Abstract
The effect of pyruvate dehydrogenase kinase-4 (PDK4) deficiency on glucose homeostasis was studied in mice fed a high-fat diet. Expression of PDK4 was greatly increased in skeletal muscle and diaphragm but not liver and kidney of wild-type mice fed the high-fat diet. Wild-type and PDK4−/− mice consumed similar amounts of the diet and became equally obese. Insulin resistance developed in both groups. Nevertheless, fasting blood glucose levels were lower, glucose tolerance was slightly improved, and insulin sensitivity was slightly greater in the PDK4−/− mice compared with wild-type mice. When the mice were killed in the fed state, the actual activity of the pyruvate dehydrogenase complex (PDC) was higher in the skeletal muscle and diaphragm but not in the liver and kidney of PDK4−/− mice compared with wild-type mice. When the mice were killed after overnight fasting, the actual PDC activity was higher only in the kidney of PDK4−/− mice compared with wild-type mice. The concentrations of gluconeogenic substrates were lower in the blood of PDK4−/− mice compared with wild-type mice, consistent with reduced formation in peripheral tissues. Diaphragms isolated from PDK4−/− mice oxidized glucose faster and fatty acids slower than diaphragms from wild-type mice. Fatty acid oxidation inhibited glucose oxidation by diaphragms from wild-type but not PDK4−/− mice. NEFA, ketone bodies, and branched-chain amino acids were elevated more in PDK4−/− mice, consistent with slower rates of oxidation. These findings show that PDK4 deficiency lowers blood glucose and slightly improves glucose tolerance and insulin sensitivity in mice with diet-induced obesity.
Keywords: pyruvate dehydrogenase kinase-4-knockout mice, diet-induced obesity, glucose oxidation, fatty acid oxidation, branched-chain amino acids
the pyruvate dehydrogenase complex (PDC) is required for the oxidative disposal of dietary carbohydrate. Decarboxylation of pyruvate produces acetyl-CoA for oxidation by the citric acid cycle or conversion to fat. Since acetyl-CoA cannot be converted back to glucose, tight control of flux through PDC is required for maintenance of euglycemia during starvation. PDC activity has to be increased after meals but reduced during fasting. PDC activity is regulated by feedback inhibition by its products acetyl-CoA and NADH and by a balance between inactivation by pyruvate dehydrogenase kinases (PDKs) and activation by pyruvate dehydrogenase phosphatases (PDPs). In the well-fed state, PDC is relatively dephosphorylated and active for disposal of excess glucose. In the fasted state, PDC is highly phosphorylated and inactive so that three-carbon compounds can be converted back to glucose (50, 51). Four PDK isoenzymes (PDK1–4) and two PDP isoenzymes (PDP1 and -2) are expressed in mammalian tissues (9, 20). PDK4 is markedly induced in most tissues by starvation (23, 51) and high dietary fat (16, 17, 40), suggesting that PDK4 may be the most important means of regulation of PDC activity during starvation and in diet-induced obesity. Indeed, blood concentrations of glucose, lactate, pyruvate, and alanine are low in PDK4-knockout (PDK4−/−) mice in the starved state (23), consistent with an important role for PDK4 in maintaining glucose levels during starvation.
The hypothesis that increased expression of PDK4 contributes to the hyperglycemia in diet-induced obesity was tested in this study with wild-type and PDK4−/− mice fed a high-fat diet. Fasting blood glucose levels were lower, glucose tolerance was slightly improved, and insulin sensitivity was slightly greater in PDK4−/− mice compared with wild-type mice.
MATERIALS AND METHODS
Animals.
Experimental protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. The procedure we used to produce the PDK-knockout mice (PDK4−/−) was described in detail previously (23). The genetic background was stabilized by backcrossing heterozygous (PDK4+/−) mice with C57BL/6J wild-type mice for eight generations. Groups of 12 wild-type male mice and 12 PDK4−/− male mice were housed with two mice per cage under controlled temperature (23 ± 2°C) and lighting (12:12-h light-dark cycle, 0700–1900 and 1900–0700), with free access to water and a high-fat diet (16% protein, 59.5% fat, 24.5% carbohydrate by calories, catalog no. F3282; Bio-Serv, Frenchtown, NJ). According to the supplier, the protein component of this diet is casein, the fat component is lard, which is rich in oleate, palmitate, stearate, and linoleate, and the carbohydrate component is a mixture of sucrose and starch. To establish whether PDK4 expression was induced by the high-fat diet, as expected from previous studies (16, 17, 40), three additional wild-type male mice were fed a standard rodent chow diet (catalog no. 7071; Harlan) that was high in carbohydrate and low in fat for the duration of the experiment. Mice were weaned and started on the diets at 4 wk of age. Body weights of the mice on the high-fat diet were monitored weekly. Food consumption was monitored during the 8th wk of the feeding period. The experiment was terminated after the mice had been on the diets for 18 wk. One-half of the mice in the two groups fed the high-fat diet were killed in the fed state (between 7:00 and 8:00 AM). The remaining animals of each group were killed after being fasted overnight (from 5:00 PM to 9:00 AM). The single group of three chow-diet fed wild-type mice were killed in the fed state (between 7:00 and 7:30 AM). Prior to their being killed, tail blood was collected from both groups of mice fed the high-fat diet for the measurement of glucose and lactate. The mice were then anesthetized with Nembutal (60 mg/kg body wt administered ip) for drawing blood from the inferior vena cava and harvesting of tissues. Gastrocnemius muscle, kidney, diaphragm, and liver were removed as rapidly as possible in the order given, immediately freeze-clamped with Wollenberger tongs at the temperature of liquid nitrogen, powdered under liquid nitrogen with a mortar and pestle, and stored at −85°C for analysis.
Glucose tolerance and insulin sensitivity tests.
Glucose tolerance tests were conducted after the mice were maintained on the high-fat diet for 16 wk. Mice were fasted overnight (16 h, 5:00 PM to 9:00 AM) before the glucose challenge. Glucose (2 g/kg body wt) was administrated by intraperitoneal injection. Tail blood glucose was monitored at given times with a glucometer (Accu-Chek; Roche, Indianapolis, IN). Blood for insulin measurement was drawn from the tail at 0, 15, and 30 min after glucose challenge. Insulin was measured according to the manufacturer's instructions (Ultrasensitive Mouse Insulin ELISA; Mercodia, Winston-Salem, NC). Insulin tolerance test was conducted after the mice were maintained on the high-fat diet for 17 wk. The mice were deprived of food for 6 h prior to the test (9:00 AM to 3:00 PM). Insulin (2 U insulin/kg body wt, Humulin R; Eli Lilly, Indianapolis, IN) was administrated by intraperitoneal injection.
Measurement of metabolites.
