Abstract
In 2004, Sininger and Cone-Wesson examined asymmetries in the signal-to-noise ratio (SNR) of otoacoustic emissions (OAE) in infants, reporting that distortion-product (DP)OAE SNR was larger in the left ear, whereas transient-evoked (TE)OAE SNR was larger in the right. They proposed that cochlear and brainstem asymmetries facilitate development of brain-hemispheric specialization for sound processing. Similarly, in 2006 Sininger and Cone-Wesson described ear asymmetries mainly favoring the right ear in infant auditory brainstem responses (ABRs). The present study analyzed 2640 infant responses to further explore these effects. Ear differences in OAE SNR, signal, and noise were evaluated separately and across frequencies (1.5, 2, 3, and 4 kHz), and ABR asymmetries were compared with cochlear asymmetries. Analyses of ear-canal reflectance and admittance showed that asymmetries in middle-ear functioning did not explain cochlear and brainstem asymmetries. Current results are consistent with earlier studies showing right-ear dominance for TEOAE and ABR. Noise levels were higher in the right ear for OAEs and ABRs, causing ear asymmetries in SNR to differ from those in signal level. No left-ear dominance for DPOAE signal was observed. These results do not support a theory that ear asymmetries in cochlear processing mimic hemispheric brain specialization for auditory processing.
I. INTRODUCTION
Hemispheric specialization is evident in a variety of tasks, including those that involve auditory function, but its relation to reports of ear asymmetry in auditory measurements is unclear. Transient-evoked (TE) otoacoustic emissions (OAEs) have higher energy in the right than the left ear in twins (McFadden et al., 1996) and in infants (Aidan et al., 1997). There is a higher prevalence of spontaneous (S) OAEs in the right ears of adults, 5–12 year old children (Bilger et al., 1990), and infants (Burns et al., 1992). The signal-to-noise ratio (SNR) of distortion-product (DP) OAEs is larger in the right ear of children (age range 5.2–7.9 years) at DPOAE f2 test frequencies of 1.9, 3, 3.8, and 6 kHz (Keogh et al., 2001). Each of these reports is consistent with right-ear dominance at the level of cochlear mechanics. Larger auditory brainstem response (ABR) amplitudes and lower ABR thresholds have been reported for right-ear stimulation (Levine and McGaffigan, 1983; Levine et al., 1988; Sininger et al., 1998), although the neural asymmetry may not always favor the right ear (Ballachanda et al., 1994). Sininger and Cone-Wesson (2006) found larger wave V amplitudes and mostly shorter latencies in the right ear of infants. Thus, the prevailing trends in both cochlear and lower brainstem neural response data support the view that auditory responses, in general, favor the right ear.
In contrast, Sininger and Cone-Wesson (2004) reported evidence of stimulus-specific specialization at the cochlear level in neonates, in which the right ear produced larger TEOAE SNRs in response to a click stimulus, and the left ear produced larger DPOAE SNRs in response to two tonal stimuli with slightly different frequencies. This left-ear DPOAE dominance contrasts with the above-mentioned studies, in which TEOAEs, SOAEs, and DPOAEs all appeared to favor the right ear. They considered a possible brainstem role via feedback to the outer hair cells in the cochlea by the medial olivocochlear efferent system. This role was not studied for OAE, inasmuch as the OAE measurements did not include conditions to elicit a change in the functional feedback provided by the medial olivocochlear efferent system.
Sininger and Cone-Wesson proposed that hemispheric specialization is facilitated at the cochlea by providing “greater amplification to stimuli that will also be preferentially processed in the auditory areas of the contralateral hemisphere.”
One ambiguity in their study is that their conclusions regarding stimulus-specific, ear dominance was based only on measurements of OAE SNR; they did not describe the extent to which ear asymmetries were present in either the OAE signal or the noise. One might hypothesize that SNR is the relevant “signal” variable in single-fiber neural responses to the central nervous system. However, OAE noise is dominated by electrical noise in the measurement system and physiologic noise due to respiration, subject movement, and perhaps blood circulation that manifests itself in the measured acoustic ear-canal response. Because the OAE noise is unlikely to reflect the noise in neural responses, OAE SNR is unlikely to reflect neural SNR. Parsing of the OAE SNR into OAE signal and OAE noise components may be important as the conclusion that asymmetric cochlear processing mimics hemispheric specialization would appear to be based on the expectation that the DPOAE signal is stronger in the left ear.
Additionally, it may be important to consider ear asymmetries in the middle-ear functioning of neonates in order to understand ear asymmetries in cochlear or neural responses. Ear effects of middle-ear functioning were examined in infants based on acoustic reflectance and admittance measurements in the ear canal (Keefe et al., 2000). For example, the equivalent volume, which is proportional to the imaginary part of the admittance, and the energy reflectance were larger in left ears below 1.4 kHz, whereas energy reflectance was larger in right ears between 2 and 5.7 kHz. Any ear asymmetries in middle-ear function might act as a confounding factor on measurements of ear asymmetries at all levels of the auditory pathway, including the cochlea.
Sininger and Cone-Wesson (2006) measured ABRs with and without contralateral stimulation and observed that the response was reduced in the left ear during contralateral noise presentation to a greater extent when compared to similar conditions for the right ear. They interpreted this asymmetric activation following contralateral stimulation to mean that the medial olivocochlear system may be the mechanism for the asymmetry in brainstem responses.
The purpose of the present study was to further explore the factors underlying these neonatal ear asymmetries by (1) replicating the Sininger and Cone-Wesson (2004, 2006) studies, (2) extending the study by separately evaluating OAE signal and noise levels averaged across frequency but also for individual ½ octave frequencies from 1.5 to 4 kHz, (3) evaluating ear asymmetries in ABR from the same subjects, and (4) comparing measures of acoustic reflectance and admittance in efforts to describe the influence of middle-ear function on asymmetries in sensory and neural responses.
