Abstract
The diets of Australopithecus africanus and Paranthropus robustus are hypothesized to have included C4 plants, such as tropical grasses and sedges, or the tissues of animals which themselves consumed C4 plants. Yet inferences based on the craniodental morphology of A. africanus and P. robustus indicate a seasonal diet governed by hard, brittle foods. Such mechanical characteristics are incompatible with a diet of grasses or uncooked meat, which are too tough for efficient mastication by flat, low-cusped molars. This discrepancy, termed the C4 conundrum, has led to the speculation that C4 plant underground storage organs (USOs) were a source of nutrition for hominin species. We test this hypothesis by examining the isotopic ecology of African mole rats, which consume USOs extensively. We measured δ18O and δ13C of enamel and bone apatite from fossil and modern species distributed across a range of habitats. We show that δ18O values vary little and that δ13C values vary along the C3 to C4/CAM-vegetative axis. Relatively high δ13C values exist in modern Cryptomys hottentotus natalensis and Cryptomys spp. recovered from hominin-bearing deposits. These values overlap those reported for A. africanus and P. robustus and we conclude that the USO hypothesis for hominin diets retains certain plausibility.
Keywords: hominin evolution, stable isotopes, plant underground storage organs, geophytes, Bathyergidae, Cryptomys
1. Introduction
The evolution of human diet is informed by multiple lines of evidence, including craniodental morphology, dental microwear, comparative primate ecology and stable isotope analysis (Teaford & Ungar 2000; Teaford et al. 2002). The carbon isotope composition (δ13C) of hominin tooth enamel indicates a diet heavily influenced by plants that use C4 photosynthesis or Crassulacean acid metabolism (CAM). As a result, the diet of Australopithecus africanus and Paranthropus robustus is hypothesized to have included C4 plants, such as tropical grasses and sedges, or the tissues of animals which themselves consumed C4 plants (Sponheimer et al. 2005a). Yet inferences based on the microwear and craniodental morphology of early hominins indicate a seasonal diet governed by modestly tough foods (e.g. A. africanus; Scott et al. 2005), hard, tough foods (e.g. A. anamensis; Macho et al. 2005; cf. Grine et al. 2006a), hard, brittle foods (e.g. A. afarensis; Ryan & Johanson 1989; Ungar 2004; cf. Grine et al. 2006b) or hard, abrasive foods (e.g. P. bosei and P. robustus; Demes & Creel 1988; Scott et al. 2005). Such mechanical characteristics are incompatible with a diet of C4 grass blades or uncooked meat; both foods are too fracture resistant for efficient mastication by flat, thickly enamelled, low-cusped molars (Lucas & Peters 2000; Teaford & Ungar 2000). In an effort to reconcile this discrepancy, termed the C4 conundrum, authors have suggested that the contribution of C4 tissues to hominin diets could be, in part and to different extents, derived from termites, sedges and/or plant underground storage organs (USOs; Sponheimer & Lee-Thorp 2003; Sponheimer et al. 2005a,b).
Plant USOs are starchy geophytic structures, such as corms, bulbs, rhizomes and tubers. They are relatively common in xeric habitats (Pate & Dixon 1982; Vincent 1985; Proches et al. 2006), and their importance in human evolution has received considerable theoretical attention (Robinson 1954; Coursey 1973; Hatley & Kappelman 1980; Laden & Wrangham 2005). For instance, it is hypothesized that the changes in tuber consumption facilitated the initial emergence and spread of Homo erectus out of Africa (Hawkes et al. 1998; O'Connel et al. 1999; Wrangham et al. 1999). Such arguments are challenging to test because direct evidence for USO consumption is difficult to obtain, particularly for more remote time periods. USOs themselves are perishable, as are many of the tools used to collect and process them.
Today, a diet of USOs is characteristic of human hunter gatherers in arid environments, particularly as a fallback food (Vincent 1985; Campbell 1986). Many of the USOs edible to humans are also consumed by African mole rats (Bathyergidae; Laden & Wrangham 2005), a radiation of rodents that have specialized in this food base for 40 Myr (Faulkes et al. 2004). Importantly, a survey of faunal assemblages at hominin-bearing localities reveals a statistical co-occurrence with mole rats (Laden & Wrangham 2005). This result suggests co-occupation of environments suitable for USO-bearing plants and raises the possibility that hominins and mole rats competed for similar food items. An analysis of stable carbon and oxygen isotopes is well suited to test this hypothesis.
Ratios of stable carbon isotopes (13C/12C) distinguish plants that use different photosynthetic pathways. Plants that have carbon-concentrating, or double carboxylation, mechanisms have advantages in warm, highly xeric and/or seasonal environments (Keeley & Rundel 2003). Some plants segregate carboxylation steps in time (CAM plants), while others segregate them in space with specific tissues (C4 plants; Keeley & Rundel 2003). These mechanisms lead to different isotope compositions relative to plants that use a single carboxylation pathway (C3 plants; Ehleringer & Rundel 1989). Ratios of 13C/12C are maintained in the tissues of animals that consume these plants or the animals that eat these primary consumers (Koch 1998). Accordingly, an isotopic analysis of consumer tissues can reveal the types of primary production supporting a food web. Consumption of C3 plants is indicated by relatively low 13C/12C ratios, whereas that of C4 plants is indicated by relatively high 13C/12C ratios. CAM plants tend to have intermediate values, but in many environments they are indistinguishable from C4 plants (Keeley & Rundel 2003).
Additionally, ratios of oxygen isotopes (18O/16O) are diagnostic of certain dietary characteristics and environmental conditions (Koch 1998). For instance, oxygen in the bioapatite of bones and teeth is derived from body water; oxygen in the body water of terrestrial animals is supplied chiefly by oxygen in food or drinking water. Consequently, the factors that alter the oxygen isotope composition of ingested water can influence the 18O/16O ratios of bioapatite. For instance, the oxygen isotope composition of meteoric water (water that is derived from the atmosphere) is positively correlated with annual and seasonal temperatures; the isotopic values of surface water are increased by preferential loss of 16O during evaporation. The water in plant roots and stems is similar to the source water, whereas that in leaves may be substantially enriched in 18O due to evapotranspiration. As a result, the changes in 18O/16O ratios in consumers may indicate changes in diet, although temporal or spatial variation of these ratios due to climate shifts often eclipses suspected dietary signals.
