Abstract
Neither the scale of adaptive variation nor the genetic basis for differential population responses to the environment is known for broadcast-spawning marine fishes. Using a common-garden experimental protocol, we document how larval growth, survival and their norms of reaction differ genetically among four populations of Atlantic cod (Gadus morhua). These traits, and their plastic responses to food and temperature, differed across spatial scales at which microsatellite DNA failed to detect population structure. Divergent survival reaction norms indicate that warm-water populations are more sensitive to changes in food, whereas cold-water populations are more sensitive to changes in temperature. Our results suggest that neither the direction nor the magnitude of demographic responses to environmental change need be the same among populations. Adaptive phenotypic plasticity, previously undocumented in marine fishes, can significantly influence the probability of recovery and persistence of collapsed populations by affecting their ability to respond to natural and anthropogenic environmental change.
Keywords: phenotypic plasticity, common-garden experiment, conservation biology, population differentiation, Atlantic cod, environment
1. Introduction
The persistence of a species depends on the resistance and resilience of its populations to anthropogenic and natural environmental perturbation. Correspondingly, risk of extinction is a function of the breadth of population responses to environmental change and of the spatial correspondence between the scale of the perturbation and the scale of adaptation. Knowledge of the spatial scale of population structure is thus fundamental to an understanding of population dynamics, conservation biology and sustainable harvesting practices (Hutchings & Reynolds 2004; Conover et al. 2006; Drakare et al. 2006).
In the marine environment, gene flow is thought to be relatively extensive, given the comparative lack of physical impediments to long-distance movement and dispersal (Hilbish 1996; Avise 2000). Although studies based on microsatellite DNA have detected restrictions in gene flow for fishes across smaller spatial scales than non-genetic studies might have predicted (Imsland & Jónsdóttir 2003; Knutsen et al. 2003), this analytical approach has often yielded equivocal results for the same species across the same geographical range (Ruzzante et al. 1998; Imsland & Jónsdóttir 2003; Hardie et al. 2006), due in part to its limited ability to resolve fully the population structure of species with comparatively high levels of gene flow (Waples 1998). The ability of hyper-variable DNA to delineate population structure may also be limited when differences between breeding groups are primarily the result of selection, which can generate differences at a much faster rate than observed at selectively neutral loci (Stockwell et al. 2003; Swain et al. 2004; Conover et al. 2006). Irrespective of their ability to detect gene flow among putative breeding groups, analyses of hyper-variable DNA are unable to detect population differences in phenotypic plasticity, or norms of reaction, for fitness-related traits.
Coupled with the challenges of delineating the boundaries of population differentiation and adaptive variation in the ocean are questions related to the failure of many marine fishes to recover from historically unprecedented collapses (Hutchings 2000; Hutchings & Reynolds 2004). One key question in this regard concerns the ability of marine fish populations to respond to environmental change; a second concerns the spatial scale at which adaptive responses to environmental change are realized. The former question depends on the level of phenotypic plasticity expressed within a population, whereas the latter is reflected by genetic differences in plasticity at the population level.
Phenotypic plasticity is the ability of a genotype to produce different phenotypes across an environmental gradient (Schlichting & Pigliucci 1998; Sultan & Stearns 2005). Plasticity can be heuristically and graphically described as a norm of reaction—a linear or nonlinear function that expresses how the phenotypic value of a trait for a given genotype changes with the environment. At the population level, reaction norms can be used to predict how individuals will respond, on average, to specific changes to an environmental variable. Genetic differences in reaction norms, previously undocumented in marine fishes, would reflect differences in the ability of populations to respond to environmental change and the manner in which they do so.
One of the most powerful means of assessing the genetic basis of phenotypic variation is to conduct a common-garden experiment in which individuals from putatively different groups are reared under the same environmental conditions (Imsland & Jónsdóttir 2003; Conover et al. 2006). In accordance with such an experimental protocol, group-level differences in the characters of interest, or their norms of reaction, comprise evidence that these differences have a genetic and, depending on the relationship between the trait(s) and fitness, possibly adaptive basis. Common-garden experiments on broadcast-spawning marine fishes are exceedingly rare owing to the logistical difficulties in allowing individuals to spawn undisturbed in a semi-natural environment and in rearing marine fish larvae.