Blood metabolites were measured in overnight-fasted mice. Blood lactate was measured with a Lactate Pro test meter (Arkray, Shiga, Japan). Pyruvate (11), ketone bodies (46), branched-chain amino acids (BCAAs) (27), branched-chain α-keto acids (BCKAs) (27), glycerol (13), and alanine (15) were measured in deproteinized serum by enzymatic methods. Serum nonesterified fatty acids (NEFA) and triacylglycerols (TAGs) were determined by colorimetric methods with the Half Micro Assay kit (Roche) and the L-type TG H Assay kit (Wako Chemicals, Richmond, VA), respectively. Tissue TAG extracted with isopropyl alcohol was also assayed with the latter kit. Glycogen was measured by the method of Lo et al. (28).
Glucose and palmitate oxidation.
Rates of these processes were determined with diaphragms obtained from overnight-fasted (16 h) mice by a modification of the procedure described previously (23). Hemidiaphragms were removed from the preincubation flasks, blotted, and transferred to new flasks containing 1.5 ml of Krebs-Henseleit buffer supplemented with 5 mmol/l glucose containing 60 μCi/mmol [U-14C]glucose, 1 mU/ml insulin (Humulin R), and 0.8% (wt/vol) BSA with and without 0.6 mmol/l palmitate. Flasks were flushed with 95% O2 plus 5% CO2, sealed with rubber serum caps fitted with hanging center wells, and incubated for 1 h with shaking at 37°C. Reactions were terminated by the injection of acid into the flasks and a solution of phenethylamine into the center wells. Rates of glucose oxidation were calculated as described previously (23).
To measure fatty acid oxidation, preincubated hemidiaphragms were removed from the flasks, blotted, and transferred to new flasks containing 1.5 ml of Krebs-Henseleit buffer, pH 7.4, 5 mmol/l glucose, 1 mU/ml insulin, 0.6 mmol/l palmitate containing 500 μCi/mmol [1-14C]palmitate, and 0.8% (wt/vol) BSA. 14CO2 was collected after 1-h incubations as described above. Palmitate oxidation was calculated from the sum of the amounts of radioactive CO2 and acid-soluble products produced during the incubations.
Measurement of PDC activity.
Actual PDC activity (activity of the complex as extracted from the tissue with phosphorylation state preserved) and total PDC activity (activity of the complex after dephosphorylation with a phosphatase) were measured as described previously (22).
Western blot analysis.
The levels of PDK2 and PDK4 protein were measured by Western blot analysis, as described previously (23). PDK1 antibody was purchased from the Stressgen Bioreagents (Ann Arbor, MI). PDK3 antibody was from the Abnova (Taipei, Taiwan). Voltage-dependent anion channel-1α protein was measured as a mitochondrial loading control. Protein was determined with a Bio-Rad protein assay kit (Hercules, NH), with BSA as the standard.
Statistical analysis.
The statistical significance of differences was determined by Student's t-test when two groups were compared and by the one-way ANOVA test when multiple groups were compared. Values are presented as means ± SE with the indicated number of independent samples.
RESULTS
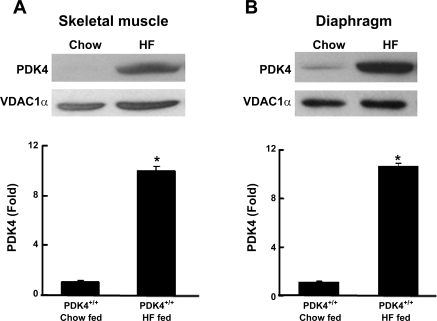
Long-term feeding of the high-fat diet greatly increased PDK4 protein expression in skeletal muscle and diaphragm but not in liver and kidney of wild-type mice.
PDK4 expression is normally low in skeletal muscle of humans (40) and rodents (50) but is increased markedly by short-term feeding of diets high in fat (16, 17, 40). As expected from the findings of those short-term studies, PDK4 protein was increased markedly in the skeletal muscle and diaphragm (10- and 11-fold, respectively) of wild-type mice fed a high-fat diet for 18 wk compared with age-matched wild-type mice fed a low-fat chow diet (Fig. 1). In complete contrast to muscle, PDK4 expression was not induced in the liver or the kidney by the high-fat diet (data not shown). PDK2 expression was not affected by the diet in any of the four tissues examined (data not shown). PDK1 protein was modestly increased in the liver (1.2-fold) and skeletal muscle (1.45-fold) but not in the kidney and diaphragm (data not shown). PDK3 protein was not detectable in diaphragm and skeletal muscle and was not altered in amount in liver and kidney by the high-fat diet (data not shown). Therefore, among the four PDKs expressed in tissues, the expression of PDK4 in muscle was most affected by feeding mice the high-fat diet.
Fig. 1.
Western blot analysis of the amounts of pyruvate dehydrogenase kinase-4 (PDK4) protein present in skeletal muscle (A) and diaphragm (B) mice fed the chow diet (Chow) and the high-fat (HF) diet for 18 wk. Mice were killed at 7:00 AM. Protein samples (50 μg) obtained from fed mice were separated on a 15% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Western blotting was carried out by a standard method. Data were obtained with 4 wild-type mice in each group. *P < 0.0001 relative to mice fed Chow. VDAC1α, voltage-dependent anion channel-1α.
PDK4 deficiency had no effect on growth, weight gain, and tissue weights of mice fed a high-fat diet.
Body weights of PDK4−/− mice did not differ from that of wild-type mice during the 18-wk feeding period on the high-fat diet (data not shown). Food consumption rates were also not different between PDK4−/− and wild-type mice during the 8th wk on the diet (2.8 ± 0.1 g/day for both groups). Obesity was induced by maintaining the mice on this diet as indicated by their appearance and final body weights (45.3 ± 1.3 vs. 45.7 ± 0.8 g for PDK4−/− and wild-type mice, respectively; n = 10/group). Wild-type mice maintained from weaning on the chow diet weighed only 28.5 ± 1.1 g (n = 3) at the same age. The weights of the liver (2.25 ± 0.22 vs. 2.38 ± 0.22 g), kidney (361 ± 15 vs. 349 ± 15 mg), heart (155 ± 9 vs. 165 ± 7 mg), and epididymal fat pads (2.09 ± 0.22 vs. 1.87 ± 0.06 g) were not significantly different between PDK4−/− and wild-type mice, respectively.
PDK4 deficiency lowered fasting blood glucose levels and improved glucose tolerance and insulin sensitivity.