In tandem, the analysis of stable carbon and oxygen isotopes can reveal important aspects of an organism's feeding ecology (Koch et al. 1994), and comparative studies of modern and fossil taxa may yield insight into the diet of extinct animals. Here, we report on the isotopic ecology of modern and Plio–Pleistocene mole rats in order to inform hypotheses on the diet of hominin species. A referential model based on mole rats is advantageous for three reasons. First, it can falsify or strengthen the hypothesis that 13C-enriched isotope values are associated with a diet of USOs. Second, it can determine whether 13C-enriched plants with USOs were temporally and spatially available to A. africanus and P. robustus. Third, a comparison of modern mole-rat dietary proclivities and stable isotope ratios may signify which types of USO, if any, were plausible food items for hominins.
2. Material and methods
(a) Sample provenience and preparation
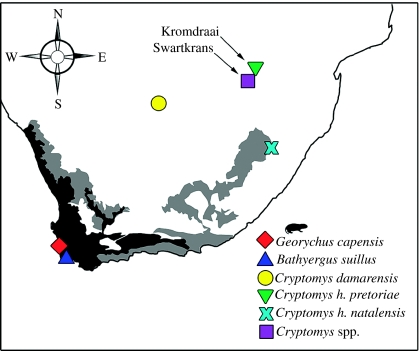
We analysed bone apatite from the mandibular ramus of five South African mole-rat taxa, two of which are different subspecies (figure 1). The species were chosen for their geographical and dietary breadth; each relies on USOs to a different extent (table 1). We also analysed enamel and bone apatite from the incisors and dentary, respectively, of Cryptomys spp. recovered from Kromdraai B and Swartkrans Member 1 (ca 1.9–1.7 Myr ago; Vrba 1985). The specimens, collectively catalogued as JY001–JY003, are housed in the Transvaal Museum, Pretoria, South Africa (n=23). We used a precision dental drill to collect approximately 5 mg apatite. The samples were ground with an agate mortar and pestle, washed in 2–3% NaOCl and soaked in 1 M acetic acid with calcium acetate buffer (pH 5.2) to remove diagenetic carbonates. Each sample was washed, dried and weighed to approximately 1.5 mg.
Figure 1.
Southern African localities where bathyergid skulls were collected; Bathyergus suillus (33°58′26.4″ S; 18°37′0.6″ E; n=11), Cryptomys damarensis (27°27′42.2″ S; 23°25′50.7″ E; n=10), Cryptomys hottentotus natalensis (29°32′02.6″ S; 29°36′29.4″ E; n=7), Cryptomys hottentotus pretoriae (25°45′2.1″ S; 28°11′9.1″ E; n=7) and Georychus capensis (33°22′41.0″ S; 18°22′41.8″ E; n=7). Specimens of Cryptomys spp. (n=23) were recovered from Kromdraai B and Swartkrans Member 1. The black (0–25%), grey (25–75%) and white (75–100%) polygons show the proportion of C4 species among grasses in South Africa (redrawn from Vogel et al. 1978).
Table 1.
Dietary characteristics of mole-rat species in the present study. (Plant genera that fix carbon via the C4 pathway are indicated in bold text; plant genera capable of fixing carbon via the CAM photosynthetic pathway are indicated with an asterisk. Cormous, bulbous and tuberous classifications follow Manning et al. (2002) or the personal observations of N. C. Bennett.)
| species | dietary characterization | source | |||
|---|---|---|---|---|---|
| general descriptive characteristics | cormous genera | bulbous genera | tuberous genera | ||
| Bathyergus suillus | >60% aerial vegetation (Cynodon dactylona); roots, small geophytes | Babiana, Cyanella, Moraea (=Homeria) | Lachenalia, Ornithogalum, Oxalis* | Othonna*, Wachendorfia | Davies & Jarvis (1986); Bennett & Jarvis (1995); Smith & Winter (1996); Sage et al. (1999); N. C. Bennett (personal observation, 2005) |
| Cryptomys damarensis | geophytes, large and dispersed | Dipcadi, Ledebouria, Ornithogalum | Acanthosicyos, Eriospermum, Talinum* | Jarvis & Bennett (1990); Bennett & Jarvis (1995); Jarvis et al. (1998) | |
| Cryptomys hottentotusb | geophytes and grass rhizomes | Cyanella, Moraea (=Hexaglottis) (=Homeria), Micranthus, Romulea | Alloteropsisc, Ornithogalum, Oxalis* | Herrea, Trachyandra, Wachendorfia | Genelly (1965); Lovegrove & Jarvis (1986); Smith & Winter (1996); Sage et al. (1999); N. C. Bennett (personal observation, 2005) |
| Georychus capensis | <15% aerial vegetation; small geophytes | Geissorhiza, Micranthus, Moraea (=Hexaglottis) (=Homeria), Ornithogalum, Romulea | Albuca, Lachenalia, Ornithogalum, Oxalis* | Herrea, Trachyandra | Du Toit et al. (1985); Lovegrove & Jarvis (1986); Smith & Winter (1996); Spinks et al. (1999); N. C. Bennett (personal observation, 2005) |
Grass blades and rhizomes.
Cryptomys hottentotus is interpreted to include the subspecies Cryptomys h. natalensis and Cryptomys h. pretoriae. Another subspecies, Cryptomys h. hottentotus, is known to consume Romulea corms (Bennett & Jarvis 1995), while some colonies consume Ornithogalum bulbs (more than 90% of diet) or Lachenalia bulbs (more than 60% of diet) selectively (Spinks et al. 1999).
A population studied by Genelly (1965) consumed the bulbs and seeds of Alloteropsis (Poaceae) nearly exclusively.
