Abstract
Defects in expression of genes of oxidative phosphorylation in mitochondria have been suggested to be a key pathophysiological feature in familial insulin resistance. We examined whether such defects can arise from lifestyle-related factors alone. Fourteen obesity-discordant (BMI difference 5.2 ± 1.8 kg/m2) and 10 concordant (1.0 ± 0.7 kg/m2) monozygotic (MZ) twin pairs aged 24–27 yr were identified among 658 MZ pairs in the population-based FinnTwin16 study. Whole body insulin sensitivity was measured using the euglycemic hyperinsulinemic clamp technique. Transcript profiles of mitochondrial genes were compared using microarray data of fat biopsies from discordant twins. Body composition of twins was determined using DEXA and maximal oxygen uptake (V̇o2max) and working capacity (Wmax) using a bicycle ergometer exercise test with gas exchange analysis. The obese cotwins had lower insulin sensitivity than their nonobese counterparts (M value 6.1 ± 2.0 vs. 9.2 ± 3.2 mg·kg LBM−1·min−1, P < 0.01). Transcript levels of genes involved in the oxidative phosphorylation pathway (GO:0006119) in adipose tissue were lower (P < 0.05) in the obese compared with the nonobese cotwins. The obese cotwins were also less fit, as measured by V̇o2max (50.6 ± 6.5 vs. 54.2 ± 6.4 ml·kg LBM−1·min−1, for obese vs. nonobese, P < 0.05), Wmax (3.9 ± 0.5 vs. 4.4 ± 0.7 W/kg LBM, P < 0.01) and also less active, by the Baecke leisure time physical activity index (2.8 ± 0.5 vs. 3.3 ± 0.6, P < 0.01). This implies that acquired poor physical fitness is associated with defective expression of the oxidative pathway components in adipose tissue mitochondria.
Keywords: body composition, cardiorespiratory fitness, spiroergometry, gene expression
obesity is associated with poor physical fitness (15) and insulin resistance (31, 32). Recent studies have suggested that genetic factors account for a substantial fraction (estimates of 50–90%) of the population variance in BMI (39, 65), insulin action (23–52%) (16, 27, 35, 46, 60) and physical activity (32–79%) (24, 41, 70). These results raise the possibility that shared genetic influences underlie the association between obesity, insulin resistance, and fitness. Recently, it has been suggested that mitochondrial dysfunction might be a key factor in development of insulin resistance (37). Several studies have reported type 2 diabetic patients and “prediabetic” insulin-resistant subjects to have impaired structure and function of mitochondria (28, 48, 53, 54, 66) and decreased expression of genes encoding key enzymes in oxidative metabolism and mitochondrial function (47, 52). Petersen et al. (54) reported reduced mitochondrial phosphorylation in insulin-resistant offspring of patients with type 2 diabetes compared with insulin-sensitive control subjects. Decreased oxidative capacity of mitochondria in insulin-resistant subjects was therefore suggested to be due to an inherited genetic defect (54).
Studies of monozygotic (MZ) twins have greatly contributed to our understanding of environmental and lifestyle factors impacting human phenotypes (43). Among genetically identical individuals, intrapair differences are caused ultimately by environmental factors and can thus be used to study relationships while controlling for genetic influences. In the present study, we studied genetically identical twins who were either concordant or discordant for obesity to determine whether lifestyle-related factors (obesity and/or physical inactivity) decrease V̇o2max and influence the transcript levels of genes involved in mitochondrial oxidative phosphorylation.
MATERIALS AND METHODS
Subjects.
The study participants were recruited from the FinnTwin16 cohort (26, 62), a population-based, longitudinal study of five consecutive birth cohorts (1975–1979) of twins and their siblings and parents.
Twin pairs to the current study were recruited on the basis of their responses to questions on weight and height at the age of 23–27 yr. After all MZ twin pairs (n = 658) had been screened, we identified 18 pairs with a reported BMI difference of at least 4 kg/m2, such that one cotwin was nonobese (mean BMI 25 kg/m2), whereas the other was obese (mean BMI 30 kg/m2) (17, 25, 58, 59). Fourteen of these pairs (8 male and 6 female pairs) participated. In addition to these discordant pairs, we studied 10 concordant MZ pairs (5 male and 5 female pairs) with a BMI difference of less than 2 kg/m2.
The subjects were healthy and normotensive and did not use any medications except for oral contraceptives. Their weight had been stable for at least 3 mo prior to the study. Two pairs had a family history of type 2 diabetes. Monozygosity was confirmed by genotyping of 10 informative genetic markers (59). The subjects provided written informed consent. The protocol was designed and performed according to the principles of the Helsinki Declaration and was approved by the Ethics Committee of the Helsinki University Central Hospital.
Study design.
