Abstract
Recent research on speciation has identified a central role for ecological divergence, which can initiate speciation when (i) subsets of a species or population evolve to specialize on different ecological resources and (ii) the resulting phenotypic modes become reproductively isolated. Empirical evidence for these two processes working in conjunction, particularly during the early stages of divergence, has been limited. We recently described a population of the medium ground finch, Geospiza fortis, that features large and small beak morphs with relatively few intermediates. As in other Darwin's finches of the Galápagos Islands, these morphs presumably diverged in response to variation in local food availability and inter- or intraspecific competition. We here demonstrate that the two morphs show strong positive assortative pairing, a pattern that holds over three breeding seasons and during both dry and wet conditions. We also document restrictions on gene flow between the morphs, as revealed by genetic variation at 10 microsatellite loci. Our results provide strong support for the central role of ecology during the early stages of adaptive radiation.
Keywords: ecological speciation, adaptive radiation, assortative mating, adaptive divergence, genetic divergence
1. Introduction
Bimodal populations, although rare, provide outstanding opportunities to study the early stages of adaptive diversification (Smith 1993; Smith & Skulason 1996; Orr & Smith 1998; Gislason et al. 1999; Rundle & Nosil 2005). We have recently described a bimodal population of the medium ground finch (Geospiza fortis) at El Garrapatero on Santa Cruz Island, Galápagos, Ecuador (figure 1). This population features birds that fall mainly into large and small beak size morphs, with relatively few intermediates, a pattern that has been confirmed statistically (Hendry et al. 2006; Huber & Podos 2006). If other G. fortis populations are any guide (Price 1987; Grant 1999; Keller et al. 2001; Grant & Grant 2006), this variation has a strong additive genetic basis and reflects selection imposed by variation in the size and hardness of seeds. The bimodality has almost certainly arisen owing to specialization by the two morphs on different food types, perhaps coupled with intra- or interspecific competition, and reflecting processes thought to have driven the adaptive radiation as a whole (Lack 1947; Boag & Grant 1981; Schluter 2000; Grant & Grant 2002; Herrel et al. 2005). Moreover, the structure of vocal mating signals (songs) of males at El Garrapatero differs between the morphs in acoustic parameters that correspond to differences in beak size and vocal performance (Huber & Podos 2006). The presence of ecologically driven bimodality in beak size, coupled with divergence in mating signals, suggests that this population might be in an early stage of speciation, a possibility that we investigate here.
Figure 1.
(a) The bimodal distribution of beak sizes for G. fortis at El Garrapatero in 2004 (white bars, females; black bars, males). Bimodality has been inferred for this population by statistical comparison of fits with unimodal and bimodal distributions (Hendry et al. 2006). (b) Representative small morph (left) and large morph (right) birds; both are mature males caught at the same time in the same mist net. (c) A large ground finch (G. magnirostris) and the same large morph G. fortis shown in (b). (d) A small ground finch (G. fuliginosa). Scale bar=5 mm. Photo credits Andrew Hendry.
We examined three factors that may influence incipient ecological speciation in El Garrapatero G. fortis: the strength of assortative pairing; the persistence of assortative pairing over time and across variable ecological conditions; and levels of gene flow between the morphs. Our study focused on breeding pairs of G. fortis during 2004–2006. Climatic conditions varied widely during these years, which allowed us to test for the strength and stability of assortative pairing under variable ecological conditions. Virtually no rain fell in 2004 and in the first two months of 2005, making this the most extreme drought in the 40-year period of record (Grant & Grant 2006). Some breeding occurred during this period but at a low rate. Heavy rains fell in March 2005, and the number of breeding pairs increased considerably. More typical rainfall prevailed in 2006.