(b) Analytical procedure
We analysed samples using a Micromass Optima dual inlet mass spectrometer located in the Departments of Earth and Planetary Sciences and Ocean Sciences, University of California, Santa Cruz. Isotope ratios for C and O are presented as δ values, where δ=1000((Rsample/Rstandard)−1) and R=either 13C/12C or 18O/16O. Reference standards are Vienna PeeDee belemnite for carbon and standard mean oceanic water for oxygen. Units are expressed as parts per thousand (‰).
To compare the modern isotopic data with those from Plio–Pleistocene specimens, carbon isotope values were corrected for the global decrease in the 13C content of atmospheric CO2 due largely to fossil fuel burning over the last 150 years (the Suess effect; Idermühle et al. 1999). Based on ice core records (Francey et al. 1999), we applied a −1.2‰ correction to all Plio–Pleistocene samples (Leuenberger et al. 1992; table 1 in the electronic supplementary material).
(c) Statistical analyses
Statistical tests were performed with JMP v. 5.0.1 for Macintosh. When possible, analysis of variance was used to assess differences in carbon and oxygen isotope composition between species. Because some data violated the assumptions of parametric statistical analysis, non-parametric Wilcoxon Z (two-sample) and Kruskal–Wallis Χ2 (multiple comparison) tests were used when necessary; further comparisons of significance were investigated with the Tukey HSD post hoc test. Orthogonal regression was used to assess correlations between the δ18O values of mole rats against modelled δ18O values of regional meteoric precipitation using the ‘OIPC: online isotopes in precipitation calculator’ of Bowen (2006) and mean annual precipitation (figures 1 and 2 in the electronic supplementary material).
3. Results
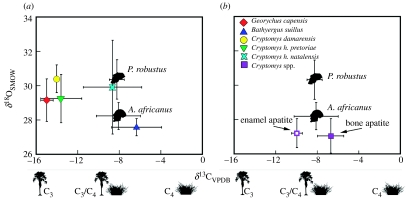
We analysed four species and two subspecies of modern mole rats (figure 1). The δ13C values varied significantly along the C3 to C4/CAM-vegetative axis (Kruskal–Wallis Χ52=36.86; p<0.0001; figure 2a). Three species (Cryptomys damarensis, Cryptomys hottentotus pretoriae and Georychus capensis) had isotopic values indicative of a C3-based diet. Two species (Bathyergus suillus and Cryptomys hottentotus natalensis) showed considerable variation and had less negative δ13C values (Tukey HSD Q=2.87; p<0.05). The δ18O values for all modern mole rats (except B. suillus) were similar, ranging from 27 to 31‰ (Kruskal–Wallis Χ52=4.80; p=0.19; figure 2a). Bathyergus suillus had δ18O values significantly less positive than other species (Tukey HSD Q=2.87; p<0.05).
Figure 2.
(a) Modern mole rats versus Plio–Pleistocene hominins: the δ13C and δ18O values of modern mole-rat tooth enamel (data available in table 1 in the electronic supplementary material) plotted with values reported for A. africanus and P. robustus (Sponheimer et al. 2005a). (b) The δ13C and δ18O values of Plio–Pleistocene Cryptomys spp. bone and tooth enamel plotted with the values reported for A. africanus and P. robustus.
The δ13C values of enamel (n=3) and bone apatite (n=23) from Plio–Pleistocene Cryptomys spp. differed (Wilcoxon Z=−2.65; p=0.008; figure 2b), indicating post-mortem alteration of the bone apatite, which is a well-known effect at these sites (Lee-Thorp & van der Merwe 1987; Lee-Thorp & Sponheimer 2003). The δ18O values of Plio–Pleistocene Cryptomys spp. bone apatite and enamel apatite were similar statistically (Wilcoxon Z=0.00; p=1.00), suggesting little or no post-mortem alteration of oxygen ratios in bone apatite. The isotopic composition of the enamel apatite indicates a diet influenced by either CAM or C4 plants, similar to that of modern C. h. natalensis. Oxygen isotope δ values of Plio–Pleistocene Cryptomys spp. were lower than those for all modern populations (F1,64=31.32; p<0.0001), except B. suillus (Wilcoxon Z=1.62; p=0.11).
Despite spatial and temporal differences, the δ13C and δ18O values of modern C. h. natalensis did not differ from those reported for A. africanus and P. robustus (δ13C values: Wilcoxon Z=−0.34; p=0.73; δ18O values: Wilcoxon Z=−0.30; p=0.77; figure 2a). Although a robust statistical analysis of Plio–Pleistocene Cryptomys spp. enamel apatite is precluded on the basis of limited samples (n=3), a non-parametric test revealed overlapping δ13C and δ18O values with those of A. africanus (δ13C: Wilcoxon Z=−0.91; p=0.36; δ18O: Wilcoxon Z=−1.82; p=0.07; figure 2b).
4. Discussion
The man who has nothing to boast of but his illustrious ancestry is like the potato—the best part under ground.
Thomas Overbury—Characters (1613)
Three conclusions are evident from this study. First, the δ13C values of modern mole rats evince a high degree of variance along the C3 to C4/CAM-vegetative axis. Second, relatively high δ13C values exist in Cryptomys spp. specimens recovered from hominin-bearing deposits. Third, the hypothesis that the diet of A. africanus and P. robustus included USOs to some extent retains certain plausibility on the basis of comparative isotopic ecology.
(a) The isotopic ecology of mole rats
The δ18O values of mole rats showed little systematic variation. We assessed whether this variation can be explained by modelled δ18O variation in meteoric water across Africa (Bowen 2006) or by mean annual rainfall. We found a negative correlation between the δ18O values of mole rats and meteoric water (r=−0.47; figure 1 in the electronic supplementary material), which suggests that mole rats may obtain their water from plants. A negative correlation between mole-rat values and regional mean annual rainfall (r=−0.43; figure 2 in the electronic supplementary material) is consistent with this suggestion, as environmental moisture influences δ18O variability in plants. The δ18O values of mole rats therefore appear to be influenced most strongly by environmental moisture levels, as mediated by the evaporative effects on plant waters, rather than the δ18O values of meteoric water itself. A similar effect of moisture level on the δ18O values of herbivores has been detected previously (Ayliffe & Chivas 1990; Levin et al. 2006).
