Abstract
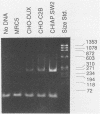
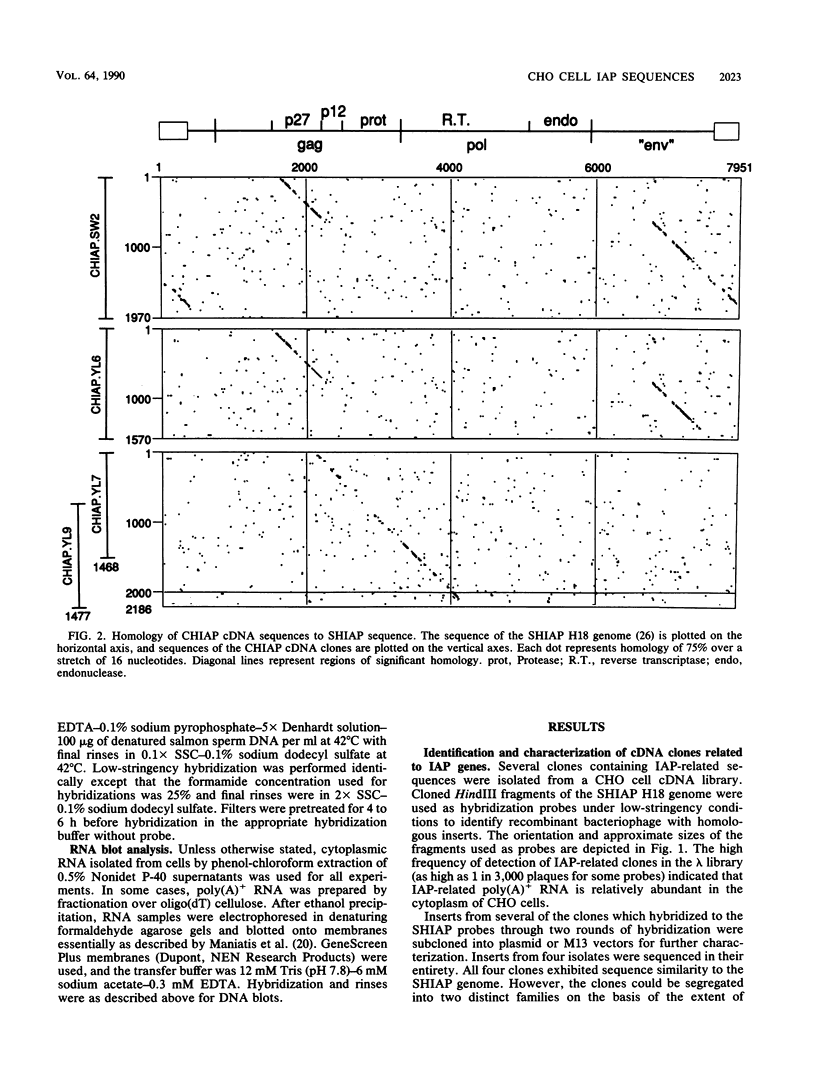
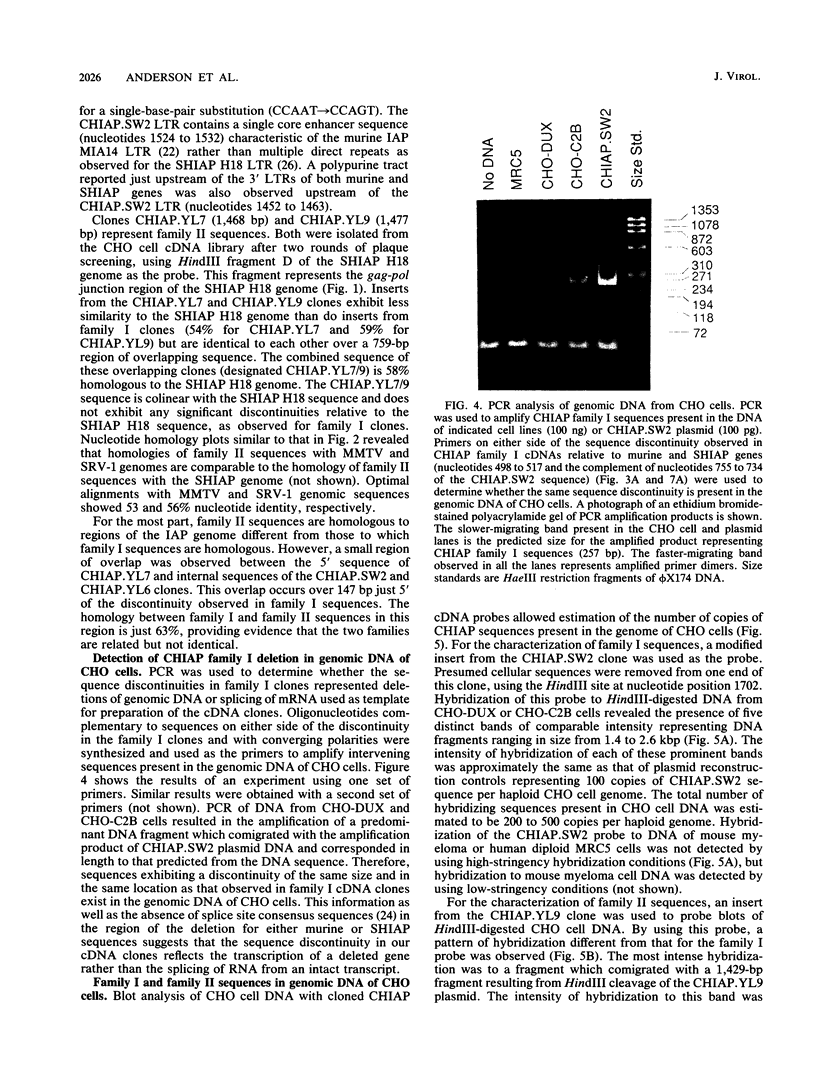
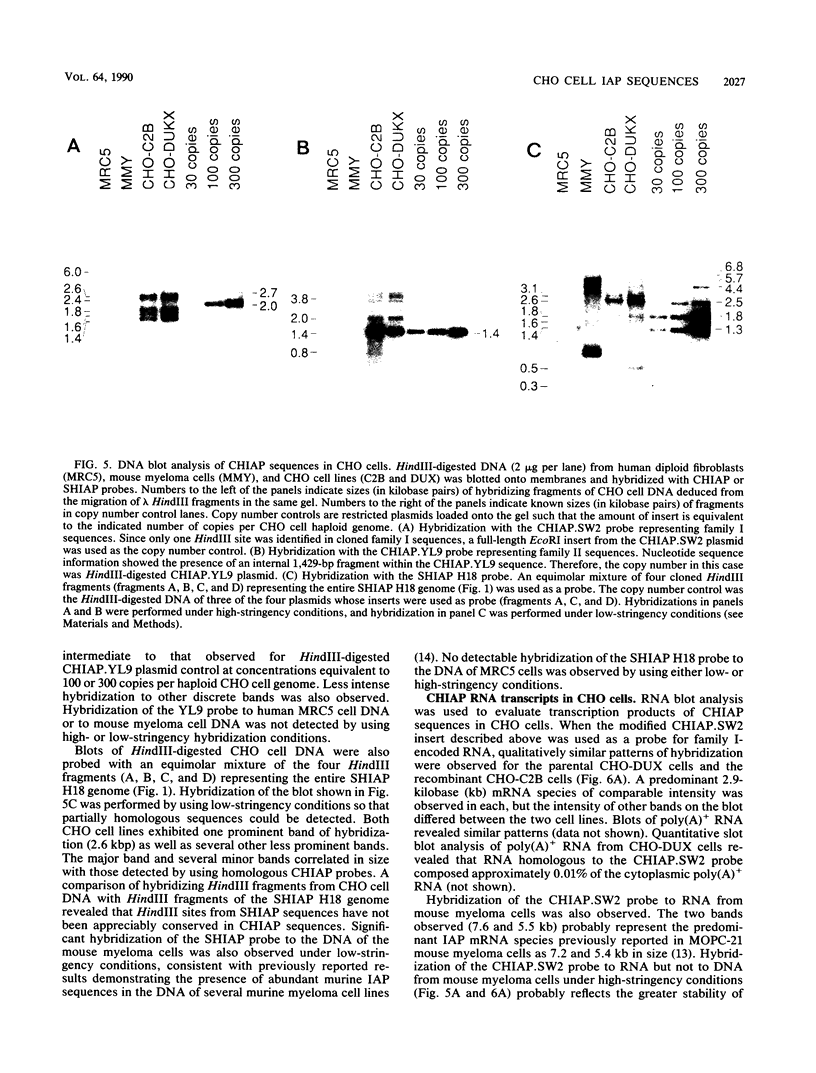
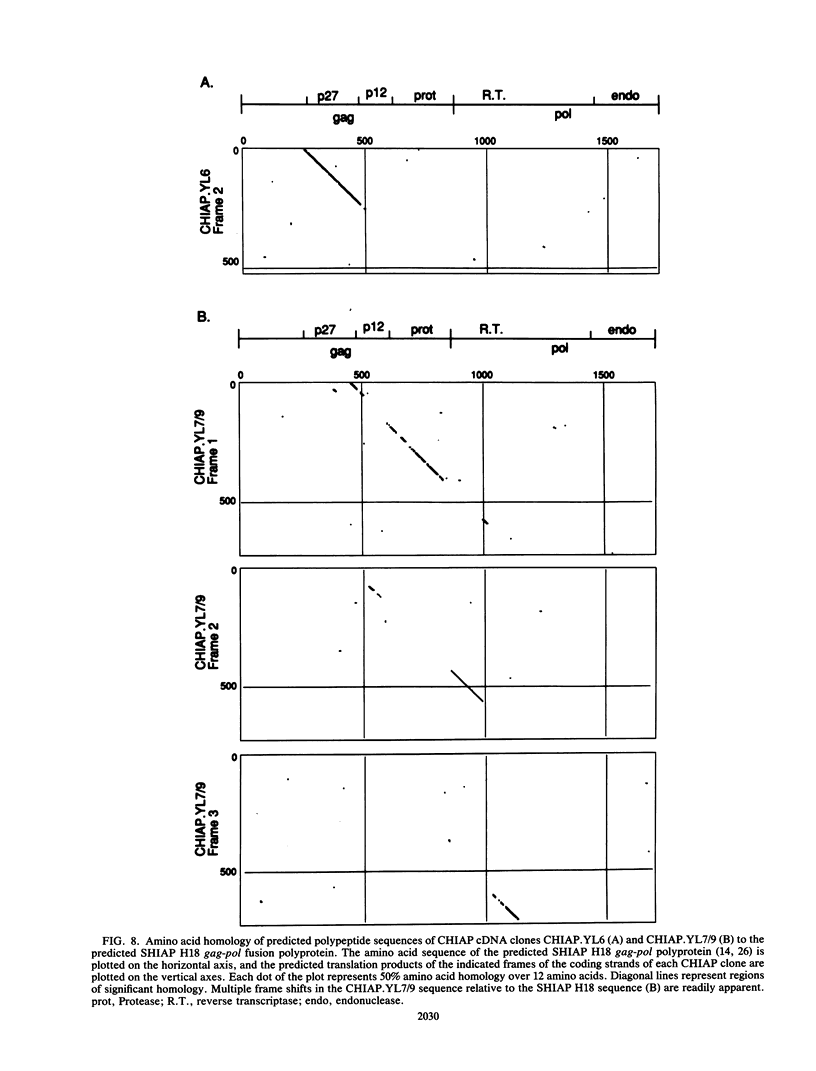
We have characterized sequences expressed in Chinese hamster ovary (CHO) cells which are related to the intracisternal A-particle (IAP) genes of mice and Syrian hamsters. Several cDNA clones homologous to Syrian hamster IAP probes have been isolated and used to evaluate the abundance and expression of these retroviruslike sequences. DNA blot analysis with homologous Chinese hamster IAP probes revealed that IAP-related sequences are present in CHO cell DNA at moderately repetitive levels (approximately 300 copies per haploid genome). Sequence analysis has revealed the existence of at least two distinct families of IAP-related sequences in CHO cell DNA. Family I sequences exhibit identical 4.5-kilobase-pair internal deletions relative to complete IAP genomes of mice or Syrian hamsters, but family II sequences showed no major sequence discontinuities relative to the IAP genes of other species. Both families are expressed as abundant cytoplasmic RNA in CHO cells, but only family II sequences produce abundant transcripts of a size consistent with that of a full-length IAP RNA. Intact gag, pol, or env open reading frames were not present in sequences of either family, although incomplete open reading frames