Abstract
Introduction
The contribution of vascular endothelial cells to prostate growth has not been investigated. We examined whether endothelial cells support growth of prostate tissue when co-inoculated with prostate epithelial cells under the renal capsule.
Methods
Vascular endothelial cells were isolated from mice and co-inoculated under the renal capsule with a prostate luminal or basal epithelial cell line. After 60 days, kidneys were examined for growth of prostate tissue. Prostatic tissues were examined by immunohistochemistry for expression of cytokeratins 5 and 8, and vascular density was determined. To determine if increased expression of VEGF-A would increase prostatic growth, transfected endothelial cells overexpressing VEGF-A were coinoculated with the prostate luminal or basal epithelial lines.
Results
Co-inoculation of endothelial cells and prostate luminal or basal epithelial cells resulted in significant growth of prostatic tissue, whereas inoculation of any of the cell lines alone resulted in little growth. The growths from co-inoculation of endothelial cells and luminal epithelial cells contained duct-like structures that stained with antibodies to cytokeratin-8, whereas those from co-inoculation of endothelial cells and basal epithelial cells contained cords of cells that stained with antibodies to cytokeratin-5. Overexpression of VEGF-A had no effect on growth of the prostatic tissues.
Conclusion
Endothelial cells contribute to the growth of prostatic epithelial cells.
Keywords: vascular endothelial growth factor, flt-1, endothelial cells
The development of the murine prostate depends on reciprocal signals between the stroma and epithelium (1,2). The importance of stromal signals has been shown in prostate reconstitution experiments in which combinations of dissociated prostate epithelial cells and undifferentiated urogenital sinus mesenchyme are inoculated under the renal capsule where they form a prostate-like organ (3-5). The urogenital sinus mesenchyme is required for formation of prostate-like structures. The stromal cells presumably provide appropriate signals that stimulate the epithelial cells to grow and organize into functional prostatic structures. In addition, the prostate stroma appears to regulate differentiation of prostate epithelium. When UGM is implanted under the renal capsule together with bladder or urethral epithelium, it directs these epithelia to develop into prostate tissue (6,7). These differentiative and organizing signals also appear to be present to some extent in mature prostate stroma (8). Prostate smooth muscle cells, fibroblasts, and even adipocytes support the development of prostate-like tissues when co-inoculated under the renal capsule with prostate epithelial cells (9-11. Another potential stromal source of signals to the prostatic epithelium is the endothelium of the blood vessels, but the ability of vascular endothelium to support prostatic epithelial growth has not been investigated.
There is ample evidence that there is a link between growth or regression of prostatic epithelium and prostatic blood vessels (12). During the involution of the prostate after castration of rats, blood vessels and epithelium regressed in parallel (13). Apoptosis of the epithelial cells correlated with apoptosis of the endothelial cells (14,15). Similarly, in castrated animals that were administered testosterone, increased DNA synthesis was observed in both prostate epithelial cells and prostate capillary endothelial cells after a one day lag (13,14). The labeling of the epithelial cells followed the same kinetics as the labeling of endothelial cells. Moreover, inhibition of vascular growth in castrated animals administered testosterone prevented the regeneration of the prostate and inhibited prostate epithelial proliferation (16,17). Together, these observations suggest that in the prostate epithelial growth is tightly coupled to vascular growth.
Considering the close relation between prostatic vascular growth and prostatic epithelial growth, it is likely that prostatic endothelial cells also contribute to prostatic epithelial growth. However, possible contribution of signals from the endothelium have not been investigated. Here we show that endothelial cells contribute to prostate epithelial growth and development.
Materials and Methods
Isolation of endothelial cells
Endothelial cell cultures were established by a modification of the methods of Folkman et al. (18) and Voyta et al. (19). Major airways were cut away from the lungs of Balb/c or FVB/N-Tg(TIE2-lacZ)182Sato/J mice, and the remaining lungs were minced into small fragments, washed in phosphate-buffered saline, and incubated in 0.15 % (w/v) collagenase (Worthington) in alpha modified Eagle's medium (αMEM) for 20 min at 37°C. The tissue suspension was pipetted against the side of the tube to break it into smaller pieces and centrifuged at 200 g for 5 min. The pellet was resuspended in αMEM containing 10 % fetal bovine serum and 5 ng/ml FGF-2 and plated onto 60 mm tissue culture dishes. After 4 h, unattached cells were washed away.
Cells from FVB/N-Tg(TIE2-lacZ)182Sato/J mice were plated at low density, and, after two days of culture, groups of cells with an endothelial cell morphology were identified and their position marked on the dish. Surrounding cells were dislodged from the dish using a drawn-out Pasteur pipet, and the remaining cells were grown in αMEM with 10% fetal bovine serum and 5 ng/ml FGF-2 until they formed a colony of approximately 200 cells. These colonies were trypsinized using cloning rings and transferred to wells of a 24-well plate. Cells from confluent wells were cloned by limiting dilution. Confluent cultures of the cloned cells were analyzed for ß-galactosidase activity. The cells were fixed for 15 min with 2% formaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline containing 2 mM MgCl2. Cultures were stained with 1 mg/ml X-gal (Sigma), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.01 % sodium deoxycholate, and 0.02% Nonidet P-40 in phosphatebuffered saline overnight at 37°C.