II. METHODS
A. Measured neonatal responses
The data described in this paper were part of a large multicenter project that focused on identification of hearing loss in neonates (Norton et al., 2000a). This is the same project from which the data were drawn for the Sininger and Cone-Wesson (2004) study, although the data reported here were from a different sample of subjects. The protocol for data collection was identical to that used in the Sininger and Cone-Wesson studies, with the exception that the protocol for the present study included measures of middle-ear function on a subset of infants. Briefly, using a computer-controlled paradigm in which test and ear order were randomized, DPOAE, TEOAE, and ABR data were collected from well babies and graduates from intensive-care nurseries. The data reported here were originally collected at three different test sites (Boys Town National Research Hospital and the University of Nebraska Medical Center in Omaha, NE and Women’s and Infant’s Hospital in Providence, RI).
OAE data were evaluated for ½ octave frequencies from 1.5 to 4 kHz. DPOAE data were collected with primary levels (L1, L2) of 65 and 50 dB SPL, whereas TEOAE data were collected in response to a click at 80 dB pSPL, using the nonlinear mode implemented on a commercial OAE measurement system. OAE data were not analyzed below 1.5 kHz because of high noise levels (and, therefore, poor SNR) at lower frequencies. Despite the fact that response measurements were restricted to the same range of stimulus frequencies for both DPOAEs and TEOAEs, it may be important to keep in mind that DPOAE measurements were made at a frequency (2f1–f2) that was about ½ octave lower than the stimulus frequency (f2). In contrast, TEOAE responses were measured at the same frequencies as the stimulus. Although this would be expected to affect estimates of signal and noise across OAE measurements, it should have no influence on comparisons across ears. DPOAEs and TEOAEs were also tested at higher stimulus levels, but the data for those stimulus conditions were not analyzed in the present study. Complete methodological details for TEOAE and DPOAE data collection can be found in Norton et al. (2000c) and Gorga et al. (2000), respectively. Signal SPL, noise SPL, and SNR were evaluated separately, and were either averaged across frequency (as in Sininger and Cone-Wesson, 2004), or analyzed separately at each frequency.
OAE responses were acquired in the original study using synchronous, time-domain averaging procedures. During acquisition of DPOAE responses, the minimum averaging included at least four 1.6-s averages at each frequency. Testing terminated at each frequency when the signal level was at least 3 dB above the mean noise level plus two standard deviations above the mean noise level. Overall, the test stopped when this definition of SNR was met at four of five frequencies (1, 1.5, 2, 3, and 4 kHz). During acquisition of TEOAE responses, at least 60 averages were obtained. Data collection continued until the SNR (defined in the more traditional way relative to mean noise level) was 3 dB or greater in the ½ octave bands centered at 1 and 1.5 kHz, and 6 dB in the ½ octave bands centered at 2, 3, and 4 kHz. For a test to stop in a given ear, the above-mentioned criteria had to be met in four of five ½ octave bands. If these stopping rules were not met, then additional rules were evaluated, but such additional rules were not relevant for data included in the present study.
ABR data were collected in response to clicks presented at both 30 and 69 dB nHL (see Sininger et al. (2000) for details on ABR data collection). Absolute ABR latencies (waves I and V), interpeak latency (IPL) differences defined as (wave V latency—wave I latency), wave V amplitude (peak to following trough at 30 and 69 dB), noise amplitude (at 30 and 69 dB), and Fsp (at 30 and 69 dB) were compared between ears. Fsp is closely related to SNR, and was originally described in the context of ABR detection by Don et al. (1984). It was used as the stopping criterion during ABR data collection as part of the multicenter clinical trial, from which the present data and those described by Sininger and Cone-Wesson (2004, 2006) were extracted.
We also collected reflectance and admittance data (Keefe et al., 2000) in a subset of the present sample of subjects (subjects at the Providence site and one of the two Omaha sites). The measurements describing middle-ear (and ear-canal) status always followed the TEOAE measurements. Middle-ear variables were selected based on previous research in terms of factors calculated using a principal components analysis (Keefe et al., 2003a). A factor accounting for the most variance in that study was highly correlated with the equivalent volume averaged from 0.25 to 1 kHz (vLo); a second factor accounting for the next most variance was highly correlated with energy reflectance averaged from 2 to 8 kHz (rHi), and was an important factor in predicting transient middle-ear dysfunction in neonates (Keefe et al., 2003b). For these reasons, these two middle-ear variables were used in the statistical analyses described in the following.
Two inclusion criteria were used for the analyses on the full data set. Criterion 1 in OAE analyses required a SNR of at least 3 dB at each of the four ½ octave frequencies in both ears for both OAE measures, and in ABR analyses required an ABR Fsp30 of at least 3.0 in both ears. Sininger and Cone-Wesson (2004) used a similar 3-dB SNR criterion for their analysis of OAE responses. Sininger and Cone-Wesson (2006) subsequently considered ear asymmetries in ABRs using a slightly more stringent inclusion criterion of Fsp=3.1, based on data collected under the same conditions as in the present study. It is not expected that the slight differences in ABR inclusion criteria would impact comparisons between the two data sets.
Our study also used an additional inclusion criterion, which was designed to be similar across OAE and ABR test types. Inclusion criterion 2 in OAE analyses equated each of the DPOAE and TEOAE tests on test specificity (90%), which was similar to a criterion on test specificity used by Norton et al. (2000b). Inclusion criterion 2 in ABR analyses used a Fsp30 that also resulted in a test specificity of 90%. This approach facilitated comparisons across measurements because it was based on the same point on the response distributions of ears with hearing within normal limits for each test type. Inclusion criterion 2 resulted in the following critical test scores: SNR ≤ 1.82 dB for DPOAE, SNR ≤ 5.81 dB for TEOAE, and Fsp30 ≤ 2.44. The apparent difference in SNR inclusion criteria for DPOAEs and TEOAEs is a consequence of the fact that, during data collection, noise levels for DPOAEs were defined as the mean noise level plus two standard deviations. The SNR criteria for the two OAE measurements are much more similar when mean noise levels are used for DPOAE measurements.
For analyses on the smaller data set that included reflectance and admittance responses along with the OAE and ABR responses, our study used an additional criterion 3, which was based on the following critical test scores: SNR ≤ 3.2 dB for DPOAE, SNR ≤ 6.4 dB for TEOAE, and Fsp30 ≤ 3.27 dB. Imposing criterion 3 resulted in a database of 693 subjects.