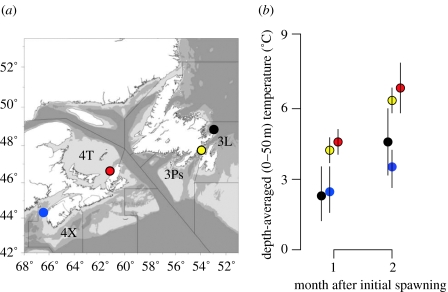
We undertook common-garden experiments on four populations of Atlantic cod to quantify population differences in larval growth, survival and their plastic responses to variation in food and temperature. North to south, cod were sampled from: Bonavista Bay, Newfoundland (Northwest Atlantic Fishery Organization (NAFO) division 3L); Placentia Bay, Newfoundland (NAFO division 3Ps); Southern Gulf of St Lawrence (NAFO division 4T); and Western Scotian Shelf (NAFO division 4X; figure 1a). Based on significant differences in reaction norms, we find that a broadcast-spawning marine fish with apparently high dispersal capabilities comprised populations that differ genetically in their responses to environmental change at spatial scales undetected by microsatellite DNA.
Figure 1.
Study populations of Atlantic cod. (a) Sampling locations of spawning adults. (b) Depth-averaged (0–50 m) water temperatures±1 s.e. for all available data from 1914 to 2003, one and two months after their initial spawning months (May: 3L, 3Ps, 4T; February: 4X).
2. Material and methods
(a) Spawning experiments
Adult cod were captured by handline (3L, 3Ps), bottom trawl (4X) or Danish seine (4T) immediately prior to, or during (3L only), their respective breeding seasons (Marcil et al. 2006a). Capture dates were as follows: 4X (5–7 January 2002, 16 January 2003); 3Ps (12 April 2002); 4T (15 May 2003); and 3L (12 June 2003). Adults from 3L and 3Ps spawned undisturbed at the Ocean Sciences Centre (OSC), Memorial University of Newfoundland, while those from 4T and 4X spawned undisturbed in the 684 m3 Pool Tank in the Aquatron Laboratory at Dalhousie University (Rowe et al. (2007) provide additional details). The number of adults in each spawning group ranged between 54 and 77 individuals. The temperature in all spawning tanks was held constant at approximately 8°C.
(b) Common-garden experiments
Eggs were sampled for the common-garden experiments approximately two weeks after they were first observed in egg collectors positioned near the surface outflows of the tanks. At this time, four batches of fertilized eggs (each batch consisted of eggs spawned over two consecutive days) were collected from each population; those from Dalhousie were transported to the OSC where the common-garden experiments were undertaken. We adopted this protocol for egg collection to increase the probability that a substantive number of families were represented within each population.
To evaluate this assumption, random samples of offspring in each population were collected from each food–temperature treatment at the end of the experiments (43 days post-hatch). Following established protocols (Hardie et al. 2006), DNA was extracted from 316, 530, 640 and 380 larvae from the 3Ps, 4T, 4X (2003) and 3L populations, respectively. The genotype of each larva was scored at the following seven microsatellite loci (Hardie et al. 2006): Gmo3, Gmo8, Gmo19, Gmo34, Gmo35, Tch5 and Mae9. The genotypes of the larvae were then compared to the genotypes, at the same seven loci, of the adults that produced these offspring (blood samples were obtained from the adults at the end of the spawning experiments). The program PAPA v. 2.0 (Duchesne et al. 2002) was used to identify the parents of each of the offspring from each of the populations. Based on these analyses, the number of families represented at the end of the common-garden experiments for populations 3Ps, 4T, 4X and 3L was 44, 31, 21 and 71, respectively.
At the OSC, eggs were incubated in 250 l flow-through tanks until hatching. Larvae were randomly sampled from each of the four batches (replicates) for each population and transferred to 30 l aquaria, one for each of the four temperature–food treatments (N=1200 larvae per replicate, four replicates for each population at each of the four treatments): low temperature–low food; low temperature–high food; high temperature–low food; and high temperature–high food. The low and high temperatures were 7±1 and 11±1°C (mean±1 s.e.), respectively (temperatures were recorded twice daily). The low food treatment was 1500 prey per litre (except for the 1000 prey per litre to which the 3Ps and 4X populations were treated in 2002; as noted below, neither the survival nor the growth data differed within treatments between the 4X groups reared in 2002 and 2003) and the high food treatment was 4500 prey per litre. Larvae were fed Isochrisis-enriched rotifers during the first 10 days after hatching, and Algomat-enriched rotifers thereafter until day 31. From days 32 through 39, larvae were fed a 1 : 1 mixture of rotifers and Artemia, and a diet consisting solely of Artemia from day 40 thereafter. Larvae were fed three times daily (morning, afternoon and evening) and were reared at a light intensity of 2000 lux. The size of larvae one day after hatching averaged 4.3, 4.3 and 4.4 mm for the 3L, 3Ps and 4T populations, respectively, and did not differ significantly from one another (one-way analysis of variance (ANOVA), p>0.05). Larvae from the 4X population were significantly larger (p=0.001) than those from the other populations, averaging 5.1 mm in length one day after hatching.
