Abstract
Postcopulatory sexual selection favours males which are strong offensive and defensive sperm competitors. As a means of identifying component traits comprising each strategy, we used an experimental evolution approach. Separate populations of Drosophila melanogaster were selected for enhanced sperm offence and defence. Despite using a large outbred population and evidence of substantive genetic variation for each strategy, neither trait responded to selection in the two replicates of this experiment. Recent work with fixed chromosome lines of D. melanogaster suggests that complex genotypic interactions between females and competing males contribute to the maintenance of this variation. To determine whether such interactions could explain our lack of response to selection on sperm offence and defence, we quantified sperm precedence across multiple sperm competition bouts using an outbred D. melanogaster population exhibiting continuous genetic variation. Both offensive and defensive sperm competitive abilities were found to be significantly repeatable only across matings involving ejaculates of the same pair of males competing within the same female. These repeatabilities decreased when the rival male stayed the same but the female changed, and they disappeared when both the rival male and the female changed. Our results are discussed with a focus on the complex nature of sperm precedence and the maintenance of genetic variation in ejaculate characteristics.
Keywords: sperm competition, genetic interactions, artificial selection, virgin effects, Drosophila
1. Introduction
Securing mates and transferring viable sperm are necessary but not sufficient to guarantee paternity. The arena of sexual selection expands after mating to include the female reproductive tract whenever the ejaculates of two or more males coincide within a female (Parker 1970; Eberhard 1996). Postcopulatory sexual selection favours males with ejaculates that outcompete sperm from previous males (sperm offence) while resisting being outcompeted by ejaculates from subsequent males (sperm defence; Parker 1970, 1984). Sperm offence is typically quantified experimentally as P2, the proportion of progeny sired by the second of two males following re-mating by a female, whereas sperm defence is measured by P1, the proportion of first-male progeny (Boorman & Parker 1976).
As sperm offence and defence abilities contribute substantially to male fitness, ejaculate characteristics might be expected to exhibit low genetic variation due to a combination of stabilizing selection on the optimal ejaculate design and intense directional selection driving to fixation of any new advantageous alleles. Nevertheless, studies have consistently found considerable genetic variation among males in sperm competitive ability (Prout & Bundgaard 1977; Gilbert & Richmond 1981; Clark et al. 1995; Hughes 1997; Radwan 1998; Civetta & Clark 2000; Hosken et al. 2001; Simmons & Kotiaho 2002). The explanation for this apparent contradiction probably relates to the multifarious nature of sperm precedence, which depends upon a suite of interacting component traits within and between individuals. Any allelic change that influences the biochemical, physiological, morphological or behavioural basis of insemination, sperm migration, sperm storage, sperm viability, fertilization or female re-mating may influence sperm precedence. Two related aspects of this complexity are likely to contribute to the maintenance of genetic variation: (i) antagonistic pleiotropy and (ii) complex interactions generating non-transitive outcomes.
Sperm size and number illustrate the potential for antagonistic pleiotropy in sperm competition traits. Both sperm size and the number of sperm produced have been demonstrated in a diversity of taxa to positively correlate with the intensity of sperm competition or with male competitive fertilization success, though exceptions do exist (reviewed by Simmons 2001; Pattarini et al. 2006). Since each of these traits bears substantive energetic costs of expression (Pitnick et al. 1995a; Wedell et al. 2002), a trade-off between them (and other life-history traits; Badyaev & Qvarnstrom 2002; Hunt et al. 2004, 2005) is expected (e.g. Parker 1982) and has been empirically demonstrated (Pitnick 1996; Oppliger et al. 1998). Similarly, even within sperm, negative genetic correlations can be found such as between flagellum length and mid-piece length in the zebra finch (Taeniopygia guttata; Birkhead et al. 2005). However, antagonistic pleiotropy of ejaculatory traits has not been well studied and its more general role in maintaining genetic variation is uncertain (Rose 1985; Curtsinger et al. 1994; Falconer & Mackay 1996).
Of perhaps greater importance for the maintenance of genetic variation is the issue of complex interactions between competing males and between males and females (Zimmering & Fowler 1968; Bishop 1996; Bishop et al. 1996; Zeh & Zeh 1996, 1997; Wilson et al. 1997; Tregenza & Wedell 2000; Mack et al. 2002; Miller & Pitnick 2002; Oh & Badyaev 2006; Pitnick et al. in press). An important series of experiments has shown that some mechanisms controlling P1 and P2 are, in fact, physiologically distinct and genetic variation in sperm offence and defence is maintained to a large extent due to the effects of genotypic interactions between individuals (Clark et al. 1995; Civetta & Clark 2000). These studies, using genetically modified, fixed-chromosome lines of Drosophila melanogaster, demonstrate that the outcome of sperm competition between males with different genotypes depends upon the genotype of the female within which they are competing (Clark & Begun 1998). Moreover, sperm precedence becomes unpredictable when the female genotype is held constant because—in a manner comparable with the ‘rock–paper–scissors’ game (Maynard-Smith 1982)—males display non-transitivity in their sperm competitive ability (Clark et al. 2000). A study using artificial insemination in domestic fowl—similarly found non-transitivity of sperm competition success (Birkhead et al. 2004).
Here, we address two outstanding issues. (i) Aside from contributing to the maintenance of genetic variation, the above interactions, because they are genetically non-additive, are predicted to limit population responses to the strong directional selection pressures experienced by males for increased sperm precedence. Yet, the striking variation observed across species and populations in the traits contributing to fertilization success suggests otherwise (Birkhead & Møller 1998). We used a novel experimental evolutionary approach to independently select males both for increased P1 and P2, as measured from sperm competition bouts between genetically variable rival males within genetically variable females. Previous experimental studies have not examined selection on the offensive and defensive sperm competition suites as a whole (i.e. the net effect of the many interacting component traits). (ii) To build upon the important findings described above, it is essential to uncover the extent to which complex genetic interactions operate under natural matings (versus artificial insemination) and conditions of continuous population genetic variation (versus conditions examining interactions between cohorts of genetically discrete ‘clonal’ populations). To this end, using an outbred D. melanogaster population exhibiting natural genotypic variation, we quantified the extent of ejaculate×female and ejaculate×ejaculate interactions on both P1 and P2 by examining the repeatabilities of individuals' sperm precedence scores across multiple consecutive sperm competition bouts. We compared repeatabilities among conditions where individual males competed multiple times against the same rival male and within the same female, where individual males competed multiple times against the same rival but within different females, and where individual males competed multiple times against different rival males and within different females.