2. Material and methods
We studied pairs of G. fortis during the breeding season in January–April 2004, January–May 2005 and January–March 2006 at El Garrapatero, Santa Cruz Island, Galápagos, Ecuador (GPS coordinates: 00°40′20″–41′20″ S; 90°13′10″–14′40″ W). Birds were captured in mist nets and banded with unique combinations of one metal and three colour bands. We took the following measurements on each bird (Grant et al. 1985): beak length; beak depth; and beak width. We then collected a small volume of blood from the ulnar vein of each bird, using a 27-gauge needle and filter paper treated with EDTA.
Focal observations of individuals were used to determine pairing status. Repeat observations of pairs were made every 3–4 days throughout the breeding season or until nestlings fledged. The occurrence of two or more of the following behaviours was used to identify mated pairs: nest building by both the male and female; copulation; mate guarding; feeding of the female by the male during incubation or courtship; feeding of nestlings; back-and-forth calling between the male and the female. This study was restricted to pairs that bred (i.e. females that laid eggs). Nests of the two morphs are fully interspersed at our study site (S.K. Huber 2005, unpublished data), and patterns of assortative pairing could thus be attributed to assortative mate choice rather than spatial segregation.
To test for assortative pairing, we first calculated a composite measure of beak size, using a principal components analysis that included beak length, depth and width (as in Grant 1999). Across all birds at El Garrapatero banded between 2004 and 2006, PC1 explained 88.3% of the variation in beak measurements (eigenvalue=2.65). Assortative pairing was then tested by plotting PC1 for males against PC1 of the females with which they were paired. Non-parametric Spearman's rank correlations were used to determine the degree of assortative pairing. These correlations were calculated based on pairs formed under dry conditions (2004 to early 2005, n=21 pairs), under very wet conditions (late 2005, n=33 pairs) and under moderately wet conditions (2006, n=26 pairs). The 2004 to early 2005 dataset did not contain any duplicate individuals. Some individuals were included in more than one of the three datasets. However, no individuals paired with multiple mates within a given year, and all birds that bred in multiple years changed mates from one year to the next.
The consequences of assortative pairing depend largely on the extent of extra-pair fertilizations (EPFs) and whether EPFs occur within or between the morphs. EPFs have been documented in G. fortis of another, small island population (Keller et al. 2001). Even low rates of EPFs would presumably eliminate any genetic differences between the morphs that accrue through assortative mating. To assess levels of intermorph gene flow, we divided the birds into small and large beak size classes, between which we examined patterns of genetic variation across 10 microsatellite loci. If genetic differences are present, then EPFs between the morphs are either absent or do not contribute substantially to genetic exchange between the morphs.
Total DNA was extracted from blood samples collected in 2004 and 2005 using a modified proteinase K phenol–chloroform protocol (Sambrook et al. 1989). Fragments were amplified by polymerase chain reaction (PCR) for 10 unlinked dinucleotide microsatellites (Petren 1998). PCR products were analysed using a multi-capillary sequencer ABI 3100. Genetic work was carried out at Naos Molecular Laboratories at the Smithsonian Tropical Research Institute in Panama.
Genetic comparisons were made between small and large beak size classes within each year. Birds were assigned to these classes by performing a principal components analysis of beak length, depth and width for all banded birds in a given year. We then placed individuals into two groups (small or large) based on a cluster analysis of PC1 (SPSS v. 12.0, 2003). Individuals that were within 0.5 s.d. of the small/large ‘cut-off’ were considered intermediate and removed from the analysis (n=47). These intermediate birds were encompassed by both the upper tail of the small beak morph distribution and the lower tail of the large beak morph distribution, and thus could not be assigned reliably to either class. If gene flow is not influenced by beak size (the null expectation), then removing birds with intermediate beak sizes should have no effect on our results.
Measures of genetic variation, including allelic diversity, frequency and heterozygosity, were calculated using Genepop v. 3.4 (Raymond & Rousset 1995), Fstat v. 2.9.3.2 and Arlequin v. 3.0 (table 1). We tested for Hardy–Weinberg equilibrium using the Markov chain method as implemented in Genepop v. 3.4 (dememorization=10 000, batches=100, iterations per batch=5000; table 1). We tested for linkage disequilibrium between pairs of loci using Genepop v. 3.4 (table 2).