In each subject, whole body insulin sensitivity, body composition, and cardiorespiratory fitness were measured. The measurement of whole body insulin sensitivity was performed using the euglycemic insulin clamp technique after an overnight fast. Prior to the clamp, a needle biopsy of abdominal subcutaneous adipose tissue was taken from 13 discordant pairs, as previously described (57). The sample used for microarray analysis was immediately frozen in liquid nitrogen and stored at −80°C until isolation of RNA.
Whole body insulin sensitivity.
Whole body insulin sensitivity was measured using the euglycemic hyperinsulinemic clamp technique as previously described (10, 58). The rate of the continuous insulin infusion was 1 mU·kg−1·min−1.
Gene expression in adipose tissue.
Total RNA was prepared from frozen fat tissue (on average 250 mg) using the RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer's protocol. Quality of RNA was analyzed using the 2100 Bioanalyzer platform (Agilent Technologies). Two micrograms of total RNA was treated according to the conventional Affymetrix eukaryotic RNA labeling protocols (Affymetrix, Santa Clara, CA). Fifteen micrograms of biotin-labeled cRNA was fragmented according Affymetrix eukaryotic sample protocol. Hybridization, staining, and washing of the Affymetrix U133 Plus 2.0 chips were performed using the Affymetrix Fluidics Station 450 and Hybridization Oven 640 under standard conditions. Preprocessing of expression data was done using the GC-RMA algorithm. Prior to analysis, the expression values were cotwin normalized, which involved dividing the obese twin's expression values with those of the thin cotwin to correct for the identical genetic background.
The pathway analysis was done with an in-house nonparametric analysis algorithm, where the objective is to find the optimal regulated pathway compositions without prior criteria for significance for individual genes' significant regulation or arbitrary P value/fold change cutoffs, as described previously (57). Briefly, the probe sets were ranked by the median fold change values between obese and lean twins to “upregulated” and “downregulated” vectors. The Affymetrix ProbeSetIDs were mapped to human genes using cross-references in the Ensembl database, and the genes were queried for their GO annotations. The topology of the GO tree (DAG tree) was fully utilized by enumerating all available routes toward the root of the GO tree and adding all encountered vertexes as GO annotations of the given gene. For detecting the affected GO gene groups (“pathways”), an iterative hypergeometric distribution P value-based calculation was used, where the objective is to find the optimum P value for a set of genes that belong to the same annotated GO gene collection (maximal regulation for the pathway). Furthermore, the data were exhaustively permuted by randomizing the gene ranks for each GO category, and an empirical P value was interpreted from the distribution of 10,000 permutation cycles. The oxidative phosphorylation pathway (GO:0006119) was queried as part of these pathway analyses (57) of the genome-wide microarray expression profiles. The global pathway analyses evaluate the significance of all individual GO categories while taking into account the structure of the tree.
Gene transcription was compared using previously reported real-time PCR data (25, 56) and current Affymetrix data. The relationships were as follows: 11β-hydroxysteroid dehydrogenase-1 (11β-HSD-1; r = 0.68, P = 0.005), glucocorticoid receptor-α (r = 0.45, P = 0.11), adiponectin [r = 0.06, not significant (NS)], lipoprotein lipase (LPL; r = 0.63, P = 0.012), peroxisome proliferator-activated receptor-γ (PPARγ; r = 0.77, P = 0.0001), leptin (r = 0.52, P = 0.01), CD68 (r = 0.09, NS), IL-6 (r = −0.26, NS), and TNF-α (r = −0.05, NS).
Body composition measurements.
Body composition, including fat mass, lean body mass (LBM), and percent whole body fat was measured by dual-energy X-ray absorptiometry (DEXA; Lunar Prodigy, Madison, WI; software version 2.15) (44).
Cardiorespiratory exercise test.
Work-conducted maximal exercise test with gas exchange analysis (spiroergometry) was performed using an electrically braked bicycle ergometer (900 ERG Ergometer; Marquette Hellige, Marquette Medical Systems) and by using a breath-by-breath gas exchange analysis system (Vmax 229, Sensormedics) with the test subject sitting upright. The initial workload was 40 W for women and 50 W for men. It was then increased by 40 and 50 W, respectively, at 3-min intervals until exhaustion [19–20/20 on the Borg scale for perceived exertion or respiratory quotient (RQ = V̇co2/V̇o2) of at least 1.1].
Blood pressure was measured manually using a stethoscope and a sphygmomanometer (Erka) from the left arm before, during, and 6–10 min after exercise. A 12-lead ECG (Mason-Likar) was continuously monitored and recorded during the exercise test using a computerized device (Case12, Marquette). For measurement of respiratory gases, a tightly attached face mask (Rudolph series 7910, Hans Rudolph) with a dead space of 185 ml was used. Arterial O2 saturation (Sao2) was assessed noninvasively with two pulse oxymeters (Datex-Ohmeda 3900 and Datex-Ohmeda 3800; Datex-Ohmeda). One sensor was attached to the earlobe and one to the left middle finger.