Endothelial cells from Balb/c mice were isolated based on their ability to take up acetylated low density lipoprotein (19). Cells harvested from the lungs were plated at high density on 60 mm dishes and incubated for one week in αMEM containing 10% fetal bovine serum and 5 ng/ml FGF-2. The cultures were incubated for 4 h in medium containing 10 μg/ml acetylated low density lipoprotein labeled with 1,1′-dioctadecyl- 3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (Biomedical Technologies, Inc., Stoughton, MA). Cells were washed with phosphate-buffered saline, trypsinized, and sorted on a FACScalibur fluorescence activated cell sorter (Becton Dickinson) as described (19). Cells in the upper 5% of fluorescence intensity were collected and plated at 5 × 103 cells/ well in a 24-well plate. After expansion, the cells were cloned by limiting dilution and verified as endothelial by staining with antibodies to von Willebrand factor.
Western blots
Endothelial cells were extracted in a buffer containing 0.5% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, 30 μg/ml of aprotinin and 10 mM EDTA. In other experiments, confluent cultures of endothelial cells transfected with an expression vector for VEGF-A or with the vector alone were incubated for 24 h in serum-free αMEM, and the conditioned medium was collected. EDTA was added to the conditioned medium to a final concentration of 10 mM, and the medium was concentrated 20-fold with Centricon-YM10 centrifugal filter devices (Millipore). Equal amounts of cell protein or concentrated conditioned medium were separated by electrophoresis on an 8% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose membrane (Schleicher & Schuell Protran, BioSciences) by electroblotting (Bio-Rad Laboratories, Hercules, CA). The membrane was blocked with 5% (wt/vol) fatfree milk powder, 0.5% (vol/vol) Tween 20 in phosphate-buffered saline overnight at 4°C and incubated with rabbit anti-human von Willebrand factor antibody (Dako), rabbit anti- Tie-2 (Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti-human VEGF-A (Santa Cruz Biotechnology) at a dilution of 1:300 for 2 h at room temperature. After washing in TBS, blots were incubated at room temperature for 1 h with a 1:2000 dilution of horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were washed three times with TBS. Immunocomplexes were developed using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Science, Inc, Boston, MA). The membrane was exposed to an X-ray film for imaging. Proteins were sized with Rainbow markers (Amersham Pharmacia Biotech).
RT-PCR
Confluent cultures of PE-L-1 cells or MLE-ßgal cells were extracted with TriZol reagent (GIBCO-BRL), and total RNA was isolated according to the manufacturer's instructions. The RNA was quantitated from its optical density, and its integrity assessed by 1.2% formaldehyde gel electrophoresis. Total RNA (2.5 μg) from each sample was reverse transcribed for 90 min at 42°C to obtain first-strand cDNAs using random hexamer primers and Maloney murine leukemia virus reverse transcriptase (Roche Diagnostics Corporation, Indianapolis, IN). The cDNA products in one-twentieth of the RT reaction mix were amplified by PCR using Thermus aquaticus DNA polymerase (Roche). The primer pair used for PCR amplification of mouse VEGFA cDNA was 5'-TGAGACCCTGGTGGACATCT-3' and 5'- CACCGCCTTGGCTTGTCAC-3'. These primers amplify the region of the VEGF-A message that is alternatively spliced and yield bands of 483, 411, 351, and 279 bp, representing messages for the 188, 164, 144, and 120 amino acid isoforms of VEGF-A. The primer pair used for amplification of mouse flk-1 cDNA was 5'- GTCATGGATCCAGATGAATTGC-3' and 5'-CGAAGTCACAGATCTTAACCAC-3' which yielded a band of 728 bp. For amplification of mouse flt-1 the primers 5'- GCACCCAGCATGTCATGCAAG-3' and 5'-TCAATCCGCTGCCTTATAGATG-3' yielded a band of 738 bp. As a control, the cDNA of α-actin was amplified using the primers 5'- ATCTGGCACCACACCTTCTACAATGAGCTGCG-3' and 5'- CGTCATACTCCTGCTTGCTGATCCACATCTGC-3' The PCR profile was as follows: 10 minutes at 95°C, followed by 30 cycles of 1 min at 94°C, 2 min at 58°C, and 3 min at 74°C. cDNA was isolated from the agarose gels using the GFX PCR DNA and gel band purification kit (Amersham Biosciences) and sequenced to verify its identity.