B. Statistical design
The first set of OAE analyses was based on the frequency-averaged SNR, OAE level (signal), and OAE noise level measured in 2,640 subjects. In this test group, 2,093 subjects were included after selection based on criterion 1 and 2,150 subjects were included based on the slightly less stringent criterion 2. Separate repeated-measures analyses of variance (ANOVA) examined associations between SNR, signal and noise levels, and a pair of independent predictors, Ear (right or left) and OAE Type (DPOAE or TEOAE). A random subject effect was included in this and subsequent models to take into account the correlation of measurements within a subject. A random site effect was considered but was not a significant source of variability; thus, it was excluded from this and subsequent models. The summaries to follow were based on the combined data from the three sites. Fixed effects included Ear, OAE Type, and their interaction. In this and other ANOVAs, effects with p<0.05 were considered statistically significant, and Tukey’s method was used to adjust p-values for multiple comparisons. The corresponding 95% confidence intervals (CI) were calculated for each mean.
The second set of OAE analyses was based on the SNR, signal and noise at each of four frequencies. Separate repeated-measures ANOVAs examined associations at each frequency. Otherwise, these analyses were the same as those used for frequency-averaged data.
Separate repeated-measures ANOVAs examined associations between ABR variables listed above and the independent predictor of ear (except that wave I latency and the IPL at 30 dB were not analyzed because wave I could not be measured reliably in many subjects at this level). Analyses of ABR data were performed using only inclusion criterion 2.
The statistical analyses on OAE responses using criteria 1 and 2 resulted in nearly identical outcomes. For this reason, the first set of analyses that compared the present results to those reported by Sininger and Cone-Wesson (2004) was based on criterion 1. The remaining results described below used criterion 2 in order to equate OAE and ABR measures on test specificity.
The analyses on OAE and ABR responses that included the admittance and reflectance test results used the middle-ear variables vLo and rHi defined earlier. These variables were included as covariates in a set of repeated-measures ANOVAs that were otherwise similar to those described previously. Criterion 3 was used to select data for inclusion in these analyses.
All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC), Version 9.1.
III. RESULTS
A. Ear effects in frequency-averaged OAEs
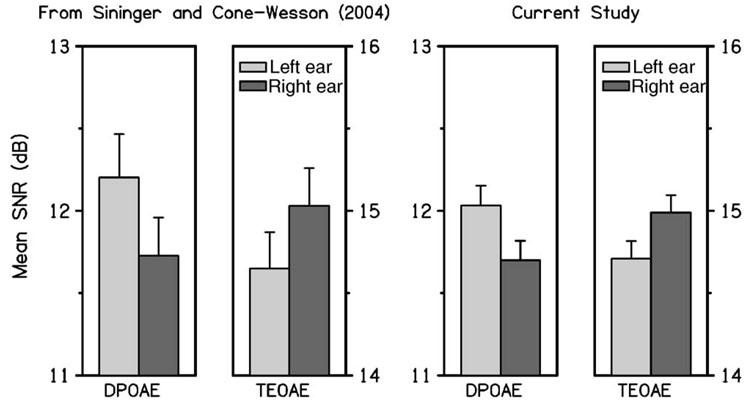
The SNR data from Sininger and Cone-Wesson (2004) showed a (statistically) significant ear × type interaction and significant ear differences for TEOAE SNR (right ear larger) and DPOAE SNR (left ear larger). Figure 1 shows the mean and CI of the frequency-averaged DPOAE and TEOAE SNR reported by Sininger and Cone-Wesson (left panels), and the present study (right panels) using similar inclusion criteria. The interaction in our analyses between OAE type and ear was significant (p=0.0004), just as it was in the Sininger and Cone-Wesson study. The DPOAE SNR was significantly larger in the left than the right ear. The mean TEOAE SNR was larger in the right than the left ear, but this difference was not significant in the present analyses. Consistent with results in Sininger and Cone-Wesson, we observed that TEOAE SNR was significantly larger than DPOAE SNR in both ears (note the difference in scales on the vertical axis for TEOAE and DPOAE SNRs). As stated earlier, however, this observation relates mainly to differences in the way noise level was defined for the two OAE measures. In one case (TEOAE), it was defined as the mean noise level, whereas in the other (DPOAE), it was defined as the mean noise level plus two standard deviations.
FIG. 1.
The mean and upper 95% confidence interval (CI) of the frequency-averaged DPOAE SNR and TEOAE SNR reported by Sininger and Cone-Wesson (2004) are plotted in the left-hand panels, and those obtained in the present study are plotted in the right-hand panels using similar inclusion criteria. Each error bar denotes the upper half of the CI. In each pair of bar plots, the left bar (lightly shaded) represents the left-ear response and the right bar (darkly shaded) represents data from the right ear. In the current study, there was no significant difference between ears, but there was a significant ear × type interaction, with a significant difference between ears for DPOAE SNR.