Our results help to elucidate the conditions that must be met for heritable variation among males to outweigh non-heritable interaction effects between competing males and between males and females. They also highlight the complex nature of sperm precedence and the advantages and disadvantages associated with using virgin matings to extrapolate generalities about postcopulatory processes.
2. Material and methods
(a) Culturing
All experiments were conducted on D. melanogaster from a large outbred wild-type stock population (LHM, referred to herein as +/+) that had adapted to the laboratory for over 200 generations. Additionally, flies were used from an LHM-bw stock line (a replica of the base population with a brown-eye (bw) recessive marker that had been introgressed through 12–13 backcross generations into the LHM background; see Chippindale et al. (2001) for details on the origin and maintenance of these lines). Both lines were generously provided by A. K. Chippindale and had been maintained in our laboratory since their arrival in 2001 in population cages supporting more than 1000 individuals with overlapping generations on standard cornmeal–molasses–agar medium with a supplement of live yeast.
All flies for experiments were collected from 200 ml bottles seeded with a low-to-moderate density of larvae from the population cages (i.e. approx. 150 larvae per bottle). Virgin males and females were collected on the day of eclosion following light anaesthetization and, unless noted otherwise, housed in groups of no more than 10 same-sex individuals in eight-dram shell vials containing medium and live yeast. Experiments began with 4- to 6-day-old, reproductively mature flies (Pitnick et al. 1995a). Throughout the experiments, eggs, larvae and adult flies were maintained in a constant environment at 25°C and a 12 h light/dark cycle.
(b) Experimental evolution for improved sperm offence and defence
The experiment was designed to select unidirectionally (on separate lines) for improved sperm defence (P1) or offence (P2). To avoid selecting any female traits that may influence sperm precedence patterns (Clark & Begun 1998), the females used to assess sperm competitiveness of selection line males did not themselves originate from the selection lines, but rather from the stock population cage. Selection line females were used solely to provide progeny for the next generation. Rival males also originated from the stock population to ensure that selection line males competed against ejaculates from genetically diverse males every generation.
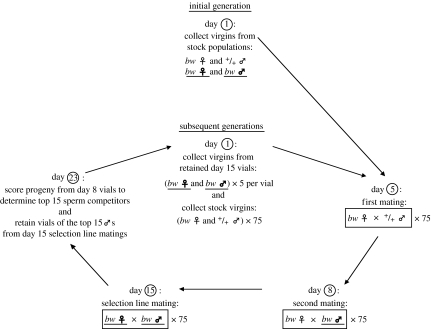
Figure 1 describes the selection protocol in detail. The complete experiment consisted of two replicates (A and B). The protocol was followed for 16 (replicate A) and 11 (replicate B) generations, with a generation time of approximately 30 days. Each replicate consisted of four lines (‘high-P1,’ ‘high-P2,’ ‘control-P1’ and ‘control-P2’). Flies from these lines are henceforth referred to as ‘selection line’ males and females. All other flies (the competitor males and the females used in the sperm competition matings on days 5 and 8) are henceforth referred to as ‘stock’ males and females.
Figure 1.
Protocol for selection on sperm competitive ability. Only the high-P2 selection experiment is illustrated; differences in protocol between this and the other lines are described below. Matings are indicated in boxes. Seventy-five recessive, brown-eye (bw) males and females were collected as larvae from the LHM-bw base population cage and were used to establish the selection line population. These individuals are referred herein as ‘selection line’ males and females and are indicated with bold and underlined text. All other flies (the competitor males and the females used in the sperm competition matings) are referred herein as ‘stock’ males and females. They were collected as larvae for each generation of the experiment from the LHM (denoted as ‘+/+’) or LHM-bw (bw) base population cages. Selection line and stock flies were collected on day 1 of the initial generation. Individual stock females (bw) were paired first with a single stock male (+/+) on the morning of day 5 and then with a single selection line male (bw) on the morning of day 8. (This mating order was reversed for the high-P1 and control-P1 lines.) All copulations were observed, and females and males were separated following copulation to prevent double matings. Stock females were transferred to new vials each day until the second mating on day 8. These vials (from days 5 to 7) were saved to count progeny prior to female re-mating. Stock males were discarded following copulation on day 8, and selection line males were transferred individually to fresh vials. Females from the sperm competition experiment were discarded on day 9, one day after re-mating. On day 15, all selection line males were paired with a single female from their own line; sib matings were avoided. Progeny prior to female re-mating were counted first. Next, progeny from the day 8 vials were counted and their eye colours were scored. The day 15 selection line mating vials with progeny from the 15 males with the highest P2 scores were retained. (15 random selection line mating vials were retained for the control lines.) When the progeny from these vials emerged, five males and five females (75 virgins of each sex in all) were collected from each vial. For the control-P1 and control-P2 lines, two males and two females (30 of each sex) were collected. These flies were used to begin the next generation of selection; stock flies were again collected from the base population cages.
We identified the selection line males with the 15 highest P1 or P2 scores (for the high-P1 and high-P2 lines, respectively). The control-P1 and control-P2 lines were subjected to no selection; each generation began with 30, rather than 75, males and females. Successive control-line generations were established by randomly selecting 15 males from each line, but they were otherwise maintained in a manner identical to the selection lines. Selection was relaxed on generations 5 and 6 (replicate A) and generations 2 and 9 (replicate B). In these instances, the experimental protocol was unchanged, except that selection line progeny were chosen randomly because P1 and P2 were not quantified. Simple linear regressions relating sperm competitiveness values to the number of progeny females produced prior to re-mating and male size were not significant for the first four generations of replicate A or the first two generations of replicate B. We therefore selected directly on raw P1 and P2 (rather than residual P1 or P2 scores) in these generations, and for the remainder of the experiment.
(c) Contribution of interaction effects to variation in sperm precedence
Ejaculate×ejaculate and ejaculate×female interactions are known to influence the outcome of sperm competition experiments. To investigate these phenomena further, we estimated the repeatability of sperm precedence of individuals from treatments with males paired multiple times against a single rival male, both within a single female and across different females, and with males paired multiple times against varying rival males within varying females.
The experimental design is illustrated in table 1. Two hundred virgin bw females and 200 virgin males (100 bw and 100+/+) were collected from the stock population cages, divided into three treatment groups (‘paired’ and ‘unpaired’ (each with 50 males and females) and four ‘control’ treatments (each with 25 males and females) described below) and assigned an identification number. All flies were scheduled to mate four times in total, with re-matings scheduled on the morning of every third day (though pairs that failed to re-mate after 3 days were given another opportunity on the fourth day). All copulations were observed, and any individuals that failed to mate on schedule were removed from the experiment. Consecutive males assigned to each female alternated between bw and +/+ so that sperm precedence could be quantified for each.