A mass flow sensor of the gas exchange device (Sensormedics) was used to measure forced expiratory volume in one second before exercise, and tidal volume and minute ventilation during exercise. From the breath-by-breath recordings ventilatory and gas exchange variables were averaged at 30-s intervals using microcomputer-assisted equipment. The variables used for analysis are explained below.
Maximal oxygen uptake (V̇o2max) was defined as the peak oxygen uptake. Maximal working capacity (Wmax) was defined as the mean workload during the last 3 min of exercise. Mechanical efficiency (73) and anaerobic threshold (AT) assessed from the slope change of V̇co2/V̇o2 were also calculated.
Physical activity.
Physical activity was assessed using the Baecke questionnaire (2). Work index refers to physical activity at work, sport index to sport during leisure time, and leisure time index to physical activity during leisure time excluding sports.
Statistical analyses.
Student's paired t-test was used to compare the means between the leaner and heavier cotwins for normally distributed variables and Wilcoxon's signed rank test for the intrapair comparison of nonnormally distributed data. Statistical analyses were performed using Stata 8.2 for Windows software (StataCorp, College Station, TX). Calculation of correlation coefficients between acquired intrapair differences in body composition and intrapair differences in fitness or metabolic measurement controls for genetic and shared environmental influences within MZ pairs. Pearson correlation and linear regression was used in the intrapair and individual univariate analyses. Multiple linear regression models were used to control for confounding factors in some analyses. When using individual twins, the P values were corrected for clustered sampling of cotwins within pairs by survey methods (61). To combine male and female pairs, we used sex-specific z-transformed values (i.e., mean of 0 and SD of 1) of the study variables in all analyses.
RESULTS
Body composition.
A pairwise comparison of the critical traits for obesity-discordant and -concordant pairs is shown in Table 1. In the discordant pairs, the heavier cotwin had, on average, a 5.4 kg/m2 higher BMI, a 9.4% higher fat percentage, 12.9 kg more fat, and 2.4 kg more LBM than the leaner cotwin. In the concordant pairs, the respective differences were 1.0 kg/m2, 2.2%, 2.1 kg fat, and 0.3 kg LBM.
Table 1.
Characteristics of MZ cotwins discordant and concordant for obesity
|
Discordant Pairs |
Concordant Pairs
|
|||
|---|---|---|---|---|
| Heavier | Leaner | Heavier | Leaner | |
| No. of subjects | 14 | 14 | 10 | 10 |
| Age, yr | 25.7±1.5 | 25.6±1.5 | 25.6±1.2 | 25.7±1.2 |
| Birth weight, g | 2588±481 | 2464±526 | 2537±702 | 2581±595 |
| Height, cm | 171±9 | 171±9 | 173±8 | 173±7 |
| Body weight, kg | 89.8±9.4 | 74.5±8.8‡ | 81.5±22.1 | 78.9±20.7* |
| BMI, kg/m2 | 30.6±2.0 | 25.4±1.8‡ | 27.3±7.4 | 26.3±7.2† |
| %Body fat | 38.3±6.5 | 29.5±8.5‡ | 27.0±11.7 | 24.8±11.8* |
| Fat mass, kg | 34.0±5.1 | 21.4±5.7‡ | 21.0±10.6 | 18.9±10.3* |
| LBM, kg | 52.8±9.8 | 50.4±10.2* | 53.6±10.9 | 53.8±10.3 |
| M value/LBM, mg·kg LBM–1·min–1 | 6.1±2.1 | 9.1±2.9† | 7.5±3.2 | 7.4±2.8 |
| Blood hemoglobin, g/l | 144±11 | 140±11 | 144±12 | 145±10 |
Data shown as mean ± SD. MZ, monozygotic; LBM, lean body mass.
P < 0.05,
P < 0.01,
P < 0.001 (Student's paired t-test).
Insulin sensitivity.
The obese cotwins had significantly lower whole body insulin sensitivity (M value) than their nonobese counterparts (Table 1). Intrapair differences in whole body insulin sensitivity were associated with those in V̇o2max (β = 0.55, P < 0.05) in a model adjusting for BMI. In the obesity-concordant pairs, there were no differences between the cotwins in insulin sensitivity.
Fitness.