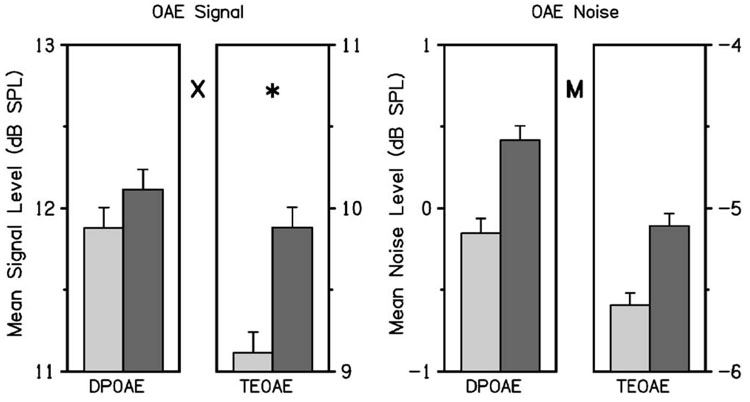
To better understand the source of the ear asymmetry in SNR, signal and noise levels were evaluated separately. Figure 2 summarizes the mean signal and noise levels in left and right ears of the subjects included in the present study, following the conventions used in Fig. 1. As in Fig. 1, these results represent averages across the four frequencies included in this analysis, only here signal and noise are presented separately. The significant effects of primary interest are shown in Fig. 2 and subsequent figures using symbols described in the figure caption. The ear × type interaction was significant for OAE signal. The OAE signal for TEOAEs was higher in the right ear, but the OAE signal for DPOAEs did not vary with ear. The interaction between OAE ear and type was not significant for OAE noise. There was a main effect for OAE noise, which was significantly higher in the right ear (by approximately 0.5 dB) regardless of OAE type. There was no significant interaction between ear and type in the OAE noise results. Thus, the higher noise level reduced the SNR in the right ear for both OAE types. The fact that DPOAE noise was significantly higher (by approximately 5.5 dB) than TEOAE noise is of lesser interest, because it relates to differences in how noise was defined for the OAE types. There was also a main effect for OAE signal of Type, with DPOAE levels larger than TEOAE levels, and this asymmetry was significant in each ear. This effect was not of interest in the present study because differing source mechanisms may result in different DPOAE and TEOAE signal levels.
FIG. 2.
The frequency-averaged OAE signal and noise SPL are plotted in the left- and right-hand halves, respectively, following the same conventions as those used in Fig. 1. The DPOAE and TEOAE SPL are separately plotted for signal and noise. Each error bar denotes the upper half of the 95% CI. Between each pair of bar plots in each panel, the letter X denotes that the ear × type interaction was significant for OAE signal, and the letter M represents that the main effect of ear was significant for OAE noise. When the interaction was significant, an asterisk denotes that ear was significant for a particular ear type; in this case, the OAE signal for TEOAE was higher in the right ear.
B. Ear effects in frequency-specific OAEs
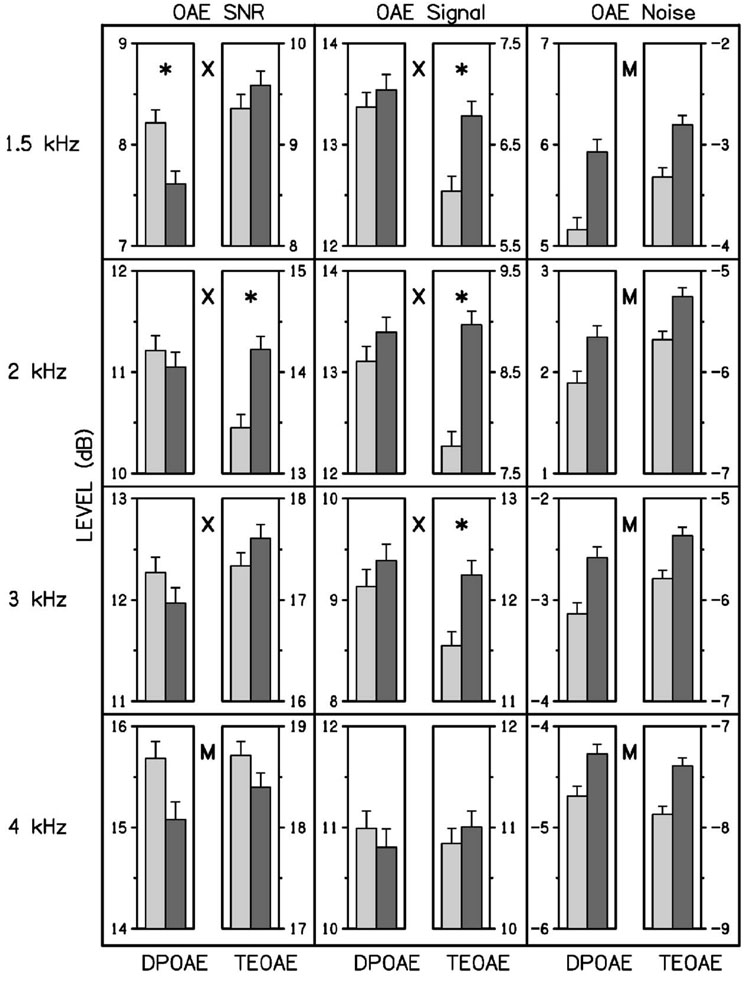
Ear asymmetries in OAEs were next investigated on a frequency-specific basis (DPOAEs at f2=1.5, 2, 3, and 4 kHz, and TEOAEs in ¼ octave bands centered at the same frequencies) by studying the significance of the ear × type interaction, and the significance of main effects of OAE SNR, signal, and noise levels. We report main effects for ear only when the ear × type interaction lacks significance, but do not report results indicating main effects for OAE type, which would simply identify frequencies at which DPOAE and TEOAE variables were unequal. Figure 3 summarizes the mean and the upper half of the 95% confidence interval data for SNR, signal, and noise in left, middle, and right columns, respectively, with data for a different frequency shown in each row.
FIG. 3.
The mean and 95% CI of the OAE SNR, and the OAE signal and noise SPL are plotted in the left, middle, and right columns of plots, respectively. The DPOAE and TEOAE SPL are separately plotted for signal and noise for each ear, with a plotting convention similar to that in Fig. 2. Between each pair of bar plots in each panel, the letter X denotes that the ear × type interaction was significant, and the letter M represents that the main effect of ear was significant. When the interaction was significant, an asterisk denotes that ear was significant for a particular ear type (DPOAE or TEOAE).
In OAE SNR analyses (left column), the ear × type interaction was significant at 1.5, 2, and 3 kHz. The ear asymmetries at these frequencies that were significant for either DPOAE SNR or TEOAE SNR were as follows: the DPOAE SNR was larger in the left ear at 1.5 kHz, and the TEOAE SNR was larger in the right ear at 2 kHz. The ear × type interaction was not significant at 4 kHz. The corresponding main effect at 4 kHz was significant, with the SNR larger in the left ear. The averaging across frequency (as shown in Fig. 1), obscured some of these differences, e.g., the results for SNR at 4 kHz. However, the general trends in the frequency-specific analyses were similar to those observed when data were collapsed across frequency.