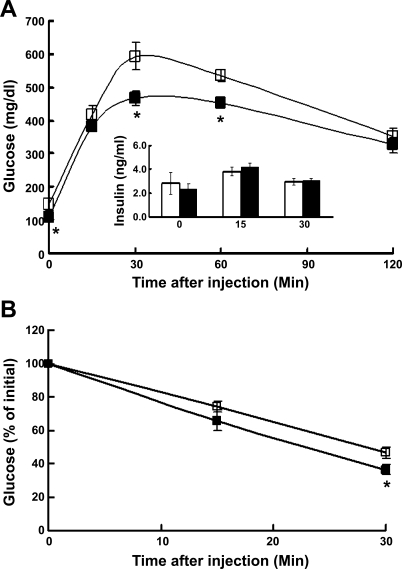
No differences in blood metabolites were found when fed animals were compared (data not shown). Significant differences emerged when blood samples from overnight-fasted mice were analyzed. Throughout the 18-wk feeding period, fasting blood glucose levels were lower in the PDK4−/− mice compared with wild-type mice. For example, in mice maintained on the high-fat diet for 6 wk, blood glucose levels after overnight fasting were 3.8 ± 0.2 and 3.2 ± 0.1 mmol/l in wild-type and PDK4−/− mice (n = 12/group, P < 0.005), respectively. Serum insulin levels, however, were comparable (0.42 ± 0.09 vs. 0.72 ± 0.21 ng/ml; n = 6/group) in the two groups of mice. In mice fed the high-fat diet for 16 wk, blood glucose levels after overnight fasting were 8.2 ± 0.8 and 5.9 ± 0.3 mmol/l in wild-type and PDK4−/− mice (n = 8/group, P < 0.05), respectively. Serum insulin levels were markedly higher than after 6 wk on the diet but again comparable in the two groups of mice (2.8 ± 0.9 vs. 2.3 ± 0.5 ng/ml; n = 5/group), consistent with the development of insulin resistance in both groups of mice. PDK4−/− mice were also slightly but significantly more glucose tolerant than wild-type mice after 16 wk on the diet (area under the curves: 40,170 ± 1,010 vs. 36,410 ± 620 min·mg·dl−1; n = 5/group, P < 0.02; Fig. 2A). Insulin levels increased slightly during the test but were not significantly different between the two groups (Fig. 2A). PDK4−/− mice were slightly but significantly more insulin sensitive after 17 wk on the diet (n = 6/group, P < 0.05; Fig. 2B).
Fig. 2.
Glucose tolerance test (GTT) and insulin tolerance test (ITT) in PDK4−/− (▪) and wild-type (□) mice fed the HF diet. A: GTT. Glucose (2 g/kg body wt) was injected intraperitoneally (ip) into overnight-fasted mice after 16 wk on the HF diet. Blood glucose was measured in tail blood obtained at indicated times after glucose administration. Insulin was measured in serum prepared from tail blood at the indicated times after glucose administration. Data are means ± SE obtained with 8 mice in each group. *P < 0.05 relative to wild-type mice. B: ITT. Insulin (2 U/kg body wt) was injected ip into 6-h-fasted mice that had been fed the HF diet for 17 wk. Data are means ± SE obtained from 6–12 mice/group. *P < 0.05 relative to wild-type mice.
PDK4 deficiency lowered blood levels of gluconeogenic precursors.
After 18 wk on the high-fat diet, blood glucose levels were 21% lower in the PDK4−/− mice compared with wild-type mice (Table 1). The sum of the concentrations of the gluconeogenic precursors lactate, pyruvate, and alanine was 26% lower in the blood of PDK4−/− mice. Blood levels of BCKAs were also lower in PDK4−/− mice, but BCAAs, NEFA, TAG, 3-hydroxybutyrate, and acetoacetate were higher. The ratio of [BCAA]/[BCKA] was significantly greater in the blood of PDK4−/− mice (5.7 ± 0.3 vs. 2.7 ± 0.3; n = 5/group, P < 0.005).
Table 1.
Fasting blood metabolite levels of wild-type and PDK4−/− mice fed the high-fat diet for 18 wk
| Measurementa |
Genotype of Mice |
|
|---|---|---|
| Wild type | PDK4−/− | |
| Glucose, mmol/l | 6.1±0.4 | 4.8±0.2** |
| Lactate, mmol/l | 2.7±0.1 | 2.1±0.2* |
| Pyruvate, mmol/l | 0.16±0.02 | 0.07±0.01** |
| Alanine, mmol/l | 0.25±0.02 | 0.12±0.01** |
| 3-Hydroxybutyrate, mmol/l | 0.98±0.26 | 2.29±0.44* |
| Acetoacetate, mmol/l | 0.18±0.04 | 0.48±0.08* |
| NEFA, mmol/l | 0.40±0.04 | 0.77±0.06** |
| Glycerol, mmol/l | 0.54±0.05 | 0.49±0.07 |
| BCAA, mmol/lb | 0.23±0.03 | 0.31±0.01* |
| BCKA, mmol/lc | 0.085±0.008 | 0.054±0.005* |
| TAG, mmol/l | 0.62±0.05 | 1.00±0.07** |
Data are means ± SE for values obtained with 4–6 mice/genotype. PDK4, pyruvate dehydrogenase kinase-4; NEFA, nonesterified fatty acids; BCAA, branched-chain amino acids; BCKA, branched-chain α-keto acids; TAG, triacylglycerol.
Glucose and lactate were measured in whole blood collected from the tail of overnight-fasted mice that were not anesthetized. Other analytes were measured in serum prepared from blood collected from the vena cava of overnight-fasted mice that were anesthetized with Nembutal. Since commercial preparations of Nembutal contain ethanol and the metabolism of ethanol by the liver generates NADH that reduces pyruvate to lactate, the values given for serum pyruvate are low. Since pyruvate and alanine should also be in thermodynamic equilibrium, the values given for serum alanine are likewise low.
This value corresponds to the sum of leucine, isoleucine, and valine.
This value corresponds to the sum of α-ketoisocaproate, α-keto-β-methylvalerate, and α-ketoisovalerate.
P < 0.05 and
P < 0.01 relative to wild-type mice by Student's t-test.
PDK4 deficiency lowered liver glycogen level in the fasted state.
Since glycogen synthesis is important for blood glucose disposal in the fed state (47, 48) and its degradation contributes to maintenance of blood glucose levels during fasting (33), muscle and liver glycogen levels were measured in fed and fasted mice (Table 2). Skeletal muscle glycogen levels were the same regardless of whether the two groups of mice were fed or fasted (Table 2). Liver glycogen levels were likewise the same in the fed state in the two groups of mice (Table 2). After overnight fasting, however, liver glycogen levels were significantly lower (48%) in the PDK4−/− mice (Table 2), indicating that glycogen stores were utilized more rapidly during fasting in these mice.
Table 2.
