Abstract
Many new mechanisms for alcoholic steatosis have been suggested by work reported in the last five years. These include alterations of transcriptional controls of lipid metabolism, better understanding of the effects of abnormal methionine metabolism on the endoplasmic reticulum stress response, unraveling of the basis for sensitization of the Kupffer cell to lipopolysaccharide, a better understanding of the role of cytokines and adipokines in alcoholic liver disease, and implication of the innate immune and complement systems in responses to alcohol. Much of this work has been facilitated by work with knockout mice. Undoubtedly, there are interrelationships among these various pathogenic mechanisms that ultimately will provide a more cohesive picture of how heavy alcohol use deranges hepatic lipid metabolism.
Keywords: ethanol, cytokine, triglyceride, fatty acid oxidation, fatty acid synthesis, innate immunity, endotoxin, endoplasmic reticulum stress
one of the best known biological effects of heavy ethanol consumption is the production of fatty liver. Studies on this effect date from the 1960s in humans and rodents. Original hypotheses regarding the mechanism for this effect included redox shifts generated by the oxidation of ethanol by alcohol and aldehyde dehydrogenases, oxidative stress, and mobilization of peripheral triglyceride from the adipose tissue to the liver. Additional research has suggested that these mechanisms are insufficient to explain the origin and perpetuation of alcoholic fatty liver. For instance, redox shifts in baboon liver are attenuated over time, although the liver remains steatotic. Antioxidant treatment of alcoholic liver disease has been disappointing. The source of triglyceride in the livers of alcohol-fed animals may be stored adipose lipid, be derived from dietary fat, or be synthesized in the liver de novo. Over the past decade, a number of newer pathways regulating the synthesis, export, and oxidation of lipids have been discovered, and ethanol has been found to interact with these basic control systems. We have gained a better understanding of the role of the innate immune system in the liver and its effects on lipid metabolism and uncovered a number of circulating factors that can influence the response of the liver to ethanol. We have thus developed a more comprehensive understanding of the multiple effects that ethanol has on the liver and other parts of the body and how this leads to the development of fatty liver.
Effects of Ethanol on Fatty Acid Oxidizing Systems
Disorders of fatty acid oxidation, such as peroxisome defects, and inherited abnormalities in the enzymes of acyl-CoA oxidation lead to the development of fatty liver, and one of the original mechanisms for alcoholic steatosis was the inhibition of β-oxidation caused by accumulation of NADH and product inhibition of the mitochondrial fatty acid-oxidizing dehydrogenases. Recent work has revealed additional ways that heavy ethanol use blocks fatty acid oxidation, through inhibition of peroxisome proliferator-activated receptor-α (PPARα) and inhibition of AMP-activated protein kinase (AMPK).
PPARα
PPARα is a nuclear hormone receptor involved in regulating fatty acid oxidation and transport. When activated, it binds as a heterodimer with retinoid X receptor (RXR) to peroxisome proliferator response elements in genes involved in the fatty acid oxidation pathways. Ethanol feeding decreased the binding of PPARα/RXR to DNA and expression of several PPARα-regulated genes. These effects appear to be mediated by acetaldehyde, as blocking aldehyde dehydrogenase (ALDH) increased the effects, whereas blocking alcohol dehydrogenase (ADH) prevented them (21). The molecular mechanism for the effect of ethanol is unknown. PPARα levels were unchanged and RXR was decreased in ethanol-fed mice; direct exposure of PPARα/RXR to acetaldehyde in vitro reduced DNA binding (18). These data suggest that posttranslational modification of PPARα or RXR might be involved. Treatment of ethanol-fed animals with PPARα agonists has been shown to inhibit these effects of ethanol and reverse the hepatic fat accumulation (18) PPARα is also involved in regulating the fatty acid export machinery. Microsomal triglyceride transfer protein (MTP) is required for the assembly of very-low-density lipoproteins (VLDL) prior to export. MTP was decreased in livers of ethanol-fed animals, and treatment with a PPARα agonist upregulated MTP and increased export of VLDL (4, 41, 53).