The growth rate experienced by cod larvae in each of the temperature–food treatments was quantified as the mean length attained at 29 days post-hatching. These means were estimated from random samples obtained from each of the four replicates per temperature–food treatment. Thus, the number of larvae sampled per replicate was: 10 for each of the 3Ps, 4T and 4X (2003) experiments; 6 for the 3L experiment; and 5 for the 4X (2002) experiment. High mortality experienced by larvae in the low temperature–low food treatment resulted in data being available for only three replicates for each of the 4T and 4X (2002) experiments. Based on the results of one-way ANOVAs, there were no differences in length within treatments between years for the 4X population (p>0.05). As a consequence, length data were pooled between years for 4X larvae. This resulted in sample sizes (N) for larval growth rate estimates for each treatment ranging between 24 and 45 for cod sampled from the 3L and 4X populations, respectively.
(c) Statistical analyses
The effects of food, temperature and population on larval growth and survival were examined using three-way factorial analyses of variance. After initially fitting all main effects and their interactions, we simplified each model by sequentially removing non-significant parameters. We initially compared all four populations separately. Then, given the significant influence that temperature can have on the growth and survival of larval marine fishes (Pepin 1991), notably Atlantic cod (Nissling 2004; Folkvold 2005), we grouped populations based on similarities in the temperatures that larvae would be expected to experience during their first few months of life in the wild (figure 1b). The cold-water populations included spring-spawning cod from Bonavista Bay (3L) and winter-spawning Western Scotian Shelf (4X) cod, for which temperatures experienced by larvae 1–2 months after fertilization are approximately one-half of those experienced by larvae from the warm-water, spring-spawning populations (Placentia Bay (3Ps) and Southern Gulf (4T)). Water temperature data were obtained from the ocean science database maintained by the Canadian Department of Fisheries and Oceans (DFO 2006).
Effects of population, treatment and their interactions on length were tested using a nested ANOVA, with batch nested within population treated as a random effect and all other terms treated as fixed effects. Type III sums of squares were used owing to unequal sample sizes among groups. Owing to unequal sample sizes, Satterthwaite's approximation (Sokal & Rohlf 1981) was used to construct the denominator mean square for tests of population effects.
Larval survival was measured as the number of larvae alive in each temperature–food replicate 43 days post-hatching (i.e. at metamorphosis) relative to the number originally present in each replicate (N=1200 per replicate). Based on the results of one-way ANOVAs, there were no differences in survival within treatments between years for the 4X population (p>0.05). As a consequence, survival data were pooled between years for 4X larvae.
3. Results
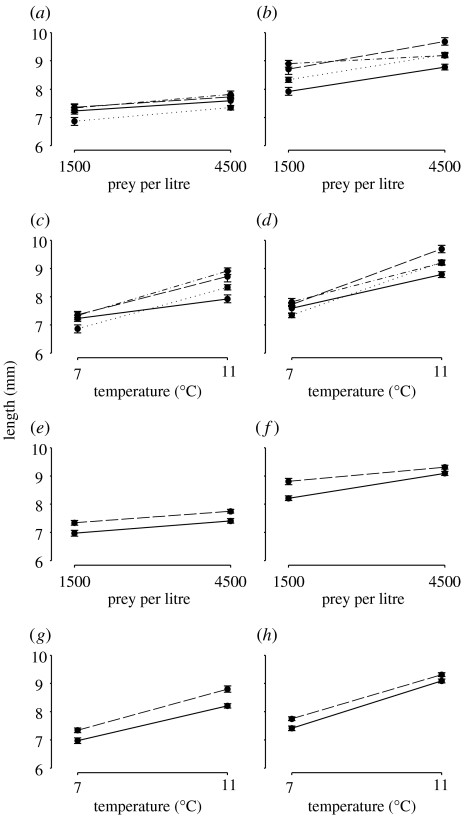
Larval growth, survival and plasticity in these traits differed among populations, although the magnitude of these differences depended on the level of comparison. Among all four populations, growth increased with food supply and temperature (figure 2a–d and table 1). Evidence of genetic differences in growth plasticity was revealed by significant population×temperature×food and population×temperature interactions. Differences in larval growth were also evident between warm- and cold-water populations (table 1). Larvae produced by the warm-water populations were consistently larger than their cold-water counterparts at both levels of temperature and food (figure 2e–h).