Transfection of cell lines
The cDNA for the 164 amino acid form of mouse VEGF-A (a gift from Genentech, Inc, South San Francisco, CA) was inserted between the BamH1 and HinD III sites of the pZeo SV vector (Invitrogen). Correct orientation of the insert was determined by restriction digestion, and identity of the insert was confirmed by DNA sequencing, MLE-ßgal cells at 40% confluence were transfected with pZeo SV vector containing the VEGF-A insert or with the empty vector using Lipofectamine (Invitrogen). Two days after transfection, the cells were trypsinized and replated at a 1:3 dilution in αMEM containing 10% fetal bovine serum, 5 ng/ml FGF-2, and 250 μg/ml Zeocin (Invitrogen). Colonies that survived Zeocin selection were analyzed by RT-PCR for VEGF-A expression. Clones that expressed 5 to 10-times more VEGF-A than nontransfected MLE-ßgal cells were selected for further study. Western blot analysis confirmed that MLE-ßgal cells transfected with the VEGF-A expression vector secreted 5 to 10-times more VEGF-A protein than MLE-ßgal cells transfected with the empty vector. There was no difference in growth rate between MLE-ßgal cells transfected with the VEGF-A expression vector and MLE cells transfected with the empty vector when plated in αMEM with 5 %, 1 %, or 0.2% serum.
Growth of prostatic tissues under the renal capsule
Prostatic luminal (PE-L-1) and basal (PE-B-1) epithelial cell lines established from p53 null C57BL/6 mice were described previously (20,21). Cells were harvested by trypsin treatment when approximately 80% confluent, and 2.5 × 105 PE-L-1 or PE-B-1 cells were mixed with 5 × 105 MLE-ßgal endothelial cells. Cells were pelleted and resuspended in 20 μl of type I collagen (Vitrogen-100 collagen from Collagen Corporation, Palo Alto, CA), and the collagen was allowed to gel at 37°C for 15 min. Collagen gels were also prepared containing 7.5 × 105 PE-L-1, PE-B-1, or MLE-ßgal cells alone or 1.5 × 106 MLE-ßgal cells alone. The gels were grafted beneath the renal capsule of 6-week-old male athymic mice (National Cancer Institute, Frederick, MD) as described (9). Each cell combination was implanted under the renal capsule into at least five kidneys for each experiment. Mice were sacrificed 6 weeks after gel implantation and the dimensions of each graft were measured.
Immunocytochemistry
At the time of sacrifice, kidneys were removed and cut with a scalpel through the highest part of the tissue growths under the renal capsule. Half of each kidney was embedded in OCT, and snap-frozen. The remainder of the kidney was fixed in formalin. Vascular density was determined in frozen sections using antibodies to PECAM-1, and differentiation of the implanted epithelial cells was determined using antibodies to cytokeratins 5 and 8. 8-μm-thick-frozen sections were post fixed in acetone for 10 minutes, treated with 0.3% hydrogen peroxide/PBS for 15 minutes, blocked in 5% normal rabbit serum or goat serum for 30 minutes, and biotin/avidin blocking reagents for 15 minutes each (Vector Laboratories, Inc.). The sections were then incubated rat anti-mouse PECAM-1 antibodies (diluted 1:200; Pharmingen) for 1 h at room temperature. The antibody-antigen complexes were visualized by the avidin/biotin method (Vectastain Elite ABC Kit, Vector Laboratories, Inc., Burlingame, CA) according to the manufacturer's instructions using diaminobenzidine as a substrate (Vector Laboratories, Inc.). The sections were counterstained with Mayer's hematoxylin (Sigma), dehydrated in graded concentrations of alcohol, and coverslipped with resin (Permount, Fisher Scientific Co.). Control sections were incubated with non-immune rabbit immunoglobulins using the same working dilution as the primary antibody. Vessels in the growths were counted in three non-adjacent sections per kidney using a Leica DMLB microscope equipped with an ocular grid.
The tissue fixed in formalin was embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin. Formalin-fixed sections were also stained with antibodies to cytokeratin 8 or cytokeratin 5 and counter-stained with Mayer's hematoxylin as described above.
Results
Establishment of mouse endothelial cell cultures
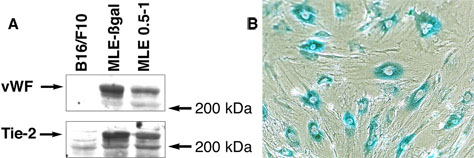
Endothelial cells were cultured from lungs of FVB/N-Tg(TIE2-lacZ)182Sato/J mice. These mice express ß-galactosidase under control of the endothelial cell-specific tie-2 promoter. Primary digests of the tissues were plated at low density, and clones of endothelial cells were selected based on their morphology (18) and grown to confluence in medium containing 10 % fetal calf serum and 5 ng/ml FGF-2. Confluent cultures of these cells (MLE-ßgal) were verified as endothelial by their expression of ß-galactosidase (Fig. 1B) and the endothelial cell-specific markers von Willebrand factor, Tie-2 (Fig. 1A), and PECAM-1 (data not shown). Cells with similar morphology were isolated from the lungs of wild type Balb/c mice (MLE cells) by sorting for cells that took up fluorescent labeled acetylated low density lipoprotein, a characteristic of endothelial cells (19). These cells also expressed von Willebrand factor, Tie-2 (Fig. 1A), and PECAM-1 (data not shown) at similar levels. Both lines have maintained expression of these markers for over 50 passages. Neither line is tumorigenic as no growths were detected 60 days after 5 × 106 cells are implanted subcutaneously in athymic mice.
Figure 1.