Table 1.
Overall genetic diversity for large and small morph G. fortis sampled at El Garrapatero (small morph, n=197; large morph, n=59). (Shown are the number of birds analysed (N), number of alleles (NA), observed heterozygosities (HO), expected heterozygosities (HE), FIS and p values from a Hardy–Weinberg test for heterozygote deficits across all birds. Italicized p values indicate those that remained significant after a sequential Bonferroni correction.)
| locus | N | NA | HO | HE | FIS | p |
|---|---|---|---|---|---|---|
| Gf03 | 254 | 14 | 0.818 | 0.854 | 0.041 | 0.017 |
| Gf04 | 254 | 5 | 0.472 | 0.474 | −0.002 | 0.502 |
| Gf05 | 254 | 10 | 0.677 | 0.681 | 0.006 | 0.255 |
| Gf07 | 245 | 19 | 0.861 | 0.870 | 0.010 | 0.007 |
| Gf08 | 253 | 24 | 0.885 | 0.925 | 0.043 | 0.004 |
| Gf09 | 256 | 15 | 0.578 | 0.601 | 0.039 | 0.266 |
| Gf11 | 243 | 29 | 0.844 | 0.937 | 0.100 | <0.001 |
| Gf12 | 252 | 16 | 0.885 | 0.901 | 0.017 | 0.204 |
| Gf13 | 255 | 13 | 0.878 | 0.870 | −0.010 | 0.751 |
| Gf16 | 255 | 11 | 0.808 | 0.789 | −0.025 | 0.673 |
Table 2.
Pairs of loci that showed significant (p<0.05) linkage disequilibrium across all loci. (Italicized p values indicate those that remain significant after sequential Bonferroni corrections.)
| loci | α2 | d.f. | p |
|---|---|---|---|
| Gf05 & Gf11 | infinity | 2 | <0.0001 |
| Gf04 & Gf13 | 19.44 | 2 | <0.0001 |
| Gf03 & Gf05 | 8.24 | 2 | 0.016 |
| Gf05 & Gf08 | 7.04 | 2 | 0.030 |
| Gf04 & Gf08 | 6.95 | 2 | 0.031 |
| Gf08 & Gf11 | 6.67 | 2 | 0.036 |
| Gf07 & Gf08 | 6.37 | 2 | 0.041 |
Genetic differences between beak size classes were analysed in several ways. First, we tested for significant differences in allele frequencies by using the ‘genic differentiation’ option in Genepop v. 3.4. Second, we estimated genetic distances using F-statistics (FST) and R-statistics (RST; Weir & Cockerham 1984; Slatkin 1995). These analyses used 10 000 randomizations. For FST across all loci (table 3; FST=0.017), the 95% confidence interval was computed to be 0.011–0.024 using Fstat.
Table 3.
Genetic differences between small and large beak size classes. (Genic differentiation p values were obtained using Genepop. Italicized p values indicate those that remained significant after a sequential Bonferroni correction.)
| locus | FST | RST | genic differentiation (p) |
|---|---|---|---|
| Gf03 | 0.026 | 0.032 | 0.005 |
| Gf04 | 0.030 | 0.016 | 0.006 |
| Gf05 | 0.012 | 0.038 | <0.001 |
| Gf07 | 0.038 | 0.001 | <0.0001 |
| Gf08 | 0.015 | 0.051 | 0.0001 |
| Gf09 | 0.005 | −0.000 | 0.009 |
| Gf11 | 0.017 | 0.050 | <0.0001 |
| Gf12 | 0.007 | 0.041 | 0.064 |
| Gf13 | 0.006 | 0.049 | 0.006 |
| Gf16 | 0.012 | 0.012 | 0.006 |
| overall | 0.017 | 0.040 | <0.0001 |
Multilocus genotypes were also used to assess population structuring. First, we used factorial correspondence analysis in Genetix v. 4.05 (Belkhir et al. 2004) to determine the similarity of allelic states between the morphological classes. We used a t-test for differences between beak morphs in scores for factors 1 and 2. Second, we used the Bayesian approach implemented in Structure v. 2.1 (Pritchard et al. 2000). We ran five simulations for each putative number of clusters (K=1–5). In each case, we used the admixture model with burn-in of 100 000 and Monte Carlo Markov chain iteration value of 500 000. The most probable number of clusters was always K=1, but visual inspection suggested some differences between the clusters. We therefore tested for significant differences in cluster placement when K=2. Specifically, the results of a Structure analysis give a probability between one and zero that an individual belongs to cluster 1 or cluster 2 (the sum of probabilities for both clusters is one). Probabilities were arcsine square root transformed, and a t-test was used to compare values between the two putative clusters for each morphs in all five iterations. This analysis revealed whether morphs were being randomly placed into the two clusters.