Figure 2.
Norms of reaction for larval growth (mean±1 s.e. for each treatment). Reaction norms for each of the four populations at (a) low and (b) high temperature and at (c) low and (d) high food supply. Solid lines, 4X cod; dotted lines, 3L cod; dashed lines, 3Ps cod; dot-dashed lines, 4T cod. Reaction norms for warm- (3Ps and 4T cod) and cold-water (4X and 3L cod) populations at (e) low and (f) high temperature and at (g) low and (h) high food supply. Solid lines, cold-water populations; dashed lines, warm-water populations.
Table 1.
Influence of population, temperature and food on larval cod growth and survival.
| variable | no. groupsa | model termb | d.f. | F | p |
|---|---|---|---|---|---|
| growth | four | pop | 3 | 2.38 | 0.108 |
| batch(pop) | 16 | 10.05 | <0.001 | ||
| temp | 1 | 615.72 | <0.001 | ||
| food | 1 | 107.09 | <0.001 | ||
| pop×temp | 3 | 8.50 | <0.001 | ||
| temp×food | 1 | 8.29 | 0.004 | ||
| pop×temp×food | 6 | 2.39 | 0.027 | ||
| residuals | 599 | ||||
| two | pop | 1 | 7.82 | 0.012 | |
| batch(pop) | 18 | 8.41 | <0.001 | ||
| temp | 1 | 728.33 | <0.001 | ||
| food | 1 | 117.40 | <0.001 | ||
| temp×food | 1 | 8.53 | 0.004 | ||
| residuals | 608 | ||||
| survival | four | food | 1 | 12.55 | <0.001 |
| residuals | 78 | ||||
| two | pop | 1 | 39.93 | <0.001 | |
| food | 1 | 21.31 | <0.001 | ||
| temp | 1 | 1.75 | 0.190 | ||
| pop×food | 1 | 8.68 | 0.004 | ||
| pop×temp | 1 | 8.10 | 0.006 | ||
| residuals | 74 |
Four-group comparisons included the Bonavista Bay (3L), Placentia Bay (3Ps), Southern Gulf of St Lawrence (4T) and Western Scotian Shelf (4X) populations; two-group comparisons differentiated cold-water populations (Bonavista Bay and Western Scotian Shelf) from warm-water populations (Placentia Bay and Southern Gulf of St Lawrence).
Model terms: pop, population; temp, temperature; batch(pop), batch nested within population.
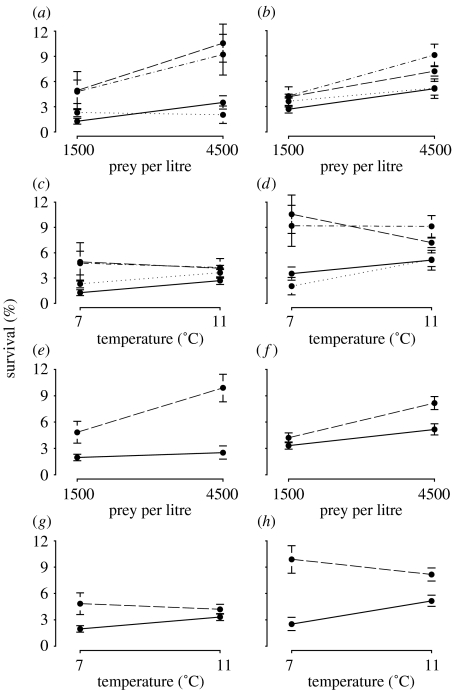
The faster growth experienced by warm-water populations may have contributed to their higher survival at both levels of food and temperature (figure 3). The influence of food and temperature on survival differed between warm- and cold-water populations, indicative of genetic differences in the shapes of their reaction norms (table 1). For example, at the lower temperature, survival of warm-water cod increased with food supply, whereas that of cold-water cod was unaffected (figure 3a,e); at the warmer temperature, survival increased marginally with increases in food in both populations (figure 3b,f). By contrast, irrespective of food supply, temperature had no demonstrable impact on the survival of warm-water cod, whereas survival increased significantly with temperature among cold-water cod (figure 3c,d,g,h).
