Abstract
Macrolide antibiotics penetrate cells, but the mechanism by which this occurs is unclear. The objective of this study was to characterize mechanisms of clarithromycin uptake by gingival fibroblasts and oral epithelium. Cultured human gingival fibroblasts and SCC-25 cells were incubated with [3H]-clarithromycin. Clarithromycin transport was assayed by measuring cell-associated radioactivity over time. Fibroblasts and epithelial cells rapidly accumulated clarithromycin, attaining steady-state intracellular concentrations within 15 minutes. Incubation in medium containing 2 μg/ml clarithromycin yielded steady-state intracellular concentrations of 75.8 μg/ml in fibroblasts and 6.6 μg/ml in SCC-25 cells. Clarithromycin transport exhibited Michaelis-Menten kinetics and was inhibited below 37° C. The Michaelis constants for fibroblasts and SCC-25 cells were 78.4 and 227 μg/ml, respectively, while the maximum transport velocities were 264 and 381 ng/min/106 cells, respectively. Thus, both types of cells take up clarithromycin via a concentrative active transport system. By increasing intracellular clarithromycin levels, this system may enhance the effectiveness of clarithromycin against invasive periodontal pathogens.
Keywords: macrolides, antimicrobial chemotherapy, aggressive periodontitis
INTRODUCTION
The goals of non-surgical periodontal therapy (scaling and root planing) are debridement of bacterial plaque and removal of bacterial products from root surfaces. While this approach is usually successful in arresting periodontal attachment loss, periodontal breakdown may continue to progress in some patients. This unsatisfactory outcome is often related to persistent infection by invasive subgingival bacteria (Bragd et al., 1987). The periodontal pathogens Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis are exceptionally difficult to eliminate by debridement alone (Chen and Slots, 1993). A. actinomycetemcomitans is strongly associated with aggressive periodontitis and recurrent adult periodontitis, while P. gingivalis is associated with severe chronic periodontitis, failing guided tissue regeneration, and acute periodontal abscesses. Both pathogens are capable of evading the body’s immune/defense system by invading epithelial cells lining periodontal pockets (Saglie et al., 1986). A. actinomycetemcomitans can invade epithelial cells and pass into the underlying connective tissue (Christersson et al., 1987; Fives-Taylor et al., 1995), while P. gingivalis can invade epithelial cells and linger inside (Lamont et al., 1995). Antimicrobial chemotherapy is frequently used as an adjunct to enhance the elimination of pathogens from patients who undergo progressive periodontal attachment loss after scaling and root planing (Chadwick and Mellersh, 1987; van Winkelhoff et al., 1996). Use of systemic antibiotics in conjunction with scaling and root planing significantly enhances gains in clinical attachment level in comparison to treatment with scaling and root planing alone (Haffajee et al., 2003). Penicillins do not readily cross the plasma membrane, which limits their effectiveness in the treatment of intracellular infections (Schentag et al, 1985). In contrast, macrolides penetrate cells to gain access to intracellular pathogens (Bosnar et al., 2005). The newer macrolide agents azithromycin and clarithromycin are highly effective against A. actinomycetemcomitans and P. gingivalis (Pajukanta et al., 1992; Pajukanta, 1993; Piccolomini et al., 1998; Goldstein et al., 1999). Both agents exhibit good activity against Eikenella corrodens, Prevotella species, fusobacteria, and other anaerobic and facultative oral pathogens (Sefton et al., 1996; Goldstein et al, 1999; Merriam et al., 2006). In addition to their favorable antimicrobial spectrum, both agents have a low incidence of gastrointestinal toxicity and are administered with a once- or twice-daily dosing interval that promotes patient compliance (Moore, 1999).
