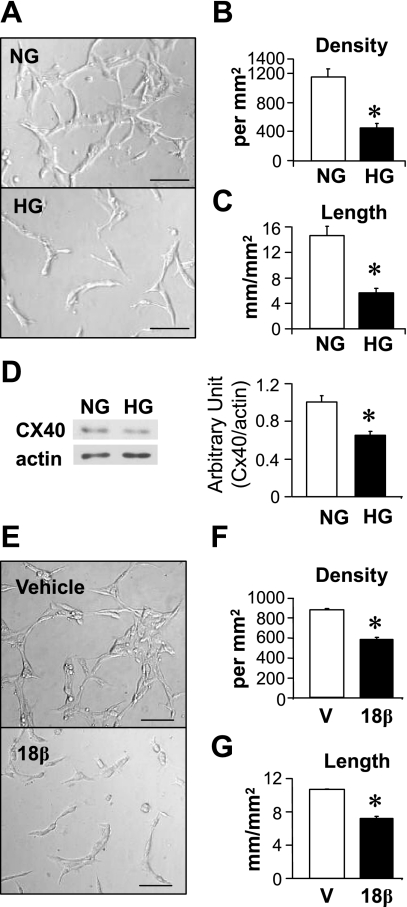
To test the effect of hyperglycemia on EC capillary network formation in vitro, rat vascular ECs were seeded on four-well chamber slide coated with Matrigel and cultured for 24 h. Figure 8, A–C, shows that ECs exposed to HG were unable to form tube-like structure. In addition, Cx40 protein expression in ECs was significantly decreased by hyperglycemia during tube formation (Fig. 8D). To determine the potential effect of GJIC on capillary network formation, 50 μM 18β-GA was added into the medium during tube formation. Figure 8, E–G, demonstrates that inhibition of GJIC with 18β-GA significantly reduced capillary network formation ability in ECs, indicating that GJIC plays an important role in capillary network formation. The results shown in Fig. 8 suggest that decreased ability of ECs for repair and revascularization may be one of the causes for decreased capillary density in the heart of diabetic mice and that reduced GJIC resulting in decreased Cx40 contributes to this endothelial cell dysfunction.
Fig. 8.
High-glucose (HG) treatment attenuates EC capillary network formation and downregulates Cx40 expression. A: representative images showing capillary network formation in control (NG) and HG-treated ECs. Bar = 100 μm. B and C: summarized data of network density (B) and total tube length (C) in NG (n = 5) and HG (n = 5)-treated ECs. *P < 0.05 vs. NG treated ECs. D: Western blot analysis showing protein expression level of Cx40 in ECs treated with NG (n = 4) and HG (n = 4). *P < 0.05 vs. NG-treated ECs. E: representative images showing capillary network formation in ECs treated with vehicle (V, 0.25% EtOH) and 18β-GA (18β). Bar = 100 μm. F and G: averaged data (n = 4, respectively) show that 18β-GA significantly decreases network density (F) and total tube length (G). *P < 0.05 vs. vehicle-treated ECs. Data are expressed as means ± SE.
Overexpression of Cx40 restores capillary network formation attenuated by hyperglycemia.
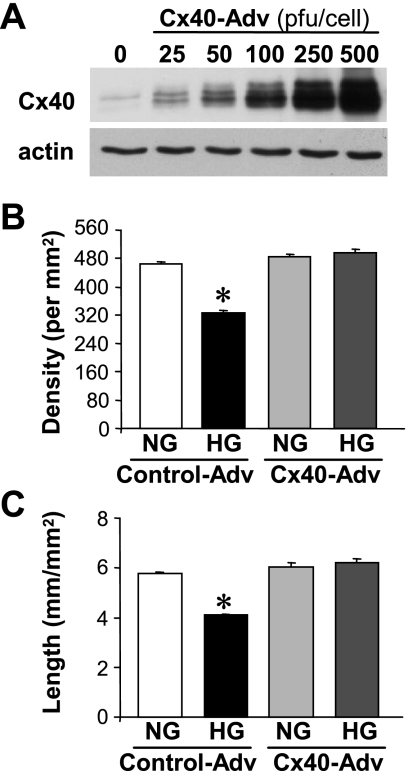
To test whether Cx40 regulates capillary network formation under hyperglycemia, human coronary ECs were transfected with Cx40 adenovirus or an empty adenovirus (as a control). Three days after infection, cells were used for capillary network formation assay. Overexpression of Cx40 (Fig. 9A) significantly inhibited a reduction of capillary network formation induced by HG treatment (Fig. 9, B and C), indicating that hyperglycemia induced attenuation of capillary network formation is, at least in part, mediated by decreased Cx40 protein expression by hyperglycemia.