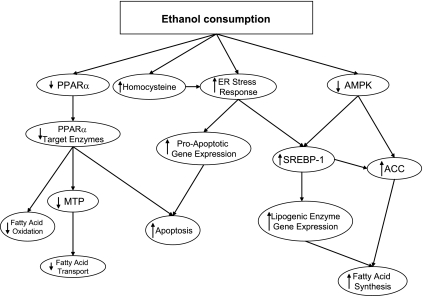
The role of PPARα in alcoholic fatty liver has been extended through studies of knockout mice. PPARα-null mice have impaired fatty acid oxidation with hypoglycemia, hypoketonemia, increased serum free fatty acids, and fatty liver with fasting. The ethanol-fed PPARα-null mice have worse hepatomegaly and hepatocyte damage, along with decreased glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase, and increased lipid peroxides compared with wild-type controls. They also demonstrated increased levels of TGF-α, TGF-β1, and other inflammatory markers compared with wild-type mice (40). Additionally, apoptosis was increased in the PPARα-null mice, associated with decreased Bcl-2 and Bcl-xL. Thus, PPARα can activate both fatty acid oxidation and export and thereby protect against the accumulation of triglyceride, improve the enzymatic defenses against oxidative stress, and reduce the apoptotic response. The effects of ethanol on this system promotes fat accumulation as shown in Fig. 1.
Fig. 1.
Interactions between ethanol, transcriptional controls of lipid metabolism, and endoplasmic reticulum (ER) stress in the liver. PPARα, peroxisome proliferator-activated receptor-α; MTP, microsomal triglyceride transfer protein; AMPK, AMP-activated protein kinase; SREBP-1, sterol response element-binding protein-1; ACC, acetyl-CoA carboxylase. Consumption of ethanol inhibits regulatory systems that are needed to promote the oxidation of fatty acids (PPARα and AMPK) and activates the systems that stimulate fatty acid synthesis (SREBP-1, in part via activation of the ER stress response). The ER stress response also increases the likelihood that hepatocytes will undergo apoptosis.
AMPK
AMPK is a master regulator of metabolism that senses cellular stresses (such as oxidative stress and reduced energy charge) and increases the activity of the major energy-generating pathways (glycolysis and fatty acid oxidation) and downregulates energy-demanding processes (fatty acid and cholesterol synthesis, protein synthesis). Activation of AMPK increases fatty acid oxidation and inhibits synthesis, whereas inhibition of AMPK blocks fatty acid oxidation and promotes fatty acid synthesis. The key regulator of this switch is malonyl-CoA and its effects on mitochondrial uptake of long-chain acyl-CoA. It regulates lipid synthesis both by direct effects on sterol regulatory element-binding protein (SREBP)-1c and through phosphorylation and inhibition of acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (downstream targets of SREBP-1 and SREBP-2, respectively, discussed below). It has been suggested that AMPK directly inhibits SREBP-1c by decreasing its stability (16, 56, 71). Administration of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), an activator of AMPK, to rats reduced the level of SREBP-1c (56). Inhibition of ACC by AMPK leads to decreased levels of malonyl-CoA. Malonyl-CoA is a precursor for the synthesis of fatty acids and an inhibitor of carnitine palmitoyltransferase I (CPT I), the rate-limiting enzyme for fatty acid oxidation. AMPK also activates malonyl-CoA decarboxylase (MCD), which degrades malonyl-CoA (48). Taken together, these effects of AMPK lead to decreased fatty acid synthesis and increased fatty acid oxidation.
In ethanol-fed rodents, AMPK activity was decreased (23, 70). This led to decreased phosphorylation and increased activity of ACC, increased levels of malonyl-CoA, and decreased activity of CPT I (23, 70), Treatment with metformin, another activator of AMPK, returned the phosphorylation levels of ACC toward those of controls (70). Decreased AMPK activity seen with chronic ethanol feeding also allows SREBP-1c to accumulate in the liver (Fig. 1) (68). Metformin and AICAR can block the ability of ethanol to activate SREBP-1 (70). This effect of AMPK on SREBP-1c may mediate actions of adiponectin (63) (see below). Thus, ethanol's effects on AMPK appear to play a role in both the decreased fatty acid oxidation and the increased fatty acid synthesis seen in alcoholic liver disease.
Effects of Ethanol on Fatty Acid Synthesis
Under certain experimental conditions, the de novo synthesis of fatty acids in the liver is increased by ethanol feeding. This was correlated with induction of a number of the rate-limiting enzymes in fatty acid synthesis by ethanol feeding. With the discovery of SREBP-1, it became obvious that ethanol might cause fatty liver by acting through this transcription factor. This observation was subsequently integrated into the newly discovered endoplasmic reticulum (ER) stress response.