3. Results
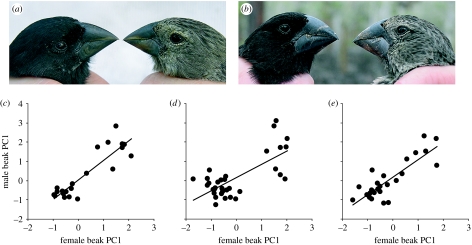
We found strong positive assortative pairing by beak size (figure 1a,b) for dry conditions in 2004 and early 2005 (r=0.742, p=0.001; figure 2c), very wet conditions in late 2005 (r=0.390, p=0.025; figure 2d) and moderately wet conditions in 2006 (r=0.705, p<0.001; figure 2e).
Figure 2.
Assortative pairing by beak size in pairs of G. fortis at El Garrapatero. (a) A breeding pair of small morph individuals (photo credit Eric Hilton) and (b) a breeding pair of large morph individuals (photo credit Sarah Huber) photos not to scale. (c) Assortative pairing under dry conditions (2004, early 2005). (d) The pattern under very wet conditions (late 2005) and (e) the pattern under moderately wet conditions (2006). Male and female ‘beak PC1’ values are scores along the first principal component based on beak length, depth and width.
Multiple lines of evidence, consistent across loci, indicate restricted gene flow between the large and small morphs. First, we found several signatures of population admixture (Hardy–Weinberg deficits and linkage disequilibrium) when pooling all of the birds (tables 1 and 2). Second, allele frequencies differed significantly between beak size classes at nine of the ten loci (table 3). Third, genetic divergence measures between the large and small beak size classes were non-trivial (FST=0.017; RST=0.040) and differed significantly from zero (table 3).
Some population structure was also evident based on multilocus genotypes. In particular, Genetix revealed significant differences between the large and small beak size classes on each of the first two factors (factor 1: t=−2.30, p=0.02; factor 2: t=2.56, p=0.01). Structure found few differences between the morphs (with a single cluster always being most likely; mean ln(p)=−10318.92), but this is expected when groups are only moderately differentiated (Pritchard et al. 2000). Yet, the assignment of individuals to clusters when K=2 was not random with respect to beak size for large morph individuals in two of the five iterations (iteration 1: t=−3.24, p=0.001; iteration 2: t=2.47, p=0.014) and for small morph individuals in one of the five iterations (t=5.65, p<0.001).
4. Discussion
Our genetic data reveal that the large and small beak morphs at El Garrapatero represent two partially distinct gene pools. Our behavioural data suggest that this genetic divergence can be attributed, at least in part, to females' choice of males with similar beak sizes. With this evidence, we are in a position to consider factors that might promote and maintain the observed bimodality.
One possible factor promoting bimodality is disruptive selection in sympatry (Rueffler et al. 2006), in this case against birds with intermediate beak sizes. Indeed, we have found that intermediate birds survive at lower rates between years in comparison with large and small morphs (A. P. Hendry & J. Podos 2006, unpublished data). Such disruptive selection could, in principle, lead to a purely sympatric origin of reproductive isolation (Higashi et al. 1999; Kondrashov & Kondrashov 1999; Ryan et al. 2007). This process is especially probable under two conditions. The first condition is that traits under divergent selection (here beak size) are the same as, or are genetically or phenotypically linked to, traits that influence mate choice (here beak size, a visual cue and song, a vocal mating signal; see Ratcliffe & Grant 1983; Podos 2001). The second condition is that mating is assortative with respect to those traits (Grant et al. 2000), as shown here.