Fig. 9.
Overexpression of Cx40 restores capillary network formation attenuated by hyperglycemia. A: Western blot analysis showing protein expression level of Cx40 in ECs after adenoviral transfection of Cx40 in a dose-dependent manner. B and C: averaged data showing network density (B) and total tube length (C) in control or Cx40 adenovirus (Adv)-transfected cells, which are treated with NG (5 mM) or HG (35 mM). *P < 0.05 vs. control-Adv-transfected NG-treated ECs. Data are expressed as means ± SE.
Hyperglycemia decreases Sp1 expression in ECs in vitro and in vivo.
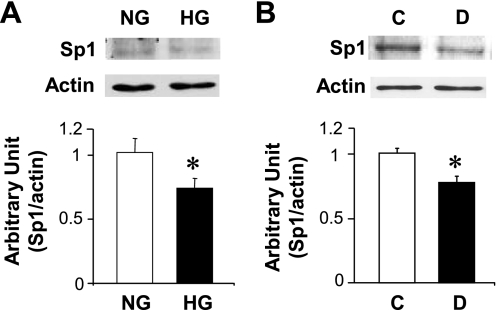
Transcription of Cx40 gene is positively controlled by the transcription factor Sp1. To elucidate the potential factors that may be involved in Cx40 downregulation under hyperglycemia, HG or mannitol (as a control) was added to the cells during tube formation, and Sp1 protein expression was measured. As shown in Fig. 10A, treatment of ECs with HG significantly decreased Sp1 expression. In line with this result, Sp1 protein expression was decreased in coronary ECs isolated from diabetic (D) mice compared with those from control (C) mice (Fig. 10B). These results suggest that downregulation of Cx40 in diabetic coronary ECs may be due, at least in part, to decreased Sp1 protein expression.
Fig. 10.
Hyperglycemia decreases Sp1 protein expression in vivo and in vitro. A: HG treatment for 24 h downregulates Sp1 protein expression in rat vascular ECs. n = 4 experiments in ECs treated with NG and HG, respectively. *P < 0.05 vs. NG-treated cells. Data are expressed as means ± SE. B: coronary ECs isolated from diabetic mice (D, n = 6) exhibits significantly lower Sp1 protein than ECs from control mice (C, n = 7). *P < 0.05 vs. control. Data are expressed as means ± SE.
DISCUSSION
It is becoming evident that GJIC may be closely linked to overall vascular physiology or pathophysiology. A number of pathologies in which intercellular communication is disrupted are known where the gap-junction proteins Cxs are modified (11, 21, 27, 28, 31, 41, 53). In the present report, we first demonstrate that Cx37 and Cx40 protein expression were significantly lowered in coronary ECs isolated from diabetic mice when compared with ECs from control (Fig. 1B). Other investigators reported altered expression of Cx43 or Cx45 in the cardiomyocyte (27, 31) or no change of any Cx expression in mesenteric artery (36, 39) in diabetic animals. A major reason for the divergent results might come from the variety of Cx isoforms and different expression levels between different species, organs, and vessel segments (9, 50, 52). As shown in Fig. 1C, Cx40 localizes only in the coronary vascular ECs, but Cx37 expresses in both ECs and SMCs, although the Cx37 protein expression level in CA is much lower than that in cardiomyocytes. These data suggest that Cx40 plays a more important role than Cx37 in GJIC between coronary ECs; the decrease in Cx40 expression in ECs would significantly affect homocellular GJIC among ECs. Supporting to these data, Fig. 2 shows direct evidence that GJIC is significantly inhibited in coronary ECs isolated from diabetic mice.