Table 1.
Truncated design of the “repeatability of sperm precedence” experiment. Bold denotes +/+ flies; all others are bw. (Progeny were sorted by eye colour and counted to quantify P1 and P2 after mating numbers 2, 3 and 4. Actual sample size was 50 males and females per treatment. See text for further explanation.)
| female | treatment | first mating | second mating | third mating | fourth mating |
|---|---|---|---|---|---|
| 1 | paired | male A | male B | male A | male B |
| 2 | male B | male A | male B | male A | |
| 3 | male C | male D | male C | male D | |
| 4 | male D | male C | male D | male C | |
| 5 | unpaired | male E | male J | male H | male K |
| 6 | male G | male L | male E | male M | |
| 7 | male J | male O | male N | male E | |
| 8 | male K | male E | male I | male F |
P1 (sperm defence) and P2 (sperm offence) scores were obtained by counting all progeny that emerged between copulations. Females were transferred to fresh vials daily, until 2 days after the final mating. P1 and P2 were calculated after the second, third and fourth copulations. In table 1, for example, male E's P2 score from the second copulation results from its sperm competition bout with male K. Male E's P2 score, for this mating, is identical to that of female 8’s P2 score. Male E's P1 score from this mating, however, results from his sperm competition bout with male I, and it is identical to the P1 score of female 8’s third mating. Extending this example one step further, the P2 and P1 scores from male E's third copulation result from competitions with the ejaculates of males L and M, respectively, and they are quantified from the progeny following female 6’s third and fourth matings, respectively. In total, three P2 scores and three P1 scores were obtained for each male and female.
Note that in the ‘paired’ treatment, ejaculates from the same two males (e.g. males A and B in table 1) were competed solely against each other, and on multiple occasions. This allowed each male's offensive and defensive abilities to be quantified multiple times against an unchanging competitor male, thus providing for examination of ejaculate×ejaculate interactions. Further, each pair of males in this treatment competed multiple times within each of two different females (females 1 and 2, in this case), thus providing for examination of ejaculate×female interactions. In the ‘unpaired’ treatment, females never mated with the same male twice, and males never competed against the same rival male twice. In rare instances, two males shared two female mating partners in common, but in these cases, the males' ejaculates only competed (i.e. mated consecutively) within one of the two females. Our four ‘control’ treatments confirmed this (table 2). The schedule of matings for these treatments was identical to that described above except that females were not assigned mates with alternating eye colours. Instead, one of the four males had an eye colour unique from that of the other three males. This enabled us to track the extent to which sperm from early copulations contributed to the progeny of later copulations. The results established that the P1 and P2 scores we report correctly represent competition between the ejaculates of only the most recent two males to mate with a female (less recent ejaculates only contributed between 0 and 1% of the offspring).
Table 2.
Control treatments demonstrate that ejaculates from early matings do not confound sperm precedence scores from later matings. (‘Unique’ males are those of the minority eye colour. Standard errors are given in parentheses.)
| male mating order (control treatments) | n | proportion of ‘unique’ males progeny after each sperm competition bout | ||
|---|---|---|---|---|
| first bout | second bout | third bout | ||
| +/+, bw, bw, bw | 24 | 0.071 (0.011) | 0.006 (0.004) | 0.000 (0.000) |
| bw, +/+, bw, bw | 14 | 0.896 (0.018) | 0.096 (0.024) | 0.007 (0.006) |
| bw, +/+, +/+, +/+ | 20 | 0.114 (0.024) | 0.006 (0.003) | 0.000 (0.000) |
| +/+, bw, +/+,+/+ | 19 | 0.904 (0.024) | 0.081 (0.016) | 0.010 (0.005) |
Repeatability (R) is equivalent to the intra-class correlation coefficient and was calculated using variance components derived from a one-way analysis of variance (ANOVA) procedure (Sokal & Rohlf 1981)
where s2A is the among-group variance component and s2 is the within-group (i.e. error) component. The groups in our analyses consisted of pairs of an individual male's or female's P scores (either P1 or P2). Standard errors of repeatabilities, which were used in one-tailed t-tests to identify the effects of genotypic interactions on the repeatabilities of P scores, were calculated as described by Becker (1992)
where k is the number of P1 or P2 scores per group and n is the number of groups. Comparable standard error estimates were found using the bootstrap method with replacement (number of replications=1000).
3. Results
(a) Experimental evolution for improved sperm offence and defence
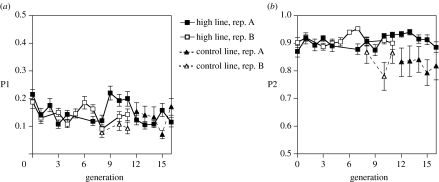
Linear regressions of mean P1 and P2 score on cumulative selection differential were non-significant for both replicates (high-P1: p=0.330 (replicate A), p=0.461 (replicate B); high-P2: p=0.194 (replicate A), p=0.729 (replicate B)). Since we selected male-specific sperm precedence trait, the slopes of these regressions can be doubled to estimate the realized heritability, h2, of the traits (Falconer & Mackay 1996). The h2 of P1 was estimated to be −0.024 and −0.030 in replicates A and B, respectively, and 0.042 and 0.022, respectively, for P2.
The response to selection for both increased P1 and P2 as a function of generation time is illustrated in figure 2. These regressions, along with our estimates of h2, indicate that selection for high-P1 and high-P2 was unsuccessful and either additive genetic variation (VA) for each trait is low or environmental variation for each trait is very high (see below). We did not quantify sperm competitiveness in the control lines until generations 12 and 8 in replicates A and B, respectively. There was no divergence between the control-P1 and high-P1 lines of either replicate. The high-P2 lines, however, do appear to have been more competitive than the control-P2 lines in some generations. Unpaired t-tests confirmed that these competitive differences were significant in generations 12, 13 and 15 (but not 14 and 16) of replicate A and in generation 10 (but not 8 and 11) of replicate B. However, the high-P2 lines did not show any signs of increasing, which would have been expected if they were evolving to be more competitive.
Figure 2.
Response in (a) the high-P1 and control-P1 and (b) the high-P2 and control-P2 selection lines of both replicates as a function of generation time. See text for heritability estimates.