Our analysis of δ13C values in mole-rat tissues permits increased dietary resolution among populations. As many as three species of mole rat live in the same habitat (Lovegrove & Jarvis 1986), hence niche partitioning is expected. A reliance on USOs does vary between species (table 1), and some species, such as B. suillus, consume aboveground resources. The relatively 13C-enriched values of B. suillus are consistent with a previous report based on bone collagen (Sealy & van der Merwe 1986) and with observations of foraging behaviour. The diet of B. suillus is nearly 60% Cynodon dactylon, a C4 grass, or more variably composed of stems and leaves (Reichman & Jarvis 1989; Bennett & Jarvis 1995). The δ13C values of C. damarensis and C. h. pretoriae are also consistent with previous reports of dietary specialization; they consume the corms and bulbs of C3 plants (table 1) despite living in an environment where most grass species are C4 (figure 1). In contrast, C. h. natalensis—which also relies on bulbs, corms and grass rhizomes in a C4-dominated system—has elevated δ13C values. Specimens of B. suillus and G. capensis co-occur along the southwestern Cape, but they have very different δ13C values, indicating divergent feeding strategies. This analysis reveals that the mere presence of mole rats in a C4 ecosystem cannot predict the availability of C4 USOs (cf. Laden & Wrangham 2005). Additionally, mole rats that live in the same habitat can display distinctly different isotopic signatures.
The genus Cryptomys has been a member of the southern African ecosystem for ca 17 Myr ago (Faulkes et al. 2004). Based on the retention of specialized morphological characteristics known to correspond with a diet of USOs, such food items were likely available for a comparable period. In fact, consumption of C4 resources has been documented in non-USO specialist rodents from Late Pliocene South African deposits (Hopley et al. 2006). Our analysis of Cryptomys from Kromdraai B and Swartkrans Member 1 demonstrates that it consumed C4 or CAM foods 1.9–1.7 Myr ago. This result suggests that the USOs of C4 or CAM plants—bulbs and corms in particular—were available to hominins recovered from the same localities, and thus could have contributed to the elevated δ13C values of A. africanus and P. robustus.
(b) USOs and the C4 conundrum
Uncertainty exists as to whether C4 USOs existed in quantities sufficient to result in the elevated δ13C values of A. africanus and P. robustus. This uncertainty stems from differing opinions on the habitats available to foraging hominins. Peters & Vogel (2005) observed that edible C4 plants are typically restricted to marshes, wetlands and disturbed ground, and that environmental models available for early South African hominin sites do not indicate such habitats. Yet habitat models based on mammalian faunal assemblages suggest a moist grassland characterized by trees, streams and rivers (Reed 1997; Avery 2001), and recent theoretical considerations have suggested that wet river margins and associated habitats were critical to hominin origins (Conklin-Brittain et al. 2002; Wrangham 2005). Such environments tend to be rich in USOs (Copeland 2004); furthermore, CAM plants (which may have δ13C values similar to C4 plants) occur across the African habitats regardless of surface moisture. CAM plants range from forests and edaphic grasslands to semiarid savannahs and desert environments, and frequently have USOs (Manning et al. 2002). Importantly, chimpanzees living in relatively dry habitats consume little or no C4 plant food (Schoeninger et al. 1999; Sponheimer et al. 2006a), which limits to some extent the value of modelling hominin diets on the foraging behaviour of chimpanzees in savannah-like environments.
Other chemical analyses of food webs in Africa bear on the USO question. Sr/Ca ratios may indicate consumption of USOs because underground plant tissues are thought to have unique Sr/Ca patterns (Sillen et al. 1995; Sponheimer et al. 2005b). An analysis of Sr/Ba ratios revealed relatively high values in Cryptomys hottentotus when compared with other fauna in South Africa (Sponheimer & Lee-Thorp 2006). Although promising, more research and larger sample sizes are needed to better understand how Sr/Ca and Sr/Ba ratios fractionate within modern ecosystems, particularly among the USO-bearing plant groups and the animals known to consume them. Isotopic subsampling of the dental perikymata of P. robustus has revealed highly variable δ13C values, which has been interpreted as evidence for seasonal foraging on very different foods (Sponheimer et al. 2006b). Alternatively, because much of this variation is within the range exhibited by USO specialists, this result may instead indicate foraging on different proportions of seasonally abundant USO-bearing plants.
(c) Converging lines of evidence for USO usage
The behaviour of living primates suggests that hominins probably selected foods on the basis of specific mechanical and nutritional properties. Mechanically, the craniodental morphology and the microwear of early hominin species indicate a diet characterized frequently or occasionally by relatively hard, gritty foods (Teaford et al. 2002). Some USOs would appear to fit these criteria, yet relevant studies are few. When chacma baboons (Papio ursinus) consume USOs during the dry season, their molar microwear bears a resemblance to the extensive pitting observed on specimens of P. robustus (Daegling & Grine 1999). This study suggests that USOs are abrasive; however, an analysis of the mechanical characteristics of putative hominin foods, particularly corms and bulbs, may yield further insights. For instance, the tubers of Kirkia wilmsii are two to nine times more puncture resistant than the bulbs of Cyperus usitatus (Peters & Maguire 1981). In this regard, Stahl (1984, p. 156) may have been prescient: ‘it would seem important to distinguish types of storage organs in discussing their potential as food sources for early hominids’.