Coronary blood flow and coronary vascular resistance are tightly controlled by vascular tone and vascular density. EC serves as a major player in regulation of vascular tone and density; endothelial dysfunction is thus considered a risk factor of cardiovascular complications in many diseases, including diabetes. Diabetic CAs exhibit attenuated endothelium-dependent relaxation (EDR) (Fig. 3A). To confirm that the inhibited EDR in diabetic CAs involves the contribution of a non-NO/prostacyclin mediator of EDH, we conducted a series of pharmacological experiments using the combination of Apa and ChTX in the presence of l-NAME and Indo. When NOS and COX were blocked, ACh-induced coronary vasodilatation was almost abolished in diabetic mice, whereas ACh still caused dose-dependent relaxation in control mice (Fig. 3B). The difference of ACh-induced coronary vasodilatation between control and diabetic mice, in the presence of l-NAME and Indo, was likely due to the difference of the EDR independent of NO and prostacyclin. Inhibition of calcium-dependent K (KCa) channels with ChTX + Apa, in the presence of l-NAME and Indo, abolished ACh-induced relaxation in control CAs and eliminated the difference of ACh-induced coronary vasodilatation between control and diabetic mice (Fig. 3C). In the absence of l-NAME and Indo, the magnitude of ACh-induced relaxation in CAs treated with ChTX + apamin (Fig. 3D) or TEA alone (Fig. 3E) was almost the same between control and diabetic mice. These data suggest that ACh-induced EDH is attenuated in diabetic CAs.
There is accumulating evidence showing that EDH is impaired in diabetic animal models and diabetic patients, although some studies suggest that EDH is enhanced in diabetes (8, 10, 20, 22, 33, 36, 48). These inconsistent results imply that the changes in vascular relaxation mediated by EDH in diabetes may vary depending on the tissues studied, the diabetic period, and the type of diabetic models. Inhibition of direct cell-to cell coupling by Cx-mimetic peptides (e.g., Gap26 and Gap27) and GA derivatives attenuate EDH and vasodilatation (14, 46). Inhibition of GJIC with 18β-GA significantly decreased EDR in control CAs (Fig. 4), whereas we were unable to evaluate the effect of 18β-GA in diabetic CAs since vehicle treatment (0.25% EtOH) interfered with EDR in diabetic CAs. The mechanism that induces inhibitory effect by EtOH on EDR in diabetic CAs is not clear and needs further study.
Cx mimetic peptides are effective in diminishing GJIC by simulating crucial extracellular loop sequences and interacting prematurely with unpaired connexons in the plasma membrane, thus disrupting the docking of the complementary hemichannels and reducing the number of operational gap junctions (17). The density of functional Cxs on the surface membrane can be influenced by modulation of transport along cytoskeletal elements, and a gap junction's resident time in the surface membrane is likely to be short because of the rapid turnover of Cxs (1–5 h) (18, 30). Thus Cx mimetic peptides are usually exposed to the tissues at least for 1 h to block not only the Cxs in the surface membrane but also renewed Cxs from cytosol. In contrast, some reports demonstrated different inhibitory mechanisms by Cx mimetic peptides in different cell types. In A7r5 cells, Martin et al. (34) reported that Cx-mimetic peptides (e.g., 37,43GAP26 and 37,43GAP27) had no effect on Cx trafficking or the de novo formation of gap plaques. Furthermore, in coupled cell pairs under cell culture condition, Cx-mimetic peptides are also capable of blocking electric coupling within 5 min (35). These observations indicate that the Cx-mimetic peptide might exert the inhibitory effect on gap junction via multiple mechanisms. As shown in Fig. 5, 300 μM 40GAP27 completely inhibited EDR in diabetic CAs but not in control CAs. Twice higher concentration of 40GAP27 was needed to partially inhibit EDR in control CAs. These data suggest that the total amount of Cx40 in diabetic CAs is much less than that in control CAs, which is in line with the result of Western blot analysis of Cx40 in coronary ECs (Fig. 1B). When taken together, the data from current study demonstrate that EDR is attenuated in diabetic CAs because of downregulated expression (and/or decreased function) of Cx40.