SREBP-1
SREBPs are transcription factors regulating fatty acid, triglyceride, and cholesterol synthesis. SREBPs are bound as precursors to the ER and nuclear envelope. When activated, they are released from the membrane and escorted to the Golgi by SREBP cleavage-activating protein (SCAP) and then cleaved by specific proteases and translocated to the nucleus, where they bind to sterol response elements and activate transcription. Overexpression of SREBP-1 in transgenic mice led to the development of massive fatty livers. The levels of the mature, active SREBP-1c, but not SREBP-2, are increased in the livers of chronically ethanol-fed mice, an effect that was suggested by in vitro experiments to be mediated by acetaldehyde (69). Acute exposure to ethanol leads to upregulation of the mRNA for SREBP-1 and its targets (65). Downstream targets of SREBP-1c were increased with acute and chronic ethanol feeding: fatty acid synthase (FAS), steroyl-CoA desaturase (SCD), malic enzyme (ME), ATP citrate lyase (ACL), and ACC (Fig. 1). All of these enzymes contribute to de novo synthesis of fatty acids. SREBP-1c knockout mice were partially protected against ethanol-induced fatty liver (35).
The activity of SREBP-1 is controlled by several different pathways. AMPK has already been discussed. Lipopolysaccharide (LPS) and TNF-α have been reported to induce the level of SREBP-1 in liver (14). Another major pathway of activation of SREBP is through the induction of the ER stress response detailed in subsequent paragraphs.
Homocysteine and the ER Stress Response
Ethanol feeding alters hepatic methionine metabolism. Methionine is normally converted to S-adenosylmethionine (SAM) by methionine adenosyltransferase (MAT). SAM is then converted to S-adenosylhomocysteine (SAH) by donating a methyl group to an accepting molecule, and SAH is then converted to homocysteine. Homocysteine is converted back to methionine by methionine synthase (MS) using N-5-methyl-THF (5-methyl-tetrahydrofolate), or by betaine-homocysteine methyltransferase (BHMT), using betaine as a methyl donor. Homocysteine is elevated in alcoholics and in chronic ethanol-fed animal models, due in part to folate deficiency and to decreased activity of methionine synthase (7, 8, 25, 57, 60).
Homocysteine, increased SAH levels, and decreased SAM/SAH ratios induce the ER stress response (15). Accumulation of unfolded and misfolded proteins triggers the “unfolded protein response,” or UPR, which may eventually lead to activation of the ER stress response. This leads to a number of effects, including increased levels of proapoptotic proteins and increased lipid synthesis to repair cell membranes (Fig. 1). Lipid synthesis induced by the ER stress response involves the activation of SREBP-1. In micropigs fed ethanol as part of a folate-deficient diet, the levels of the active form of SREBP-1c were correlated positively with SAH and homocysteine and negatively with SAM/SAH ratios (15) SAM supplementation normalized SREBP-1c levels, possibly by preventing the ER stress response (16). Although SAM might normalize SREBP-1c levels by increasing adiponectin levels (16), betaine also normalized SREBP-1c levels (33). Mice fed ethanol intragastrically showed an increase in SREBP-1c independent of changes in AMPK activity (31), which was prevented by betaine supplementation. All of these reports point to a prominent role for ER stress induction in the pathogenesis of alcoholic steatosis.
The ER stress response also activates apoptosis by upregulating a number of proteins that participate in the apoptotic pathway [glucose-regulated protein (GRP)78, GRP94, C/EBP homology protein (CHOP), and caspase 12] (33). The release of GRP78 from the ER membrane activates transcription factors (e.g., CHOP) and cleavage and activation of caspase 12 (35). CHOP-null mice fed ethanol had reduced apoptosis despite the presence of fatty liver and necroinflammation (34). SREBP-1c knockout mice fed ethanol had the expected levels of proapoptotic factors, suggesting that CHOP, and the ER stress response, independently of SREBP-1c, play the major role in ethanol-induced apoptosis. Although there are reported correlations between TNF-α levels and SAH/SAM ratios after chronic ethanol feeding (43), TNF-α receptor (TNFR1) knockout mice were not protected against the ER stress response and responded to betaine administration the same as wild-type mice (32). Thus, homocysteine/ER stress pathways probably play a significant role in ethanol-induced liver disease synergistically with TNF-α.