Figure 3.
Norms of reaction for larval survival (mean±1 s.e. for each treatment). Reaction norms for each of the four populations at (a) low and (b) high temperature and at (c) low and (d) high food supply. Solid lines, 4X cod; dotted lines, 3L cod; dashed lines, 3Ps cod; dot-dashed lines, 4T cod. Reaction norms for warm- (3Ps and 4T cod) and cold-water (4X and 3L cod) populations at (e) low and (f) high temperature and at (g) low and (h) high food supply. Solid lines, cold-water populations; dashed lines, warm-water populations.
4. Discussion
We find that a widely distributed, broadcast-spawning marine fish with apparently high dispersal capabilities comprised populations that differ genetically in their responses to the environment at spatial scales (approx. 600–800 km) undetected by microsatellite DNA. Population variability in the shapes of reaction norms implies that Atlantic cod do not respond demographically to similar changes in the environment in a similar manner. Increased water temperature, for example, is predicted to have a positive influence on the survival, and thus recruitment, of cold-water populations, but a negligible, and possibly negative, influence on the larval survival of warm-water populations. The interactive effect that temperature and food have on survival also differs among populations. Survival within warm-water populations is predicted to increase with food abundance, irrespective of water temperature, whereas any positive influence on the survival of cold-water larvae may only be evident at warmer temperatures. Thus, neither the direction nor the magnitude of demographic responses to environmental change need be the same among populations nor be related to coarse spatial metrics such as latitude.
Genetic differences in fitness-related traits and their plasticity may reflect adaptations by cod to their local environments. This hypothesis is based on the premise that individuals are likely to experience better growth and survival under environmental conditions most similar to those experienced in the wild (Haugen & Vøllestad 2000). Our experimental temperatures were similar to those that larvae from the warm-water populations would be expected to experience in nature. The observation that growth and survival of cod from these populations was consistently higher than those from the cold-water populations is consistent with the hypothesis that genetic differences in these strong correlates of fitness are adaptive. Our data are also consistent with, and provide an explanation for, observations that increasing temperature enhances survival in cod populations that experience comparatively cold temperatures (Planque & Frédou 1999; Worm & Myers 2003; Ottersen et al. 2006) but not in populations that experience comparatively warm temperatures (Planque & Frédou 1999; Beaugrand et al. 2003; Cook & Heath 2005). In this regard, our work represents a natural extension of previous research that has identified a genetic basis to geographical differences in Atlantic cod growth (Purchase & Brown 2000; Imsland & Jónsdóttir 2003; Salvanes et al. 2004), antifreeze proteins (Goddard et al. 1999) and growth responses to light (Puvanendran & Brown 1998).
We interpret population differences in growth, survival and plasticity to be a consequence of genetic rather than maternal effects. To eliminate the latter, and other potential pre-fertilization effects, ideally we would have reared cod for two or more generations, but this was not possible owing to the long generation time of Atlantic cod. In fishes, maternal effects are generated primarily by differences in egg size, which are then manifested by differences in size at hatch (Conover & Schultz 1995). We think it unlikely that the population differences documented here can be attributed to maternal effects. Size at hatching was not positively associated with either larval survival or growth; although Western Scotian Shelf (4X) larvae were significantly larger at hatch, they experienced the highest mortality and attained the smallest size at 29 days.
In accordance with recent studies on body morphometry (Marcil et al. 2006a,b), our data reveal significant genetic differences among populations of Atlantic cod at a spatial scale at which disjunctions in gene flow were not detectable using traditional population genetic markers (Hardie et al. 2006). The common-garden experiments demonstrated genetic differences between cod sampled from Placentia Bay and Southern Gulf of St Lawrence and those sampled from Western Scotian Shelf. By contrast, based on the analyses of seven hyper-variable markers, there were no significant differences in allelic identity among the three populations, as indicated by non-significant (p>0.05) pair-wise Fst values of less than 0.001 (Hardie et al. 2006). Although studies based on selectively neutral markers have revealed restrictions in gene flow at spatial scales similar to (e.g. Ruzzante et al. 1998) or smaller than those documented here (e.g. Knutsen et al. 2003), the degree to which these differences reflect differential selection pressures is not known; however, studies of genetic differentiation based on the pantophysin locus in Atlantic cod (e.g. Pogson & Fevolden 2005) are compelling in this regard. Our observations underscore the point that genetic divergence can develop much more rapidly in adaptive than in selectively neutral traits despite levels of gene flow that would prevent or eliminate differentiation at selectively neutral loci (Hard 1995; Conover et al. 2006).