Cardiorespiratory exercise tests did not show any pathological circulatory or respiratory changes in any measured and monitored variable. Sao2 remained normal (>96%) in all subjects throughout the test. The cotwins of the concordant pairs did not differ from each other with respect to any of the cardiorespiratory variables (Table 2). In the discordant pairs, the obese cotwin had a significantly lower V̇o2max when expressed per kilogram body weight (V̇o2max/kg, P < 0.001) or kilogram LBM (V̇o2max/LBM, P < 0.05) than the nonobese cotwin. Maximal working capacity, expressed per kilogram LBM (Wmax/LBM), was significantly lower in the obese than in the nonobese cotwin (P < 0.01). The obese cotwin also had lower mechanical efficiency than the nonobese one (P < 0.05). Regarding physical activity, the obese cotwin had a lower leisure time index than the nonobese one (2.8 vs. 3.3, obese vs. lean, P < 0.01). Work or sport indexes did not differ between the cotwins (2.4 vs. 2.4 and 3.1 vs. 3.0, respectively). Correlations between V̇o2max/LBM and the physical and biochemical characteristics are shown in Table 3.
Table 2.
Cardiorespiratory exercise test: characteristics of MZ twin pairs discordant and concordant for obesity
|
Discordant Pairs |
Concordant Pairs
|
|||
|---|---|---|---|---|
| Heavier | Leaner | Heavier | Leaner | |
| No. of subjects | 14 | 14 | 10 | 10 |
| Wmax, W | 197±41 | 215±63 | 226±65 | 234±56 |
| Wmax/LBM, W/kg LBM | 3.9±0.5 | 4.4±0.6† | 4.2±0.8 | 4.4±0.7 |
| V̇o2max, l/min | 2.60±0.59 | 2.67±0.70 | 2.81±0.81 | 2.88±0.62 |
| V̇o2max/BW, ml·kg BW–1·min–1 | 29.2±4.7 | 36.0±7.4‡ | 35.9±12.5 | 38.2±10.8 |
| V̇o2max/LBM, ml·kg LBM–1·min–1 | 50.8±6.2 | 54.2±6.1* | 52.1±9.0 | 53.8±6.9 |
| Mechanical efficiency, % | 22.0±1.4 | 23.1±1.3* | 23.2±1.1 | 23.3±1.4 |
| RQ | 1.1±0.04 | 1.1±0.1 | 1.2±0.1 | 1.1±0.03 |
| AT, l/min | 1.3±0.5 | 1.5±0.5 | 1.3±0.3 | 1.3±0.3 |
| AT/LBM, ml·kg LBM–1·min–1 | 25.3±7.2 | 30.2±6.0* | 25.0±5.1 | 25.8±6.7 |
| Resting heart rate, min−1 | 78±9 | 75±11 | 75±12 | 71±13 |
| Maximal heart rate, min−1 | 187±7 | 187±10 | 187±9 | 185±7 |
| Resting systolic BP, mmHg | 133±15 | 126±15* | 120±7 | 123±11 |
| Resting diastolic BP, mmHg | 71±12 | 68±8 | 64±4 | 65±10 |
| Maximal systolic BP, mmHg | 193±30 | 190±24 | 191±21 | 187±18 |
Data shown as mean ± SD. V̇o2max, maximal O2 uptake, Wmax, maximal working capacity; BW, total body weight; RQ, respiratory quotient (V̇co2max/V̇o2max); AT, anaerobic threshold; BP, blood pressure.
P < 0.05,
P < 0.01,
P < 0.001 (Student's paired t-test).
Table 3.
Correlations between V̇o2max/kg LBM and physical and biochemical characteristics in all MZ twins (n = 48)
| Correlation Coefficient | |
|---|---|
| Birth weight, g | −0.15 |
| Height, cm | −0.15 |
| Body weight, kg | −0.44† |
| BMI, kg/m2 | −0.42† |
| %Body fat | −0.16 |
| Fat mass, kg | −0.22 |
| M value/LBM, mg·kg LBM−1·min–1 | 0.45† |
| Resting energy expenditure, kJ/day | −0.26 |
| Mean adipose tissue gene expression of oxidative phosphorylation pathway (mean-centroid)a | 0.42* |
n = 22 (twins of discordant pairs for whom both gene expression data and V̇o2max were available).
P < 0.05,
P < 0.01 (Pearson correlation corrrected for clustered twin data).
Transcript levels.