This leaves two possible explanations for the apparent difference in competitive ability between the control-P2 and high-P2 lines. First, founder effects could be responsible. Even though the flies used to establish, each selection line were randomly collected from the stock population at generation 0, the subpopulation of flies used for both control-P2 lines could have been genetically inferior at offensive sperm competition. Given that the effective population sizes were smaller for the ‘control’ lines than for the ‘high’ lines, the hypothesis that genetic drift occurred cannot be discounted. Second, though sib matings were carefully avoided, the control-P2 lines could have lost competitive vigour as a result of inbreeding depression. If inbreeding depression were occurring, however, we would expect sperm competitiveness to have decreased over time rather than stay relatively constant. In summary, we do not interpret the differences between the high-P2 and control-P2 lines to be the result of the ‘high’ lines responding to selection.
(b) Contribution of interaction effects to variation in sperm precedence
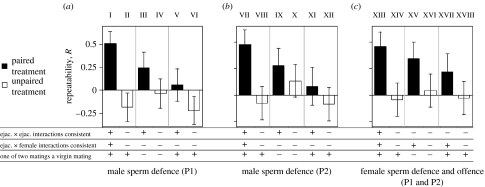
Repeatabilities were estimated for pair-wise comparisons of P1 or P2 scores both within and between treatments (figure 3). This approach enabled us to analyse varied effects of ejaculate×female and ejaculate×ejaculate interactions, as well as to evaluate whether virgin effects influenced the repeatability of sperm precedence scores. For instance, each male's virgin mating corresponds to its first P1 score, but the second and third P1 scores represent non-virgin matings (table 1). Similarly, the first P2 scores of males resulted from non-virgin matings, but the second males' ejaculates were competing against rival ejaculates inseminated during virgin matings. Figure 3 illustrates these effects for each repeatability estimate.
Figure 3.
Effect of genotypic interactions on the repeatability of P1 and P2. Open bars (unpaired treatment) and closed bars (paired treatment) represent repeatability estimates for (a) male P1 (b) male P2 and (c) female P1 and P2. Roman numerals are referred in table 3 to describe statistical analyses. The table below the graphs summarizes whether the ejaculate×ejaculate and ejaculate×female interactions present were consistent (+) or not consistent (−) within each set of pairs of P1 or P2 scores analysed (mating protocol is described in the text and in table 1). Ejaculate×ejaculate interaction are considered consistent when a male competed against the same rival male for each of the two scores analysed. Female×ejaculate interactions are considered consistent when a male mated with the same female for each of the two scores analysed. Instances in the paired treatment where the same two males mated with a female, but in reverse order, are recorded here as inconsistent ejaculate×ejaculate interactions. The table also summarizes whether or not one of the P1 or P2 scores in each repeatability estimate involved a virgin mating (see text for further discussion). The Roman numerals are used to identify those repeatability estimates compared in table 3. Error bars, which represent one standard error, were obtained using the bootstrap method with replacement (number of replications=1000). Across- and within-treatment statistical comparisons of repeatabilities are summarized in table 3.
The magnitude of each repeatability estimate was predicted, a priori, to be dictated by the extent to which interactions between individuals and ejaculates were consistent. This prediction was met. We review the male analyses first. No interaction was consistent in the unpaired treatment, meaning that males never mated twice with the same female or competed twice against the same rival male. Consistent with our prediction, repeatabilities of P1 and of P2 always appear to be lower among males in the unpaired treatment (open bars in figure 3a,b) relative to males in the paired treatment (closed bars). However, these across-treatment differences were statistically significant (table 3) only for the repeatabilities where both ejaculate×ejaculate and ejaculate×female interactions were held constant in the paired treatment (i.e. the comparison of the left-hand open and closed bars in figure 3a,b). The paired-treatment males’ P1 and P2 scores from these analyses resulted from sperm competition bouts with the same rival male within the same female. The repeatabilities for the paired treatment decreased in the comparisons for which only the effects of ejaculate×ejaculate interactions were consistent. It is interesting to note that this decrease was statistically significant when comparing (in figure 3a,b) the left- and right-hand closed bars, but not the left-hand and middle closed bars, though these comparisons were not significant when we controlled for the false discovery rate as described by Benjamini & Hochberg (1995) (table 3). The sharper declines observed in the left- and right-hand closed bars suggest that virgin effects could be yet another component of the complexity of sperm precedence, however, the experimental design prevents us from distinguishing between virgin effects and age effects (because all flies were older as non-virgins than they were as virgins).
Table 3.
Summary of one-tailed t-tests comparing repeatabilities of sperm precedence scores within and across treatments (Roman numerals correspond to those found in figure 3).
| sex | strategy | predicted relationship of repeatabilities, R | t (d.f.) | p |
|---|---|---|---|---|
| male | P1 | I>III | 1.231 (64) | 0.111 |
| I>V | 2.055 (64) | 0.022 | ||
| I>II | 3.333 (71) | 0.001a | ||
| III>IV | 1.220 (71) | 0.113 | ||
| V>VI | 1.192 (71) | 0.119 | ||
| P2 | VII>IX | 0.913 (62) | 0.183 | |
| VII>XI | 1.737 (62) | 0.044 | ||
| VII>VIII | 2.549 (71) | 0.006a | ||
| IX>X | 0.624 (71) | 0.267 | ||
| XI>XII | 0.708 (71) | 0.241 | ||
| female | P1 and P2 | XIII>XV | 0.542 (64) | 0.295 |
| XIII>XVII | 1.068 (64) | 0.145 | ||
| XIII>XIV | 2.305 (73) | 0.012 | ||
| XV>XVI | 1.328 (73) | 0.094 | ||
| XVII>XVIII | 1.073 (73) | 0.143 |
significant when controlling for false discovery rate, described by Benjamini & Hochberg (1995).
Unlike males, whose ‘P1 sperm’ and ‘P2 sperm’ are transferred to different females in consecutive copulations, females' P1 and P2 scores are determined from the same clutch of offspring. P1 and P2 scores for each female clutch are therefore interdependent, and they always add up to 1. This means that repeatability estimates of P1 and P2 for females are identical. As such, they are presented together in figure 3c and table 3. The same repeatability comparisons were made for females as were described for males (above). The patterns revealed were similar, with one exception in the paired treatment: the highest repeatability estimates (left-hand closed bars) were not significantly different than the lowest estimates (right-hand closed bars).