Another factor that may promote bimodality is initial divergence during a period of allopatry. The El Garrapatero G. fortis morphs may have originated at different places on the same island, or on different islands, under distinct ecological conditions and divergent selection regimes. Following secondary contact, these differences could have led to assortative mating and reduced gene flow through the sympatric processes described above. Indeed, this scenario of initial allopatric divergence followed by further sympatric divergence mirrors a widely accepted model of speciation in many taxa, including Darwin's finches (Grant 1999; Schluter 2000).
Yet another factor potentially influencing bimodality is introgression with other Darwin's finch species. At our study site, G. fortis is sympatric with a smaller ground finch species, Geospiza fuliginosa, and a larger ground finch species, Geospiza magnirostris. Perhaps large G. fortis historically hybridized with G. magnirostris or small G. fortis hybridized with G. fuliginosa. We have identified at least one instance of hybridization in our population, in which a large morph G. fortis female mated with a G. magnirostris male. This sort of interspecies mating could increase phenotypic and genetic variation in G. fortis, which might then facilitate the emergence of bimodality (Seehausen 2004). Indeed, the Galápagos ground finches may be a promising system for determining how hybridization facilitates speciation (Mallet 2007) rather than just hampering it (Grant & Grant 2002).
In conjunction with previous work on Darwin's finches, our results support the role of ecologically mediated phenotypic divergence as an important driving force in the early stages of adaptive radiation. Divergence is initiated when variation in food types, food availability or competition imposes divergent selection (in allopatry) or disruptive selection (in sympatry) on beak morphology (Boag & Grant 1981; Grant 1999). Resulting adaptive divergence then imposes secondary consequences on the evolution of mating signals (Ratcliffe & Grant 1983; Podos 2001; Podos & Nowicki 2004; Podos et al. 2004; Huber & Podos 2006). This divergence in mating signals may then cause assortative mating and thus help maintain reproductive isolation in sympatry. Beak morphology in Darwin's finches may therefore be regarded as one of the elusive ‘magic traits’ of speciation (Gavrilets 2004), given that it is both a target of divergent selection and a component in mating signals that drive assortative mating (Grant & Grant 1997; Podos & Hendry 2006). We have identified a population that seems to be in the early stages of this process.
It is uncertain whether or not the two morphs at El Garrapatero will ultimately diverge to the level of well-defined species. For instance, immigration from a nearby unimodal population (Hendry et al. 2006), which might itself have considerable gene flow between birds with large and small beaks, could hamper further divergence between the morphs at El Garrapatero. Additionally, environmental conditions may eventually change to the extent that ‘hybrid’ offspring no longer have reduced fitness, as has been the case for established species of Darwin's finches (Grant & Grant 1996). Regardless, our data support the hypothesis that the early stages of assortative mating and reproductive isolation are driven by ecological divergence.
Acknowledgments
The collection of data in this study was done in concordance with Animal use Protocols approved by the University of Massachusetts Amherst.
We thank the Galápagos National Park and the Charles Darwin Research Station for providing support to this research. Anthony Herrel, Ana Gabela, Eric Hilton, Haldre Rogers and Steve Johnson provided assistance in the field. Funding was provided by National Science Foundation grant IBN 0347291 to J.P., and National Science Foundation grant DDIG 0508730 to J.P. and S.K.H. Additional funding for S.K.H. was provided by an American Ornithologists' Union Student Research grant, an Explorer's Club grant, a Sigma Xi Grant-in-Aid of Research, an Animal Behaviour Society Student Research grant and a University of Massachusetts Woods Hole Fellowship. Support for L.F.D. was provided by Instituto para la Formación y Aprovechamiento de Recursos Humanos and Secretaria Nacional de Ciencia, Tecnología e Innovación.