In OAE signal analyses (middle column), the ear × type interaction was significant at 1.5, 2, and 3 kHz. The significant ear asymmetries in signal at these frequencies were as follows: the TEOAE signal was larger in the right ear at 1.5, 2, and 3 kHz. At 4 kHz, there was no ear × type interaction and no main effect for ear asymmetry. Despite the trends favoring DPOAE level in the right ear as well at 1.5, 2, and 3 kHz, no statistically significant ear asymmetries were observed.
In OAE noise analyses (right column), the ear × type interaction was not significant at any frequency. Consistent with frequency-averaged noise results, OAE noise was significantly larger in the right ear at every frequency, regardless of OAE type. The possible reasons for the observation of higher noise levels for stimulus conditions involving the right ear are further discussed below.
Like the previous findings in which data were averaged across frequency, these results bring into question the view that stimulus-driven ear asymmetries facilitate hemispheric specialization. Rather, the TEOAE signal at 1.5, 2, and 3 kHz in the present results is consistent with previous TEOAE (and SOAE) results showing a right-ear advantage.
C. Effect of middle-ear covariates on OAEs
With only two exceptions, the OAE ANOVA results including vLo and rHi as middle-ear covariates had the same pattern of significance as the ANOVAs described previously without middle-ear covariates. One exception was that TEOAE SNR was higher in the right ear at 2 kHz (p=0.021) when the middle-ear covariates were included but was not significant otherwise. Second, a marginal main effect for ear in the SNR data at 4 kHz for the basic model (in this reduced data set of 693 subjects) was not significant when middle-ear covariates were included. The middle-ear covariates did not change the pattern of significance in any of the OAE signal and noise level analyses. The fact that the OAE models including middle-ear covariates performed nearly identically to OAE models without covariates means that middle-ear functioning, as encoded in vLo and rHi, did not explain ear differences in OAEs, even though ear differences existed in vLo and rHi. Such inter-ear differences in admittance and reflectance responses were described in Keefe et al. (2000). Further, rHi was important for identifying middle-ear dysfunction in infants who did not pass a newborn hearing screening test based on OAE/ABR testing, yet who were subsequently found to have hearing within normal limits (Keefe et al., 2003a, b).
D. Ear effects in ABR results
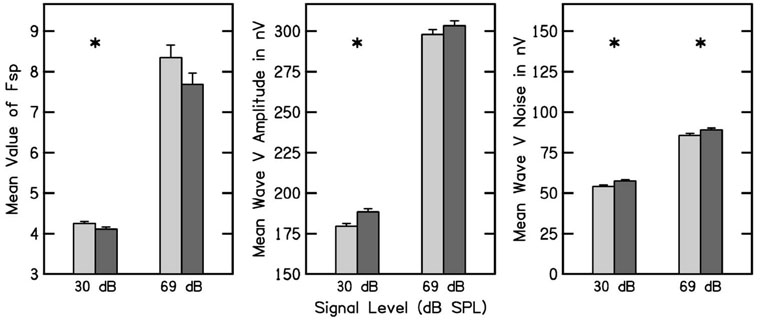
Imposing the ABR criterion 2 resulted in an inclusion of 2221 subjects. The ABR results are organized into two groups: (1) Fsp, and response and noise amplitudes; and (2) absolute response latencies and interpeak latency differences. Figure 4 plots Fsp, wave V amplitude and noise amplitude measured using click stimulus levels of 30 and 69 dB nHL. Figure 4 follows the convention used to designate ears in other figures. Consistent with previously reported data (Sininger and Cone-Wesson, 2006), the Fsp at 30 dB nHL was significantly smaller in the right ear, but Fsp at 69 dB did not differ across ears. Note that the error bars were smaller for the Fsp at 30 dB, compared to the 69 dB condition. This occurred because the stopping rule during data collection at 30 dB nHL included both the Fsp and observer-based criteria, whereas at 69 dB, only the observer-based criterion was used, resulting in greater Fsp variability at the end of testing for the 69 dB condition. Although the absolute difference in Fsp was smaller at 30 dB, it is likely that it was more than counterbalanced by the reduced variability, which resulted in a significant outcome at this level.
FIG. 4.
The mean and 95% CI of the ABR Fsp, wave V signal amplitude, and wave V noise amplitude are plotted in the left, middle, and right panels, respectively. The left pair of bar plots in each panel shows data obtained at 30 dB nHL click stimulus level, and the right pair are data obtained at 69 dB nHL. In each pair of bar plots, ear is designated following the conventions used in previous figures. An asterisk denotes that ear was significant for a particular ABR response.
The wave V amplitude at 30 dB was larger for the right ear, but wave V amplitude at 69 was not statistically significantly different between ears. The ABR noise levels at both 30 and 69 dB were significantly higher in the right ear. These results indicate that the larger wave V amplitude in the right ear at 30 dB was associated with a larger noise amplitude in this ear, so that the Fsp, which functions as a SNR measure in an ABR test, was smaller in the right ear. The wave V amplitudes at 30 dB are consistent with some of the OAE findings, namely that DPOAE and TEOAE signals at 1.5 kHz and the TEOAE signal at 2 kHz were greater in the right ear. The noise sources for OAE and ABR measurements are different and perhaps statistically independent. Our observations are similar to those of Sininger and Cone-Wesson (2006), although they found statistically significant differences at both stimulus levels, with larger responses following right-ear stimulation. Sininger and Cone-Wesson (2006) did not report ABR noise levels.