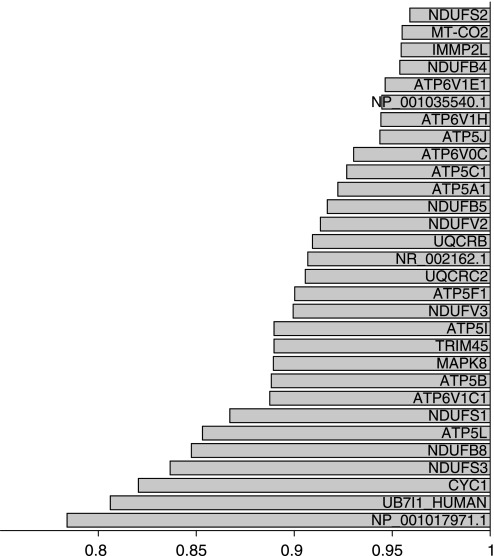
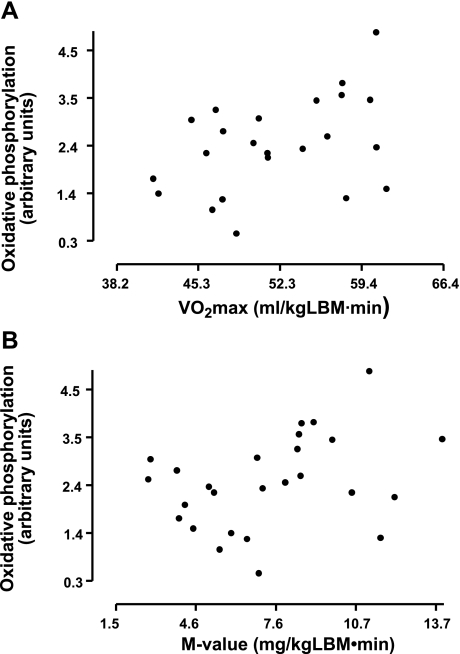
The genome-wide transcript profiling of fat biopsies showed that genes involved in the mitochondrial oxidative phosphorylation pathway were significantly downregulated in the obese compared with the nonobese cotwins (nominal P < 0.001, permuted P < 0.05). The rank of the oxidative phosphorylation pathway was 123 among 184 significantly downregulated pathways. Among 68 genes in the pathway, 31 were responsible for the optimum pathway composition (Fig. 1). The mean centroid [describing the average activity of the regulated part of the pathway, as described by Mootha et al. (47)] was calculated for the oxidative phosphorylation pathway and used to examine possible correlation with V̇o2max. The oxidative phosphorylation pathway was found to significantly correlate with V̇o2max and the M value in the twin subjects (Fig. 2). These associations also remained significant after adjusting for percent body fat in a multiple regression model (β = 0.45, P < 0.05; β = 0.29, P < 0.05, respectively).
Fig. 1.
Transcription of the genes in the oxidative phosphorylation pathway (GO:0006119) was coordinately downregulated in the obese cotwins' adipose tissue compared with transcription in the nonobese cotwins' adipose tissue in 13 monozygotic (MZ) twin pairs discordant for obesity. The nonobese cotwins' gene expression values are set as 1.0. The x-axis shows the ratio of the obese to non-bese cotwins' expression values. On the y-axis are the 30 most differentially expressed genes until optimum pathway composition was reached.
Fig. 2.
Relationships between mean oxidative phosphorylation pathway activity and maximal O2 consumption (V̇o2max; r = 0.42, P < 0.05; A) and the M value (r = 0.35, P < 0.05; B). LBM, lean body mass. Scale unit is 1 SD, and the sample mean is in the middle of the scale.
DISCUSSION
The present study shows that, independent of genetic factors, obesity is associated with poor fitness, low insulin sensitivity, and decreased transcript levels of genes involved in mitochondrial oxidative phosphorylation. This conclusion is based on a study of a group of genetically identical MZ twin pairs discordant or concordant for BMI. MZ twin pairs discordant for obesity offer a unique opportunity to study effects of acquired obesity independently of confounding genetic influences while matching for age, sex, and many socioeconomic, childhood, and familial factors. Monozygosity in the present study was confirmed by genotyping of 10 informative genetic markers (59). This study design controls in an ideal way for the genetic effect on obesity. Obese cotwins were shown to have lower whole body insulin sensitivity, V̇o2max, and Wmax relative to their LBM than their nonobese counterparts. These physiological changes were accompanied by the significantly lower transcript levels of genes involved in the mitochondrial oxidative phosphorylation pathway in adipose tissue.
V̇o2max was reduced in the obese compared with the nonobese twin when expressed per kilogram LBM and per kilogram body weight but not when expressed as milliliters per minute. The finding that V̇o2max per minute is not negatively influenced by obesity is consistent with several studies (9, 18, 36, 40, 42, 63), as is the finding that V̇o2max per kilogram total body weight is lower in obese than in nonobese subjects (9, 12, 18, 22, 36, 42, 63, 64). Since muscle tissue is the main site of V̇o2 during maximal exertion, V̇o2max divided by LBM seems the most unbiased way of comparing cardiorespiratory fitness of individuals of different sizes (11, 72). Previous studies expressing V̇o2max per kilogram LBM have shown either decreased (9, 22, 23, 67, 69) or unchanged (12, 18, 34, 40) V̇o2max in obese compared with nonobese subjects. Possibly, genetic differences as well as those in age, sex, family environment, and physical activity could, together with small sample sizes, have confounded comparison of obese and nonobese subjects in the latter studies (12, 18, 34, 40).