spanning putative p27 and protease regions of IAP genes were observed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Kuff E. L., Lueders K. K., Birkenmeier E. Genetic relationship between the Mus cervicolor M432 retrovirus and the Mus Musculus intracisternal type A particle. J Virol. 1981 Dec;40(3):901–911. doi: 10.1128/jvi.40.3.901-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D., Stassen J. M., Marafino B. J., Jr, Builder S., De Cock F., Ogez J., Tajiri D., Pennica D., Bennett W. F., Salwa J. Biological properties of human tissue-type plasminogen activator obtained by expression of recombinant DNA in mammalian cells. J Pharmacol Exp Ther. 1984 Oct;231(1):146–152. [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Egrie J. C., Browne J., Lai P., Lin F. K. Characterization of recombinant monkey and human erythropoietin. Prog Clin Biol Res. 1985;191:339–350. [PubMed] [Google Scholar]
- Gotoh O. An improved algorithm for matching biological sequences. J Mol Biol. 1982 Dec 15;162(3):705–708. doi: 10.1016/0022-2836(82)90398-9. [DOI] [PubMed] [Google Scholar]
- Heine U. I., Kramarsky B., Wendel E., Suskind R. G. Enhanced proliferation of endogenous virus in Chinese hamster cells associated with microtubules and the mitotic apparatus of the host cell. J Gen Virol. 1979 Jul;44(1):45–55. doi: 10.1099/0022-1317-44-1-45. [DOI] [PubMed] [Google Scholar]