References
- Belkhir, K., Chikhi, L., Raufaste, N. & Bonhomme, F. 2004 Genetixlogiciel sous WindowsTM pour la génétique des populations, 4.05. Montpellier, France: Laboratoire Génome, Populations, Interactions CNRS UMR 5000, Université de Montpellier II.
- Boag P.T, Grant P.R. Intense natural selection in a population of Darwin's finches (Geospizinae) in the Galápagos. Science. 1981;214:82–85. doi: 10.1126/science.214.4516.82. doi:10.1126/science.214.4516.82 [DOI] [PubMed] [Google Scholar]
- Gavrilets S. Princeton University Press; Princeton, NJ: 2004. Fitness landscapes and the origin of species. [Google Scholar]
- Gislason D, Ferguson M.M, Skulason S, Snorrason S.S. Rapid and coupled phenotypic and genetic divergence in Icelandic Arctic char (Salvelinus alpinus) Can. J. Fish. Aquat. Sci. 1999;56:2229–2234. doi:10.1139/cjfas-56-12-2229 [Google Scholar]
- Grant P.R. Princeton University Press; Princeton, NJ: 1999. Ecology and evolution of Darwin's finches. [Google Scholar]
- Grant B.R, Grant P.R. High survival of Darwin's finch hybrids: effects of beak morphology and diets. Ecology. 1996;77:500–509. doi:10.2307/2265625 [Google Scholar]
- Grant P.R, Grant B.R. Mating patterns of Darwin's finch hybrids determined by song and morphology. Biol. J. Linn. Soc. 1997;60:317–343. doi:10.1006/bijl.1996.0103 [Google Scholar]
- Grant P.R, Grant B.R. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. doi:10.1126/science.1070315 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. doi:10.1126/science.1128374 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Abbot I, Schluter D, Curry R.L, Abbott L.K. Variation in the size and shape of Darwin's finches. Biol. J. Linn. Soc. 1985;25:1–39. [Google Scholar]
- Grant P.R, Grant B.R, Petren K. The allopatric phase of speciation: the sharpbeaked ground finch (Geospiza difficilis) on the Galápagos islands. Biol. J. Linn. Soc. 2000;69:287–317. doi:10.1006/bijl.1999.0382 [Google Scholar]
- Hendry A.P, Grant P.R, Grant B.R, Ford H.A, Brewer M.J, Podos J. Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proc. R. Soc. B. 2006;273:1887–1894. doi: 10.1098/rspb.2006.3534. doi:10.1098/rspb.2006.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel A, Podos J, Huber S.K, Hendry A.P. Bite performance and morphology in a population of Darwin's finches: implications for the evolution of beak shape. Funct. Ecol. 2005;19:43–48. doi:10.1111/j.0269-8463.2005.00923.x [Google Scholar]
- Higashi M, Takimoto G, Yamamura N. Sympatric speciation by sexual selection. Nature. 1999;402:523–526. doi: 10.1038/990087. doi:10.1038/990087 [DOI] [PubMed] [Google Scholar]
- Huber S.K, Podos J. Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis) Biol. J. Linn. Soc. 2006;88:489–498. doi:10.1111/j.1095-8312.2006.00638.x [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Heritability of morphological traits in Darwin's finches: misidentified paternity and maternal effects. Heredity. 2001;87:325–336. doi: 10.1046/j.1365-2540.2001.00900.x. doi:10.1046/j.1365-2540.2001.00900.x [DOI] [PubMed] [Google Scholar]
- Kondrashov A.S, Kondrashov F.A. Interactions among quantitative traits in the course of sympatric speciation. Nature. 1999;400:351–354. doi: 10.1038/22514. doi:10.1038/22514 [DOI] [PubMed] [Google Scholar]
- Lack D. Cambridge University Press; Cambridge, UK: 1947. Darwin's finches. [Google Scholar]
- Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. doi:10.1038/nature05706 [DOI] [PubMed] [Google Scholar]