Effects of Ethanol on Intrahepatic Inflammatory Pathways
The pioneering work of Ron Thurman's laboratory clearly established that the Kupffer cell plays an important role in the development of both fatty liver and liver inflammation. Mechanisms by which ethanol activates the Kupffer cells have been elucidated in the last 5 to 10 years.
Role of Kupffer Cells and TNF-α in Alcoholic Liver Disease
Dysregulated cytokine signaling, particularly of those released from the Kupffer cells, occurs with chronic ethanol use. The cytokine most studied in alcoholic liver disease is TNF-α. A multitude of studies have confirmed the role of TNF-α in alcoholic liver disease. Livers depleted of Kupffer cells have been shown to have markedly decreased liver damage in response to chronic ethanol feeding (1). TNF-α antibody prevented ethanol-induced liver injury (29), and TNFR1 knockout mice were protected against ethanol-induced liver injury (66). Alcoholics have higher circulating levels of TNF-α compared with nondrinkers (13, 37). Chronic ethanol ingestion increased circulating levels of LPS (10, 19, 42), secondary to increased intestinal permeability (55). This effect of ethanol may differentiate alcoholics with and without liver disease (36). Kupffer cells from ethanol-fed animals are sensitized to LPS, responding with increased TNF-α production (13, 37). The mechanisms for this sensitization have been elucidated recently (Fig. 2).
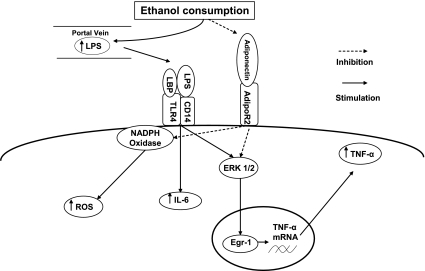
Fig. 2.
Effects of ethanol consumption on cytokine synthesis by Kupffer cells. LPS, lipopolysaccharide; LBP, LPS-binding protein; TLR-4, toll-like receptor 4; CD14, cellular determinant 14; adipoR2, adiponectin receptor 2; ROS, reactive oxygen species; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α; ERK1/2, extracellular signal-regulated kinase-1/2; Egr-1, early-growth response factor 1. Ethanol consumption increases absorption of LPS from the GI tract into the portal vein. Kupffer cells are sensitized to LPS as a result of increased expression of TLR-4 and increased levels of transcription factor Egr-1, leading to secretion of increased amounts of TNF-α and other cytokines such as IL-6. Adiponectin can counteract these effects, but circulating levels of adiponectin are reduced with heavy ethanol intake.
Toll-like receptor 4.
Toll-like receptor 4 (TLR-4) is the receptor on macrophages that binds LPS, stimulating transcription of the genes for TNF-α and other cytokines. Uesugi's group demonstrated that C3H/HeJ mice (which do not express TLR-4 due to a naturally occurring mutation) have decreased levels of TNF-α and reduced liver damage after chronic ethanol feeding (59). Therefore, ethanol's effects on the other known TLRs were surveyed. There was increased expression of TLR-1, -2, -4, -6, -7, -8, and -9, and there was increased TNF-α secretion when mice fed an ethanol-containing diet were exposed to ligands specific for each receptor (24). DPI (diphenyleneiodonium chloride), an inhibitor of NADPH oxidase, prevented the ethanol-induced increase in TLR-2, -4, -6, and -9 (24). Thus, chronic ethanol exposure may increase the expression of some of the TLRs, particularly TLR-4, through production of reactive oxygen species. Park et al. (44) showed that direct interaction of NADPH oxidase isoenzyme 4 with TLR-4 is involved in LPS-mediated reactive oxygen species production. LPS itself was not responsible for the increased levels of the TLRs. The implication of this work is that there may be additional factors besides LPS that are activating the Kupffer cells in alcoholic patients (viral products, gram-positive wall materials, etc.) that synergize with LPS in increasing cytokine production in the liver.