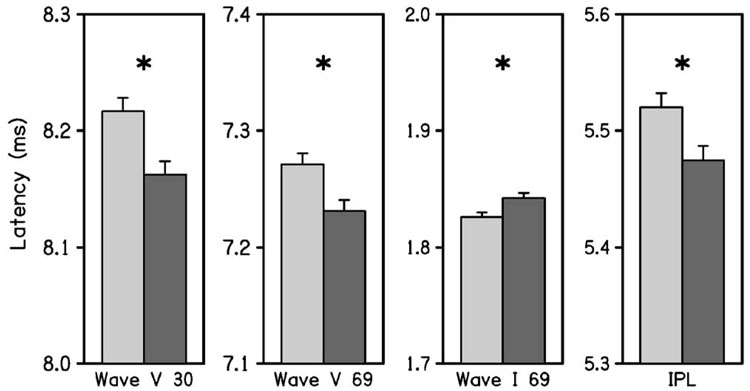
Figure 5 plots absolute latencies and IPL differences (i.e., wave V latency minus wave I latency). Wave I latency, which was analyzed only at a 69 dB click level, was longer in the right ear. This result is further discussed in the next section, which considers the effects of middle-ear covariates on ABR responses. Wave V latency was significantly shorter on the right side at both stimulation levels, which is an effect in the opposite direction, compared to Wave I latency at 69 dB nHL. As a consequence, the IPL (which is reported only at the 69-dB click level for reasons described above) was significantly shorter on the right side.
FIG. 5.
The mean and 95% CI of the ABR wave latencies are plotted in each panel. The latency responses from left to right across these panels are for wave V at 30 dB nHL, wave V at 69 dB nHL, wave I at 69 dB nHL, and the IPL (i.e., wave V latency minus wave I latency) at 69 dB nHL. In each pair of bar plots, ear is designated following the conventions used in previous figures. An asterisk denotes that ear was significant for a particular ABR latency.
E. Effect of middle-ear covariates on ABRs
When middle-ear covariates were included in the model for the subsample of responses in the 693 subjects on whom middle-ear testing was performed and inclusion criterion 3 was satisfied, there was no ear asymmetry in wave I latency. Removing the middle-ear covariates from the ANOVA in this subsample resulted in a longer wave I latency for the right ear, which is the same result obtained for the full sample (as described above). As wave I reflects the first neural response along the auditory pathway, these results are consistent with an absence of inter-ear differences at the lowest neural level of the auditory system if the influence of the middle ear is taken into account. In this same subsample, the wave V latencies at both stimulus levels were shorter in the right ear whether the middle-ear covariates were present or absent from the ANOVA. Further, the IPL was shorter in the right ear for both cases. These wave V latency and IPL results in the subsample replicated the results in the full sample, and were insensitive to presence or absence of the middle-ear covariates.
The absence of ear asymmetry for wave I latencies when middle-ear covariates were included suggests that the ear-asymmetry effect, which was represented by the shorter IPL for the right ear, occurred above the level of the cochlea. It is likely that the effect of forward transmission through the middle ear were not fully described by the middle-ear covariates. We might expect an ear asymmetry in middle-ear transmission to result in an asymmetry in wave I latency with no asymmetry in IPL. Middle-ear transmission would influence wave I and wave V latencies in a similar manner, and, because the IPL is the difference in these latencies, the IPL would not be affected.
IV. DISCUSSION
These results show that the interaction of OAE type and ear was significant for both criteria 1 and 2. There was no ear asymmetry in the TEOAE SNR for either criterion, but the DPOAE SNR for each criterion was larger in the left ear. This pattern in OAE SNR asymmetry was at least qualitatively similar to that reported by Sininger and Cone-Wesson (2004). Based on ear asymmetries in OAE SNR, they stated that the cochlea provides greater amplification to stimuli that would also be preferentially processed in the contralateral hemisphere of the brain. However, when SNR was separated into signal and noise level components, our frequency-averaged results showed no ear asymmetry in DPOAE signal, but a larger TEOAE signal level in the right ear. This suggests that, regardless of stimulus, OAE signal levels were either equal between ears or greater in the right ear. In the frequency-averaged results, there was no stimulus condition for which the left ear produced the larger OAE signal level.
Frequency-averaged results revealed an ear asymmetry in OAE noise of ~0.5 dB. It is difficult to understand how an asymmetry in peripheral acoustic noise relates to differential processing in the auditory areas of the cortex (i.e., favoring tones eliciting DPOAEs or clicks eliciting TEOAEs) or how this might relate to stimulus-specific ear asymmetries in cochlear amplification. Because OAE signal is related to the strength of cochlea excitation that also drives neural encoding, OAE signal level has a more direct representation of stimulus encoding within the cochlea than does OAE SNR. OAE noise levels were higher in the right ear at each frequency and in the average across frequency. TEOAE signal was higher at 1.5, 2, and 3 kHz in the right ear, with no ear asymmetry at any frequency in DPOAE signal. OAE signal levels following right-ear stimulation were either equal to or greater than those observed following left-ear stimulation regardless of OAE type and, therefore, the stimulus eliciting the response. No left-ear advantage for OAE signal was observed for any combination of OAE type and stimulus frequency.
The absence of a left-ear advantage for OAE signal across OAE type and stimulus frequency contrasts with the larger left-ear DPOAE SNR reported by Sininger and Cone-Wesson (2004) and replicated in our results. Because Sininger and Cone-Wesson reported results only for SNR, the extent to which this SNR effect was influenced by ear asymmetries in DPOAE signal, noise, or some combination thereof cannot be known from their report. In our analyses, the dominant effect producing the SNR result was revealed by the presence of a higher noise level in the right ear (of approximately 0.5 dB) that was independent of OAE type. This is because TEOAE signal was larger in the right ear for several conditions, whereas DPOAE signal was similar across ears. The effect of higher noise in the right ear produced an ear asymmetry in OAE SNR, controlled by the reduction of the right-ear dominance of TEOAE signal compared to TEOAE SNR, and by the presence of a left-ear dominance for DPOAE SNR due only to the larger DPOAE noise in the right ear. One possible explanation for the higher noise level in the right ear is that a potential source of the noise, related to blood flow, is closer in the right than the left ear. This is consistent with Rubens et al. (2007), who described effects on TEOAE measurements of an anatomical asymmetry in newborns for which the right innominate vein is closer to the right inner ear than is the left innominate vein to the left inner ear (Rohen et al., 2002).