There have been no previous studies to characterize mechanisms of clarithromycin uptake by human gingival fibroblasts and oral epithelial cells. However, previous studies show that azithromycin and clarithromycin attain significantly higher concentrations in gingiva than in serum (Malizia et al., 1997; Blandizzi et al., 1999; Burrell and Walters, 2008). Moreover, clarithromycin reportedly reaches significantly higher steady-state concentrations at human gingivitis sites than at healthy sites (Burrell and Walters, 2008). These reports suggest that active transporters could mediate cellular uptake of clarithromycin. This issue is clinically relevant, because clarithromycin transport into pocket epithelial cells could facilitate elimination of invasive periodontal pathogens. Clarithromycin transport and accumulation by fibroblasts, which are abundant in gingival connective tissue, could potentially help sustain clarithromycin levels in the gingiva. In this report, cultured gingival fibroblasts and an oral epithelial cell line were studied to test the hypothesis that these cells possess an active transport system for accumulating clarithromycin.
MATERIALS AND METHODS
Cell culture
SCC-25 epithelial cells (CRL-1628, obtained from ATCC), originally derived from oral mucosa (Rheinwald and Beckett, 1981), were seeded into 75 cm2 tissue culture flasks and cultured in a 37°C incubator containing 5% CO2. Cells were grown in 50% Dulbecco’s modified Eagle’s medium/50% Ham’s F12 medium (Invitrogen Corp, Grand Island, NY, USA) containing 2 mM L-glutamine, 10% heat-inactivated fetal bovine serum, and 0.4 μg/ml hydrocortisone. They were fed every three days until they formed a confluent monolayer.
A fibroblast strain previously isolated from healthy (non-edematous, non-bleeding, pink) interproximal papillae under an IRB-approved protocol (Mariotti and Cochran, 1990) was seeded into 75 cm2 tissue culture flasks and cultured in a 37°C incubator containing 5% CO2. Cells were grown in minimal essential medium (Invitrogen Corp) containing 2 mM L-glutamine and 10% heat-inactivated fetal bovine serum. They were fed every three days until the cells formed a confluent monolayer.
Assay of clarithromycin transport
Cultured SCC-25 cells and fibroblasts monolayers were washed three times with Hanks Balanced Salts Solution (HBSS), harvested by brief treatment with HBSS containing 0.5 mg/mL trypsin and 0.2 mg/mL EDTA, counted with a hemocytometer, and suspended in HBSS at a density of 106 cells/ml. Transport was assayed by measuring changes in cell-associated radioactivity over time. Aliquots of cell suspension were incubated at 37° C with [3H]-clarithromycin (10 μg/ml for time course assays, 2 μg/ml for determination of the ratio of intracellular to extracellular concentrations, and 8–50 μg/ml in kinetic assays to determine the Michaelis constant (Km) and maximal velocity of transport (Vmax). After the indicated interval (3 minutes for kinetic assays and 2–15 minutes for uptake time courses), 0.5 ml aliquots of cell suspension were rapidly withdrawn, layered over 0.4 ml of a mixture of canola oil/dibutylphthalate (3:10) and centrifuged for 30 seconds at 15,000 × g in a microcentrifuge (Walters et al., 1999). After removal of aqueous and oil layers, cell pellets were recovered by cutting off the ends of the microcentrifuge tubes. The pellets were lysed for liquid scintillation counting by agitating in 0.8 ml of water for 12 hrs. Lineweaver-Burk analysis was used to determine Km and Vmax.
Assay of clarithromycin efflux
Cells were loaded to a steady-state intracellular clarithromycin concentration by incubation for 20 minutes at 37°C in HBSS containing 10 μg/ml [3H]-clarithromycin. To trigger efflux of intracellular clarithromycin stores, the concentration of clarithromycin in the extracellular medium was abruptly diluted 11:1 with 37°C HBSS. The decrease in intracellular clarithromycin concentration was monitored for 60 minutes.