Glycogen content of liver and skeletal muscle of wild-type and PDK4−/− mice fed the high-fat diet for 18 wk
| Measurement (Nutritional State) |
Genotype |
|
|---|---|---|
| Wild type | PDK4−/− | |
| Liver glycogen, μmol/g wet wt | ||
| Feda | 320±21 | 360±21 |
| Fastedb | 48±7 | 25±3* |
| Muscle glycogen, μmol/g wet wt | ||
| Fed | 30±3 | 26±4 |
| Fasted | 13±1 | 13±1 |
| Liver TAG, mg/g wet wt (fasted) | 103±5 | 143±6** |
| Heart TAG, mg/g wet wt (fasted) | 5.9±1.9 | 6.9±0.6 |
| Muscle TAG, mg/g wet wt (fasted) | 57±7 | 67±4 |
Data are means ± SE for values obtained with 4 mice/genotype.
Mice with continuous access to food were killed at 7 AM for this measurement.
Mice that had been fasted overnight were killed at 9 AM for this measurement.
P < 0.02 and
P < 0.01 relative to wild-type mice by Student's t-test.
PDK4 deficiency increased TAG concentration in liver but not in heart and muscle in the fasted state.
Since serum TAG levels were greater in PDK4−/− mice than in wild-type mice (Table 1), the TAG contents of heart, skeletal muscle, and liver were measured. TAG content of the heart and skeletal muscle of the two groups of mice did not differ significantly (Table 2). Liver TAG content was 39% greater in the liver of PDK4−/− mice compared with that of wild-type mice (Table 2).
In fed mice, PDK4 deficiency increased PDC activity in the skeletal muscle and diaphragm but not in the liver and kidney.
PDC activities were measured in four tissues harvested from PDK4−/− and wild-type mice when the mice were in the fed state after 18 wk on the high-fat diet (Table 3). Total PDC activities (activity of the complex after dephosphorylation by phosphatase treatment) for any given tissue did not differ between the two groups of mice (Table 3). Likewise, no differences were found in actual PDC activities (activity of the complex as extracted from the tissue) for the liver and kidney between wild-type and PDK4−/− mice (Table 3). On the other hand, PDK4 deficiency caused much higher actual PDC activities in skeletal muscle and diaphragm (Table 3). These tissue-specific findings are consistent with the observed induction of PDK4 protein by the high-fat diet in skeletal muscle and diaphragm but not in the liver and kidney (Fig. 1). Compared with actual PDC activities reported previously with wild-type mice fed chow diet (Table 5 in Ref. 23), actual PDC activities were lower in skeletal muscle (0.53 ± 0.04 vs. 0.25 ± 0.04 U/g wet wt) and diaphragm (2.46 ± 0.16 vs. 1.00 ± 0.07 U/g wet wt) of wild-type mice fed the high-fat diet (Table 3), again consistent with the observed induction of PDK4 protein in these tissues by the high-fat diet.
Table 3.
Actual and total PDC activities in organs of wild-type and PDK4−/− mice fed high-fat diet for 18 wk
| Tissue (Nutritional State) |
Actual PDC |
Total PDC
|
||
|---|---|---|---|---|
| Wild type | PDK4−/− | Wild type | PDK4−/− | |
| Liver | ||||
| Feda | 0.19±0.02 | 0.22±0.05 | 2.01±0.01 | 2.01±0.05 |
| Fastedb | 0.14±0.01 | 0.15±0.01 | 2.23±0.05 | 2.19±0.02 |
| Skeletal Muscle | ||||
| Fed | 0.25±0.04 | 1.25±0.04* | 3.41±0.03 | 3.62±0.05 |
| Fasted | 0.11±0.01# | 0.16±0.01# | 3.37±0.04 | 3.58±0.08 |
| Kidney | ||||
| Fed | 1.72±0.16 | 1.65±0.18 | 5.56±0.22 | 5.65±0.23 |
| Fasted | 1.02±0.21# | 1.66±0.09* | 5.35±0.08 | 5.40±0.05 |
| Diaphragm | ||||
| Fed | 1.00±0.07 | 2.17±0.26* | 6.15±0.08 | 6.26±0.09 |
| Fasted | 0.53±0.07 | 0.83±0.01# | 6.18±0.05 | 6.29±0.29 |
Data are means ± SE and in U/g wet wt for values obtained with 3 mice/genotype. PDC, pyruvate dehydrogenase complex.
Organs were collected from fed mice killed at 7 AM.
Organs were collected after overnight fasting of the mice.
Significantly different from fed state of each genotype (P < 0.05) by 1-way ANOVA test;
significantly different from wild-type mice in the same nutritional state (P < 0.05) by 1-way ANOVA test.
In fasted mice, PDK4 deficiency increased PDC activity in kidney but not skeletal muscle, liver, and diaphragm.
As we expected, overnight fasting induced no difference in total PDC activity of any of the four tissues examined from wild-type and PDK4−/− mice (Table 3). On the basis of previous studies, we had expected that overnight fasting would further reduce actual PDC activities below that caused by the feeding of the high-fat diet. Indeed, a trend in this direction was observed in all tissues of the wild-type mice, although the magnitude of the decrease was only large enough to be statistically significant for the skeletal muscle and kidney. In PDK4−/− mice, fasting induced large decreases in actual PDC activities in the skeletal muscle and diaphragm but had no effect in the kidney and only a trend for a lower value in the liver (Table 3). Among the four tissues examined, PDK4 deficiency resulted in a higher actual PDC activity only in the kidney. A trend for higher actual PDC activities was apparent for diaphragm and skeletal muscle but not the liver of PDK4−/− mice compared with the wild-type mice.
Effect of fasting on expression of the PDKs in tissues of wild-type and PDK4−/− mice.