Although reaction norms can be heritable (Schlichting & Pigliucci 1998; Sultan & Stearns 2005), plasticity selection has rarely been documented in vertebrates. In fishes, the most compelling evidence of selection responses in plasticity is provided by genetic changes in life-history reaction norms among populations of European grayling (Thymallus thymallus) over a period of 9–22 generations (Haugen 2000; Haugen & Vøllestad 2000). Population variation in phenotypic plasticity raises the question of whether reaction norms might respond evolutionarily to fishing-induced selection pressures, as suggested for life-history traits such as age and size at maturity and growth rate (Law 2000; Swain et al. 2007). Differential mortality brought about by fishing may select for changes in either the shape or the elevation of reaction norms (Hutchings 1993, 2004; Reznick 1993; Haugen & Vøllestad 2000), both of which would be expected to have consequences for the mean and/or variance in population growth rate and, thus, recovery potential and stock productivity.
The breadth of genetic variation in phenotypic plasticity documented here has almost certainly contributed to the ability of Atlantic cod to persist in an ever-changing natural environment, the population consequences of which have been exacerbated by fishing (Hutchings & Reynolds 2004; Mora et al. 2007). The collapse of many of the world's fisheries underscores a pressing need to study the adaptive variation in commercially exploited marine species with the aim of establishing recovery strategies at biologically meaningful and genetically relevant spatial scales. In this regard, our work underscores the merit in undertaking common-garden experiments in conjunction with studies of population structure based solely on allelic variability, particularly for genes thought to be under natural selection.
Acknowledgments
The research protocol was approved by the Dalhousie University's Committee on Laboratory Animals, in accordance with the guidelines provided by the Canadian Council on Animal Care.
We thank P. Avendaño, R. Gillett, M. Jones, J. Marcil, C. Smith, S. Thompson and staff at the Ocean Sciences Centre, Memorial University of Newfoundland and at the Aquatron Laboratory, Dalhousie University for their technical assistance. We gratefully acknowledge J. Batt, W. Scott (Live Fish Ltd) and C. d'Entremont (Inshore Fisheries Ltd) for the collection of 4X cod, Fisheries and Oceans Canada (Gulf Region) for the collection of 4T cod, Earl Johnson for the collection of 3Ps cod and Darrin Cooper for the collection of 3L cod. Two anonymous referees provided very helpful comments on an earlier version of the manuscript. The work was supported by a Strategic grant awarded to J.A.H., D.P.S. and J.A.B. by the Natural Sciences and Engineering Research Council (NSERC) of Canada and by an NSERC Postgraduate Scholarship to S.R.
References
- Avise J.C. Harvard University Press; Cambridge, MA: 2000. Phylogeography. [Google Scholar]
- Beaugrand G, Brander K.M, Lindley J.A, Souissi S, Reid P.C. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426:661–664. doi: 10.1038/nature02164. doi:10.1038/nature02164 [DOI] [PubMed] [Google Scholar]
- Conover D.O, Schultz E.T. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 1995;10:248–252. doi: 10.1016/S0169-5347(00)89081-3. doi:10.1016/S0169-5347(00)89081-3 [DOI] [PubMed] [Google Scholar]
- Conover D.O, Clarke L.M, Munch S.B, Wagner G.N. Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. J. Fish Biol. 2006;69:21–47. doi:10.1111/j.1095-8649.2006.01274.x [Google Scholar]
- Cook R.M, Heath M.R. The implications of warming climate for the management of North Sea demersal fisheries. ICES J. Mar. Sci. 2005;62:1322–1326. doi:10.1016/j.icesjms.2005.04.023 [Google Scholar]
- DFO 2006 Oceanographic databases: spatial-temporal query applications to ocean science databases. Department of Fisheries and Oceans, Ottawa. URL (www.mar.dfo-mpo.gc.ca/science/ocean/database/data_query.html.)