Although our data indicate that Cx40 is an important gap junction peptide required for normal coronary vascular function, it has to be notified that multiple gap junction peptides may be required to completely block endothelium-dependent hyperpolarization (6). More study, using combinations of different Cx mimetic peptides, is needed to further reveal the identity of the gap junction proteins involved in coronary vascular dysfunction in diabetic mice. Furthermore, it has to be emphasized that changes in Cx expression and function may not be the sole contributors to endothelial dysfunction, and multiple mechanisms contribute to the inhibited coronary vasodilatation in diabetic mice. In addition to the impaired GJIC by decreased Cx40 expression, we believe that the inhibited endothelium-dependent hyperpolarization in diabetic CA may also involve other mechanisms: for example, 1) dysfunctional coupling of Cx peptides and their spatial co-association with K+ channels (12, 42), and 2) changes in expression and activity K+ channels in the vascular smooth muscle cells (13, 26).
In addition to regulation of vascular tone, ECs also play an important role in revascularization in the capillary network. The data shown in Fig. 7 demonstrate that capillary density in LV myocardium was significantly decreased in diabetic mice compared with that in control mice. Decreased density of capillaries is due to loss of existing capillary network and to attenuated regeneration of new capillaries. EC apoptosis and occlusion of arteriole and capillaries both contribute to microvascular rarefaction. Indeed, we observed apoptosis of capillary ECs in the LV myocardium of diabetic mice; the apoptotic rate in capillary ECs was higher in diabetic mice (5.8 ± 0.7%; P < 0.05) than that in control mice (2.6 ± 0.6%). Our results are consistent with the observations by other investigators (5, 55).
Regeneration of new EC monolayer and formation of new vessels attribute to the migration and proliferation of neighboring mature ECs and to the mobilization of circulating endothelial progenitor cells in sites of vascular wall where ECs are injured and/or lost. The results from our study show that ECs treated with HG had significantly impaired ability to form capillary network in vitro (Fig. 8, A–C), implying that hyperglycemia may attenuate ECs to form new capillary network, subsequently decreasing capillary density. In addition, 18β-GA significantly attenuated capillary network formation (Fig. 8, E–G), which indicates that GJIC is required for EC-derived capillary network formation. Interestingly, we also found that Cx40 protein expression was decreased by HG treatment during network formation (Fig. 8D). Furthermore, we demonstrated that overexpression of Cx40 gene in ECs restored capillary network formation that was attenuated by hyperglycemia (Fig. 9). These data support the contention that hyperglycemia downregulates Cx40 protein expression, which makes ECs dysfunctional to form new capillary network and subsequently decreases capillary density in the heart.
Participation of gap junctions in vascular development and control of cell proliferation are reported but in a highly specific manner. In cultured ECs, wound-induced migration is associated with an increased Cx43 expression and a downregulated Cx37 expression, but with no change in Cx40 expression (29). Thromboxane A2-induced reduction of endothelial tube formation is mediated by impairment of GJIC (1). After mechanical injury in SMCs, Cx43 expression is upregulated in the neointima of rat carotid artery, whereas no change of Cx43 is observed in rabbit iliac artery (40, 54). This evidence suggests that vascular Cxs seems to be essential to the coordination of cell proliferation, migration, and angiogenesis.
Mouse Cx40 gene contains a number of potential transcription factor binding sites (e.g., for AP-2, Sp1, and p53) (37, 43). It has been shown that Sp1 positively regulates Cx40 gene transcription in mice, rats, and humans (4, 16, 32). We demonstrate here that Sp1 expression was decreased in coronary ECs isolated from diabetic mice and in ECs treated with HG for 24 h compared with that in respective controls (Fig. 10). These data suggest that reduction of Cx40 expression by hyperglycemia might be caused by downregulation of Sp1 protein expression and/or attenuation of Sp1 function. Functional change of Sp1 by O-GlcNacylation are well studied compared with the change in protein expression of Sp1 in the diabetic state (7, 15, 38, 51), although those data reached inconsistent results. Yang et al. (51) showed that O-GlcNAcylation of Sp1 represses Sp1-mediated transcription. In contrast, Du et al. (15) demonstrated that O-GlcNAcylated Sp1 activates transcription of responsive genes. Our laboratory showed that HG treatment increases Sp1 O-GlcNAcylation and decreases SERCA promoter activity in cardiomyocytes (7). Further study is needed to investigate whether O-GlcNAcylation of the Sp1, which regulates Cx40 gene transcription, is involved in downregulating Cx40 expression and subsequently causing coronary endothelial dysfunction in diabetes.