Several studies have reported impaired mitochondrial activity (28, 48, 53–55, 66) or decreased transcript levels of genes involved in mitochondrial oxidative metabolism (47, 52) in skeletal muscle of insulin-resistant subjects. Kelley et al. (28) reported smaller mitochondria and reduced activity of the electron transport chain in obese subjects and type 2 diabetic patients compared with normal-weight, insulin-sensitive control subjects. Patti et al. (52) and Mootha et al. (47) both found reduced expression of genes encoding proteins involved in mitochondrial oxidative metabolism in skeletal muscle from insulin-resistant subjects. Reduced rates of mitochondrial phosphorylation (54), impaired insulin-stimulated ATP synthesis (55), reduced mitochondrial density (48), and prolonged phosphocreatine recovery half-time (66) have also been found to characterize skeletal muscle of type 2 diabetic patients. These results supported the concept that mitochondrial dysfunction and subsequent impairments in the oxidative metabolism characterize insulin-resistant subjects, possibly representing critical etiological factors (37). Very recently, however, Boushel et al. (7) suggested that mitochondrial function is normal in type 2 diabetes. They showed that, although overall muscle oxidative capacity was lower in the type 2 diabetic patients, their mitochondrial function was unimpaired. They thus concluded that the impairment of oxidative phosphorylation and electron transport can be attributed to diminished mitochondrial content in the diabetic muscle.
When pursuing the explanation for the discrepant findings in the above studies, physical activity/fitness of the study groups needs to be considered. In the study by Mootha et al. (47), subjects with impaired glucose tolerance as well as those with type 2 diabetes had significantly lower V̇o2max than those with normal glucose tolerance. This could indicate a lower level of physical activity in the insulin-resistant study groups compared with the control group, although this was not specifically assessed. Schrauwen-Hinderling et al., on the other hand, found no significant difference in V̇o2max between obese subjects with type 2 diabetes and similarly obese subjects with normal glucose tolerance (66). Since there was a trend toward better V̇o2max in the non-insulin-resistant subjects, the nonsignificant result might simply reflect the small study sample (12 diabetic subjects and 9 nondiabetic control subjects). In only two of the above studies (54, 55) were the study groups matched for physical activity. The method of physical activity assessment was not, however, explained in the studies by Petersen et al. (54, 55). Additionally, two studies investigated only subjects defined as sedentary (48, 52) but presented no further data on their physical activity. In conclusion, none of the studies reporting mitochondrial dysfunction in insulin-resistant subjects properly measured physical activity and/or fitness, although exercise training is known to increase mitochondrial content and oxidative capacity of skeletal muscle fibers (1, 19–21) as well as to enhance insulin sensitivity (6, 8, 49, 50, 68) and prevent progression of impaired glucose tolerance to type 2 diabetes (13, 30, 33, 51).
In the present study, we found significant reduction of transcript levels of genes encoding components of mitochondrial oxidative phosphorylation in adipose tissue in obese monozygotic cotwins. We recognize the limitation of the use of adipose tissue instead of skeletal muscle tissue. However, our results of fat biopsies are in line with previous studies reporting reduced oxidative metabolism and downregulation of genes involved in oxidative phosphorylation in obese and insulin-resistant subjects' muscle (28, 47, 48, 52–55, 66). The finding that this is also true in obese and nonobese subjects with identical genes is novel. It shows that lifestyle-related factors, mainly physical inactivity and acquired obesity, are enough to significantly impair expression of genes encoding proteins involved in mitochondrial oxidative phosphorylation. This is in agreement with the findings of Boushel et al. (7) as well as intervention studies reporting improvements of mitochondrial function in response to physical activity (45, 71).
Although we found that the reduced transcript levels of genes encoding mitochondrial oxidative phosphorylation in obesity is influenced by environmental and acquired factors, it does not exclude the possibility that genetic factors contribute to regulation of mitochondrial oxidative metabolism, as has been shown for V̇o2max (4, 5, 14, 29, 38), physical activity (24, 41), and the V̇o2max response to physical training (3). The heritability estimates for V̇o2max vary between 50 and 67% in the two most recent studies (4, 38).
In conclusion, acquired obesity decreases physical fitness, whole body insulin sensitivity, and expression of mitochondrial genes involved in oxidative phosphorylation independently of genetic influences. These data suggest that physical inactivity may have contributed to the defects in mitochondrial oxidative phosphorylation described in type 2 diabetic patients and prediabetic subjects. The latter possibility, however, needs to be tested in an intervention study.
GRANTS
Data collection and analyses have been supported by the National Institute on Alcohol Abuse and Alcoholism (Grants AA-08315, AA-00145, and AA-12502), the European Union Fifth Framework Program (QLRT-1999-00916, QLG2-CT-2002-01254), the Academy of Finland (Grants 44069, 100499, and 201461), and Helsinki University Central Hospital grants. L. Mustelin and K. Pietiläinen were supported by the Juho Vainio Foundation and K. Pietiläinen by the Yrjö Jahnsson Foundation. L. Peltonen and J. Kaprio were supported by the Center of Excellence in Common Disease Genetics of the Academy of Finland.