One potential confounding issue is the extent to which the measurement-based stopping rules in the DPOAE and TEOAE tests, which were fully described in the original studies that were the source of the data (Gorga et al., 2000; Norton et al., 2000a, b, c; Sininger et al., 2000) and brieflysummarized in Sec. II, might affect the interpretation of OAE signal, noise, and SNR. Specifically, given a stopping rule based on SNR, is it valid to evaluate levels of signal and noise as well as SNR? If it were not possible to interpret the measured OAE signal SPL as a measure of the magnitude of the cochlear source strength due to the presence of measurement noise, it would also not be possible to interpret the measured SNR as a measure of the magnitude of the cochlear source strength. In that case, it would not be possible to utilize OAE SNR as a measure of asymmetric cochlear processing. In fact, our use of SNR and Signal and Noise separately is identical to how these quantities were described in the aforementioned original studies.
Further, the OAE measurements were not unduly contaminated by the SNR-based stopping rules used to acquire the data. Suppose the noise level was stationary. A longer averaging time would increase the SNR and decrease the noise level, but the time-synchronous measurement of signal level would only be slightly affected if the SNR were sufficiently large. The stopping rules were relevant to responses with SNRs in the range of 3–6 dB or less, but would have less of an effect for responses with larger SNRs. The inclusion criteria excluded ears with small SNR, i.e., criterion 1 required a SNR of at least 3 dB; criterion 2 required a SNR of at least 1.82 dB for DPOAEs, and 5.81 dB for TEOAEs. As stated earlier, the statistical results for ear asymmetries in OAEs were nearly identical using either criterion 1 or 2. The insensitivity of the analyses to choice of criterion suggests that any confounding factor of ears with low SNR was minimal. This is evident in the measured SNRs across frequency, which were on average 12 dB for DPOAEs and 15 dB for TEOAEs (see Fig. 1). Most ears included in the analysis had SNRs higher than the limits required by the stopping rules. Because of these factors, it was possible to interpret OAE signal levels as providing information on the cochlear source strengths.
The ABR results showed right-ear dominance for some of the response and noise amplitudes, and never showed left-ear dominance, findings that, on balance, are consistent with other reports (e.g., Sininger and Cone-Wesson, 2006). A left-ear dominance for Fsp at 30 dB nHL was observed, but it is likely, although not certain, that this effect resulted from larger ear differences in noise amplitude than in wave V amplitude. In addition, a right-ear dominance in the full sample was observed for the IPL and for the wave I and wave V latencies. The observation of right-ear dominance for IPL, but not for wave I latency, when the middle-ear covariates were taken into account suggests that the source(s) of asymmetry are at least partly neural in origin, rather than residing solely in the peripheral mechanics. These results also show that the middle-ear covariates encode some information relevant to middle-ear transmission. The fact that energy reflectance at high frequencies is larger in the right ear of neonates (Keefe et al., 2000) is consistent with a reduced forward transmission through the right middle ear, and a reduced middle-ear transmission would be expected to increase the wave I latency in the right ear. This prediction is consistent with our data.
Ear asymmetries in OAEs, which are pre-neural responses, were also present in the latency of wave I, which is the first neural response. Because the underlying ear-canal measurements did not directly measure forward transmission through the middle ear, a detailed explanation of this result would be confounded by the possibility of an ear asymmetry in forward transmission through the middle ear that was not represented by the middle-ear covariates. However, the right-ear dominance of IPL would not be expected to be confounded by middle-ear transmission, and is consistent with the right-ear dominance of TEOAE signal for some conditions. Our results support the findings referenced in the Introduction of a right-ear dominant effect in cochlear mechanics and neural responses, but do not support the finding of a left-ear dominance in DPOAE signal. Thus, they do not support the view that there is a stimulus-driven, peripherally based factor in these interaural effects of auditory processing that might influence the functional maturation of hemispheric specialization in the brain.
Our data set of OAE and ABR responses did not allow an evaluation of the potential contribution to ear asymmetries due to feedback by the medial olivocochlear efferent system, although this was explored by Sininger and Cone-Wesson (2006) in their evaluation of ABR asymmetries. Further study with more refined stimulus conditions is needed to assess the extent to which these efferent effects contribute to ear asymmetries in OAE responses.
The significant ear asymmetries in OAE and ABR responses reported herein have too small an effect size to carry clinical importance in newborn hearing screening programs, even though they do reveal subtle aspects of cochlear and neural processing. Based on the present results, the OAE and ABR test criteria, which are used in such hearing screening programs to identify infants at risk for sensorineural hearing loss, should not incorporate information on response asymmetry between right and left ears.
V. CONCLUSIONS
Our observations are consistent with the preponderance of earlier studies showing a right-ear dominance for TEOAE signal and ABR measures. Both OAE and ABR analyses revealed that noise was larger in the right than the left ear. The middle-ear covariates had little effect on the OAE analyses but were useful in interpreting the ear asymmetries in ABR wave I latencies. Our observations are inconsistent with the conclusions of Sininger and Cone-Wesson (2004), inasmuch as we found no evidence of left-ear dominance in DPOAE measures, and, in particular, no left-ear dominance in DPOAE signal. We did not observe a stimulus-guided asymmetry in OAE responses (i.e., different ear dominance for click-elicited TEOAE and tone-elicited DPOAE signals). Such an asymmetry would appear to be essential to a theory that asymmetric cochlear processing mimics hemispheric specialization.
ACKNOWLEDGMENTS
We thank Betty Vohr, M.D., for allowing us to include data from Women and Infants Hospital, Providence, RI, which was one of the sites for the multicenter clinical trial. We appreciate the assistance of James C. Lynch, Ph.D. of the University of Nebraska Medical Center, who consulted on the statistical analyses. The present research was supported by the NIH grant nos. DC003784 and DC002251 using a database of responses collected with support by the NIH grant no. DC01958. We thank Associate Editor Brenda Lonsbury-Martin and two anonymous reviewers for comments on an earlier version of this report.
Footnotes
Portions of this work were presented in “Ear asymmetries in middle ear, cochlear and brainstem responses in infants,” 2005 Midwinter Meeting of the Association for Research in Otolaryngology, New Orleans, LA, 2005.