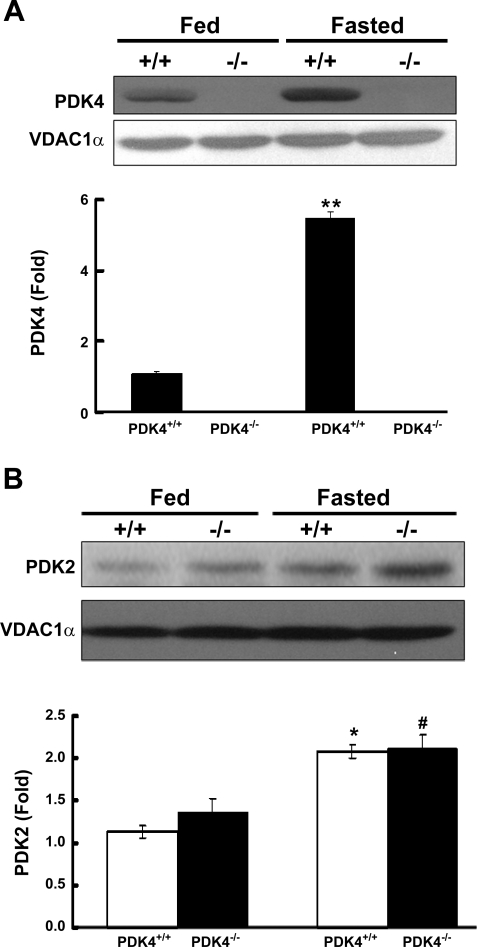
Although PDK4 was already markedly induced in the skeletal muscle of wild-type mice fed the high-fat diet (Fig. 1), PDK4 protein was increased further (5.5-fold) by fasting the animals overnight (Fig. 3A). Although consistent with the lower muscle PDC activity induced by fasting (Table 3), nearly the same decrease in muscle PDC activity was induced by fasting in PDK4−/− mice (Table 3) despite the complete absence of PDK4 (Fig. 3A). PDK2, on the other hand, was not induced in skeletal muscle in response to fasting in either wild-type or PDK4−/− mice (data not shown), ruling out a compensatory response by this isozyme. The amount of PDK1 protein was increased significantly in response to fasting in wild-type mice (1.5-fold, P < 0.01) but not significantly in PDK4−/− mice (1.2-fold, P = 0.08). In the kidney, PDK2 protein was increased twofold by fasting in both wild-type and PDK4−/− mice (Fig. 3B). PDK4 protein increased eightfold in the kidney of wild-type mice (data not shown), consistent with the greater decrease in actual PDC activity in these mice compared with that of the PDK4−/− mice. In the liver, PDK2 protein increased approximately twofold in response to fasting in both wild-type and PDK4−/− mice, whereas PDK4 protein was induced approximately threefold in the wild-type mice (data not shown). PDK1 and PDK3 did not differ significantly in the liver and the kidney in wild-type and PDK4−/− mice in the fed or fasted states. These findings indicate altered expression of PDK1, -2, and -3 do not compensate for the lack of PDK4 in any of the tissues of PDK4−/− mice examined.
Fig. 3.
Western blot analysis of the amounts of PDK4 protein present in the skeletal muscle (A) and the amount of PDK2 protein present in kidney (B) of wild-type mice and PDK4−/− mice fed the HF diet. Fed mice were killed at 7:00 AM. Fasted mice were killed at 9:00 AM after being fasted overnight (food removed at 5 PM). Western blot analysis was carried out as described in the legend to Fig. 1. Data were obtained with 3 mice in each group. **P < 0.001 relative to fed state of wild-type mice; *P < 0.01 relative to fed state of wild-type mice; #P < 0.01 relative to fed state of PDK4−/− mice.
PDK4 deficiency increased glucose oxidation and decreased palmitate oxidation by isolated diaphragms.
Diaphragms from PDK4−/− mice oxidized glucose at a rate 50% greater than diaphragms from wild-type mice (Table 4). Less pyruvate accumulated in the incubation medium with diaphragms from PDK4−/− mice (0.57 ± 0.05 vs. 1.48 ± 0.05 μmol·h−1·g−1; n = 3/group, P < 0.005). Palmitate inhibited glucose oxidation by 14% in diaphragms from wild-type mice but exerted no inhibition with diaphragms from PDK4−/− mice (Table 4), indicating that inhibition of glucose oxidation by palmitate requires induction of PDK4. The rate of palmitate oxidation was 25% lower in diaphragm isolated from PDK4−/− mice relative to wild-type mice (Table 4).
Table 4.
Effect of palmitate on rates of glucose oxidation and palmitate oxidation by isolated diaphragms from wild-type mice and PDK4−/− mice fed high-fat diet
| Measurementsa | Addition, mmol/l |
Genotype |
|
|---|---|---|---|
| Wild type | PDK4−/− | ||
| Glucose oxidation | None | 2.01±0.17 | 3.02±0.06* |
| Glucose oxidation | Palmitate (0.6) | 1.74±0.15** | 3.06±0.18* |
| Palmitate oxidation | Glucose (5.0) | 0.44±0.03 | 0.33±0.01* |
Data are means ± SE and in μmol·h−1·g wet wt−1; n = 3 in each group.
Diaphragms were obtained from overnight-fasted mice. Glucose oxidation was measured by 14CO2 production from [U-14C]glucose, palmitate oxidation by 14CO2, and radioactive acid-soluble product formation from [1-14C]palmitate.
P < 0.05 relative to diaphragms from wild-type mice by Student's unpaired t-test;
P < 0.05 relative to diaphragms from wild-type mice incubated without palmitate by Student's paired t-test.
DISCUSSION
PDC is inhibited during starvation and in diabetes by phosphorylation of the α-subunits of its E1 component (4) mediated by greater PDK activity, as observed in both animal models (3, 18, 51) and humans (10, 28, 40), and reduced PDP activity (21). Upregulation of PDK4 contributes to the increase in PDK activity (49–51), but its importance is uncertain relative to stimulation of PDK activity by acetyl-CoA and NADH (38) produced by the increase in fatty acid oxidation in starvation and diabetes. PDK4−/− mice have lower than normal blood glucose levels during starvation because higher PDC activity results in limitation of substrates for gluconeogenesis (23). These findings led us to examine the effects of PDK4 deficiency on glucose homeostasis in mice rendered obese and insulin resistant with a high-fat diet. As expected (19, 32), feeding wild-type mice such a diet caused obesity, fasting hyperglycemia, elevated fasting serum insulin levels, fasting glucose intolerance, and insulin resistance. PDK4 expression was increased in the skeletal muscle of these mice, in agreement with short-term effects of high-fat diets in rodents (16, 17) and humans (40). Like wild-type mice, PDK4−/− mice maintained on the high-fat diet became obese with elevated insulin levels. However, fasting blood glucose levels were significantly lower, glucose tolerance was a little better, and insulin sensitivity was slightly greater compared with wild-type mice.