Intracellular Signaling Pathways Mediating Increased Reactivity of Kupffer Cells
The response to LPS is regulated by the transcription factors early-growth response factor-1 (Egr-1), nuclear factor-κB (NF-κB), and activator protein-1 (AP-1) (58, 64). In macrophage-like cell lines exposed chronically to ethanol, LPS stimulation resulted in increased Egr-1 binding to the TNF-α promoter, whereas NF-κB binding activity was decreased, and AP-1 was unchanged (51). A dominant-negative Egr-1 construct blunted the LPS-induced TNF-α mRNA accumulation in ethanol-naïve cells and prevented the sensitization seen with chronic ethanol exposure (51). These results were confirmed in vivo with increased expression and DNA binding of Egr-1 in response to LPS in mice chronically fed ethanol (39). Furthermore, ethanol-fed Egr-1 knockout mice were protected against steatosis, increased alanine aminotransferase (ALT), and increased TNF-α levels (39), despite similar plasma LPS levels. Extracellular signal-regulated kinase 1/2 (ERK1/2) regulates Egr-1 expression, and ethanol exposure increased phosphorylated ERK1/2 and Egr-1 in macrophage cell lines (51). Kinase-dead ERK1/2 prevented the ethanol-induced increase in Egr-1 binding to the TNF-α promoter, and a dominant negative ERK1/2 blocked the increase in TNF-α synthesis by ethanol-treated cells (51). Thus, the ability of ethanol to both increase portal vein LPS levels, and increase the sensitivity of the Kupffer cells to LPS stimulation appears to be central to the development of alcoholic steatosis and liver injury (Fig. 2).
Roles of Other Factors in the Development of Alcoholic Liver Disease
A number of other factors, some circulating (adiponectin, plasminogen activator inhibitor, complement), and some produced locally in the liver (IL-6 and osteopontin), have been reported to play a role in the response of the liver to heavy ethanol use. The mechanisms by which these factors lead to steatosis or inflammation are just beginning to be understood.
Adiponectin.
Adiponectin is an adipokine, a cytokine-like protein secreted by adipocytes. Two receptors for adiponectin have been identified, AdipoR1 and AdipoR2, with AdipoR2 the predominant receptor in the liver (52, 62). Ethanol feeding decreases plasma adiponectin levels as well as liver AdipoR1 mRNA (16), and treatment of chronically ethanol-fed animals with adiponectin has been shown to prevent development of liver injury (61). Adiponectin action is mediated in part by PPARα and AMPK (67). In H4IIEC3 hepatoma cells, PGC-1α (PPARγ coactivator-1) activated PPARα reporter activity, and adiponectin increased PGC-1α in these cells (67). This suggested that induction of PGC-1α mediated the activation of PPARα by adiponectin. Increased adiponectin levels correlated with increased AMPK activity in hepatocytes of mice fed diets high in saturated fat plus ethanol (67), possibly explaining earlier reports of the protective effect of saturated fat against the development of alcoholic steatosis. The mechanism by which the AdipoR2 receptor signals increased AMPK activity is unknown (63).
Adiponectin also has anti-inflammatory effects. Plasma adiponectin levels inversely correlated with TNF-α levels, and administration of adiponectin reduced TNF-α levels in ethanol-fed mice (61). Adiponectin inhibited LPS-stimulated release of TNF-α by Kupffer cells due to suppression of degradation of IκB (an inhibitor of NF-κB), of phosphorylation of ERK1/2, and of DNA binding activity of Egr-1 (44). Adiponectin also reduced the levels of reactive oxygen species in Kupffer cells of ethanol-fed rats. Thus, reduced circulating levels of adiponectin may accentuate the effects of ethanol on Kupffer cell (Fig. 2).
Osteopontin.
Osteopontin is a matricellular protein implicated in alcoholic liver disease. It is a chemoattractant molecule that increases neutrophil localization to the peritoneal cavity after local injection. In studies with human Hep G2 hepatoma cells, ethanol and LPS induced higher levels of osteopontin expression than ethanol alone (46). Elevated osteopontin levels correlated with neutrophil infiltration (6), and neutralizing antibody against osteopontin prevented neutrophil infiltration and ethanol-induced liver injury (5). Therefore, osteopontin, like IL-8, probably plays a role in the characteristic neutrophilic infiltrate in alcoholic hepatitis. Whether it makes a contribution to the development of the fatty liver stage is unknown at present.
IL-6.