Compellingly, USOs also vary in their nutritional properties. Bulbs and corms are a relatively rich source of starch carbohydrates when compared with tubers (Orthen 2001; Schoeninger et al. 2001), and Conklin-Brittain et al. (2002) suggested that a dietary shift to USOs among australopith-grade hominins led to a reduction in fibre intake and an overall improvement in nutritional quality. In fact, the fibre content of wild USOs in the diet of modern Cryptomys is much lower than that of fruit and pith in the diet of chimpanzees (Bennett & Jarvis 1995). Typically, a fibrous diet favours a digestive system capable of caeco-colic fermentation (Alexander 1993), which is characteristic of hominoid primates (Lambert 1998). A mole-rat species with a similar isotopic composition to hominins, Bathyergus suillus, is a caeco-colic fermenting herbivore, rather than a caecal fermenter, as is the case in most rodent species (Kotze et al. 2006). Such an anatomical resemblance suggests that the digestive kinetics of the hominoid gut would not preclude an adaptive shift towards the consumption of USOs.
In conclusion, our isotopic analysis of modern and Plio–Pleistocene mole rats reveals the inclusion of 13C-enriched foods in their diets. Since mole rats are USO specialists, this enriched isotopic signature is certain to be derived to a large extent from the USO-bearing plants. Accordingly, the consumption of USOs by early hominins cannot be refuted on the basis of δ13C values, although it is apparent that other food objects could have contributed to the 13C-enriched values of hominin teeth, such as termites and the flesh of grazing animals (Backwell & d'Errico 2001; Peters & Vogel 2005; Sponheimer et al. 2005b), or, perhaps, the flesh of mole rats themselves (Henshilwood 1997). Finally, although we have focused on C4 USOs, it is important to emphasize that CAM plants may also have contributed to the 13C-enriched values of A. africanus and P. robustus. Many South African CAM plants are geophytic; indeed, 20% of the Cape flora is characterized as such (Proches et al. 2006). In some areas, geophytic plants represent 40% of the total flora (Snijman & Perry 1987; Manning et al. 2002). Perhaps not surprisingly, accumulations of cormous tunics are widespread in the Early Holocene archaeological record of the western Cape (Deacon 1976), indicating the importance of USOs, or corms and bulbs specifically, as an important food resource. Future directions will include a study of δ13C values among geophytic species and an analysis of USO mechanical characteristics.
Acknowledgments
We are grateful to J. Luus, S. Potze, J. F. Thackeray and the Transvaal Museum for facilitating access to research specimens. P. W. Lucas, K. Fox-Dobbs, S. D. Newsome, M. Sponheimer, R. W. Wrangham, the Duckitt Family, D. Codron and an anonymous reviewer contributed intellectual exchange that inspired or improved this study. D. Andreasen provided technical assistance. E. R. Vogel provided statistical advice. Transportation and examination of specimens were approved by the US Fish and Wildlife Service and the Chancellor's Animal Research Committee of UC-Santa Cruz (approval no. 122204). Funding was received from the American Philosophical Society, the UC-Santa Cruz Committee on Research, the UC-Santa Cruz Division of Social Sciences and the Wenner-Gren Foundation (to N.J.D.), and a grant from the National Science Foundation (NSF EAR-0309383) (to P.L.K.).
Supplementary Material
The δ<13C and δ<18O values of bone and enamel apatite of Cryptomys spp. recovered from Kromdraai B and Swartkrans Member 1 and the δ<13C and δ<18O values of bone apatite for five species of modern mole-rat. The Suess effect is a correction for the global decrease in the <13C content of atmospheric carbon dioxide due to fossil fuel burning over the past 150 years. We applied a −1.2‰ correction to all data from Plio–Pleistocene specimens (see methods for details).
Orthogonal regressions between modern mole-rat spp. mean δ<18O values versus δ<18O values modeled for meteoric precipitation (Bowen 2006) (r=−0.47).
Orthogonal regressions between modern mole-rat spp. mean δ<18O values versus regional mean annual precipitation (mm) (r=−0.43)
References
- Alexander R.M. The relative merits of foregut and hindgut fermentation. J. Zool. 1993;231:391–401. [Google Scholar]
- Avery D.M. The Plio–Pleistocene vegetation and climate of Sterkfontein and Swartkrans, South Africa, based on micromammals. J. Hum. Evol. 2001;41:113–132. doi: 10.1006/jhev.2001.0483. doi:10.1006/jhev.2001.0483 [DOI] [PubMed] [Google Scholar]
- Ayliffe L.K, Chivas A.R. Oxygen isotope composition of bone phosphate of Australian kangaroos—potential as a paleoenvironmental recorder. Geochim. Cosmochim. Acta. 1990;54:2603–2609. doi:10.1016/0016-7037(90)90246-H [Google Scholar]
- Backwell L.R, d'Errico F. Evidence of termite foraging by Swartkrans early hominids. Proc. Natl Acad. Sci. USA. 2001;98:1358–1363. doi: 10.1073/pnas.021551598. doi:10.1073/pnas.021551598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N.C, Jarvis J.U.M. Coefficients of digestibility and nutritional values of geophytes and tubers eaten by southern African mole-rats (Rodentia: Bathyergidae) J. Zool. 1995;236:189–198. [Google Scholar]
- Bowen, G. J. 2006 Online isotopes in precipitation calculator (OIPC). See http://wateriso.eas.purdue.edu/waterisotopes/pages/data_access/oipc.html
- Campbell A. The use of wild food plants, and drought in Botswana. J. Arid Environ. 1986;11:81–91. [Google Scholar]
- Conklin-Brittain N.L, Wrangham R.W, Smith C.C. A two-stage model of increased dietary quality in early hominid evolution: the role of fiber. In: Ungar P.S, Teaford M.F, editors. Human diet: its origin and evolution. Bergin & Garvey; Westport, CT: 2002. pp. 61–76. [Google Scholar]
- Copeland, S. R. 2004 Paleoanthropological implications of vegetation and wild plant resources in modern savanna landscapes, with applications to Plio–Pleistocene Olduvai Gorge, Tanzania. PhD dissertation, Rutgers University.