Determination of intracellular clarithromycin concentration
Aliquots of suspended cells were loaded to steady-state with [3H]-clarithromycin, and intracellular clarithromycin content was assayed as described above. The intracellular volume of identical cell aliquots was measured by incubation for 20 minutes at 37°C with [3H]-water (5 aCi/ml, NEN Life Science Products). Intracellular clarithromycin concentrations were calculated by dividing cell content by cell volume.
RESULTS
SCC-25 cells and gingival fibroblasts rapidly accumulated clarithromycin, but exhibited signs of saturation after 10 minutes (Figure 1). In comparison to fibroblasts, SCC-25 cells appeared to saturate earlier and at a lower level of clarithromycin content per cell. Clarithromycin transport activity by SCC-25 cells and fibroblasts was directly correlated with temperature between 4° and 37°C (Figure 2, upper panel). The slope of the regression line was steeper for fibroblasts, suggesting that transport by these cells is more sensitive to temperature.
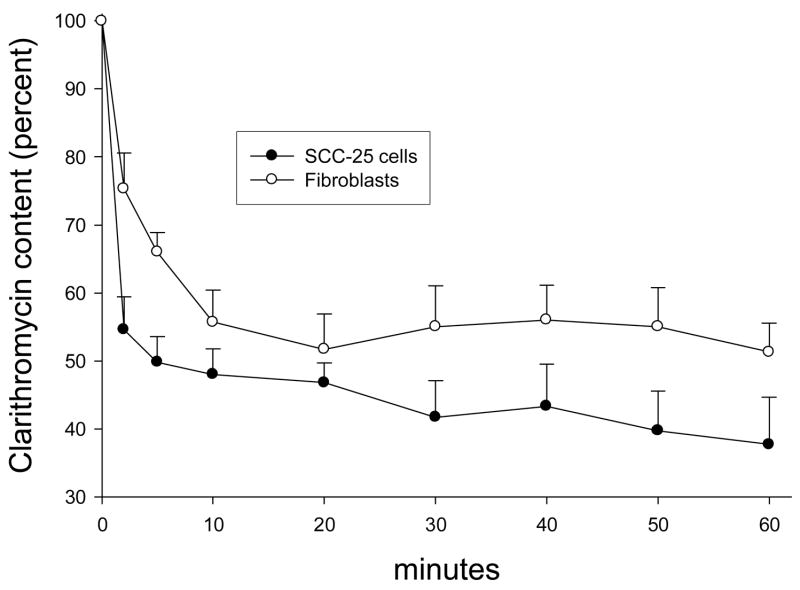
Figure 1.
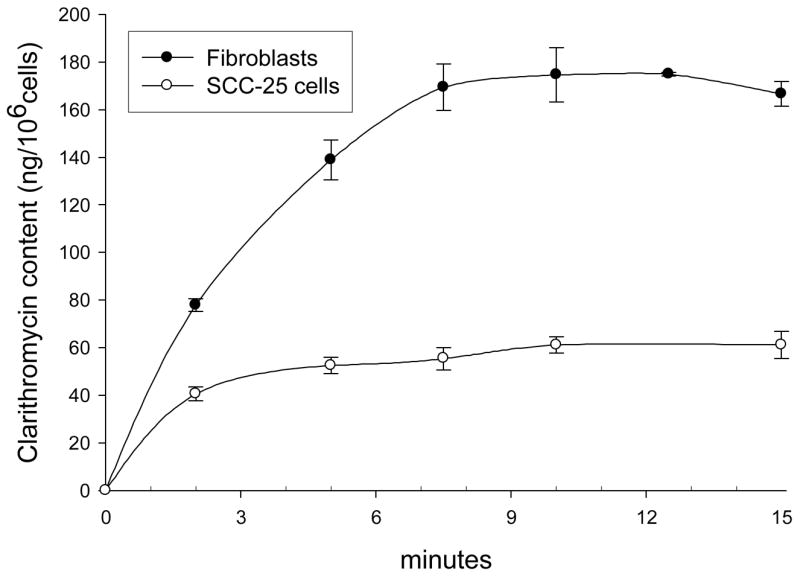
Time course of clarithromycin accumulation by SCC-25 cells and gingival fibroblasts. After cell preincubation at 37°C, 10 μg/ml [3H]-clarithromycin was added and uptake was monitored over the indicated time intervals. The data represent the mean ± SEM of six experiments with SCC-25 cells and four experiments with gingival fibroblasts.