Characterization of endothelial cell lines. A. Expression of von Willebrand factor and Tie-2 by mouse endothelial cells. Proteins were extracted from confluent cultures of lung endothelial cells from wild-type mice (MLE 0.5-1) or from mice expressing ß-galactosidase under control of the tie-2 promoter (MLE-ßgal). As a control, proteins were extracted from cultures of B16 melanoma cells. Equal amounts of protein were separated by electrophoresis on an 8% polyacrylamide SDS gel and blotted onto nitrocellulose. Blots were probed with antibodies to von Willebrand factor (vWF) or Tie-2. B. Confluent cultures of MLE-ßgal cells were lightly fixed with 0.2 % glutaraldehyde and stained for ß-galactosidase activity using the X-gal substrate.
Endothelial cells support growth of prostate tissue in vivo
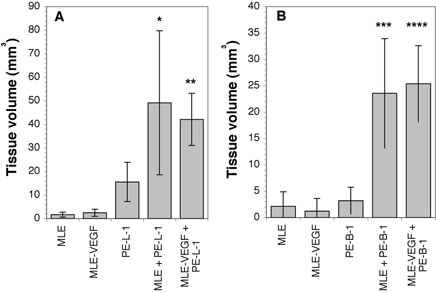
To determine if endothelial cells support the growth of prostate epithelial cells cells in vivo, we adapted the in vivo co-culture system in which combinations of prostatic cells inoculated under the renal capsule form prostatic tissues (9). In these experiments, 2.5 × 105 MLE-ßgal endothelial cells were co-inoculated with 5 × 105 PE-L-1 prostate luminal epithelial cells under the renal capsule. When inoculated alone, 7.5 × 105 or 1.5 × 106 MLE-ßgal cells did not grow, and 7.5 × 105 PE-L-1 cells grew to a minor extent (Fig. 2A). However, coinoculation of PE-L-1 cells with endothelial cells led to significant growth of prostatic tissue (Fig. 2A). Growths were not as large as those obtained when smooth muscle cells were co-inoculated with PE-L-1 cells (9). The growths contained large, epitheliallined cavities reminiscent of prostatic ducts (Fig. 3A) that often contained secretory products (not shown).
Figure 2.
Growth of prostatic cells under the renal capsule. MLE-ßgal mouse endothelial cells (2.5 × 105 cells) transfected with an expression vector encoding VEGFA (MLE-VEGF) or with the vector alone (MLE) were co-inoculated under the renal capsule with 5 × 105 PE-L-1 prostate luminal epithelial cells (A) or PE-B-1 prostate basal epithelial cells (B). As a control 7.5 × 105 MLE, MLE-VEGF, PE-L-1, or PE-B-1 cells were implanted alone. After 60 days, the mice were sacrificed, and the size of the prostatic growth measured. * p < 0.01 compared to MLE alone and p < 0.05 compared to PE-L-1 alone; ** p < 0.01 compared to MLE-VEGF alone or PE-L-1 alone; *** p < 0.01 compared to MLE alone or PE-B-1 alone; **** p < 0.01 compared to MLE-VEGF alone or PE-B-1 alone.
Figure 3.
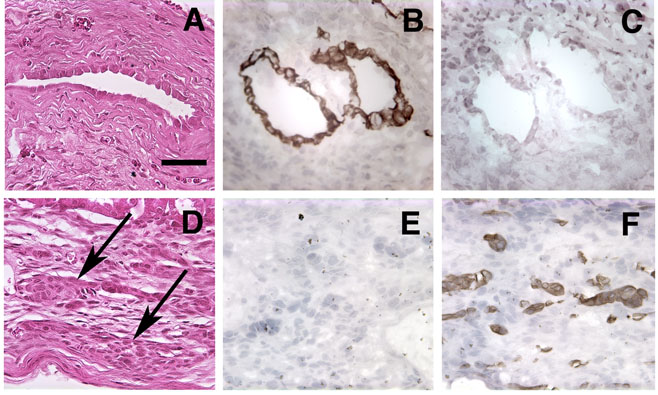
Histological and immunohistochemical analysis of growth under the renal capsule. Growths arising from the implantation of 2 × 105 MLE-ßgal cells and 5 × 105 PE-L-1 cells (A to C) or PE-B-1 cells (D to F) were harvested after 60 days, and 5 μm sections were stained with hematoxylin and eosin (A and D). Sections were also immunostained with antibodies to cytokeratin 8 (B and E) or cytokeratin 5 (C and F). Arrows in D point out cords of epitheloid cells. Bar in A equals 50 μm. All panels have the same magnification.
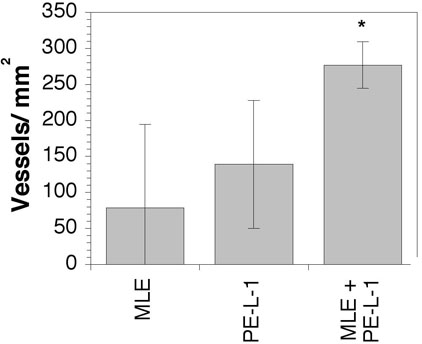
The effect of co-inoculation of endothelial cells with PE-L-1 cells on the vascularity of the prostatic growths was also investigated. Vascular density in the growths resulting from co-inoculation of MLE-ßgal cells and PE-L-1 cells was approximately double that in the small growths that occurred after inoculation of either cell type alone (Fig. 4). Using RT-PCR the mRNA for bacterial ß-galactosidase was detected in the prostatic growths, indicating that the implanted MLE-ßgal cells survived and incorporated into the growths. However, the MLE-ßgal cells could not be localized in the vessels of the growths by staining with the X-gal substrate for ß-galactosidase or immunostaining with antibodies to ß-galactosidase. Failure to detect the MLE-ßgal cells was probably due to the low level of expression of ß-galactosidase in these cells.