- Coursey D.G. Hominid evolution and hypogeous plant foods. Man. 1973;8:634–635. [Google Scholar]
- Daegling D.J, Grine F.E. Terrestrial foraging and dental microwear in Papio ursinus. Primates. 1999;40:559–572. doi:10.1007/BF02574831 [Google Scholar]
- Davies K.C, Jarvis J.U.M. The burrow systems and burrowing dynamics of the mole-rats Bathyergus suillus and Cryptomys hottentotus in the fynbos of the south-western Cape, South Africa. J. Zool. 1986;209:125–147. [Google Scholar]
- Deacon, H. J. 1976 Where hunters gathered South African Archaeological Society monograph 1.
- Demes B, Creel N. Bite force, diet, and cranial morphology of fossil hominids. J. Hum. Evol. 1988;17:657–670. doi:10.1016/0047-2484(88)90023-1 [Google Scholar]
- Du Toit J.T, Jarvis J.U.M, Louw G.N. Nutrition and burrowing energetics of the Cape mole-rat Georychus capensis. Oecologia. 1985;66:81–87. doi: 10.1007/BF00378556. doi:10.1007/BF00378556 [DOI] [PubMed] [Google Scholar]
- Ehleringer J.R, Rundel P.W. Stable isotopes: history, units and instrumentation. In: Rundel P.W, Ehleringer J.R, Nagy K.A, editors. Stable isotopes in ecological research. Springer; New York, NY: 1989. pp. 1–16. [Google Scholar]
- Faulkes C.G, Verheyen E, Verheyen W, Jarvis J.U.M, Bennett N.C. Phylogeographical patterns of genetic divergence and speciation in African mole-rats (Family: Bathyergidae) Mol. Ecol. 2004;13:613–629. doi: 10.1046/j.1365-294x.2004.02099.x. doi:10.1046/j.1365-294X.2004.02099.x [DOI] [PubMed] [Google Scholar]
- Francey R.J, Allison C.E, Etheridge D.M, Trudinger C.M, Enting I.G, Leuenberger M, Langenfelds R.L, Michel E, Steele L.P. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B. 1999;51:170–193. doi:10.1034/j.1600-0889.1999.t01-1-00005.x [Google Scholar]
- Genelly R.E. Ecology of the common mole-rat (Cryptomys hottentotus) in Rhodesia. J. Mammal. 1965;46:647–665. doi:10.2307/1377935 [Google Scholar]
- Grine F.E, Ungar P.S, Teaford M.F. Was the early Pliocene hominin ‘Australopithecus’ anamensis a hard object feeder? S. Afr. J. Sci. 2006a;102:301–310. [Google Scholar]
- Grine F.E, Ungar P.S, Teaford M.F, El-Zaatari S. Molar microwear in Praeanthropus afarensis: evidence for dietary stasis through time and under diverse paleoecological conditions. J. Hum. Evol. 2006b;51:297–319. doi: 10.1016/j.jhevol.2006.04.004. doi:10.1016/j.jhevol.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Hatley T, Kappelman J. Bears, pigs, and Plio–Pleistocene hominids: a case for exploitation of belowground food resources. Hum. Ecol. 1980;8:371–387. doi:10.1007/BF01561000 [Google Scholar]
- Hawkes K, O'Connell J.F, Blurton Jones N.G, Alvarez H, Charnov E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. doi:10.1073/pnas.95.3.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshilwood C.S. Identifying the collector: evidence for human processing of the Cape Dune mole-rat, Batheyrgus suillus, from Blombos Cave, southern Cape. J. Archaeol. Sci. 1997;24:659–662. doi:10.1006/jasc.1996.0148 [Google Scholar]
- Hopley P.J, Latham A.G, Marshall J.D. Palaeoenvironments and palaeodiets of mid-Pliocene micromammals from Makapansgat Limeworks, South Africa: a stable isotope and dental microwear approach. Palaeogeo. Palaeoclim. Palaeoeco. 2006;233:235–251. doi:10.1016/j.palaeo.2005.09.011 [Google Scholar]
- Idermühle A, et al. Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica. Nature. 1999;398:121–126. doi:10.1038/18158 [Google Scholar]
- Jarvis J.U.M, Bennett N.C. The evolutionary history, population biology, and social structure of African mole-rats: family Bathyergidae. In: Nevo E, Reig O.A, editors. Evolution of subterranean mammals at the organismal and molecular levels. Wiley Liss; New York, NY: 1990. pp. 97–128. [PubMed] [Google Scholar]
- Jarvis J.U.M, Bennett N.C, Spinks A.C. Food availability and foraging by wild colonies of damaraland mole-rats (Cryptomys damarensis): implications for sociality. Oecologia. 1998;113:290–298. doi: 10.1007/s004420050380. doi:10.1007/s004420050380 [DOI] [PubMed] [Google Scholar]
- Keeley J.E, Rundel P.W. Evolution of CAM and C4 carbon-concentrating mechanisms. Int. J. Plant Sci. 2003;164(Suppl. 3):S55–S57. doi:10.1086/374192 [Google Scholar]
- Koch P.L. Isotopic reconstruction of past continental environments. Annu. Rev. Earth Planet. Sci. 1998;26:573–613. doi:10.1146/annurev.earth.26.1.573 [Google Scholar]
- Koch P.L, Fogel M.L, Tuross M. Tracing the diets of fossil animals using stable isotopes. In: Lajtha K, Michener B, editors. Stable isotopes in ecology and environmental science. Blackwell Press; Oxford, UK: 1994. pp. 63–92. [Google Scholar]
- Kotze S.H, van der Merwe E.L, O'Riain M.J. The topography and gross anatomy of the gastrointestinal tract of the Cape dune mole-rat (Bathyergus suillus) Anat. Histol. Embryol. 2006;35:259–264. doi: 10.1111/j.1439-0264.2005.00676.x. doi:10.1111/j.1439-0264.2005.00676.x [DOI] [PubMed] [Google Scholar]