PDK4 deficiency had no effect on postprandial blood glucose concentrations in mice on the high-fat diet. We expected this, since PDK4 deficiency affects the supply of substrates for hepatic gluconeogenesis, and the contribution that gluconeogenesis makes to maintenance of blood glucose is minimized when mice are in the absorptive state. On the other hand, we did not expect that the largest differences in PDC activities would occur in the skeletal muscle and diaphragm of well-fed mice. Presumably because expression of PDK4 was greatly promoted by the high-fat diet in these tissues of the wild-type mice, actual PDC activity was five times greater in the skeletal muscle and twice greater in the diaphragm of PDK4−/− mice compared with wild-type mice. Although it seems that such a large difference in PDC activity should have been reflected in a difference in blood glucose levels, differences in the eating patterns of the mice within the groups may have precluded the finding of such a difference because of marked variation in the blood glucose levels. In contrast, with mice in the postabsorptive state, we always found that blood glucose levels were significantly lower (15–25%) in the PDK4−/− mice. Lactate, pyruvate, and alanine levels were also lower, consistent with substrate limitation for gluconeogenesis being responsible for the lower blood glucose levels. These findings are similar to our previous findings with PDK4−/− mice fed chow diet (23) and to the findings with the PDK inhibitor dichloroacetate, which also reduces gluconeogenic substrates (7) and glucose in starved (7, 37) and diabetic rats (2, 24, 35). It should be noted, however, that the effects of PDK4 deficiency on PDC activity were surprisingly subtle. Of the four tissues examined, only the kidney demonstrated a significantly higher PDC activity in PDK4-deficient mice compared with wild-type mice. Trends for higher PDC activities were noted in skeletal muscle and diaphragm, but the values were not statistically significant. Despite this, isolated diaphragms from PDK4−/− mice oxidized [U-14C]glucose to 14CO2 at a faster rate and accumulated less pyruvate than diaphragms from wild-type mice, suggesting greater PDC activity in diaphragms from PDK−/− mice. These findings suggest that surprisingly small changes in PDC activity may induce significant effects upon the levels of gluconeogenic precursors and therefore the level of blood glucose. Alternatively, larger increases in actual PDC activity may occur in response to PDK4 deficiency in tissues not examined in this study, e.g., heart, adipose tissue, and brain.
Liver glycogen levels were comparable in fed PDK4−/− and wild-type mice but significantly lower in PDK4−/− mice compared with the wild-type mice after overnight fasting. This can be explained by greater reliance on glycogenolysis for blood glucose maintenance in PDK4−/− mice because hepatic gluconeogenesis is reduced for want of three-carbon substrates.
This study suggests that PDK4 contributes to setting the activity states of PDC in some but not all tissues of fasted insulin-resistant mice. PDK4 is more important in muscle and kidney than in liver, in agreement with the levels of PDK4 expression in these tissues. On the other hand, the finding that PDC remains largely inactive and therefore heavily phosphorylated in all tissues of fasted PDK4−/− mice indicates that the other PDKs play major roles in setting the phosphorylation state of PDC under these conditions. Three additional PDKs are expressed in tissues (9), and activities of the PDKs are collectively regulated by the concentrations of pyruvate, acetyl-CoA, and NADH (24, 38). Dephosphorylation of PDC also depends upon the activity of two PDPs, which are also subject to regulation at the level of expression and by small molecule effectors (21).
PDK4−/− mice fed the high-fat diet were modestly more glucose tolerant than wild-type mice fed the same diet. Both were insulin resistant, as evidenced by high fasting levels of insulin and the amounts of insulin that had to be injected to lower blood glucose levels. However, PDK4−/− mice were slightly more insulin sensitive, which may contribute to their better glucose tolerance. Other contributing factors include greater rates of glucose and pyruvate oxidation because of higher PDC activity and lower rates of gluconeogenesis because of reduced cycling of three carbon gluconeogenic compounds back to the liver.
The finding that PDK4 deficiency slightly increased insulin sensitivity was surprising. We had anticipated finding greater insulin resistance in PDK4−/− mice. Stimulation of glucose oxidation by greater PDC activity suppresses fatty acid oxidation (Table 4 and Ref. 23). Reduced fatty acid oxidation would be expected to result in greater tissue accumulation of TAG and proinflammatory lipids that activate the serine/threonine stress kinases that are responsible for inactivation of components of the insulin-signaling cascade (45) and hence, insulin resistance. Although the mechanism responsible for inhibition of fatty acid oxidation in PDK4 deficiency remains to be established, greater PDC activity would be expected to lead to an increase in malonyl-CoA, inhibitor of carnitine palmitoyltransferase I (CPT I), the rate-limiting enzyme in fatty acid oxidation. Since long-chain acyl-CoA esters are converted to long-chain acyl carnitine esters by CPT I, inhibition of CPT I by malonyl-CoA increases long-chain acyl-CoA esters, which in turn increase diacylglycerol and ceramide, activators of the stress kinases that induce insulin resistance. Indeed, inhibition of acetyl-CoA carboxyalase and activation of CPT I protects mice against diet-induced insulin resistance (26). Despite what seems should happen, our studies with PDK4−/− mice indicate that PDK4 deficiency also affords some protection against diet-induced insulin resistance. Although this dilemma is difficult to explain, findings published recently with malonyl-CoA decarboxylase-knockout mice (26) challenge the current view on the mechanism by which fatty acids induce inflammation and insulin resistance. Theoretically, elevated levels of malonyl-CoA caused by a deficiency of malonyl-CoA decarboxylase should inhibit CPT I, resulting in elevated concentrations of long-chain acyl-CoA esters, diacylglycerol, and ceramide that should induce insulin resistance by activation of the stress kinases. Instead, malonyl-CoA decarboxylase-knockout mice were found protected against the development of insulin resistance. On the basis of these findings, Koves et al. (26) proposed that high levels of fatty acids may induce a mitochondrial overload or stress by exceeding the capacity of the mitochondria for fatty acid oxidation. Mitochondrial overload may induce the accumulation of “incomplete products of fatty acid oxidation” that function as proinflammatory compounds responsible for activation of the stress kinases. Although no attempt was made to measure inflammatory lipids in this study, TAG levels were not significantly more elevated in the skeletal muscle and hearts of PDK4−/− mice compared with wild-type mice. Interestingly, TAG levels were elevated in the liver of PDK4−/− mice, but the liver may be less important in clearing glucose in response to insulin than muscle under the conditions of these experiments.
The mechanism responsible for the increase in insulin sensitivity in PDK4−/− mice is beyond the scope of this study. However, it should be pointed out that insulin works by driving cells of the body to use glucose rather than fat to meet their need for energy. Despite the bad press that fat receives, insulin is a “fat-sparing” “at the expense of glucose” hormone. Likewise, insulin clearly spares BCAAs for protein synthesis at the expense of glucose. This is relevant to the present studies because activation of PDC by knocking out PDK4 also drives cells of the body to use glucose rather than fat and BCAAs. Although insulin promotes glucose utilization by affecting the activities of many metabolic enzymes, activation of PDC, in part by downregulation of PDK4 expression, is clearly among the most important of the actions of insulin. This action of insulin on PDC, like PDK4 deficiency, has significant ramifications on both glucose and fat metabolism. Perhaps, therefore, the insulin-like effect of PDK4 deficiency is a contributing factor to the slight increase in the effectiveness of insulin in PDK4−/− mice.
Feeding mice a high-fat diet caused a marked increase in PDK4 expression in skeletal muscle and diaphragm compared with chow-fed mice. This is presumably due to insulin resistance induced by the accumulation of body fat. The activity state of PDC was markedly reduced in skeletal muscle in the fed state as a consequence of greater PDK4 expression, and the activity state of PDC in skeletal muscle and the diaphragm was much higher in the fed state of PDK4−/− mice because PDK4 could not be induced.