Figure 4.
Vascularity of prostatic growths under the renal capsule. Sections from the prostatic growth were stained with antibodies to PECAM-1 and vessel segments were counted in the prostatic growths. * p < 0.05 compared to MLE alone or PE-L-1 alone.
We also examined if endothelial cells would support the growth of prostate basal epithelial cells in vivo. When MLE-ßgal cells were co-inoculated with the PE-B-1 basal epithelial cell line under the renal capsule, significant growths of prostate-like tissue occurred (Fig. 2B). These growths contained small nests and cords of epitheloid cells (Fig. 3D) that did not have easily identifiable lumens. No growth was observed if MLEßgal cells or PE-B-1 cells were inoculated alone (Fig. 2B).
Endothelial cells support differentiation of prostatic cells
To determine if endothelial cells also support the differentiation of the prostate cells, we determined if the cells in the implants expressed luminal or basal cell-specific cytokeratins. In prostatic growths resulting from the implant of MLE-ßgal cells with PE-L-1 cells, all epithelial cells lining the duct-like structures stained positive for cytokeratin 8, a prostate luminal cell-specific cytokeratin (Fig. 3B). No expression of the basal cell-specific cytoketatin, cytokeratin 5, was observed (Fig. 3C). Similarly, in prostatic growths resulting from the implant of MLE-ßgal cells and PE-B-1 cells, all cells in the cord-like structures stained positive for basal cell-specific cytokeratin 5 (Fig. 3F), and expression of cytokeratin 8 was not observed (Fig. 3E). Thus, co-inoculation with endothelial cells supported continued expression of differentiated markers by the inoculated luminal and basal epithelial cells but did not promote transdifferentiation from one phenotype to the other.
Prostate epithelial cell growth is not stimulated by overproduction of VEGF-A
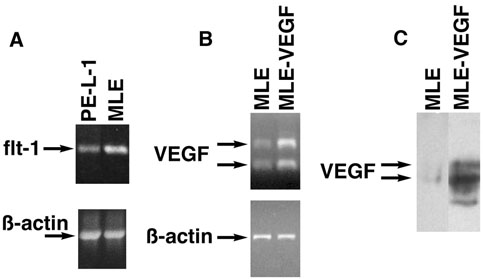
As it has been reported that prostate tumor cells express receptors for VEGF (22-28), the expression of VEGF receptors by the prostate luminal epithelial cell line, PE-L-1, was investigated. RT-PCR revealed that the PE-L-1 cells expressed mRNA for VEGF receptor-1 (flt-1) but at a lower level than in the cultured endothelial cells (Fig. 5A). There was no detectable message for VEGF receptor-2 (flk-1) (data not shown).
Figure 5.
Characterization of cell lines. A. Flt-1 expression in PE-L-1 cells. RNA isolated from PE-L-1 cells or MLE-ßgal cells was analyzed by RT-PCR with primers specific for flt-1. As a control, the same samples were analyzed by RT-PCR with primers specific for ß-actin. B. VEGF expression in transfected MLE-ßgal cells. RNA isolated from MLE-ßgal cells transfected with an expression vector for VEGF-A (MLEVEGF) or with the vector alone (MLE) was analyzed by RT-PCR with primers specific for VEGF or ß-actin. C. VEGF protein expression in transfected MLE-ßgal cells. Conditioned medium from confluent cultures of MLE-ßgal cells transfected with an expression vector for VEGF-A (MLE-VEGF) or with the vector alone (MLE) was concentrated 20-fold, run on an 8% polyacrylamide gel, and blotted onto nitrocellulose. Blots were probed with antibodies to VEGF-A.
We hypothesized that increased expression of VEGF might lead to increased growth of prostatic cell implants either by direct stimulation of PE-L-1 cell growth or by increased recruitment of endothelial cells. To examine if altered VEGF-A expression modified prostate epithelial growth in vivo, the MLE-ßgal cells were transfected with a pZeo SV VEGF-A expression vector and clones that expressed approximately 10-times more VEGF-A than cells transfected with the vector alone were chosen (Fig. 5B-C). PE-L-1 cells were implanted under the renal capsule with endothelial cells overexpressing VEGF-A or endothelial cells transfected with vector alone. Overexpression of VEGF-A did not alter the growth of MLE-ßgal cells in culture or when inoculated alone in vivo. Co-inoculation of PE-L-1 cells with the MLE-ßgal cells overexpressing VEGF-A under the renal capsule resulted in the same amount of prostatic growth as obtained when PE-L-1 cells were co-inoculated with MLE-ßgal cells transfected with the vector alone (Fig. 2A). Similarly, co-inoculation of PE-B-1 cells with MLE cells over-expressing VEGF-A resulted in the same amount of prostatic growth as when PE-B-1 cells were co-inoculated with MLE-ßgal cells transfected with the vector alone (Fig. 2B). Thus, increased VEGF-A expression by endothelial cells had no effect on growth of prostatic cells under the renal capsule. Increased expression of VEGF-A by the implanted endothelial cells also did not alter the number of blood vessels surrounding the prostatic growths (data not shown).