In conclusion, the results of this study demonstrate that hyperglycemia may contribute to the development of diabetic coronary vascular complications by selectively downregulating the Cx40 protein expression in coronary arterial ECs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-66917.
Acknowledgments
We thank Dr. Atsushi Miyanohara for making the adenoviral vector constructed with Cx40 gene.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ashton AW, Yokota R, John G, Zhao S, Suadicani SO, Spray DC, Ware JA. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). J Biol Chem 274: 35562–35570, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bastide B, Neyses L, Ganten D, Paul M, Willecke K, Traub O. Gap junction protein connexin40 is preferentially expressed in vascular endothelium and conductive bundles of rat myocardium and is increased under hypertensive conditions. Circ Res 73: 1138–1149, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Berne RM Regulation of coronary blood flow. Physiol Rev 44: 1–29, 1964. [DOI] [PubMed] [Google Scholar]
- 4.Bierhuizen MF, van Amersfoorth SC, Groenewegen WA, Vliex S, Jongsma HJ. Characterization of the rat connexin40 promoter: two Sp1/Sp3 binding sites contribute to transcriptional activation. Cardiovasc Res 46: 511–522, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Camp TM, Tyagi SC, Senior RM, Hayden MR. Gelatinase B(MMP-9) an apoptotic factor in diabetic transgenic mice. Diabetologia 46: 1438–1445, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chaytor AT, Martin PE, Edwards DH, Griffith TM. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am J Physiol Heart Circ Physiol 280: H2441–H2450, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278: 44230–44237, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol 31: 641–649, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE. Gap junction protein phenotypes of the human heart and conduction system. J Cardiovasc Electrophysiol 6: 813–822, 1995. [DOI] [PubMed] [Google Scholar]
- 10.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Diep HK, Vigmond EJ, Segal SS, Welsh DG. Defining electrical communication in skeletal muscle resistance arteries: a computational approach. J Physiol 568: 267–281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, Triggle C. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol 146: 1110–1118, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dora KA, Martin PE, Chaytor AT, Evans WH, Garland CJ, Griffith TM. Role of heterocellular Gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochem Biophys Res Commun 254: 27–31, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97: 12222–12226, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupays L, Mazurais D, Rucker-Martin C, Calmels T, Bernot D, Cronier L, Malassine A, Gros D, and Theveniau-Ruissy M. Genomic organization and alternative transcripts of the human Connexin40 gene. Gene 305: 79–90, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 29: 606–612, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Evans WH, Martin PE. Gap junctions: structure and function (Review). Mol Membr Biol 19: 121–136, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther 111: 384–399, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Feletou M, Vanhoutte PM. EDHF: new therapeutic targets? Pharmacol Res 49: 565–580, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Firouzi M, Kok B, Spiering W, Busjahn A, Bezzina CR, Ruijter JM, Koeleman BP, Schipper M, Groenewegen WA, Jongsma HJ, de Leeuw PW. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens 24: 325–330, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald SM, Kemp-Harper BK, Tare M, Parkington HC. Role of endothelium-derived hyperpolarizing factor in endothelial dysfunction during diabetes. Clin Exp Pharmacol Physiol 32: 482–487, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Gross ML, Heiss N, Weckbach M, Hansen A, El-Shakmak A, Szabo A, Munter K, Ritz E, Amann K. ACE-inhibition is superior to endothelin A receptor blockade in preventing abnormal capillary supply and fibrosis of the heart in experimental diabetes. Diabetologia 47: 316–324, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int 60: 190–201, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Sohmiya K, Ukimura A, Endoh S, Mori T, Shimomura H, Okabe M, Terasaki F, Kitaura Y. Angiotensin II receptor blockade prevents microangiopathy and preserves diastolic function in the diabetic rat heart. Heart 89: 1236–1242, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2275–H2284, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Howarth FC, Nowotny N, Zilahi E, El Haj MA, Lei M. Altered expression of gap junction connexin proteins may partly underlie heart rhythm disturbances in the streptozotocin-induced diabetic rat heart. Mol Cell Biochem 305: 145–151, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Kansui Y, Fujii K, Nakamura K, Goto K, Oniki H, Abe I, Shibata Y, Iida M. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol 287: H216–H224, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kwak BR, Pepper MS, Gros DB, Meda P. Inhibition of endothelial wound repair by dominant negative connexin inhibitors. Mol Biol Cell 12: 831–845, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 273: 67–72, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, Ogawa K, Imanaga I, Tribulova N. Alterations of connexin 43 in the diabetic rat heart. Adv Cardiol 42: 243–254, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Linhares VL, Almeida NA, Menezes DC, Elliott DA, Lai D, Beyer EC, Campos de Carvalho AC, Costa MW. Transcriptional regulation of the murine Connexin40 promoter by cardiac factors Nkx2–5, GATA4 and Tbx5. Cardiovasc Res 64: 402–411, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino A, Ohuchi K, Kamata K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br J Pharmacol 130: 549–556, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin PE, Wall C, Griffith TM. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br J Pharmacol 144: 617–627, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matchkov VV, Rahman A, Bakker LM, Griffith TM, Nilsson H, Aalkjaer C. Analysis of effects of connexin-mimetic peptides in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol 291: H357–H367, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto T, Miyamori K, Kobayashi T, Kamata K. Specific impairment of endothelium-derived hyperpolarizing factor-type relaxation in mesenteric arteries from streptozotocin-induced diabetic mice. Vascul Pharmacol 44: 450–460, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta 1719: 6–23, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Pan X, Solomon SS, Borromeo DM, Martinez-Hernandez A, Raghow R. Insulin deprivation leads to deficiency of Sp1 transcription factor in H-411E hepatoma cells and in streptozotocin-induced diabetic ketoacidosis in the rat. Endocrinology 142: 1635–1642, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Pannirselvam M, Ding H, Anderson TJ, Triggle CR. Pharmacological characteristics of endothelium-derived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur J Pharmacol 551: 98–107, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Polacek D, Bech F, McKinsey JF, Davies PF. Connexin43 gene expression in the rabbit arterial wall: effects of hypercholesterolemia, balloon injury and their combination. J Vasc Res 34: 19–30, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Rummery NM, McKenzie KU, Whitworth JA, Hill CE. Decreased endothelial size and connexin expression in rat caudal arteries during hypertension. J Hypertens 20: 247–253, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat 209: 689–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seul KH, Tadros PN, Beyer EC. Mouse connexin40: gene structure and promoter analysis. Genomics 46: 120–126, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Shahab A Why does diabetes mellitus increase the risk of cardiovascular disease? Acta Med Indones 38: 33–41, 2006. [PubMed] [Google Scholar]
- 45.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Tare M, Coleman HA, Parkington HC. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am J Physiol Heart Circ Physiol 282: H335–H341, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Tasca C, Stefaneanu L, Vasilescu C. The myocardial microangiopathy in human and experimental diabetes mellitus. (A microscopic, ultrastructural, morphometric and computer-assisted symbolic-logic analysis). Endocrinologie 24: 59–69, 1986. [PubMed] [Google Scholar]
- 48.Triggle CR, Ding H, Anderson TJ, Pannirselvam M. The endothelium in health and disease: a discussion of the contribution of non-nitric oxide endothelium-derived vasoactive mediators to vascular homeostasis in normal vessels and in type II diabetes. Mol Cell Biochem 263: 21–27, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res 91: 104–111, 2002. [DOI] [PubMed] [Google Scholar]
- 50.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol 112: 479–486, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci USA 98: 6611–6616, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh HI, Dupont E, Coppen S, Rothery S, Severs NJ. Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J Histochem Cytochem 45: 539–550, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Yeh HI, Lu CS, Wu YJ, Chen CC, Hong RC, Ko YS, Shiao MS, Severs NJ, Tsai CH. Reduced expression of endothelial connexin37 and connexin40 in hyperlipidemic mice: recovery of connexin37 after 7-day simvastatin treatment. Arterioscler Thromb Vasc Biol 23: 1391–1397, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Yeh HI, Lupu F, Dupont E, Severs NJ. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol 17: 3174–3184, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111: 2073–2085, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Zhao X, Lu X, Feng Q. Deficiency in endothelial nitric oxide synthase impairs myocardial angiogenesis. Am J Physiol Heart Circ Physiol 283: H2371–H2378, 2002. [DOI] [PubMed] [Google Scholar]