Serum NEFA, TAG, and ketone bodies were elevated in fasted PDK4−/− mice. Glycerol levels were not increased, suggesting that adipose tissue lipolysis may not be increased. The rate of fatty acid oxidation was reduced in diaphragms from PDK4−/− mice, presumably because the capacity for glucose and pyruvate oxidation was increased. NEFA may have been increased in the serum because fatty acid oxidation was inhibited by greater glucose and pyruvate oxidation in peripheral tissues. Greater delivery of NEFA to the liver may account for the higher hepatic TAG content and increased serum TAG. The increase in serum ketone bodies likely reflects greater synthesis in the liver plus inhibition of their oxidation by glucose and pyruvate oxidation in peripheral tissues. The latter is suggested by our finding that the rate of ketone body oxidation is significantly reduced in diaphragms isolated from PDK4−/− mice compared with diaphragms isolated from wild-type mice (Jeoung NH and Harris RA, unpublished studies).
Inhibition of glucose oxidation by fatty acids is an important feature of the glucose-fatty acid cycle (36). The increase in serum levels of NEFA during starvation and in diabetes results in an increase in fatty acid oxidation that inhibits glucose oxidation in peripheral tissues. Inhibition of PDC activity by phosphorylation surely contributes to the mechanism by which fatty acids inhibit glucose oxidation (24). The present study shows that upregulation of PDK4 is important for this effect. Palmitate inhibited glucose oxidation by diaphragms from wild-type but not by diaphragms from PDK4−/− mice. Indeed, glucose oxidation inhibited fatty acid oxidation in diaphragms from PDK4−/− mice. Although the mechanism responsible for this effect was not determined, inhibition of CPT I by malonyl-CoA (39, 43) is an attractive possibility. However, thiolase I can be a limiting step for β-oxidation (14), and the activities of PDC and thiolase I are controlled by both CoA and acetyl-CoA (5, 34). They have similar Km for CoA (5–10 μM) (25, 39), but PDC is less sensitive to inhibition by acetyl-CoA (Ki of 3.9 μM vs. 40 μM, respectively) (25, 34). A greater pyruvate oxidation rate because of greater PDC activity in PDK4−/− mice may decrease CoA and increase acetyl-CoA, resulting in inhibition of β-oxidation. Regardless of the mechanism, elevated serum NEFA levels and inhibition of fatty acid oxidation in muscle did not translate into elevated TAG levels in skeletal muscle and heart of PDK4−/− mice. TAG accumulation is considered a harbinger for the development of insulin resistance in these tissues (8, 12, 44). Indeed, insulin sensitivity was slightly but significantly greater in PDK4−/− mice relative to wild-type mice fed a high-fat diet.
BCAAs were elevated in the blood of fasted PDK4−/− mice. This likely reflects less synthesis of alanine in peripheral tissues as a consequence of reduced availability of pyruvate due to a greater rate of pyruvate oxidation by PDC. Less transamination of pyruvate with glutamate to produce alanine results in less availability of α-ketoglutarate for transamination of BCAAs. The mechanism is corroborated by the observed decrease in BCKAs in PDK4−/− mice.
Upregulation of PDK4 conserves substrates for gluconeogenesis, begging the question of whether PDK4 and the other PDKs are possible targets for therapeutic intervention in diabetes. This study showed that in mice lacking PDK4, insulin sensitivity was increased and overnight fasting glucose levels were lower in a diet-induced obese mouse model of insulin resistance. The relatively modest effects that lack of PDK4 has on blood glucose levels during fasting and starvation suggest that hypoglycemia will not be a problem with a compound that specifically inhibits PDK4. Dichloroacetate has not proven useful for the treatment of hyperglycemia due to its relatively low potency, specificity, and the neuropathy resulting from long-term treatment (41, 52). More potent PDK inhibitors have been developed recently. SDZ048-619 increases PDC activity in tissues of the hyperglycemic Zucker diabetic fatty rat and reduces blood lactate but, surprisingly, not blood glucose (1, 6). However, AZD7545, a specific PDK2 inhibitor, markedly lowers blood glucose in hyperglycemic Zucker diabetic fatty rats (30, 31). Studies analogous to those reported here are currently being conducted with PDK2−/− and PDK2/PDK4 double-knockout mice.
GRANTS
This work was supported by grants to R. A. Harris from the American Diabetes Association and the U. S. Public Health Service (DK-47844) and a postdoctoral fellowship award (to N. H. Jeoung) from the Midwest Affiliate of the American Heart Association.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aicher TD, Anderson RC, Gao J, Shetty SS, Coppola GM, Stanton JL, Knorr DC, Sperbeck DM, Brand LJ, Vinluan CC, Kaplan EL, Dragland CJ, Tomaselli HC, Islam A, Lozito RJ, Liu X, Maniara WM, Fillers WS, DelGrande D, Walter RE, Mann WR. Secondary amides of (R)-3,3,3-trifluoro-2-hydroxy-2-methylpropionic acid as inhibitors of pyruvate dehydrogenase kinase. J Med Chem 43: 236–249, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Karounos D, Yoneyama T, Hollingsworth JW. Dichloroacetate-induced changes in liver of normal and diabetic rats. Proc Soc Exp Biol Med 149: 814–821, 1975. [DOI] [PubMed] [Google Scholar]
- 3.Bajotto G, Murakami T, Nagasaki M, Qin B, Matsuo Y, Maeda K, Ohashi M, Oshida Y, Sato Y, Shimomura Y. Increased expression of hepatic pyruvate dehydrogenase kinases 2 and 4 in young and middle-aged Otsuka Long-Evans Tokushima Fatty rats: induction by elevated levels of free fatty acids. Metabolism 55: 317–323, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barrera CR, Namihira G, Hamilton L, Munk P, Eley MH, Linn TC, Reed LJ. α-Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys 148: 343–358, 1972. [DOI] [PubMed] [Google Scholar]
- 5.Batenburg JJ, Olson MS. Regulation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. J Biol Chem 251: 1364–1370, 1976. [PubMed] [Google Scholar]
- 6.Bebernitz GR, Aicher TD, Stanton JL, Gao J, Shetty SS, Knorr DC, Strohschein RJ, Tan J, Brand LJ, Liu C, Wang WH, Vinluan CC, Kaplan EL, Dragland CJ, DelGrande D, Islam A, Lozito RJ, Liu X, Maniara WM, Mann WR. Anilides of (R)-trifluoro-2-hydroxy-2-methylpropionic acid as inhibitors of pyruvate dehydrogenase kinase. J Med Chem 43: 2248–2257, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Blackshear PJ, Holloway PA, Alberti KG. The metabolic effects of sodium dichloroacetate in the starved rat. Biochem J 142: 279–286, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 329: 191–196, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chokkalingam K, Jewell K, Norton L, Littlewood J, van Loon LJ, Mansell P, Macdonald IA, Tsintzas K. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: an important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 92: 284–292, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Czok R, Lamprecht W. Pyruvate, phosphoenolpyruvate and d-glycerate-2-phosphate. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Academic, 1974.