Interleukins have also been shown to play a part in ethanol-induced liver injury. IL-6 knockout mice are highly susceptible to ethanol-induced steatosis and apoptosis (22, 27, 38). Induction of Bcl-2 and Bcl-x(L) in liver were seen in ethanol-fed control mice, but this was not observed in ethanol-fed IL-6–/– mice. Expression of Bax proteins was elevated in both genotypes. Injection of IL-6 markedly induced Bcl-2 and Bcl-x(L) but not Bax. Ethanol inhibited IL-6-activated antiapoptotic signals, but this could be overcome with increasing the concentrations of IL-6. IL-6 injections for 10 days decreased steatosis in ethanol-fed mice, as well as in ob/ob mice and mice fed a high-fat diet, which correlated with increased mitochondrial fatty acid oxidation and fat export and increased expression of PPARα (28); IL-6 also increased PPARα mRNA in human muscle (2).
Perfusion of steatotic rat livers with IL-6 markedly improved the graft survival when the livers were subsequently transplanted (54), although this effect was less pronounced with livers from ethanol-fed rats than from Zucker fatty rats (which develop fatty liver due to insulin resistance and obesity), consistent with the inhibitory effect of chronic ethanol-feeding on IL-6 responses (22). It was reported that ME3738, an inducer of IL-6, improved histology, liver ATP, and ALT levels in rats fed ethanol for 8 wk, although the effects were of borderline statistical significance (20). These data suggest that ethanol exposure renders the livers less responsive to IL-6. There are also reports of the involvement of IL-10, an anti-inflammatory cytokine, in the response of the liver to ethanol (38). Administration of ethanol to IL-10 knockout mice induced steatosis to the same degree as in wild type mice, but the inflammatory effect of LPS was greater in the IL-10 knockouts (26).
Plasminogen Activator Inhibitor 1
Plasminogen activator inhibitor 1 (PAI-1) is the main inhibitor of fibrinolysis and has recently been implicated in alcoholic steatosis. Plasma PAI-1 levels correlated with liver PAI-1 expression and the degree of liver steatosis but not with adipose tissue PAI-1 levels (3). PAI-1 levels were increased in response to acute and chronic ethanol intake by mice (9). Metformin, an activator of AMPK that protects against fatty liver, blunted the effect of ethanol on PAI-1 levels and on lipid accumulation in the liver. PAI-1 knockout mice had less lipid accumulation induced by ethanol feeding, and metformin had no effect on lipid accumulation in the knockout animals, suggesting that metformin acts via reducing expression of PAI-1 (9). PAI-1 may also play a role in ethanol-induced liver inflammation, as knockout mice showed decreased inflammation (9). The mechanism of this action has not yet been investigated. There is also a relationship between TNF-α and PAI-1: TNF-α increases levels of PAI-1, and TNF-α knockout mice had a blunted increase in PAI-1 with ethanol administration (17, 49). It remains to be seen how these observations can be integrated with the other pathophysiological pathways previously discussed.
Complement
The complement system has been implicated in many different liver diseases, including viral infection, liver regeneration, ischemic/reperfusion effects after transplantation, and alcoholic liver disease (reviewed by Ref. 47). Early studies examined complement deposition in livers of ethanol-fed rats. Both C3 and C8 deposition were increased, but C1 deposition was minimal (30), suggesting activation of complement through either the alternate pathway or the lectin pathway. LPS is a known inducer of the alternate pathway. There is also an ethanol-induced decrease in CD59 and Crry (the rat equivalent of DAF/CD55), which are inhibitory membrane proteins involved in the control of the complement system (30). It is possible that the following effects of complement represent additional downstream consequences of increased LPS in the portal vein. Hepatocytes in fact express receptors for C5a (50).
Two groups have examined responses of C3 knockout mice to ethanol feeding. One group found decreased steatosis, increased levels of adiponectin, and decreased levels of AdipoR2 in the knockout mice fed ethanol compared with wild-type mice (11). A second report confirmed reduction of steatosis in C3 knockout mice fed ethanol and also reported that this was associated with increased ALT, TNF-α, IL-6, and interferon-γ (IFNγ) (45). Thus, C3 may play a role in promoting alcoholic steatosis but surprisingly might also have anti-inflammatory properties. A breakdown product of C3, acylation-stimulating protein (ASP), may stimulate fatty acid uptake in the liver and contribute to steatosis in this manner (45). In addition, C3 knockout mice had increased phospholipase D1 (PLD1). PLD1 is involved in the assembly of VLDL, and an increase in this molecule likely would increase VLDL secretion and prevent fat accumulation (11).