4. Discussion
We describe the proximate and ultimate effects of ejaculate×ejaculate and ejaculate×female interactions in an outbred D. melanogaster population displaying natural genetic variation. We were unable to experimentally evolve males for enhanced offensive or defensive sperm competitive ability, two traits with immense influence on male fitness. Sperm precedence is a multifarious trait, dependent upon a suite of interacting component traits within and between individuals. Some of these are likely to be sperm performance traits (e.g. motility) that are associated with the mitochondria and therefore inherited maternally (Frank & Hurst 1996; Gemmel et al. 2004). Such traits may be limited in their ability to respond to selection on males. Other components of the ejaculate are known to be antagonistically pleiotropic, further limiting directional change. For instance, in D. melanogaster, both sperm size and sperm quantity contribute independently to male fertilization success, but they also interact with one another within species (Pattarini et al. 2006) and show a negative correlation across species (Pitnick 1996).
The results of our selection experiment (figure 2), coupled with the work by Hughes (1997), could be interpreted to suggest that low heritabilities of P1 and P2 are common across D. melanogaster populations, despite the notion that all quantitative traits are likely to exhibit significant VA (Roff 1997). Low VA in sperm competitive ability could mean that selection on this trait is so intense that alleles contributing to P1 and P2 are fixed, or approaching fixation, leaving populations with little capacity to respond. However, genetic variation for each of these traits remains high (Clark et al. 1995; Hughes 1997; Civetta & Clark 2000); they are not approaching fixation. Recent work by Van Homrigh et al. (2007) with D. bunnanda revealed ample amounts of VA in sexually selected cuticular hydrocarbons, but interestingly, this variation was not in the direction of selection. A similar phenomenon could be limiting the directional evolution of sperm competitive ability.
Additionally, a response to selection may have been prevented (figure 2) because the competitive environment (i.e. female reproductive tracts and rival ejaculates) was dynamic and evolutionarily independent of the selection line males. By design, we did not assess sperm precedence with females or rival males from within the selection lines because we were interested in eliminating the complexity of higher level, coevolutionary processes in an attempt to investigate ‘root,’ ejaculate-level adaptations that contribute universally to offensive and defensive sperm competitive abilities. We conclude that there may be substantive VA for many of the component traits that contribute to sperm offence and defence (e.g. sperm length ; Miller & Pitnick 2002; Pattarini et al. 2006), but that sperm offence and defence, per se, exhibit high levels of non-heritable variation. A recent series of experiments using genetically discrete D. melanogaster lines indicates that this non-heritable variation is largely maintained by ejaculate×ejaculate and ejaculate×female interactions (reviewed above and in Clark 2002). The results of our selection experiment suggest that the evolution of sperm competitiveness may ultimately be regulated by these interactions.
Our repeatability experiment was designed to quantify the proximate effects that ejaculate×ejaculate and ejaculate×female interactions have on sperm precedence in a population displaying continuous, natural genetic variation. We found both the components of sperm competitiveness (i.e. offence and defence) to be highly repeatable only when these interactions were consistent; that is, only when each male competed each time against the same rival male and within the same female (figure 3). These repeatabilities declined when the rival male stayed the same but the female changed, and they disappeared when males competed each time against different rival males within different females. The repeatability experiment and the selection experiment were performed under the same conditions with flies from the same base population. Taken together, these experiments indicate that the non-heritable genetic variation in sperm competitive ability is indeed maintained to a larger extent by genotypic interaction effects in a naturally variable population. Importantly, this variation outweighs the heritable genetic variation of individual ejaculate traits in our study populations.
As expected of traits subject to intense sexual selection (Eberhard 1985; Andersson 1994), ejaculatory traits exhibit rapid and dramatic evolutionary divergence. In Drosophila, this pattern is observed for both sperm and accessory gland proteins (Acps). Many of the genes encoding Acps show signs of positive selection, with their evolution so rapid that clear differences exist even among sibling species (Whalen & Wilson 1986; Aguade et al. 1992; Civetta & Singh 1995, 1998; Tsaur & Wu 1997; Aguade 1999; Begun et al. 2000; Swanson et al. 2001; Panhuis et al. 2003; Kern et al. 2004; Kohn et al. 2004; Stevison et al. 2004; Mueller et al. 2005). With respect to gross morphology of sperm, sperm length varies by more than 400-fold within the genus Drosophila, frequently differing between sibling species (Joly 1987; Pitnick & Markow 1994; Pitnick et al. 1995a,b) and even among geographical populations within species (Pitnick et al. 2003).
Numerous recent studies have demonstrated the importance of ejaculate×female interactions in determining differential male fertilization success (Bishop 1996; Bishop et al. 1996; Rice 1996; Wilson et al. 1997; Howard et al. 1998; Clark et al. 1999; Cordoba-Aguilar 1999; Hosken et al. 2001; Mack et al. 2002; Miller & Pitnick 2002; Tregenza & Wedell 2002; Amitin & Pitnick 2006; Pitnick et al. in press). Furthermore, it is recognized that female physiology and behaviour is modified following copulation, with the physical act of copulation and/or the presence of Acps or sperm serving as the proximate trigger for such changes (reviewed in Eberhard 1996; see also: Wolfner 1997, 2002; Tram & Wolfner 1998; Chapman 2001; Heifetz et al. 2001; Swanson & Vacquier 2002; Gillott 2003; Kubli 2003; Fazeli et al. 2004; McGraw et al. 2004; Snook & Hosken 2004; Georgiou et al. 2005; Peng et al. 2005). Delving deeper into the mechanisms underlying these interactions between the sexes is critical for furthering our understanding of postcopulatory sexual selection (Simmons 2001; Birkhead & Pizzari 2002) and its significance for diversification and speciation (Markow 1997; Howard 1999; Eady 2001).
Sperm precedence patterns have been shown to break down after more than two matings in pseudoscorpions (Cordylochernes scorpioides; Zeh & Zeh 1994) and orb-web spiders (Nephila plumipes; Elgar et al. 2003). Recent work by Morrow et al. (2005) analysed mean sperm precedence scores and revealed no such effect of mating history on P1 or P2 in D. melanogaster, though they considered female mating history alone. The design of our repeatability experiment allowed us to compare sperm precedence scores from matings involving males and females when they were virgins and non-virgins. Our results suggest that virgin effects on the repeatability of competitive fertilization success do exist. Repeatabilities of P1 and P2 scores appeared to be higher in our ‘paired’ treatment only when non-virgin matings were considered, as opposed to non-virgin and virgin matings (figure 3). Field-caught females have been estimated to store sperm from a mean of 1.82 to 2.44 males in D. melanogaster (Harshman & Clark 1998; Jones & Clark 2003). This figure refers to the number of rival ejaculates present in females at any given time and cannot be used to determine the total number of mates per female or the mean time interval between matings. Nonetheless, females in the wild clearly re-mate frequently and, as a consequence, only a small proportion of matings in nature are likely to involve virgin flies. Virgin flies were used in our experimental evolution study of sperm precedence. It would be interesting to investigate the extent to which virgin effects might contribute to the high amounts of non-heritable variation detected in sperm offence and defence.