- Laden G, Wrangham R.W. The rise of the hominids as an adaptive shift in fallback foods: underground storage organs (USOs) and australopith origins. J. Hum. Evol. 2005;49:482–498. doi: 10.1016/j.jhevol.2005.05.007. doi:10.1016/j.jhevol.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Lambert J.E. Primate digestion: interactions among anatomy, physiology, and feeding ecology. Evol. Anthropol. 1998;7:8–20. doi:10.1002/(SICI)1520-6505(1998)7:1<8::AID-EVAN3>3.0.CO;2-C [Google Scholar]
- Lee-Thorp J.A, Sponheimer M. Three case studies used to reassess the reliability of fossil bone and enamel isotope signals for paleodietary studies. J. Anthropol. Archaeol. 2003;22:208–216. doi:10.1016/S0278-4165(03)00035-7 [Google Scholar]
- Lee-Thorp J.A, van der Merwe N.J. Carbon isotope analysis of fossil bone apatite. S. Afr. J. Sci. 1987;83:712–715. [Google Scholar]
- Leuenberger M, Siegenthaler U, Langway C.C. Carbon isotope composition of atmospheric CO2 during the last ice age from an Antarctic ice core. Nature. 1992;357:488–490. doi:10.1038/357488a0 [Google Scholar]
- Levin N.E, Cerling T.E, Passey B.H, Harris J.M, Ehleringer J.R. A stable isotope aridity index for terrestrial environments. Proc. Natl Acad. Sci. USA. 2006;103:11 201–11 205. doi: 10.1073/pnas.0604719103. doi:10.1073/pnas.0604719103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove B.G, Jarvis J.U.M. Coevolution between mole-rats (Bathyergidae) and a geophyte, Micranthus (Iridaceae) Cimbebasia. 1986;8:79–85. [Google Scholar]
- Lucas P.W, Peters C.R. Function of postcanine tooth shape in mammals. In: Teaford M.F, Smith M.M, Ferguson M.W.J, editors. Development, function and evolution of teeth. Cambridge University Press; Cambridge, UK: 2000. pp. 282–289. [Google Scholar]
- Macho G.A, Shimizu D, Jiang Y, Spears I.R. Australopithecus anamensis: a finite-element approach to studying the function adaptations of extinct hominins. Anat. Rec. A. 2005;283:310–318. doi: 10.1002/ar.a.20175. doi:10.1002/ar.a.20175 [DOI] [PubMed] [Google Scholar]
- Manning J, Goldblatt P, Snijman D. Timber Press; Portland, OR: 2002. The color encyclopedia of cape bulbs. [Google Scholar]
- O'Connel J.F, Hawkes K, Blurton Jones N.G. Grandmothering and the evolution of Homo erectus. J. Hum. Evol. 1999;36:461–485. doi: 10.1006/jhev.1998.0285. doi:10.1006/jhev.1998.0285 [DOI] [PubMed] [Google Scholar]
- Orthen B. A survey of the polysaccharide reserves in geophytes native to the winter-rainfall region of South Africa. S. Afr. J. Bot. 2001;67:371–375. [Google Scholar]
- Pate J.S, Dixon K.W. University of Western Australia Press; Nedlands, Australia: 1982. Tuberous, cormous and bulbous plants: biology of an adaptive strategy in Western Australia. [Google Scholar]
- Peters C.R, Maguire B. Wild plant foods of the Makapansgat area: a modern ecosystems analogue for Australopithecus africanus adaptations. J. Hum. Evol. 1981;10:565–583. doi:10.1016/S0047-2484(81)80048-6 [Google Scholar]
- Peters C.R, Vogel J.C. Africa's wild C4 plant foods and possible early hominid diets. J. Hum. Evol. 2005;48:219–236. doi: 10.1016/j.jhevol.2004.11.003. doi:10.1016/j.jhevol.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Proches S, Cowling R.M, Goldblatt P, Manning J.C, Snijman D.A. An overview of Cape geophytes. Biol. J. Linn. Soc. 2006;87:27–43. doi:10.1111/j.1095-8312.2006.00557.x [Google Scholar]
- Reed K.E. Early hominid evolution and ecological change through the African Plio–Pleistocene. J. Hum. Evol. 1997;32:289–322. doi: 10.1006/jhev.1996.0106. doi:10.1006/jhev.1996.0106 [DOI] [PubMed] [Google Scholar]
- Reichman O.J, Jarvis J.U.M. The influence of three sympatric species of fossorial mole-rats (Bathyergidae) on vegetation. J. Mammal. 1989;70:763–771. doi:10.2307/1381710 [Google Scholar]
- Robinson J.T. Prehominid dentition and hominid evolution. Evolution. 1954;8:324–334. doi:10.2307/2405779 [Google Scholar]
- Ryan A.S, Johanson D.C. Anterior dental microwear in Australopithecus afarensis: comparisons with human and nonhuman primates. J. Hum. Evol. 1989;18:235–268. doi:10.1016/0047-2484(89)90051-1 [Google Scholar]
- Sage R.F, Meirong L, Monson R.K. The taxonomic distribution of C4 photosynthesis. In: Sage R.F, Monson R.K, editors. C4 plant biology. Academic Press; San Diego, CA: 1999. pp. 561–584. [Google Scholar]
- Schoeninger M.J, Moore J, Sept J.M. Subsistence strategies of two “savanna” chimpanzee populations: the stable isotopic evidence. Am. J. Primatol. 1999;49:297–314. doi: 10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N. doi:10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Schoeninger M.J, Bunn H.T, Murray S.S, Marlett J.A. Composition of tubers used by Hadza foragers of Tanzania. J. Food Comp. Anal. 2001;14:15–25. doi:10.1006/jfca.2000.0961 [Google Scholar]
- Scott R.S, Ungar P.S, Bergstrom T.S, Brown C.A, Grine F.E, Teaford M.F, Walker A. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436:693–695. doi: 10.1038/nature03822. doi:10.1038/nature03822 [DOI] [PubMed] [Google Scholar]
- Sealy J.C, van der Merwe N.J. Isotope assessment and the seasonal-mobility hypothesis in the southwestern Cape of South Africa. Curr. Anthropol. 1986;27:135–150. doi:10.1086/203404 [Google Scholar]
- Sillen A, Hall G, Armstrong R. Strontium calcium ratios (Sr/Ca) and strontium isotopic ratios (87Sr/86Sr) of Australopithecus robustus and Homo sp. from Swartkrans. J. Hum. Evol. 1995;28:277–285. doi:10.1006/jhev.1995.1020 [Google Scholar]