- 12.Dubé J, Goodpaster BH. Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr Opin Clin Nutr Metab Care 9: 553–559, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Eggstein M, Kuhlmann E. Triglycerids and glycerol: determination after alkaline hydrolysis. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Academic, 1974.
- 14.Fong JC, Schulz H. On the rate-determining step of fatty acid oxidation in heart. Inhibition of fatty acid oxidation by 4-pentenoic acid. J Biol Chem 253: 6917–6922, 1978. [PubMed] [Google Scholar]
- 15.Grassl M l-Alanine: determination with GPT and LDH. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Academic, 1974.
- 16.Holness MJ, Kraus A, Harris RA, Sugden MC. Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes 49: 775–781, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Holness MJ, Smith ND, Bulmer K, Hopkins T, Gibbons GF, Sugden MC. Evaluation of the role of peroxisome-proliferator-activated receptor alpha in the regulation of cardiac pyruvate dehydrogenase kinase 4 protein expression in response to starvation, high-fat feeding and hyperthyroidism. Biochem J 364: 687–694, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans 31: 1143–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Howarth FC, Qureshi MA, Gbewonyo AJ, Tariq S, Adeghate E. The progressive effects of a fat enriched diet on ventricular myocyte contraction and intracellular Ca2+ in the C57BL/6J mouse. Mol Cell Biochem 273: 87–95, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem 273: 17680–17688, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Huang B, Wu P, Popov KM, Harris RA. Starvation and diabetes reduce the amount of pyruvate dehydrogenase phosphatase in rat heart and kidney. Diabetes 52: 1371–1376, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeoung NH, Sanghani PC, Zhai L, Harris RA. Assay of the pyruvate dehydrogenase complex by coupling with recombinant chicken liver arylamine N-acetyltransferase. Anal Biochem 356: 44–50, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J 397: 417–425, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J 154: 327–348, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohn MC, Achs MJ, Garfinkel D. Computer simulation of metabolism in pyruvate-perfused rat heart. III. Pyruvate dehydrogenase. Am J Physiol Regul Integr Comp Physiol 237: R167–R173, 1979. [DOI] [PubMed] [Google Scholar]
- 26.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Livesey G, Lund P. Determination of branched-chain amino and keto acids with leucine dehydrogenase. Methods Enzymol 166: 3–10, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Lo S, Russell JC, Taylor AW. Determination of glycogen in small tissue samples. J Appl Physiol 28: 234–236, 1970. [DOI] [PubMed] [Google Scholar]
- 29.Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol Genet Metab 65: 181–186, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Mayers RM, Butlin RJ, Kilgour E, Leighton B, Martin D, Myatt J, Orme JP, Holloway BR. AZD7545, a novel inhibitor of pyruvate dehydrogenase kinase 2 (PDHK2), activates pyruvate dehydrogenase in vivo and improves blood glucose control in obese (fa/fa) Zucker rats. Biochem Soc Trans 31: 1165–1167, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Mayers RM, Leighton B, Kilgour E. PDH kinase inhibitors: a novel therapy for Type II diabetes? Biochem Soc Trans 33: 367–370, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology 44: 1191–1205, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Newsholme EA Carbohydrate metabolism in vivo: regulation of the blood glucose level. Clin Endocrinol Metab 5: 543–578, 1976. [DOI] [PubMed] [Google Scholar]
- 34.Olowe Y, Schulz H. Regulation of thiolases from pig heart. Control of fatty acid oxidation in heart. Eur J Biochem 109: 425–429, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Park R, Radosevich PR, Leach WJ, Seto P, Arieff AI. Metabolic effects of dichloroacetate in diabetic dogs. Am J Physiol Endocrinol Metab 245: E94–E101, 1983. [DOI] [PubMed] [Google Scholar]
- 36.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963. [DOI] [PubMed] [Google Scholar]
- 37.Randle PJ, Sugden PH, Kerbey AL, Radcliffe PM, Hutson NJ. Regulation of pyruvate oxidation and the conservation of glucose. Biochem Soc Symp: 47–67, 1978. [PubMed]
- 38.Ravindran S, Radke GA, Guest JR, Roche TE. Lipoyl domain-based mechanism for the integrated feedback control of the pyruvate dehydrogenase complex by enhancement of pyruvate dehydrogenase kinase activity. J Biol Chem 271: 653–662, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 268: 25836–25845, 1993. [PubMed] [Google Scholar]
- 40.Sparks LM, Xie H, Koza RA, Mynatt R, Bray GA, Smith SR. High-fat/low-carbohydrate diets regulate glucose metabolism via a long-term transcriptional loop. Metabolism 55: 1457–1463, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Staack H, Binstock JF, Schulz H. Purification and properties of a pig heart thiolase with broad chain length specificity and comparison of thiolases from pig heart and Escherichia coli. J Biol Chem 253: 1827–1831, 1978. [PubMed] [Google Scholar]
- 42.Stacpoole PW, Henderson GN, Yan Z, Cornett R, James MO. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev 30: 499–539, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Stanley WC, Hernandez LA, Spires D, Bringas J, Wallace S, McCormack JG. Pyruvate dehydrogenase activity and malonyl CoA levels in normal and ischemic swine myocardium: effects of dichloroacetate. J Mol Cell Cardiol 28: 905–914, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Stannard SR, Johnson NA. Insulin resistance and elevated triglyceride in muscle: more important for survival than “thrifty” genes? J Physiol 554: 595–607, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson DH, Mellanby J, Krebs HA. Enzymic determination of D(−)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J 82: 90–96, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woerle HJ, Meyer C, Dostou JM, Gosmanov NR, Islam N, Popa E, Wittlin SD, Welle SL, Gerich JE. Pathways for glucose disposal after meal ingestion in humans. Am J Physiol Endocrinol Metab 284: E716–E725, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Woerle HJ, Szoke E, Meyer C, Dostou JM, Wittlin SD, Gosmanov NR, Welle SL, Gerich JE. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 290: E67–E77, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys 381: 1–7, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 48: 1593–1599, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J 329: 197–201, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yount EA, Felten SY, O'Connor BL, Peterson RG, Powell RS, Yum MN, Harris RA. Comparison of the metabolic and toxic effects of 2-chloropropionate and dichloroacetate. J Pharmacol Exp Ther 222: 501–508, 1982. [PubMed] [Google Scholar]