PACS number(s): 43.64.Jb, 43.64.Ri, 43.64.Ha, 43.71.Rt [BLM]
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
Contributor Information
Douglas H. Keefe, Boys Town National Research Hospital, 555 North 30th Street, Omaha, Nebraska 68131.
Michael P. Gorga, Boys Town National Research Hospital, 555 North 30th Street, Omaha, Nebraska 68131
Walt Jesteadt, Boys Town National Research Hospital, 555 North 30th Street, Omaha, Nebraska 68131.
Lynette M. Smith, College of Public Health, 984375 Nebraska Medical Center, Omaha, Nebraska 68198-4375
References
- Aidan D, Lestang P, Avan P, Bonfils P. Characteristics of transient-evoked otoacoustic emissions (TEOEs) in neonates. Acta Oto-Laryngol. 1997;117:25–30. doi: 10.3109/00016489709117986. [DOI] [PubMed] [Google Scholar]
- Ballachanda BB, Rupert A, Moushegian G. Asymmetric frequency-following responses. J. Am. Acad. Audiol. 1994;5:133–137. [PubMed] [Google Scholar]
- Bilger RC, Matthies ML, Hammel DR, Demorest ME. Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions. J. Speech Hear. Res. 1990;33:418–432. doi: 10.1044/jshr.3303.418. [DOI] [PubMed] [Google Scholar]
- Burns EM, Arehart KH, Campbell K. Prevalence of spontaneous otoacoustic emissions in neonates. J. Acoust. Soc. Am. 1992;91:1571–1575. doi: 10.1121/1.402438. [DOI] [PubMed] [Google Scholar]
- Don M, Elberling C, Waring M. Objective detection of averaged auditory brainstem responses. Scand. Audiol. 1984;13:219–228. doi: 10.3109/01050398409042130. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Norton SJ, Sininger YS, Cone-Wesson B, Folsom RC, Vohr BR, Widen JE. Identification of neonatal hearing impairment: Distortion product otoacoustic emissions during the perinatal period. Ear Hear. 2000;21:400–424. doi: 10.1097/00003446-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Folsom RC, Gorga MP, Vohr BR, Bulen JC, Norton SJ. Identification of neonatal hearing impairment: Ear-canal measurements of acoustic admittance and reflectance in neonates. Ear Hear. 2000;21:443–461. doi: 10.1097/00003446-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Gorga MP, Neely ST, Zhao F, Vohr B. Ear-canal acoustic admittance and reflectance effects in human neonates. II. Predictions of middle-ear dysfunction and sensorineural hearing loss. J. Acoust. Soc. Am. 2003b;113:407–422. doi: 10.1121/1.1523388. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Zhao F, Neely ST, Gorga MP, Vohr B. Ear-canal acoustic admittance and reflectance effects in human neonates. I. Predictions of otoacoustic emission and auditory brainstem responses. J. Acoust. Soc. Am. 2003a;113:389–406. doi: 10.1121/1.1523387. [DOI] [PubMed] [Google Scholar]
- Keogh T, Kei Rl, Criscoll C, Smyth V. Distortion-product otoacoustic emissions in schoolchildren: Effects of ear asymmetry, handedness, and gender. J. Am. Acad. Audiol. 2001;12:506–513. [PubMed] [Google Scholar]
- Levine RA, Liederman J, Riley P. The brainstem auditory evoked potential asymmetry is replicable and reliable. Neuropsychologia. 1988;26:603–614. doi: 10.1016/0028-3932(88)90116-9. [DOI] [PubMed] [Google Scholar]
- Levine RA, McGaffigan PM. Right-left asymmetries in the human brainstem: Auditory evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1983;55:532–537. doi: 10.1016/0013-4694(83)90163-3. [DOI] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC, Pasanen EG. Additional findings on heritability and prenatal masculinization of cochlear mechanisms: Click-evoked otoacoustic emissions. Hear. Res. 1996;97:102–119. [PubMed] [Google Scholar]
- Norton SJ, Gorga MP, Widen JE, Folsom RC, Sininger YS, Cone-Wesson B, Vohr BR, Fletcher K. Identification of neonatal hearing impairment: A multicenter investigation. Ear Hear. 2000a;21:348–356. doi: 10.1097/00003446-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Norton SJ, Gorga MP, Widen JE, Folsom RC, Sininger YS, Cone-Wesson B, Vohr BR, Mascher K, Fletcher K. Identification of Neonatal Hearing Impairment: Evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brain stem evoked response test performance. Ear Hear. 2000b;21:508–528. doi: 10.1097/00003446-200010000-00013. [DOI] [PubMed] [Google Scholar]
- Norton SJ, Gorga MP, Widen JE, Vohr BR, Folsom RC, Sininger YS, Cone-Wesson B, Fletcher K. Identification of neonatal hearing impairment: Transient evoked otoacoustic emissions during the perinatal period. Ear Hear. 2000c;21:425–442. doi: 10.1097/00003446-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Yokochi C, Lutjen-Drecoll E. Color Atlas of Anatomy. 5th ed. Lippincott: Williams & Wilkins, Philadelphia; 2002. p. 257. [Google Scholar]
- Rubens DD, Vohr BR, Tucker R, O’Neil A, Chung W. Newborn oto-acoustic emission hearing screening tests. Early Hum. Dev‥. 2007 doi: 10.1016/j.earlhumdev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B. Asymmetric cochlear processing mimics hemispheric specialization. Science. 2004;305:1581. doi: 10.1126/science.1100646. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B. Lateral asymmetry in the ABR of neonates: Evidence and mechanisms. Hear. Res. 2006;212:203–211. doi: 10.1016/j.heares.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B, Abdala C. Gender distinctions and lateral asymmetry in the low-level auditory brainstem response of the human neonate. Hear. Res. 1998;126:58–66. doi: 10.1016/s0378-5955(98)00152-x. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B, Folsom RC, Gorga MP, Vohr BR, Widen JE, Ekelid M, Norton SJ. Identification of neonatal hearing impairment: Auditory brain stem responses in the perinatal period. Ear Hear. 2000;21:400–424. doi: 10.1097/00003446-200010000-00006. [DOI] [PubMed] [Google Scholar]