Figure 2.
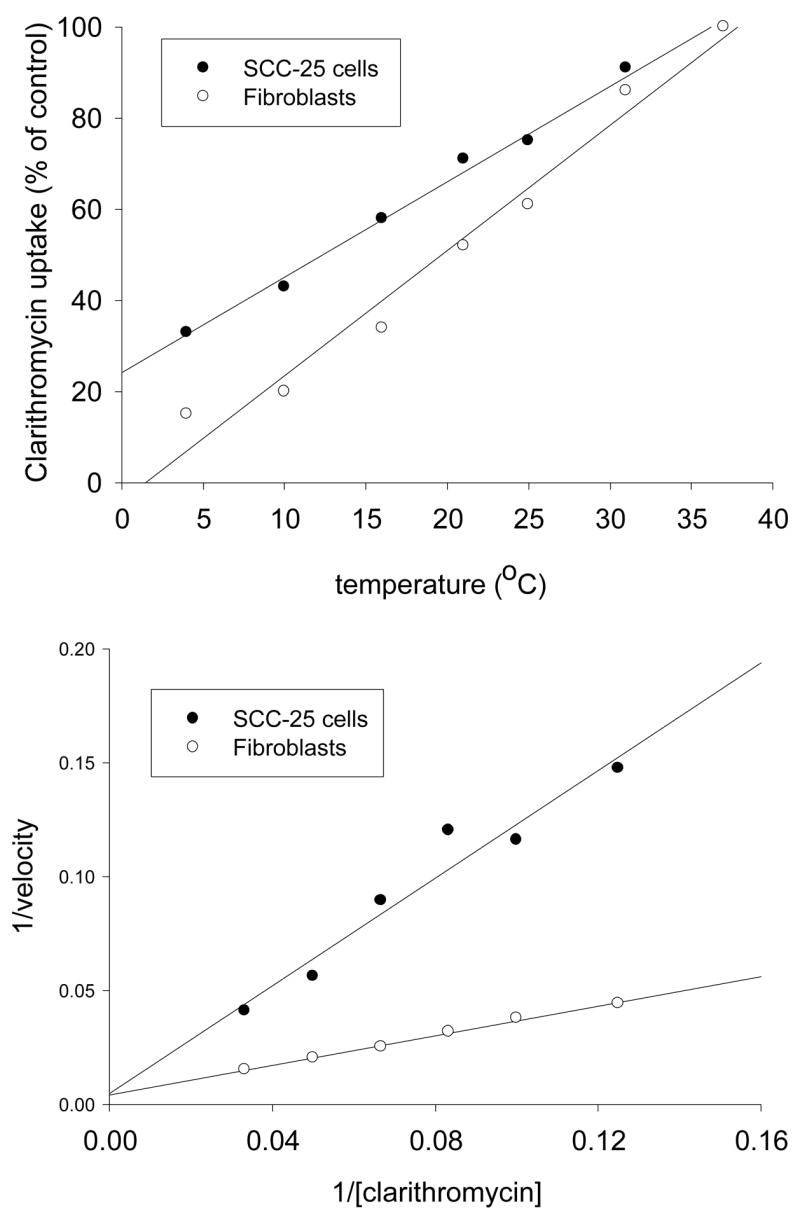
Characteristics of clarithromycin transport by SCC-25 cells and gingival fibroblasts. Upper panel: Temperature-dependence of clarithromycin accumulation. Cells were pre-incubated at the specified temperature between 4° and 37° C, then 10 μg/ml clarithromycin was added and uptake was monitored over a three minute interval. The SCC-25 data represent the mean of 4 experiments. The gingival fibroblast data represent the mean of 3 experiments. Lower panel: Representative Lineweaver-Burk plots of clarithromycin transport. Both panels were derived from four experiments with SCC-25 cells and three experiments with gingival fibroblasts.