Discussion
We have found that vascular endothelial cells are able to support the growth and maintenance of differentiation of prostatic epithelium in vivo. Substantial evidence supports a close relation between endothelial growth or regression and epithelial growth or regression in the prostate. This close relationship may partly be the result of secretion of angiogenic factors by prostatic epithelium. Prostatic epithelial cells produce several angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factors, and the angiopoietins (29-34). Synthesis of VEGF is regulated by androgens in prostatic luminal epithelial cells (31,35-38). Thus, castration may decrease the balance of pro-angiogenic factors produced by the epithelium, resulting in regression of the vasculature. Vascular regression would starve the epithelium of essential nutrients, resulting in epithelial regression. Similarly, restoration of androgen to castrated animals would stimulate VEGF production by prostate epithelial cells leading to expansion of the vasculature. The growing vasculature would supply nutrients to support the expansion of the epithelial compartment.
VEGF seems to play an essential role in the regulation of vascular growth in response to androgens as blocking VEGF signals with soluble VEGF receptors reduced vascular growth in the prostates of castrated mice administered testosterone (17,39) and inhibited regeneration of the prostate (16,17). However, in the experiment presented here, increased expression of VEGF did not alter the ability of endothelial cells to potentiate prostate tissue growth. VEGF is highly expressed in the kidney (40). Thus, it is likely that the kidney already produced adequate amounts of VEGF to support endothelial cell expansion in the implants, and increased amounts were superfluous.
The relationship between prostatic epithelial cell and blood vessel health has been used to suppress prostate tumor growth. As in other tumors, prostate tumors regulate their vascularization by the release of angiogenesis factors that stimulate the surrounding vasculature to sprout new capillaries that grow into the tumor. Factors that target angiogenic factors or endothelial cells themselves block the recruitment of new blood vessels and thereby inhibit prostate tumor growth (41-47). These anti-vascular therapies are believed to act by starving the prostatic tumors of nutrients supplied by the blood vessels.
However, in the experiments presented here, endothelial cells themselves, without being organized into vessels, were able to support the establishment and growth of prostatic tissue in vivo. These results may be related to the observation that the vascular endothelium appears to have a direct role in supporting growth of some epithelial tissues. In mice that do not develop blood vessels because of the knockout of the endothelial cell specific receptor, VEGF receptor-2, liver does not develop (48). Similarly, the islets of Langerhans do not develop in Xenopus embryos in which the early dorsal aorta has been ablated, thereby blocking further vascular development (49). The development of these tissues is blocked at a stage before blood begins to flow in the embryo, suggesting that there is a direct requirement for endothelium rather than the nutrients delivered through the blood. Development of these tissues can be rescued in vitro by addition of endothelial cells. Thus, development of some epithelia requires factors directly supplied by endothelial cells. Even in adult animals, liver responds to factors secreted by the endothelium. Administration of VEGF to mice stimulates liver growth by stimulating growth of sinusoidal endothelial cells (50). Growth of the endothelium stimulates the growth of the liver epithelium. Epithelial growth is partly due to hepatocyte growth factor (HGF) produced by the endothelial cells (50). A recent study reported that in mice deficient in the angiogenesis inhibitor, pigment epithelium-derived factor, there is overgrowth of blood vessels and hyperplasia of prostatic epithelium, suggesting that prostatic epithelium may also respond to direct signals from endothelial cells (51).
Acknowledgments
The authors thank Gilbert Kim and Xiao-li Liu for their expert technical assistance.
This work was supported by grants DK52644, CA90593, and CA132641 from the National Institutes of Health.