- Drakare S, Lennon J.J, Hillebrand H. The imprint of the geographical, evolutionary and ecological context on species–area relationships. Ecol. Lett. 2006;9:215–227. doi: 10.1111/j.1461-0248.2005.00848.x. doi:10.1111/j.1461-0248.2005.00848.x [DOI] [PubMed] [Google Scholar]
- Duchesne P, Godbout M.H, Bernatchez L. PAPA (package for the analysis of parental allocation): a computer program for simulated and real parental allocation. Mol. Ecol. Notes. 2002;2:191–193. doi:10.1046/j.1471-8286.2002.00164.x [Google Scholar]
- Folkvold A. Comparison of size-at-age of larval Atlantic cod (Gadus morhua) from different populations based on size- and temperature-dependent growth models. Can. J. Fish. Aquat. Sci. 2005;62:1037–1052. doi:10.1139/f05-008 [Google Scholar]
- Goddard S.V, Kao M, Fletcher G.L. Population differences in antifreeze production cycles of juvenile Atlantic cod (Gadus morhua) reflect adaptations to overwintering environment. Can. J. Fish. Aquat. Sci. 1999;56:1991–1999. doi:10.1139/cjfas-56-11-1991 [Google Scholar]
- Hard J.J. A quantitative genetic perspective on the conservation of intraspecific diversity. Am. Fish. Soc. Symp. 1995;17:304–326. [Google Scholar]
- Hardie D.C, Gillett R.M, Hutchings J.A. The effects of isolation and colonization history on the genetic structure of marine-relict populations of Atlantic cod (Gadus morhua) in the Canadian Arctic. Can. J. Fish. Aquat. Sci. 2006;63:1830–1839. doi:10.1139/F06-085 [Google Scholar]
- Haugen T.O. Growth and survival effects on maturation pattern in populations of grayling with recent common ancestors. Oikos. 2000;90:107–118. doi:10.1034/j.1600-0706.2000.900111.x [Google Scholar]
- Haugen T.O, Vøllestad L.A. Population differences in early life-history traits in grayling. J. Evol. Biol. 2000;13:897–905. doi:10.1046/j.1420-9101.2000.00242.x [Google Scholar]
- Hilbish T.J. Population genetics of marine species: the interaction of natural selection and historically differentiated populations. J. Exp. Mar. Biol. Ecol. 1996;200:67–83. doi:10.1016/S0022-0981(96)02645-7 [Google Scholar]
- Hutchings J.A. Reaction norms for reproductive traits in brook trout and their influence on life history evolution effected by size-selective harvesting. In: Stokes T.K, McGlade J.M, Law R, editors. The exploitation of evolving resources. Springer; Berlin, Germany: 1993. pp. 107–125. [Google Scholar]
- Hutchings J.A. Collapse and recovery of marine fishes. Nature. 2000;406:882–885. doi: 10.1038/35022565. doi:10.1038/35022565 [DOI] [PubMed] [Google Scholar]
- Hutchings J.A. The cod that got away. Nature. 2004;428:899–900. doi: 10.1038/428899a. doi:10.1038/428899a [DOI] [PubMed] [Google Scholar]
- Hutchings J.A, Reynolds J.A. Marine fish population collapses: consequences for recovery and extinction risk. Bioscience. 2004;54:297–309. doi:10.1641/0006-3568(2004)054[0297:MFPCCF]2.0.CO;2 [Google Scholar]
- Imsland A.K, Jónsdóttir Ó.D.B. Linking population genetics and growth properties of Atlantic cod. Rev. Fish. Biol. Fish. 2003;13:1–26. doi:10.1023/A:1026373509576 [Google Scholar]
- Knutsen H, Jorde P.E, André C, Stenseth N.Chr. Fine-scaled geographical population structuring in a highly mobile marine species: the Atlantic cod. Mol. Ecol. 2003;12:385–394. doi: 10.1046/j.1365-294x.2003.01750.x. doi:10.1046/j.1365-294X.2003.01750.x [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 2000;57:659–668. doi:10.1006/jmsc.2000.0731 [Google Scholar]
- Marcil J, Swain D.P, Hutchings J.A. Genetic and environmental components of phenotypic variation in body shape among populations of Atlantic cod (Gadus morhua L) Biol. J. Linn. Soc. 2006a;88:351–365. doi: 10.1098/rspb.2005.3306. doi:10.1111/j.1095-8312.2006.00656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcil J, Swain D.P, Hutchings J.A. Countergradient variation in body shape of a widespread marine fish, the Atlantic cod (Gadus morhua) Proc. R. Soc. B. 2006b;273:217–223. doi: 10.1098/rspb.2005.3306. doi:10.1098/rspb.2005.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C, Metzger R, Rollo A, Myers R.A. Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc. R. Soc. B. 2007;274:1023–1028. doi: 10.1098/rspb.2006.0338. doi:10.1098/rspb.2006.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissling A. Effects of temperature on egg and larval survival of cod (Gadus morhua) and sprat (Sprattus sprattus) in the Baltic Sea—implications for stock development. Hydrobiologia. 2004;514:115–123. doi:10.1023/B:hydr.0000018212.88053.aa [Google Scholar]