The kinetics of clarithromycin transport yielded a linear Lineweaver-Burk plot in SCC-25 cells as well as fibroblasts (Figure 2, lower panel). The estimated Km values for transport by SCC-25 cells and gingival fibroblasts were 227 μg/ml and 78.4 μg/ml, respectively, and the Vmax values were 381 ng/min/106 cells and 264 ng/min/106 cells, respectively (Table 1). Differences between the two types of cells was statistically significant (P < 0.05, t-test). At steady state, after 20 minutes of cell incubation in medium containing 2 μg/ml clarithromycin, the ratio of intracellular clarithromycin concentration to extracellular concentration was approximately 3.3 in SCC-25 cells and 38 in gingival fibroblasts (Table 1).
Table 1.
Kinetic Constantsa for Clarithromycin Transport by SCC-25 Cells and Gingival Fibroblasts
| Cell type | Km (μg/ml) | Vmax (μg/min/106) | Cellular/Extracellular concentration ratio |
|---|---|---|---|
| SCC-25 cell line | 227 ± 20 b | 381 ± 29.5 b | 3.3 ± 0.28 c |
| gingival fibroblasts | 78.4 ± 7.2 b | 264 ± 32.1 b | 37.9 ± 2.6 c |
Km (Michaelis constant) and Vmax (maximum transport velocity) were determined by Lineweaver-Burk analysis of transport activity assayed in the presence of several different concentrations of clarithromycin (8 to 50 μg/ml) during the rapid initial phase of uptake (first 3 minutes). The Cellular/Extracellular concentration ratio was determined in medium containing 2 μg/ml clarithromycin. The data represent the mean ± SEM of four experiments with SCC-25 cells and three experiments with gingival fibroblasts.
Values within columns are significantly different (P < 0.05, t-test)
Values within columns are significantly different (P < 0.05, Mann-Whitney rank sum test)
When SCC-25 cells and fibroblasts were loaded to steady state with 10 μg/ml clarithromycin and clarithromycin in the extracellular medium was then diluted to 0.9 μg/ml, clarithromycin efflux from the cells was observed (Figure 3). Loaded SCC-25 cells lost approximately half of their clarithromycin content within 5 minutes. Thereafter, the rate of efflux decreased significantly. Under similar conditions, fibroblasts lost less than half of their clarithromycin content over a 20 minute period. Clarithromycin efflux from fibroblasts was consistently slower than efflux from SCC-25 cells.
Figure 3.
Efflux of [3H]-clarithromycin from loaded SCC-25 cells and gingival fibroblasts. Cells were loaded to steady-state by incubation for 20 minutes at 37°C in medium containing 10 μg/ml clarithromycin. To trigger efflux, extracellular antimicrobial solutions were diluted 1:10 with 37°C medium. Efflux was monitored by the decrease in cell-associated [3H]. The data represent the mean ± SEM of five experiments with SCC-25 cells and three experiments with gingival fibroblasts.
DISCUSSION
The results demonstrate that gingival fibroblasts and SCC-25 cells actively accumulate clarithromycin. SCC-25 cells are a malignant variant of oral squamous epithelial cells, but share many of their functions and features. These cells can be grown in culture without an underlying fibroblast feeder layer, which greatly simplifies their use in transport assays. In fibroblasts as well as SCC-25 cells, clarithromycin accumulation exhibited saturable kinetics and was concentrative and temperature-dependent.