Literature Cited
- 1.Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- 2.Chung LW. The role of stromal-epithelial interaction in normal and malignant growth. Cancer Surv. 1995;23:33–42. [PubMed] [Google Scholar]
- 3.Norman JT, Cunha GR, Sugimura Y. The induction of new ductal growth in adult prostatic epithelium in response to an embryonic prostatic inductor. Prostate. 1986;8:209–220. doi: 10.1002/pros.2990080302. [DOI] [PubMed] [Google Scholar]
- 4.Kinbara H, Cunha GR, Boutin E, Hayashi N, Kawamura J. Evidence of stem cells in the adult prostatic epithelium based upon responsiveness to mesenchymal inductors. Prostate. 1996;29:107–116. doi: 10.1002/(SICI)1097-0045(199608)29:2<107::AID-PROS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer L, Reese BA. Epithelialmesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto K, Salm SN, Coetzee S, Xiong X, Burger PE, Shapiro E, Lepor H, Moscatelli D, Wilson EL. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells. 2006;24:1859–1868. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 8.Cunha GR, Hayward SW, Dahiya R, Foster BA. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat (Basel) 1996;155:63–72. doi: 10.1159/000147791. [DOI] [PubMed] [Google Scholar]
- 9.Takao T, Tsujimura A, Coetzee S, Salm SN, Lepor H, Shapiro E, Moscatelli D, Wilson EL. Stromal/epithelial interactions of murine prostatic cell lines in vivo: a model for benign prostatic hyperplasia and the effect of doxazosin on tissue size. Prostate. 2003;54:17–24. doi: 10.1002/pros.10147. [DOI] [PubMed] [Google Scholar]
- 10.Tokuda Y, Toda S, Masaki Z, Sugihara H. Proliferation and differentiation of rat dorsal prostatic epithelial cells in collagen gel matrix culture, focusing upon effects of adipocytes. Int J Urol. 1999;6:509–519. doi: 10.1046/j.1442-2042.1999.00099.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang SM, Chung LW. Interaction between prostatic fibroblast and epithelial cells in culture: role of androgen. Endocrinology. 1989;125:2719–2727. doi: 10.1210/endo-125-5-2719. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Is tissue mass regulated by vascular endothelial cells? Prostate as the first evidence. Endocrinology. 1998;139:441–442. doi: 10.1210/endo.139.2.5858. [DOI] [PubMed] [Google Scholar]
- 13.English HF, Drago JR, Santen RJ. Cellular response to androgen depletion and repletion in the rat ventral prostate: autoradiography and morphometric analysis. Prostate. 1985;7:41–51. doi: 10.1002/pros.2990070106. [DOI] [PubMed] [Google Scholar]
- 14.Franck-Lissbrant I, Haggstrom S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139:451–456. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
- 15.Shabisgh A, Tanji N, D'Agati V, Burchardt M, Rubin M, Goluboff ET, Heitjan D, Kiss A, Buttyan R. Early effects of castration on the vascular system of the rat ventral prostate gland. Endocrinology. 1999;140:1920–1926. doi: 10.1210/endo.140.4.6644. [DOI] [PubMed] [Google Scholar]
- 16.Lissbrant IF, Hammarsten P, Lissbrant E, Ferrara N, Rudolfsson SH, Bergh A. Neutralizing VEGF bioactivity with a soluble chimeric VEGF-receptor protein flt(1- 3)IgG inhibits testosterone-stimulated prostate growth in castrated mice. Prostate. 2004;58:57–65. doi: 10.1002/pros.10312. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Kovalenko B, Huang Y, Moscatelli D. Vascular endothelial growth factor and angiopoietin are required for prostate regeneration. Prostate. 2007;67:485–499. doi: 10.1002/pros.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkman J, Haudenschild CC, Zetter BR. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci USA. 1979;76:5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salm SN, Koikawa Y, Ogilvie V, Tsujimura A, Coetzee S, Moscatelli D, Moore E, Lepor H, Shapiro E, Sun TT, Wilson EL. Transforming growth factor-beta is an autocrine mitogen for a novel androgen-responsive murine prostatic smooth muscle cell line, PSMC1. J Cell Physiol. 2000;185:416–424. doi: 10.1002/1097-4652(200012)185:3<416::AID-JCP12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Salm SN, Koikawa Y, Ogilvie V, Tsujimura A, Coetzee S, Moscatelli D, Moore E, Lepor H, Shapiro E, Sun TT, Wilson EL. Generation of active TGF-beta by prostatic cell cocultures using novel basal and luminal prostatic epithelial cell lines. J Cell Physiol. 2000;184:70–79. doi: 10.1002/(SICI)1097-4652(200007)184:1<70::AID-JCP7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer FA, Miller LJ, Lindquist R, Kowalczyk P, Laudone VP, Albertsen PC, Kreutzer DL. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 23.Kollermann J, Helpap B. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1 in benign, premalignant, and malignant prostate tissue. Am J Clin Pathol. 2001;116:115–121. doi: 10.1309/1LBM-6X32-JH6W-ENUD. [DOI] [PubMed] [Google Scholar]
- 24.Soker S, Kaefer M, Johnson M, Klagsbrun M, Atala A, Freeman MR. Vascular endothelial growth factor-mediated autocrine stimulation of prostate tumor cells coincides with progression to a malignant phenotype. Am J Pathol. 2001;159:651–659. doi: 10.1016/S0002-9440(10)61736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn D, Simak R, Steiner GE, Handisurya A, Susani M, Marberger M. Expression of the VEGF-receptor Flt-1 in benign, premalignant and malignant prostate tissues. J Urol. 2000;164:506–510. [PubMed] [Google Scholar]