The effects of ethanol on C5 knockout mice have also been examined. After C5 is cleaved by C3b, C5b forms part of the attack complex. In knockout mice fed ethanol-containing diets, steatosis was observed, but ALT, TNF-α, IL-6, and IFNγ levels were not increased (45), suggesting that C5 is involved in alcohol-induced liver cell injury but not steatosis. The increase in inflammatory cytokines seen in C3 knockout mice might have been due to C5, as plasmin and thrombin can cleave C5 in the absence of C3 (11, 45). C6 knockout mice were also more susceptible to ethanol-induced liver damage, and thus C6 may play a protective role (12). Ethanol-fed CD55/DAF knockout mice showed both increased steatosis and increased ALT levels compared with wild-type mice fed ethanol (45). These results suggest that the complement pathway plays an important and complex role in alcoholic liver disease.
Summary
Many new mechanisms for alcoholic steatosis have been suggested by work reported in the past five years. These include alterations of transcriptional controls of lipid metabolism, a more comprehensive understanding of the effects of abnormal methionine metabolism on the ER stress response and apoptosis, unraveling of the basis for sensitization of the Kupffer cell to LPS, a better understanding of the role of additional cytokines in alcoholic liver disease, and implication of the innate immune and complement systems in responses to alcohol. Undoubtedly, there are interrelationships among these effects, which, when understood, will provide a more cohesive picture of how alcohol abuse deranges hepatic lipid metabolism and results in steatosis. The sheer number of new reported participants in the body's responses to ethanol suggests that additional factors will be discovered.
GRANTS
This work was performed with support from the Indiana Alcohol Research Center (PHS P60 AA-07611-20) and R01 AA-15070-04 to D. W. Crabb.
Acknowledgments
We are grateful for Dr. Suthat Liangpunsakul for assistance in reviewing this manuscript.
REFERENCES
- 1.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20: 453–460, 1994. [PubMed] [Google Scholar]
- 2.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Alessi MC, Bastelica D, Mavri A, Morange P, Berthet B, Grino M, Juhan-Vague I. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol 23: 1262–1268, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Ameen C, Edvardsson U, Ljungberg A, Asp L, Akerblad P, Tuneld A, Olofsson SO, Linden D, Oscarsson J. Activation of peroxisome proliferator-activated receptor alpha increases the expression and activity of microsomal triglyceride transfer protein in the liver. J Biol Chem 280: 1224–1229, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol 208: 473–485, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee A, Burghardt RC, Johnson GA, White FJ, Ramaiah SK. The temporal expression of osteopontin (SPP-1) in the rodent model of alcoholic steatohepatitis: a potential biomarker. Toxicol Pathol 34: 373–384, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res 17: 552–555, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Barak AJ, Beckenhauer HC, Tuma DJ, Badakhsh S. Effects of prolonged ethanol feeding on methionine metabolism in rat liver. Biochem Cell Biol 65: 230–233, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology 130: 2099–2112, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 4: 8–14, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Bykov I, Jauhiainen M, Olkkonen VM, Saarikoski ST, Ehnholm C, Junnikkala S, Vakeva A, Lindros KO, Meri S. Hepatic gene expression and lipid parameters in complement C3−/− mice that do not develop ethanol-induced steatosis. J Hepatol 46: 907–914, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Bykov IL, Vakeva A, Jarvelainen HA, Meri S, Lindros KO. Protective function of complement against alcohol-induced rat liver damage. Int Immunopharmacol 4: 1445–1454, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases LPS-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats: respective roles of MAPKs and NF-kappaB. Biochem Biophys Res Commun 294: 849–853, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp Biol Med (Maywood) 232: 614–621, 2007. [PubMed] [Google Scholar]
- 15.Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol 289: G54–G63, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res 31: 1231–1239, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fearns C, Loskutoff DJ. Induction of plasminogen activator inhibitor 1 gene expression in murine liver by lipopolysaccharide. Cellular localization and role of endogenous tumor necrosis factor-alpha. Am J Pathol 150: 579–590, 1997. [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 12: 162–169, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Fukumura A, Tsutsumi M, Tsuchishima M, Hayashi N, Fukura M, Yano H, Ozaki K, Takase S. Effect of the inducer of interleukin-6 (ME3738) on rat liver treated with ethanol. Alcohol Clin Exp Res 31: S49–S53, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem 276: 68–75, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Gao B Therapeutic potential of interleukin-6 in preventing obesity- and alcohol-associated fatty liver transplant failure. Alcohol 34: 59–65, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Villafranca J, Guillen A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie 90: 460–466, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 43: 989–1000, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Halsted CH, Villanueva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvili L, James SJ, Poirier L. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology 23: 497–505, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Hill DB, D'Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res 26: 74–82, 2002. [PubMed] [Google Scholar]