While the values for the Km of clarithromycin transport suggest that it is taken up with low affinity, there was evidence of substantial concentration of this agent inside SCC-25 cells as well as fibroblasts. When incubated with clarithromycin at a concentration similar to that attained in human gingiva during antimicrobial chemotherapy (2–3 μg/g, Burrell and Walters, 2008), SCC-25 cells achieved steady-state intracellular clarithromycin levels that were approximately 3.3-fold higher than extracellular levels. A similar degree of intracellular concentration was observed in kidney epithelial cells by Bosnar et al. (2005). Under similar conditions, gingival fibroblasts attained intracellular concentrations that were almost 40-fold higher than extracellular levels. The difference in intracellular concentration between fibroblasts and SCC-25 cells was greater than expected based on the observed kinetic constants for transport. Although fibroblasts transport clarithromycin with significantly higher affinity than SCC-25 cells, their maximum transport velocity is lower. It is possible that some of the differences in uptake kinetics could be related to differences in the transformation states of fibroblasts and SCC-25 cells. The efficiency of clarithromycin transport by fibroblasts, as estimated by the ratio Vmax/Km, is only about twice that of SCC-25 cells. Previous reports suggest that exposure to clarithromycin concentrations of greater than 7.5 μg/ml is cytocidal to human gingival epithelial cells (Inoue et al., 2004), but does not disrupt vital activities in human periodontal ligament fibroblasts (Maizumi et al., 2002). It is possible that the cytocidal effects of clarithromycin could inhibit transport in epithelial cells, thereby limiting maximum steady-state intracellular drug levels.
Our findings suggest that clarithromycin accumulation by cells is mediated by an active transport system. As indicated by the linear Lineweaver-Burk plots, the kinetics of clarithromycin transport are saturable and obey the Michaelis-Menten equation. Clarithromycin is a weak base, so it could potentially be taken up by transporters that accept organic cation substrates. The two major transport systems for organic cations are the organic cation transporter (OCT) family and the organic anion transporting polypeptide (OATP) family. Consistent with a possible role for OATP, a recent study demonstrated that clarithromycin inhibits the uptake of certain drugs that are known substrates for transport by OATP family members OATP1B1 and OATP1B3 (Seithel et al., 2007).
While clarithromycin has not been evaluated in clinical trials as an adjunct to periodontal therapy, it is more effective in vitro against P. gingivalis and Prevotella intermedia than azithromycin (Goldstein et al., 1999) and inhibits A. actinomycetemcomitans with similar potency (Piccolomini et al., 1998; Pajukanta et al., 1992). Several studies have examined the effectiveness of azithromycin in periodontal applications. A placebo-controlled randomized clinical trial has shown that azithromycin enhances the reduction of pocket depth and bleeding on probing by scaling and root planing in adults with severe recurrent periodontitis (Smith et al., 2002). A second randomized trial demonstrated that use of azithromycin in combination with scaling and root planing enhances pocket reduction and attachment gain in smokers with moderate to advanced attachment loss (Mascarenhas et al., 2005). Azithromycin is a recommended therapy for acute periodontal abscesses with systemic manifestations in individuals with an allergy to beta-lactam drugs (Pallasch, 1996).
Transporters are capable of moving their substrates in the forward or reverse direction to maintain equilibrium between intracellular and extracellular concentrations. Since gingival fibroblasts comprise a relatively large volume of the gingival connective tissue (Schroeder and Listgarten, 1997), their ability to take up, accumulate and release clarithromycin could allow them to function as reservoirs for this agent in the gingiva. Consistent with this role, clarithromycin efflux from clarithromycin-loaded fibroblasts and SCC-25 cells was observed when antibiotic levels were experimentally decreased in the extracellular medium. Forward transport into cells presumably occurs in vivo when clarithromycin levels in tissue are increasing. As antibiotic levels in the blood and interstitial fluid decrease from their peak values, the direction of transport may reverse in a manner that maintains relatively high antimicrobial levels in gingival interstitial fluid and in gingival crevicular fluid. It is unclear how long fibroblasts could potentially sustain clarithromycin levels in the gingiva. However, a recent study demonstrated that the concentration of clarithromycin in gingival connective tissue can be several-fold higher than the level in serum approximately four hours after serum levels start to decrease from their peak concentration (Burrell and Walters, 2008). Doxycycline, which is also accumulated by gingival fibroblasts, yields therapeutic concentrations that are more sustained and less variable in gingival crevicular fluid than in blood serum (Lavda et al, 2004). In epithelial cells, forward transport of clarithromycin greatly enhances the intracellular concentration of this antibiotic. In summary, transport by gingival fibroblasts and pocket epithelium could potentially enhance the effectiveness of clarithromycin in periodontal therapy by sustaining their therapeutic levels in gingival connective tissue and inside epithelial cells that have been invaded by A. actinomycetemcomitans or P. gingivalis.