Acknowledgments
We thank Erjastiina Heikkinen, Katja Tuominen, and Mia Urjansson for excellent assistance, Juha Saharinen for expertise, and Anna Keski-Rahkonen for collegial help and valuable comments on the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol 88: 99–107, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36: 936–942, 1982. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 87: 1003–1008, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, Daw EW, Rice T, Perusse L, Gagnon J, Province MA, Leon AS, Rao DC, Skinner JS, Wilmore JH. Familial resemblance for V̇o2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc 30: 252–258, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C, Lesage R, Lortie G, Simoneau JA, Hamel P, Boulay MR, Perusse L, Theriault G, Leblanc C. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc 18: 639–646, 1986. [PubMed] [Google Scholar]
- 6.Boule NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care 28: 108–114, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunt JC, Salbe AD, Harper IT, Hanson RL, Tataranni PA. Weight, adiposity, and physical activity as determinants of an insulin sensitivity index in pima Indian children. Diabetes Care 26: 2524–2530, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Davies CT, Godfrey S, Light M, Sargeant AJ, Zeidifard E. Cardiopulmonary responses to exercise in obese girls and young women. J Appl Physiol 38: 373–376, 1975. [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Ekelund U, Franks PW, Wareham NJ, Aman J. Oxygen uptakes adjusted for body composition in normal-weight and obese adolescents. Obes Res 12: 513–520, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Elliot DL, Goldberg L, Kuehl KS, Hanna C. Metabolic evaluation of obese and nonobese siblings. J Pediatr 114: 957–962, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson J, Taimela S, Koivisto VA. Exercise and the metabolic syndrome. Diabetologia 40: 125–135, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Fagard R, Bielen E, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise. J Appl Physiol 70: 357–362, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Fogelholm M, Malmberg J, Suni J, Santtila M, Kyrolainen H, Mantysaari M. Waist circumference and BMI are independently associated with the variation of cardio-respiratory and neuromuscular fitness in young adult men. Int J Obes (Lond) 30: 962–969, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Freeman MS, Mansfield MW, Barrett JH, Grant PJ. Heritability of features of the insulin resistance syndrome in a community-based study of healthy families. Diabet Med 19: 994–999, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Gertow K, Pietilainen KH, Yki-Jarvinen H, Kaprio J, Rissanen A, Eriksson P, Hamsten A, Fisher RM. Expression of fatty-acid-handling proteins in human adipose tissue in relation to obesity and insulin resistance. Diabetologia 47: 1118–1125, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord 24: 841–848, 2000. [DOI] [PubMed] [Google Scholar]
- 19.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50: 817–823, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 21.Hood DA Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90: 1137–1157, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Exercise capacity in lean versus obese women. Scand J Med Sci Sports 11: 305–309, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Huttunen NP, Knip M, Paavilainen T. Physical activity and fitness in obese children. Int J Obes 10: 519–525, 1986. [PubMed] [Google Scholar]
- 24.Joosen AM, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Am J Clin Nutr 82: 1253–1259, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kannisto K, Pietilainen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Jarvinen H. Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab 89: 4414–4421, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res 5: 366–371, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, Stengard J, Kesaniemi YA. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia 35: 1060–1067, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Klissouras V, Pirnay F, Petit JM. Adaptation to maximal effort: genetics and age. J Appl Physiol 35: 288–293, 1973. [DOI] [PubMed] [Google Scholar]
- 30.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest 65: 1272–1284, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopelman PG Obesity as a medical problem. Nature 404: 635–643, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, Aunola S, Keinanen-Kiukaanniemi S, Laakso M, Valle TT, Ilanne-Parikka P, Louheranta A, Hamalainen H, Rastas M, Salminen V, Cepaitis Z, Hakumaki M, Kaikkonen H, Harkonen P, Sundvall J, Tuomilehto J, Uusitupa M. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 54: 158–165, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Lafortuna CL, Proietti M, Agosti F, Sartorio A. The energy cost of cycling in young obese women. Eur J Appl Physiol 97: 16–25, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Lehtovirta M, Kaprio J, Forsblom C, Eriksson J, Tuomilehto J, Groop L. Insulin sensitivity and insulin secretion in monozygotic and dizygotic twins. Diabetologia 43: 285–293, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Loftin M, Sothern M, Trosclair L, O'Hanlon A, Miller J, Udall J. Scaling VO(2) peak in obese and non-obese girls. Obes Res 9: 290–296, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Maes HH, Beunen GP, Vlietinck RF, Neale MC, Thomis M, Vanden EB, Lysens R, Simons J, Derom C, Derom R. Inheritance of physical fitness in 10-yr-old twins and their parents. Med Sci Sports Exerc 28: 1479–1491, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 27: 325–351, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Maffeis C, Schena F, Zaffanello M, Zoccante L, Schutz Y, Pinelli L. Maximal aerobic power during running and cycling in obese and non-obese children. Acta Paediatr 83: 113–116, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Maia JA, Thomis M, Beunen G. Genetic factors in physical activity levels: a twin study. Am J Prev Med 23: 87–91, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Marinov B, Kostianev S. Exercise performance and oxygen uptake efficiency slope in obese children performing standardized exercise. Acta Physiol Pharmacol Bulg 27: 59–64, 2003. [PubMed] [Google Scholar]