- 26.Jackson MW, Roberts JS, Heckford SE, Ricciardelli C, Stahl J, Choong C, Horsfall DJ, Tilley WD. A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res. 2002;62:854–859. [PubMed] [Google Scholar]
- 27.Chevalier S, Defoy I, Lacoste J, Hamel L, Guy L, Begin LR, Aprikian AG. Vascular endothelial growth factor and signaling in the prostate: more than angiogenesis. Mol Cell Endocrinol. 2002;189:169–179. doi: 10.1016/s0303-7207(01)00728-6. [DOI] [PubMed] [Google Scholar]
- 28.Kaliberov SA, Kaliberova LN, Stockard CR, Grizzle WE, Buchsbaum DJ. Adenovirus-mediated FLT1-targeted proapoptotic gene therapy of human prostate cancer. Mol Ther. 2004;10:1059–1070. doi: 10.1016/j.ymthe.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–167. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 30.Brown LF, Yeo KT, Berse B, Morgentaler A, Dvorak HF, Rosen S. Vascular permeability factor (vascular endothelial growth factor) is strongly expressed in the normal male genital tract and is present in substantial quantities in semen. J Urol. 1995;154:576–579. doi: 10.1097/00005392-199508000-00073. [DOI] [PubMed] [Google Scholar]
- 31.Richard C, Kim G, Koikawa Y, Salm SN, Tsujimura A, Wilson EL, Moscatelli D. Androgens modulate the balance between VEGF and angiopoietin expression in prostate epithelial and smooth muscle cells. Prostate. 2002;50:83–91. doi: 10.1002/pros.10035. [DOI] [PubMed] [Google Scholar]
- 32.Mansson PE, Adams P, Kan M, McKeehan WL. Heparin-binding growth factor gene expression and receptor characteristics in normal rat prostate and two transplantable rat prostate tumors. Cancer Res. 1989;49:2485–2494. [PubMed] [Google Scholar]
- 33.Crabb JW, Armes LG, Carr SA, Johnson CM, Roberts GD, Bordoli RS, McKeehan WL. Complete primary structure of prostatropin, a prostate epithelial cell growth factor. Biochemistry. 1986;25:4988–4993. doi: 10.1021/bi00366a003. [DOI] [PubMed] [Google Scholar]
- 34.Story MT, Sasse J, Jacobs SC, Lawson RK. Prostatic growth factor: purification and structural relationship to basic fibroblast growth factor. Biochemistry. 1987;26:3843–3849. doi: 10.1021/bi00387a016. [DOI] [PubMed] [Google Scholar]
- 35.Joseph IB, Nelson JB, Denmeade SR, Isaacs JT. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997;3:2507–2511. [PubMed] [Google Scholar]
- 36.Joseph IB, Isaacs JT. Potentiation of the antiangiogenic ability of linomide by androgen ablation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostatic cancers. Cancer Res. 1997;57:1054–1057. [PubMed] [Google Scholar]
- 37.Sordello S, Bertrand N, Plouet J. Vascular endothelial growth factor is upregulated in vitro and in vivo by androgens. Biochem Biophys Res Commun. 1998;251:287–290. doi: 10.1006/bbrc.1998.9328. [DOI] [PubMed] [Google Scholar]
- 38.Haggstrom S, Wikstrom P, Bergh A, Damber JE. Expression of vascular endothelial growth factor and its receptors in the rat ventral prostate and Dunning R3327 PAP adenocarcinoma before and after castration. Prostate. 1998;36:71–79. doi: 10.1002/(sici)1097-0045(19980701)36:2<71::aid-pros1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Wang GM, Kovalenko B, Wilson EL, Moscatelli D. Vascular density is highest in the proximal region of the mouse prostate. Prostate. 2007;67:968–975. doi: 10.1002/pros.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veenendaal LM, Jin H, Ran S, Cheung L, Navone N, Marks JW, Waltenberger J, Thorpe P, Rosenblum MG. In vitro and in vivo studies of a VEGF121/rGelonin chimeric fusion toxin targeting the neovasculature of solid tumors. Proc Natl Acad Sci U S A. 2002;99:7866–7871. doi: 10.1073/pnas.122157899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Curwen JO, Hennequin LF, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF. ZD4190: an orally active inhibitor of vascular endothelial growth factor signaling with broadspectrum antitumor efficacy. Cancer Res. 2000;60:970–975. [PubMed] [Google Scholar]
- 43.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
- 44.Sweeney P, Karashima T, Kim SJ, Kedar D, Mian B, Huang S, Baker C, Fan Z, Hicklin DJ, Pettaway CA, Dinney CP. Anti-vascular endothelial growth factor receptor 2 antibody reduces tumorigenicity and metastasis in orthotopic prostate cancer xenografts via induction of endothelial cell apoptosis and reduction of endothelial cell matrix metalloproteinase type 9 production. Clin Cancer Res. 2002;8:2714–2724. [PubMed] [Google Scholar]
- 45.Melnyk O, Zimmerman M, Kim KJ, Shuman M. Neutralizing anti-vascular endothelial growth factor antibody inhibits further growth of established prostate cancer and metastases in a pre-clinical model. J Urol. 1999;161:960–963. [PubMed] [Google Scholar]
- 46.Fox WD, Higgins B, Maiese KM, Drobnjak M, Cordon-Cardo C, Scher HI, Agus DB. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin Cancer Res. 2002;8:3226–3231. [PubMed] [Google Scholar]
- 47.Borgstrom P, Bourdon MA, Hillan KJ, Sriramarao P, Ferrara N. Neutralizing antivascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 49.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 50.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 51.Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J, Crawford SE. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]