Acknowledgments
This investigation was supported by USPHS research grants R01 DE012601 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA.
References
- Blandizzi C, Malizia T, Lupetti A, Pesce D, Gabriele M, Giuca MR, Campa M, Del Tacca M, Senesi S. Periodontal tissue disposition of azithromycin in patients affected by chronic inflammatory periodontal diseases. J Periodontol. 1999;70:960–966. doi: 10.1902/jop.1999.70.9.960. [DOI] [PubMed] [Google Scholar]
- Bosnar M, Kelneric Z, Munic V, Erakovic V, Parnham MJ. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob Agents Chemother. 2005;49:2372–2377. doi: 10.1128/AAC.49.6.2372-2377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragd L, Dahlen G, Wikstrom M, Slots J. The capability of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius to indicate progressive periodontitis; a retrospective study. J Clin Periodontol. 1987;14:95–99. doi: 10.1111/j.1600-051x.1987.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Burrell RC, Walters JD. Distribution of systemic clarithromycin to gingiva. J Periodontol. 2008;79 doi: 10.1902/jop.2008.080013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick PR, Mellersh AR. The use of a tissue culture model to assess the penetration of antibiotics into epithelial cells. J Antimicrob Chemother. 1987;19:211–220. doi: 10.1093/jac/19.2.211. [DOI] [PubMed] [Google Scholar]
- Chen C, Slots J. The current status and future prospects of altering the pathogenic microflora of periodontal disease. Curr Opin Periodontol. 1993;1:71–77. [PubMed] [Google Scholar]
- Christersson LA, Albini B, Zambon JJ, Wikesjo UM, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor P, Meyer D, Mintz K. Characteristics of Actinobacillus actinomycetemcomitans invasion of and adhesion to cultured epithelial cells. Adv Dent Res. 1995;9:55–62. doi: 10.1177/08959374950090011001. [DOI] [PubMed] [Google Scholar]
- Goldstein EJC, Citron DM, Merriam CV, Warren Y, Tyrrell K. Activities of telithromycin compared to those of erythromycin, azithromycin, clarithromycin, roxithromycin and other antimicrobial agents against unusual anaerobes. Antimicrob Agents Chemother. 1999;43:2801–2805. doi: 10.1128/aac.43.11.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kumakura S, Uchida M, Tsutsui T. Effects of eight antibacterial agents on cell survival and expression of epithelial-cell- or cell-adhesion-related genes in human gingival epithelial cells. J Periodontal Res. 2004;39:50–58. doi: 10.1111/j.1600-0765.2004.00704.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavda M, Clausnitzer CE, Walters JD. Distribution of systemic ciprofloxacin and doxycycline to gingiva and gingival crevicular fluid. J Periodontol. 2004;75:1663–1667. doi: 10.1902/jop.2004.75.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizumi N, Tamura Y, Kanai H, Tsutsui T. Quantitative comparison of the cytocidal effect of seven macrolide antibiotics on human periodontal ligament fibroblasts. J Periodontal Res. 2002;37:250–254. doi: 10.1034/j.1600-0765.2002.01616.x. [DOI] [PubMed] [Google Scholar]
- Malizia T, Tejada MR, Ghelardi E, Senesi S, Gabriele M, Giuca MR, Blandizzi C, Danesi R, Campa M, Del Tacca M. Periodontal tissue disposition of azithromycin. J Periodontol. 1997;68:1206–1209. doi: 10.1902/jop.1997.68.12.1206. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Cochran DL. Characterization of fibroblasts derived from human periodontal ligament and gingiva. J Periodontol. 1990;61:103–111. doi: 10.1902/jop.1990.61.2.103. [DOI] [PubMed] [Google Scholar]