- Ottersen G, Hjermann D.Ø, Stenseth N.Chr. Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish. Oceanogr. 2006;15:230–243. doi:10.1111/j.1365-2419.2006.00404.x [Google Scholar]
- Pepin P. Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can. J. Fish. Aquat. Sci. 1991;48:503–518. [Google Scholar]
- Planque B, Frédou T. Temperature and the recruitment of Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 1999;56:2069–2077. doi:10.1139/cjfas-56-11-2069 [Google Scholar]
- Pogson G.H, Fevolden S.-E. Natural selection and the genetic differentiation of coastal and Arctic populations of the Atlantic cod in northern Norway: a test involving nucleotide sequence variation at the pantophysin (PanI) locus. Mol. Ecol. 2005;12:63–74. doi: 10.1046/j.1365-294x.2003.01713.x. doi:10.1046/j.1365-294X.2003.01713.x [DOI] [PubMed] [Google Scholar]
- Purchase C.F, Brown J.A. Interpopulation differences in growth rates and food conversion efficiencies of young Grand Banks and Gulf of Maine Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 2000;57:2223–2229. doi:10.1139/cjfas-57-11-2223 [Google Scholar]
- Puvanendran V, Brown J.A. Effect of light intensity on the foraging and growth of Atlantic cod larvae: interpopulation difference? Mar. Ecol. Prog. Ser. 1998;167:207–214. [Google Scholar]
- Reznick D.N. Norms of reaction in fishes. In: Stokes T.K, McGlade J.M, Law R, editors. The exploitation of evolving resources. Springer; Berlin, Germany: 1993. pp. 72–90. [Google Scholar]
- Rowe S, Hutchings J.A, Skjæraasen J.E. Nonrandom mating in a broadcast spawner: mate size influences reproductive success in Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 2007;64:219–226. doi:10.1139/F06-182 [Google Scholar]
- Ruzzante D.E, Taggart C.T, Cook D. A nuclear DNA basis for shelf- and bank-scale population structure in northwest Atlantic cod (Gadus morhua): Labrador to Georges Bank. Mol. Ecol. 1998;7:1663–1680. doi:10.1046/j.1365-294x.1998.00497.x [Google Scholar]
- Salvanes A.G.V, Skjæraasen J.E, Nilsen T. Sub-populations of coastal cod with different behaviour and life-history strategies. Mar. Ecol. Prog. Ser. 2004;267:241–251. [Google Scholar]
- Schlichting C.D, Pigliucci M. Sinauer; Sunderland, MA: 1998. Phenotypic evolution: a reaction norm perspective. [Google Scholar]
- Sokal R.R, Rohlf J.F. W. H. Freeman; San Francisco, CA: 1981. Biometry. [Google Scholar]
- Stockwell C.A, Hendry A.P, Kinnison M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003;18:94–101. doi:10.1016/S0169-5347(02)00044-7 [Google Scholar]
- Sultan S.E, Stearns S.C. Environmentally contingent variation: phenotypic plasticity and norms of reaction. In: Hallgrimsson B, Hall B, editors. Variation. Elsevier; Boston, MA: 2005. pp. 303–332. [Google Scholar]
- Swain D.P, Hutchings J.A, Foote C.J. Environmental and genetic influences on stock identification characters. In: Cadrin S.X, Friedland K.D, Waldman J, editors. Stock identification methods. Academic Press; New York, NY: 2004. pp. 43–83. [Google Scholar]
- Swain D.P, Sinclair A.F, Hanson J.M. Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. doi:10.1098/rspb.2006.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples R.S. Separating the wheat from the chaff: patterns of genetic differentiation in high gene flow species. J. Hered. 1998;89:438–450. doi:10.1093/jhered/89.5.438 [Google Scholar]
- Worm B, Myers R.A. Meta-analysis of cod–shrimp interactions reveals top-down control in oceanic food webs. Ecology. 2003;84:162–173. [Google Scholar]