- Orr M.R, Smith T.B. Ecology and speciation. Trends Ecol. Evol. 1998;13:502–506. doi: 10.1016/s0169-5347(98)01511-0. doi:10.1016/S0169-5347(98)01511-0 [DOI] [PubMed] [Google Scholar]
- Petren K. Microsatellite primers from Geospiza fortis and cross-species amplification in Darwin's finches. Mol. Ecol. 1998;7:1782–1784. doi: 10.1046/j.1365-294x.1998.00518.x. [DOI] [PubMed] [Google Scholar]
- Podos J. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature. 2001;409:185–188. doi: 10.1038/35051570. doi:10.1038/35051570 [DOI] [PubMed] [Google Scholar]
- Podos J, Hendry A.P. The biomechanics of ecological speciation. In: Herrel A, Speck T, Rowe N, editors. Ecology and biomechanics: a mechanical approach to the ecology of animals and plants. CRC Press; Boca Raton, FL: 2006. pp. 301–321. [Google Scholar]
- Podos J, Nowicki S. Beaks, adaptation, and vocal evolution in Darwin's finches. Bioscience. 2004;54:501–510. doi:10.1641/0006-3568(2004)054[0501:BAAVEI]2.0.CO;2 [Google Scholar]
- Podos J, Southall J.A, Rossi-Santos M. Vocal mechanics in Darwin's finches: correlation of beak gape and song frequency. J. Exp. Biol. 2004;207:607–619. doi: 10.1242/jeb.00770. doi:10.1242/jeb.00770 [DOI] [PubMed] [Google Scholar]
- Price T. Diet variation in a population of Darwin's finches. Ecology. 1987;68:1015–1028. doi:10.2307/1938373 [Google Scholar]
- Pritchard J.K, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe L.M, Grant P.R. Species recognition in Darwin's finches (Geospiza, Gould). I. Discrimination by morphological cues. Anim. Behav. 1983;31:1139–1153. doi:10.1016/S0003-3472(83)80021-9 [Google Scholar]
- Raymond M, Rousset F. Genepop version 1.2: population genetics software for exact tests and ecumenicism. J. Hered. 1995;86:248–249. [Google Scholar]
- Rueffler C, Van Dooren T.J.M, Leimar O, Abrams P.A. Disruptive selection and then what? Trends Ecol. Evol. 2006;21:238–245. doi: 10.1016/j.tree.2006.03.003. doi:10.1016/j.tree.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Rundle H.D, Nosil P. Ecological speciation. Ecol. Lett. 2005;8:336–352. doi:10.1111/j.1461-0248.2004.00715.x [Google Scholar]
- Ryan P.G, Bloomer P, Moloney C.L, Grant T.J, Delport W. Ecological speciation in South Atlantic island finches. Science. 2007;315:1420–1423. doi: 10.1126/science.1138829. doi:10.1126/science.1138829 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E.F, Maniatis T. 2nd edn. Cold Spring Harbor Laboratory Press; New York, NY: 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- Schluter D. Oxford University Press; Oxford, UK: 2000. The ecology of adaptive radiation. [Google Scholar]
- Seehausen O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004;19:198–207. doi: 10.1016/j.tree.2004.01.003. doi:10.1016/j.tree.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Slatkin M. A measure of population subdivision based on microsatellite allele frequencies. Genetics. 1995;139:457–462. doi: 10.1093/genetics/139.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.B. Disruptive selection and the genetic basis of bill size polymorphism in the African finch Pyrenestes. Nature. 1993;363:618–620. doi:10.1038/363618a0 [Google Scholar]
- Smith T.B, Skulason S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 1996;27:111–133. doi:10.1146/annurev.ecolsys.27.1.111 [Google Scholar]
- Weir B.S, Cockerham C.C. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. doi:10.2307/2408641 [DOI] [PubMed] [Google Scholar]