- Mascarenhas P, Gapski R, Al-Shammari K, Hill R, Soehren S, Fenno JC, Giannobile WV, Wang HL. Clinical response of azithromycin as an adjunct to non-surgical periodontal therapy in smokers. J Periodontol. 2005;76:426–436. doi: 10.1902/jop.2005.76.3.426. [DOI] [PubMed] [Google Scholar]
- Merriam CV, Citron DM, Tyrrell KL, Warren YA, Goldstein EJ. In vitro activity of azithromycin and nine comparator agents against 296 strains of oral anaerobes and 31 strains of Eikenella corrodens. Int J Antimicrob Agents. 2006;28:244–248. doi: 10.1016/j.ijantimicag.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Moore PA. Dental therapeutic indications for the newer long-acting macrolide antibiotics. J Am Dent Assoc. 1999;130:1341–1343. doi: 10.14219/jada.archive.1999.0404. [DOI] [PubMed] [Google Scholar]
- Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro activity of azithromycin compared with that of erythromycin against Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1992;36:1241–1243. doi: 10.1128/aac.36.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajukanta R. In vitro antimicrobial susceptibility of Porphyromonas gingivalis to azithromycin, a novel macrolide. Oral Microbiol Immunol. 1993;8:325–326. doi: 10.1111/j.1399-302x.1993.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Pallasch TJ. Pharmacokinetic principles of antimicrobial therapy. Periodontol 2000. 1996;10:5–11. doi: 10.1111/j.1600-0757.1996.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Piccolomini R, Catamo G, Di Bonaventura G. Bacteriostatic and bactericidal in vitro activities of clarithromycin and erythromycin against periodontopathic Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1998;42:3000–3001. doi: 10.1128/aac.42.11.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Saglie FR, Smith CT, Newman MG, Carranza FA, Jr, Pertuiset JH, Cheng L, Auil E, Nisengard RJ. The presence of bacteria in the oral epithelium in periodontal disease. II. Immunohistochemical identification of bacteria. J Periodontol. 1986;57:492–500. doi: 10.1902/jop.1986.57.8.492. [DOI] [PubMed] [Google Scholar]
- Schentag JJ, Swanson DJ, Smith IL. Dual individualization-antibiotic dosage calculation from the integration of in vitro pharmacodynamics and in vivo pharmacokinetics. J Antimicrob Chemother. 1985;15(suppl A):47–57. doi: 10.1093/jac/15.suppl_a.47. [DOI] [PubMed] [Google Scholar]
- Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Sefton AM, Maskell JP, Beighton D, Whiley A, Shain H, Foyle D, Smith SR, Smales FC, Williams JD. Azithromycin in the treatment of periodontal disease. Effect on microbial flora. J Clin Periodontol. 1996;23:998–1003. doi: 10.1111/j.1600-051x.1996.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, Dorje F, Fromm MF, Konig J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35:779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- Smith SR, Foyle DM, Daniels J, Joyston-Bechal S, Smales FC, Sefton A, Williams J. A double-blind placebo-controlled trial of azithromycin as an adjunct to non-surgical treatment of periodontitis in adults: clinical results. J Clin Periodontol. 2002;29:54–61. doi: 10.1034/j.1600-051x.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Walters JD, Zhang F, Nakkula RJ. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]