- 43.Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet 17: 387–392, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51: 1106–1112, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 288: E818–E825, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Mills GW, Avery PJ, McCarthy MI, Hattersley AT, Levy JC, Hitman GA, Sampson M, Walker M. Heritability estimates for beta cell function and features of the insulin resistance syndrome in UK families with an increased susceptibility to type 2 diabetes. Diabetologia 47: 732–738, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism 54: 1472–1479, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Østergard T, Andersen JL, Nyholm B, Lund S, Nair KS, Saltin B, Schmitz O. Impact of exercise training on insulin sensitivity, physical fitness and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 290: E998–E1005, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 20: 537–544, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pietiläinen KH, Kannisto K, Korsheninnikova E, Rissanen A, Kaprio J, Ehrenborg E, Hamsten A, Yki-Järvinen H. Acquired obesity increases CD68 and TNF-(alpha) and decreases adiponectin gene expression in adipose tissue. A study in monozygotic twins J Clin Endocrinol Metab 91: 2776–2781, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Pietilainen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, Suomalainen A, Götz A, Suortti T, Yki-Jarvinen H, Orešiè M, Kaprio J, Peltonen L. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Medicine 5: e51, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pietilainen KH, Rissanen A, Kaprio J, Makimattila S, Hakkinen AM, Westerbacka J, Sutinen J, Vehkavaara S, Yki-Jarvinen H. Acquired obesity is associated with increased liver fat, intra-abdominal fat, and insulin resistance in young adult monozygotic twins. Am J Physiol Endocrinol Metab 288: E768–E774, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Pietilainen KH, Rissanen A, Laamanen M, Lindholm AK, Markkula H, Yki-Jarvinen H, Kaprio J. Growth patterns in young adult monozygotic twin pairs discordant and concordant for obesity. Twin Res 7: 421–429, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 42: 139–145, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Rao JNK, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 12: 46–60, 1984. [Google Scholar]
- 62.Rose RJ, Dick DM, Viken And RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res 25: 637–643, 2001. [PubMed] [Google Scholar]
- 63.Rowland T, Bhargava R, Parslow D, Heptulla RA. Cardiac response to progressive cycle exercise in moderately obese adolescent females. J Adolesc Health 32: 422–427, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Rowland TW Effects of obesity on aerobic fitness in adolescent females. Am J Dis Child 145: 764–768, 1991. [PubMed] [Google Scholar]
- 65.Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, Davis CJ, Fagnani C, Hjelmborg J, Kaprio J, De Lange M, Luciano M, Martin NG, Pedersen N, Pietilainen KH, Rissanen A, Saarni S, Sorensen TI, Van Baal GC, Harris JR. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res 6: 409–421, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 50: 113–120, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Seres L, Lopez-Ayerbe J, Coll R, Rodriguez O, Manresa JM, Marrugat J, Alastrue A, Formiguera X, Valle V. [Cardiopulmonary function and exercise capacity in patients with morbid obesity]. Rev Esp Cardiol 56: 594–600, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Soman VR, Koivisto VA, Deibert D, Felig P, DeFronzo RA. Increased insulin sensitivity and insulin binding to monocytes after physical training. N Engl J Med 301: 1200–1204, 1979. [DOI] [PubMed] [Google Scholar]
- 69.Souza MS, Cardoso AL, Yasbek P Jr, Faintuch J. Aerobic endurance, energy expenditure, and serum leptin response in obese, sedentary, prepubertal children and adolescents participating in a short-term treadmill protocol. Nutrition 20: 900–904, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, Kyvik KO, Rose RJ, Kujala UM, Kaprio J, Harris JR, Pedersen NL, Hunkin J, Spector TD, de Geus EJ. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS 1: e22, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toledo FG, Watkins S, Kelley DE. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab 91: 3224–3227, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Vanderburgh PM, Katch FI. Ratio scaling of V̇o2max penalizes women with larger percent body fat, not lean body mass. Med Sci Sports Exerc 28: 1204–1208, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Wasserman K, Hansen J, Sue DY, Whipp BJ. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lea & Febiger, 1994, p. 112–131.