Acknowledgments
We thank B.A. Byrnes and B. Miller for their superb technical assistance and W. D. Brown, G. T. Miller, R. A. Schmedicke, J. A. C. Uy, L. L. Wolf and two anonymous reviewers for their useful comments and suggestions involving the experimental designs. This work was supported by a National Science Foundation grant (DEB-0315008) to S.P. and A.B.
References
- Aguade M. Positive selection drives the evolution of the Acp29AB accessory gland protein in Drosophila. Genetics. 1999;152:543–551. doi: 10.1093/genetics/152.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguade M, Miyashita N, Langley C.H. Polymorphism and divergence in the Mst26A male accessory gland gene region in Drosophila. Genetics. 1992;132:755–770. doi: 10.1093/genetics/132.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitin E.G, Pitnick S. Influence of developmental environment on male- and female-mediated sperm precedence in Drosophila melanogaster. J. Evol. Biol. 2006;20:380–390. doi: 10.1111/j.1420-9101.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Badyaev A.V, Qvarnstrom A. Putting sexual traits into the context of an organism: a life-history perspective in studies of sexual selection. Auk. 2002;119:301–310. doi:10.1642/0004-8038(2002)119[0301:PSTITC]2.0.CO;2 [Google Scholar]
- Becker W.A. 5th edn. Academic Enterprises; Pullman, WA: 1992. Manual of quantitative genetics. [Google Scholar]
- Begun D.J, Whitley P, Todd B.L, Waldrip-Dail H.M, Clark A.G. Molecular population genetics of male accessory gland proteins in Drosophila. Genetics. 2000;156:1879–1888. doi: 10.1093/genetics/156.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London, UK: 1998. Sperm competition and sexual selection. [Google Scholar]
- Birkhead T.R, Pizzari T. Postcopulatory sexual selection. Nat. Rev. Genet. 2002;3:262–273. doi: 10.1038/nrg774. doi:10.1038/nrg774 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Chaline N, Biggins J.D, Burke T, Pizzari T. Nontransitivity of paternity in a bird. Evolution. 2004;58:416–420. doi:10.1554/03-488 [PubMed] [Google Scholar]
- Birkhead T.R, Pellatt E.J, Brekke P, Yeates R, Castillo-Juarez H. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–387. doi: 10.1038/nature03374. doi:10.1038/nature03374 [DOI] [PubMed] [Google Scholar]
- Bishop J.D.D. Female control of paternity in the internally fertilizing compound ascidian Diplosoma listerianum. I. Autoradiographic investigation of sperm movements in the female reproductive tract. Proc. R. Soc. B. 1996;263:369–376. doi:10.1098/rspb.1996.0057 [Google Scholar]
- Bishop J.D.D, Jones C.S, Noble L.R. Female control of paternity in the internally fertilizing compound ascidian Diplosoma listerianum. II. Investigation of male mating success using RAPD markers. Proc. R. Soc. B. 1996;263:401–407. doi:10.1098/rspb.1996.0061 [Google Scholar]
- Boorman E, Parker G.A. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol. Entomol. 1976;1:145–155. [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. doi:10.1046/j.1365-2540.2001.00961.x [DOI] [PubMed] [Google Scholar]
- Chippindale A.K, Gibson J.R, Rice W.R. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:1671–1675. doi: 10.1073/pnas.041378098. doi:10.1073/pnas.041378098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A, Clark A.G. Chromosomal effects on male and female components of sperm precedence in Drosophila. Genet. Res. 2000;75:143–151. doi: 10.1017/s0016672399004292. doi:10.1017/S0016672399004292 [DOI] [PubMed] [Google Scholar]
- Civetta A, Singh R.S. High divergence of reproductive tract proteins and their association with postzygotic reproductive isolation in Drosophila melanogaster and Drosophila virilis group species. J. Mol. Evol. 1995;41:1085–1095. doi: 10.1007/BF00173190. doi:10.1007/BF00173190 [DOI] [PubMed] [Google Scholar]
- Civetta A, Singh R.S. Sex-related genes, directional sexual selection, and speciation. Mol. Biol. Evol. 1998;15:901–909. doi: 10.1093/oxfordjournals.molbev.a025994. [DOI] [PubMed] [Google Scholar]
- Clark A.G. Sperm competition and the maintenance of polymorphism. Heredity. 2002;88:148–153. doi: 10.1038/sj.hdy.6800019. doi:10.1038/sj.hdy.6800019 [DOI] [PubMed] [Google Scholar]
- Clark A.G, Aguade M, Prout T, Harshman L.G, Langley C.H. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.G, Begun D.J. Female genotypes affect sperm displacement in Drosophila. Genetics. 1998;149:1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.G, Begun D.J, Prout T. Female × male interactions in Drosophila sperm competition. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. doi:10.1126/science.283.5399.217 [DOI] [PubMed] [Google Scholar]
- Clark A.G, Dermitzakis E.T, Civetta A. Nontransitivity of sperm precedence in Drosophila. Evolution. 2000;54:1030–1035. doi: 10.1111/j.0014-3820.2000.tb00102.x. doi:10.1554/0014-3820(2000)054[1030:NOSPID]2.3.CO;2 [DOI] [PubMed] [Google Scholar]
- Cordoba-Aguilar A. Male copulatory sensory stimulation induces female ejection of rival sperm in a damselfly. Proc. R. Soc. B. 1999;266:779–784. doi:10.1098/rspb.1999.0705 [Google Scholar]
- Curtsinger J.W, Service P.W, Prout T. Antagonistic pleiotropy, reversal of dominance, and genetic polymorphism. Am. Nat. 1994;144:210–228. doi:10.1086/285671 [Google Scholar]
- Eady P.E. Postcopulatory, prezygotic reproductive isolation. J. Zool. 2001;253:47–52. doi:10.1017/S095283690100005X [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, UK: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Eberhard W.G. Princeton University Press; Princeton, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Elgar M.A, Bruce M.J, Champion de Crespigny F.E, Cutler A.R, Cutler C.L, Gaskett A.C, Herberstein M.E, Ramamurthy S, Schneider J.M. Male mate choice and patterns of paternity in the polyandrous, sexually cannibalistic orb-web spider Nephila plumipes. Aust. J. Zool. 2003;51:357–365. doi:10.1071/ZO02079 [Google Scholar]
- Falconer D.S, Mackay T.F.C. Longman Group Ltd; Essex, UK: 1996. Introduction to quantitative genetics. [Google Scholar]