- Heine U. I., Todaro G. J. New type B retrovirus isolates associated with kinetochores and centrioles of the host cell. J Gen Virol. 1978 Apr;39(1):41–52. doi: 10.1099/0022-1317-39-1-41. [DOI] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kuff E. L., Callahan R., Howk R. S. Immunological relationship between the structural proteins of intracisternal A-particles of Mus musculus and the M432 retrovirus of Mus cervicolor. J Virol. 1980 Mar;33(3):1211–1214. doi: 10.1128/jvi.33.3.1211-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lubiniecki A. S., Dinowitz M., Nelson E., Wiebe M., May L., Ogez J., Builder S. Endogenous retroviruses of continuous cell substrates. Dev Biol Stand. 1989;70:187–191. [PubMed] [Google Scholar]
- Lubiniecki A. S., May L. H. Cell bank characterization for recombinant DNA mammalian cell lines. Dev Biol Stand. 1985;60:141–146. [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Comparison of the sequence organization of related retrovirus-like multigene families in three evolutionarily distant rodent genomes. Nucleic Acids Res. 1983 Jul 11;11(13):4391–4408. doi: 10.1093/nar/11.13.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences homologous to retrovirus-like genes of the mouse are present in multiple copies in the Syrian hamster genome. Nucleic Acids Res. 1981 Nov 25;9(22):5917–5930. doi: 10.1093/nar/9.22.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Givens J. F., Taber R. L., Zeigel R. F. Characterization of virus-like particles released from the hamster cell line CHO-K1 after treatment with 5-bromodeoxyuridine. J Gen Virol. 1978 Jun;39(3):505–517. doi: 10.1099/0022-1317-39-3-505. [DOI] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., ROBINSON A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958 Dec 1;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Servenay M., Kupiec J. J., d'Auriol L., Galibert F., Peries J., Emanoil-Ravier R. Nucleotide sequence of the Chinese hamster intracisternal A-particle genomic region corresponding to 5'LTR-GAG. Nucleic Acids Res. 1988 Aug 11;16(15):7725–7725. doi: 10.1093/nar/16.15.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Byrn R. A., Marsters S. A., Gregory T., Groopman J. E., Capon D. J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S., Fitch W. M. Comparative biosequence metrics. J Mol Evol. 1981;18(1):38–46. doi: 10.1007/BF01733210. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Kitasato H., Kawakami M., Ono M. Molecular cloning of retrovirus-like genes present in multiple copies in the Syrian hamster genome. Nucleic Acids Res. 1982 Oct 11;10(19):5733–5746. doi: 10.1093/nar/10.19.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion C., Green M. Cyclic AMP-amplified replication of RNA tumour virus-like particles in Chinese hamster ovary cells. Nat New Biol. 1973 Aug 22;244(138):227–231. doi: 10.1038/newbio244227a0. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]