- 27.Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene 21: 32–43, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 40: 933–941, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology 26: 1530–1537, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Jarvelainen HA, Vakeva A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol 105: 57–63, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol 45: 717–724, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology 40: 442–451, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124: 1488–1499, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res 29: 1496–1503, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol 21, Suppl 3: S7–S9, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol 282: G6–G15, 2002. [DOI] [PubMed] [Google Scholar]
- 38.McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 287: G497–G502, 2004. [DOI] [PubMed] [Google Scholar]
- 39.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology 128: 2066–2076, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology 40: 972–980, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther 310: 417–424, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol 142: 367–373, 1993. [PMC free article] [PubMed] [Google Scholar]
- 43.Novitskiy G, Ravi R, Potter JJ, Rennie-Tankersley L, Wang L, Mezey E. Effects of acetaldehyde and TNF alpha on the inhibitory kappa B-alpha protein and nuclear factor kappa B activation in hepatic stellate cells. Alcohol Alcohol 40: 96–101, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Park PH, Thakur V, Pritchard MT, McMullen MR, Nagy LE. Regulation of Kupffer cell activity during chronic ethanol exposure: role of adiponectin. J Gastroenterol Hepatol 21, Suppl 3: S30–S33, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology 132: 1117–1126, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, Kharbanda KK, Liu QY, Lu SC, McClain CJ, Swanson C, Zakhari S. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr 86: 14–24, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol 3: 333–340, 2006. [PubMed] [Google Scholar]
- 48.Saha AK, Ruderman NB. Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol Cell Biochem 253: 65–70, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Sawdey MS, Loskutoff DJ. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest 88: 1346–1353, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schieferdecker HL, Schlaf G, Jungermann K, Gotze O. Functions of anaphylatoxin C5a in rat liver: direct and indirect actions on nonparenchymal and parenchymal cells. Int Immunopharmacol 1: 469–481, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem 277: 14777–14785, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA 100: 14217–14222, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugimoto T, Yamashita S, Ishigami M, Sakai N, Hirano K, Tahara M, Matsumoto K, Nakamura T, Matsuzawa Y. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. J Hepatol 36: 157–162, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z, Kunos G, Diehl AM, Gao B. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology 125: 202–215, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Thurman RG Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol Gastrointest Liver Physiol 275: G605–G611, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Tomita K, Tamiya G, Ando S, Kitamura N, Koizumi H, Kato S, Horie Y, Kaneko T, Azuma T, Nagata H, Ishii H, Hibi T. AICAR, an AMPK activator, has protective effects on alcohol-induced fatty liver in rats. Alcohol Clin Exp Res 29: 240S–245S, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Trimble KC, Molloy AM, Scott JM, Weir DG. The effect of ethanol on one-carbon metabolism: increased methionine catabolism and lipotrope methyl-group wastage. Hepatology 18: 984–989, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol 20: 6084–6094, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 34: 101–108, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Villanueva JA, Halsted CH. Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology 39: 1303–1310, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem 272: 17795–17801, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Yin HQ, Kim M, Kim JH, Kong G, Kang KS, Kim HL, Yoon BI, Lee MO, Lee BH. Differential gene expression and lipid metabolism in fatty liver induced by acute ethanol treatment in mice. Toxicol Appl Pharmacol 223: 225–233, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117: 942–952, 1999. [DOI] [PubMed] [Google Scholar]
- 67.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol 287: G1–G6, 2004. [DOI] [PubMed] [Google Scholar]
- 69.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 277: 29342–29347, 2002. [DOI] [PubMed] [Google Scholar]
- 70.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]