- Fazeli A, Affara N.A, Hubank M, Holt W.V. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol. Reprod. 2004;71:60–65. doi: 10.1095/biolreprod.103.026815. doi:10.1095/biolreprod.103.026815 [DOI] [PubMed] [Google Scholar]
- Frank S.A, Hurst L.D. Mitochondria and male disease. Nature. 1996;383:224. doi: 10.1038/383224a0. doi:10.1038/383224a0 [DOI] [PubMed] [Google Scholar]
- Gemmel N.J, Metcalf V.J, Allendorf F.W. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004;19:238–244. doi: 10.1016/j.tree.2004.02.002. doi:10.1016/j.tree.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Georgiou A.S, Sostaric E, Wong C.H, Snijders A.P, Wright P.C, Moore H.D, Fazeli A. Gametes alter the oviductal secretory proteome. Mol. Cell. Proteomics. 2005;4:1785–1796. doi: 10.1074/mcp.M500119-MCP200. doi:10.1074/mcp.M500119-MCP200 [DOI] [PubMed] [Google Scholar]
- Gilbert D.G, Richmond R.C. Studies of esterase-6 in Drosophila melanogaster. VI. Ejaculate competitive abilities of males having null or active alleles. Genetics. 1981;97:85–94. doi: 10.1093/genetics/97.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. doi:10.1146/annurev.ento.48.091801.112657 [DOI] [PubMed] [Google Scholar]
- Harshman L.G, Clark A.G. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998;52:1334–1341. doi: 10.1111/j.1558-5646.1998.tb02015.x. doi:10.2307/2411303 [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Tram U, Wolfner M.F. Male contributions to egg production: the role of accessory gland products and sperm in Drosophila melanogaster. Proc. R. Soc. B. 2001;268:175–180. doi: 10.1098/rspb.2000.1347. doi:10.1098/rspb.2000.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D.J, Garner T.W.J, Ward P.I. Sexual conflict selects for male and female reproductive characters. Curr. Biol. 2001;11:489–493. doi: 10.1016/s0960-9822(01)00146-4. doi:10.1016/S0960-9822(01)00146-4 [DOI] [PubMed] [Google Scholar]
- Howard D.J. Conspecific sperm and pollen precedence and speciation. Annu. Rev. Ecol. Syst. 1999;30:109–132. doi:10.1146/annurev.ecolsys.30.1.109 [Google Scholar]
- Howard D.J, Gregory P.G, Chu J, Cain M.L. Conspeceific sperm precedence is an effective barrier to hybridization between closely related species. Evolution. 1998;52:511–516. doi: 10.1111/j.1558-5646.1998.tb01650.x. doi:10.2307/2411086 [DOI] [PubMed] [Google Scholar]
- Hughes K.A. Quantitative genetics of sperm precedence in Drosophila melanogaster. Genetics. 1997;145:139–151. doi: 10.1093/genetics/145.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Bussiere L.F, Jennions M.D, Brooks R. What is genetic quality? Trends Ecol. Evol. 2004;19:329–333. doi: 10.1016/j.tree.2004.03.035. doi:10.1016/j.tree.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Hunt J, Brooks R, Jennions M.D. Female mate choice as a condition-dependent life history trait. Am. Nat. 2005;166:79–93. doi: 10.1086/430672. doi:10.1086/430672 [DOI] [PubMed] [Google Scholar]
- Joly D. Between species divergence of cyst length distributions in the Drosophila melanogaster species complex. Jpn J. Genet. 1987;62:257–263. [Google Scholar]
- Jones B, Clark A.G. Bayesian sperm competition estimates. Genetics. 2003;163:1193–1199. doi: 10.1093/genetics/163.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A.D, Jones C.D, Begun D.J. Molecular population genetics of male accessory gland proteins in the Drosophila simulans complex. Genetics. 2004;167:725–735. doi: 10.1534/genetics.103.020883. doi:10.1534/genetics.103.020883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn M.H, Fang H.S, Wu C.-I. Inference of positive and negative selection on the 5′ regulatory regions of Drosophila genes. Mol. Biol. Evol. 2004;21:374–383. doi: 10.1093/molbev/msh026. doi:10.1093/molbev/msh026 [DOI] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. doi:10.1007/s00018-003-3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack P.D, Hammock B.A, Promislow D.E.L. Sperm competitive ability and genetic relatedness in Drosophila melanogaster: similarity breeds contempt. Evolution. 2002;56:1789–1795. doi: 10.1111/j.0014-3820.2002.tb00192.x. doi:10.1554/0014-3820(2002)056[1789:SCAAGR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Markow T.A. Assortative fertilization in Drosophila. Proc. Natl Acad. Sci. USA. 1997;94:7756–7760. doi: 10.1073/pnas.94.15.7756. doi:10.1073/pnas.94.15.7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard-Smith J. Cambridge University Press; Cambridge, UK: 1982. Evolution and the theory of games. [Google Scholar]
- McGraw L.A, Gibson G, Clark A.G, Wolfner M.F. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. doi:10.1016/j.cub.2004.08.028 [DOI] [PubMed] [Google Scholar]
- Miller G.T, Pitnick S. Sperm–female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. doi:10.1126/science.1076968 [DOI] [PubMed] [Google Scholar]
- Morrow E.H, Steward A.D, Rice W.R. Patterns of sperm precedence are not affected by female mating history in Drosophila melanogaster. Evolution. 2005;59:2608–2615. doi:10.1554/05-238.1 [PubMed] [Google Scholar]
- Mueller J.L, Ripoll C.F, Aquadro C.F, Wolfner M.F. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc. Natl Acad. Sci. USA. 2005;101:13 542–13 547. doi: 10.1073/pnas.0405579101. doi:10.1073/pnas.0405579101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K.P, Badyaev A.V. Adaptive genetic complementarity in mate choice coexists with selection for elaborate sexual traits. Proc. R. Soc. B. 2006;273:1913–1919. doi: 10.1098/rspb.2006.3528. doi:10.1098/rspb.2006.3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppliger A, Hosken D.J, Ribi G. Snail sperm production characteristics vary with sperm competition risk. Proc. R. Soc. B. 1998;265:1–8. doi:10.1098/rspb.1998.0468 [Google Scholar]
- Panhuis T.M, Swanson W.J, Nunney L. Population genetics of accessory gland proteins and sexual behavior in Drosophila melanogaster populations from Evolution Canyon. Evolution. 2003;57:2785–2791. doi: 10.1111/j.0014-3820.2003.tb01520.x. doi:10.1554/03-335 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. doi:10.1016/0022-5193(82)90225-9 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and the evolution of animal mating strategies. In: Smith R.L, editor. Sperm competition and the evolution of animal mating systems. Academic Press; Orlando, FL: 1984. pp. 1–60. [Google Scholar]