- Smith J.A.C, Winter K. Taxonomic distribution of Crassulacean acid metabolism. In: Winter K, Smith J.A.C, editors. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Springer; Berlin, Germany: 1996. pp. 427–436. [Google Scholar]
- Snijman D, Perry P. A florisitic analysis of the Nieuwoudtville Wild Flower Reserve, north-western Cape. S. Afr. J. Bot. 1987;53:445–454. [Google Scholar]
- Spinks A.C, Branch T.A, Croeser S, Bennett N.C, Jarvis J.U.M. Foraging in wild and captive colonies of the common mole-rat Cryptomys hottentotus hottentotus (Rodentia: Bathyergidae) J. Zool. 1999;249:143–152. doi:10.1111/j.1469-7998.1999.tb00752.x [Google Scholar]
- Sponheimer M, Lee-Thorp J.A. Differential resource utilization by extant great apes and australopithecines: towards solving the C4 conundrum. Comp. Biochem. Physiol. 2003;136:27–34. doi: 10.1016/s1095-6433(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Sponheimer M, Lee-Thorp J.A. Enamel diagenesis at South African australopith sites: Implications for paleoecological reconstruction with trace elements. Geochim. Cosmochim. Acta. 2006;70:1644–1654. doi:10.1016/j.gca.2005.12.022 [Google Scholar]
- Sponheimer M, Lee-Thorp J.A, de Ruiter D, Codron D, Codron J, Baugh A.T, Thackeray F. Hominins, sedges, and termites: new carbon isotope data from Sterkfontein valley and Kruger National Park. J. Hum. Evol. 2005a;48:301–312. doi: 10.1016/j.jhevol.2004.11.008. doi:10.1016/j.jhevol.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Sponheimer M, de Ruiter D, Lee-Thorp J.A, Spath A. Sr/Ca and early hominin diets revisited: new data from modern and fossil tooth enamel. J. Hum. Evol. 2005b;48:147–156. doi: 10.1016/j.jhevol.2004.09.003. doi:10.1016/j.jhevol.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Sponheimer M, Loudon J.E, Codron D, Howells M.E, Pruetz J.D, Codron J, de Ruiter D.J, Lee-Thorp J.A. Do “savanna” chimpanzees consume C4 resources? J. Hum. Evol. 2006a;51:128–133. doi: 10.1016/j.jhevol.2006.02.002. doi:10.1016/j.jhevol.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Sponheimer M, Passey B.H, de Ruiter D.J, Guatelli-Steinberg D, Cerling T.E, Lee-Thorp J.A. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Nature. 2006b;314:980–982. doi: 10.1126/science.1133827. [DOI] [PubMed] [Google Scholar]
- Stahl A.B. Hominid dietary selection before fire. Curr. Anthropol. 1984;25:151–168. doi:10.1086/203106 [Google Scholar]
- Teaford M.F, Ungar P.S. Diet and the evolution of the earliest human ancestors. Proc. Natl Acad. Sci. USA. 2000;97:13 506–13 511. doi: 10.1073/pnas.260368897. doi:10.1073/pnas.260368897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teaford M.F, Ungar P.S, Grine F.E. Paleontological evidence for the diets of African Plio–Pliestocene hominins with special reference to early Homo. In: Ungar P.S, Teaford M.F, editors. Human diet: its origin and evolution. Bergin & Garvey; Westport, CT: 2002. pp. 143–166. [Google Scholar]
- Ungar P.S. Dental topography and diets of Australopithecus afarensis and early Homo. J. Hum. Evol. 2004;46:605–622. doi: 10.1016/j.jhevol.2004.03.004. doi:10.1016/j.jhevol.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Vincent A.S. Plant foods in savanna environments: a preliminary-report of tubers eaten by the Hadza of northern Tanzania. World Archaeol. 1985;17:131–148. doi: 10.1080/00438243.1985.9979958. [DOI] [PubMed] [Google Scholar]
- Vogel J.C, Fuls A, Ellis R.P. The distribution of Kranz grasses in South Africa. S. Afr. J. Sci. 1978;74:209–215. [Google Scholar]
- Vrba E. The fossil record of African antelopes (Mammalia, Bovidae) in relation to human evolution and paleoclimate. In: Vrba E, Denton G, Burckle L, Partridge T, editors. Paleoclimate and evolution with emphasis on human origins. Yale University Press; New Haven, CT: 1985. pp. 385–424. [Google Scholar]
- Wrangham R.W. The delta hypothesis. In: Lieberman D.E, Smith R.J, Kelley J, editors. Interpreting the past: essays on human, primate, and mammal evolution. Brill Academic; Leiden, Holland: 2005. pp. 231–243. [Google Scholar]
- Wrangham R.W, Jones J.H, Laden G, Pilbeam D, Conklin-Brittain N.L. The raw and the stolen: cooking and the ecology of human origins. Curr. Anthropol. 1999;40:567–594. doi:10.1086/300083 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The δ<13C and δ<18O values of bone and enamel apatite of Cryptomys spp. recovered from Kromdraai B and Swartkrans Member 1 and the δ<13C and δ<18O values of bone apatite for five species of modern mole-rat. The Suess effect is a correction for the global decrease in the <13C content of atmospheric carbon dioxide due to fossil fuel burning over the past 150 years. We applied a −1.2‰ correction to all data from Plio–Pleistocene specimens (see methods for details).
Orthogonal regressions between modern mole-rat spp. mean δ<18O values versus δ<18O values modeled for meteoric precipitation (Bowen 2006) (r=−0.47).
Orthogonal regressions between modern mole-rat spp. mean δ<18O values versus regional mean annual precipitation (mm) (r=−0.43)