- Pattarini J.M, Starmer W.T, Bjork A, Pitnick S. Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution. 2006;60:2064–2080. doi:10.1554/06-142.1 [PubMed] [Google Scholar]
- Peng J, Chen S, Busser S, Liu H.F, Honeger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 2005;15:207–213. doi: 10.1016/j.cub.2005.01.034. doi:10.1016/j.cub.2005.01.034 [DOI] [PubMed] [Google Scholar]
- Pitnick S. Investment in testes and the cost of making long sperm in Drosophila. Am. Nat. 1996;148:57–80. doi:10.1086/285911 [Google Scholar]
- Pitnick S, Markow T.A. Male gametic strategies: Sperm size, testes size, and the allocation of ejaculate among successive mates by the sperm-limited fly Drosophila pachea and its relatives. Am. Nat. 1994;143:785–819. doi:10.1086/285633 [Google Scholar]
- Pitnick S, Markow T.A, Spicer G.S. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl Acad. Sci. USA. 1995a;92:10 614–10 618. doi: 10.1073/pnas.92.23.10614. doi:10.1073/pnas.92.23.10614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Spicer G.S, Markow T.A. How long is a giant sperm? Nature. 1995b;375:109. doi: 10.1038/375109a0. doi:10.1038/375109a0 [DOI] [PubMed] [Google Scholar]
- Pitnick S, Miller G.T, Schneider K, Markow T.A. Ejaculate–female coevolution in Drosophila mojavensis. Proc. R. Soc. B. 2003;270:1507–1512. doi: 10.1098/rspb.2003.2382. doi:10.1098/rspb.2003.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick, S., Wolfner, M. F. & Suarez, S. S. In Press. Ejaculate– and sperm–female interactions. In Sperm biology: an evolutionary perspective (eds T. R. Birkhead, D. J. Hosken & S. Pitnick). Amsterdam, The Netherlands: Elsevier Press.
- Prout T, Bunndgaard J. The population genetics of sperm displacement. Genetics. 1977;85:95–124. doi: 10.1093/genetics/85.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan J. Heritability of sperm competition success in the bulb mite Rhizoglyphus robini. J. Evol. Biol. 1998;11:321–327. doi: 10.1038/sj.hdy.6800649. doi:10.1007/s000360050091 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. doi:10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman and Hall; New York, NY: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Rose M. Life history evolution with antagonistic pleiotropy and overlapping generations. Theor. Popul. Biol. 1985;28:342–358. doi:10.1016/0040-5809(85)90034-6 [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Simmons L.W, Kotiaho J.S. Evolution of ejaculates: patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution. 2002;56:1622–1631. doi: 10.1111/j.0014-3820.2002.tb01474.x. doi:10.1554/0014-3820(2002)056[1622:EOEPOP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Snook R.R, Hosken D.J. Sperm death and dumping in Drosophila. Nature. 2004;428:939–941. doi: 10.1038/nature02455. doi:10.1038/nature02455 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W. H. Freeman and Company; San Francisco, CA: 1981. Biometry. [Google Scholar]
- Stevison L.S, Counterman B.A, Noor M.A. Molecular evolution of X-linked accessory gland proteins in Drosophila pseudoobscura. J. Hered. 2004;95:114–118. doi: 10.1093/jhered/esh027. doi:10.1093/jhered/esh027 [DOI] [PubMed] [Google Scholar]
- Swanson W.J, Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002;3:137–144. doi: 10.1038/nrg733. doi:10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- Swanson W.J, Zhang Z.H, Wolfner M.F, Aquadro C.F. Positive Darwinian selection drives the evolution of several female reproductive proteins in mammals. Proc. Natl Acad. Sci. USA. 2001;98:2509–2514. doi: 10.1073/pnas.051605998. doi:10.1073/pnas.051605998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tram U, Wolfner M.F. Seminal fluid regulation of female sexual attractiveness in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1998;95:4051–4054. doi: 10.1073/pnas.95.7.4051. doi:10.1073/pnas.95.7.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Polyandrous females avoid costs of inbreeding. Nature. 2002;415:71–73. doi: 10.1038/415071a. doi:10.1038/415071a [DOI] [PubMed] [Google Scholar]
- Tsaur S.C, Wu C.I. Positive selection and the molecular evolution of a gene of male reproduction Acp26Aa of Drosophila. Mol. Biol. Evol. 1997;14:544–549. doi: 10.1093/oxfordjournals.molbev.a025791. [DOI] [PubMed] [Google Scholar]
- Van Homrigh A, Higgie M, McGuigan K, Blows M.W. The depletion of genetic variance by sexual selection. Curr. Biol. 2007;17:528–532. doi: 10.1016/j.cub.2007.01.055. doi:10.1016/j.cub.2007.01.055 [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–314. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
- Whalen M, Wilson T.G. Variation and genomic localization of genes encoding Drosophila melanogaster male accessory glad proteins separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Genetics. 1986;114:77–92. doi: 10.1093/genetics/114.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Tubman S.C, Eady P.E, Robertson G.W. Female genotype affects male success in sperm competition. Proc. R. Soc. B. 1997;264:1491–1495. doi:10.1098/rspb.1997.0206 [Google Scholar]
- Wolfner M.F. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. doi:10.1016/S0965-1748(96)00084-7 [DOI] [PubMed] [Google Scholar]
- Wolfner M.F. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. doi:10.1038/sj.hdy.6800017 [DOI] [PubMed] [Google Scholar]
- Zeh J.A, Zeh D.W. Last-male sperm precedence breaks down when females mate with three males. Proc. R. Soc. B. 1994;257:287–292. doi:10.1098/rspb.1994.0127 [Google Scholar]
- Zeh J.A, Zeh D.W. The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc. R. Soc. B. 1996;263:1711–1717. doi:10.1098/rspb.1996.0250 [Google Scholar]
- Zeh J.A, Zeh D.W. The evolution of polyandry II: post-copulatory defenses against genetic incompatibility. Proc. R. Soc. B. 1997;264:69–75. doi:10.1098/rspb.1997.0010 [Google Scholar]
- Zimmering S, Fowler G.L. Progeny: sperm ratios and non-functional sperm in Drosophila melanogaster. Genet. Res. Camb. 1968;12:359–363. doi: 10.1017/s0016672300011940. [DOI] [PubMed] [Google Scholar]