Abstract
Angiotensin II (Ang II) stimulation of the Ang type 1 receptor (AT1R) facilitates myocardial remodeling through NADPH oxidase-mediated generation of oxidative stress. Components of the renin-angiotensin system constitute an autocrine/paracrine unit in the myocardium, including renin, which is the rate-limiting step in the generation of Ang II. This investigation sought to determine whether cardiac oxidative stress and cellular remodeling could be attenuated by in vivo renin inhibition and/or AT1R blockade in a rodent model of chronically elevated tissue Ang II levels, the transgenic (mRen2)27 rat (Ren2). The Ren2 overexpresses the mouse renin transgene with resultant hypertension, insulin resistance, and cardiovascular damage. Young (6- to 7-wk-old) heterozygous (+/−) male Ren2 and age-matched Sprague-Dawley rats were treated with the renin inhibitor aliskiren, which has high preferential affinity for human and mouse renin, an AT1R blocker, irbesartan, or placebo for 3 wk. Myocardial NADPH oxidase activity and immunostaining for NADPH oxidase subunits and 3-nitrotyrosine were evaluated and remodeling changes assessed by light and transmission electron microscopy. Blood pressure, myocardial NADPH oxidase activity and subunit immunostaining, 3-nitrotyrosine, perivascular fibrosis, mitochondrial content, and markers of activity were significantly increased in Ren2 compared with SD littermates. Both renin inhibition and blockade of the AT1R significantly attenuated cardiac functional and structural alterations, although irbesartan treatment resulted in greater reductions of both blood pressure and markers of oxidative stress. Collectively, these data suggest that both reduce changes driven, in part, by Ang II-mediated increases in NADPH oxidase and, in part, increases in blood pressure.
Keywords: cardiac remodeling, reduced nicotinamide adenine dinucleotide phosphate oxidase, oxidative stress
increased tissue renin-angiotensin system (RAS) activity likely plays a key role in the development of cardiac hypertrophy, remodeling, and hypertension. Components of the RAS, including renin, constitute an autocrine/paracrine system in the myocardium. Angiotensin (Ang) II receptors have been characterized in cardiomyocytes and cardiac fibroblasts (8, 34) as well as the endothelial lining of coronary arteries (46). Conversion of angiotensinogen to Ang I by renin is the rate-limiting step in the generation of Ang II (32). Thus, inhibiting renin activation should abrogate any direct effects of renin as well as reduce tissue Ang II levels/actions. Blockade of the Ang II type I receptor (AT1R) has cardiac protective properties, but AT1R blockade also results in increased release of renin from renal juxtaglomerular cells, which may create a feed-forward mechanism for myocardial injury through cellular actions of renin that do not depend on conversion of angiotensinogen to angiotensin peptides (25). Thus, the use of renin inhibitors, such as aliskiren, hold promise in complementing current inhibitors of RAS activity in the prevention of tissue injury and remodeling by blocking this pathway at its source (27).
Many of the cardiac pathological effects of RAS activation appear to be mediated through redox cycling of reactive oxygen species (ROS), generated primarily by a NADPH oxidase-dependent pathway (4–6). In cardiomyocytes this oxidase is comprised of a membrane-bound NOX2 and p22phox heterodimer and four regulatory subunits, p40phox, p47phox, p67phox, and the small G protein Rac1 (4–6, 9). Activation of this complex promotes hypertrophy and perivascular fibrosis (16, 44). The importance of RAS-induced NADPH oxidase activation and the generation of ROS in this cardiac pathology is supported by the fact that inhibitors of NADPH oxidase (24, 31), free radical scavengers (12, 44), and AT1R blockade (30, 44) attenuate this pathological process.
The new nonpeptide renin inhibitor, aliskiren, is a potent human and mouse renin inhibitor; the IC50 (i.e., the half-maximal inhibitory concentration) for human renin is 0.6 nmol, for mouse renin is 4.5 nmol, and for rat renin is 80 nmol (13, 19). Because of this species specificity for its substrate, aliskiren cannot be studied efficiently in conventional rat models. To circumvent this issue, we have employed the transgenic (mRen2)27 rat (Ren2), which overexpresses the mouse renin transgene in a number of tissues, including the heart (21, 47). This allows for the investigation of renin inhibition in a model of chronic (in utero onward) RAS activation with subsequent elevated tissue levels of Ang II in the heart and other tissues. We have previously utilized this model to evaluate the role of elevated cardiac RAS on NADPH oxidase generation of ROS and resulting structural/functional changes as evaluated by transmission electron microscopy (TEM), histological, immunohistochemical, and biochemical techniques (16, 38, 44). In the current investigation, we hypothesized that renin inhibition employing aliskiren administration in vivo would attenuate the excessive oxidative stress perivascular and interstitial fibrosis and mitochondrial abnormalities that have been observed in myocardial tissue of Ren2 rats (11, 16, 28).
MATERIALS AND METHODS
Animals and treatments.
All animal procedures were approved by the University of Missouri animal care and use committees and housed in accordance with National Institutes of Health (NIH) guidelines. Male Ren2 (6–7 wk of age) and age-matched male SD rats were randomly assigned to placebo (Ren2-C and SDC), aliskiren (Ren2-A and SDA) at 50 mg·kg−1·day−1 (13), or irbesartan (Ren2-I) at 30 mg·kg−1·day−1 in saline via intraperitoneal injection. Since there have been no reports of comparisons of in vivo BP effects of irbesartan and aliskerin, we estimated, on the basis of available pharmacological data, the dose of irbesartan that would provide a BP-lowering effect comparable with that achieved with alliskerin at the dose used in this study.
Systolic BP.
After several simulated systolic BP (SBP) measurements for acclimation of animals, SBP was measured in triplicate, on separate occasions throughout the day, using the tail-cuff method (Student Oscillometric Recorder, Model MC4000 RSP; Harvard Systems) prior to initiation of treatment and on days 19 or 20 prior to the rats being killed (16, 38).
Measurement of NADPH oxidase activity.
NADPH oxidase activity was determined in plasma membrane fractions, as described previously (44). Aliquots of membrane and cytosolic fractions (12.5–100 μg proteins) were incubated with NADPH (100 μM) at 37°C. NADPH activity was determined by measuring the conversion of Radical Detector (Cayman Chemical) in the absence and presence of NADPH inhibitor diphenylene iodonium sulfate (500 μM) using spectrophotometric (450 nm) techniques.
Immunostaining of NADPH oxidase subunits.
Harvested left ventricle (LV) tissue was immersed and fixed in 3% paraformaldehyde (44). After fixation, tissues were placed in histological cassettes and dehydrated with ethanol series, infiltrated with low-melting (50°C) paraplast, and embedded in high-melting (56°C) paraplast. Blocks were sectioned by 4 μm, deparaffinized in CitriSolv, and rehydrated in ethanol series and HEPES wash buffer, and epitopes were retrieved in citrate buffer. The first section was washed (3 × 15 min) with HEPES wash buffer and then mounted with Mowiol (1st control level). The second and third sections were washed and incubated with 1:100 primary and secondary antibodies in 10-fold diluted blocking agent as the second and third control levels. Subsequent sections were incubated with 1:100 goat p47phox (Santa Cruz Biotechnology, Santa Cruz, CA) as well as mouse Rac1 (Upstate Cell Signaling, Charlottesville, VA), respectively, in 10-fold diluted blocker overnight. The goat antibody-based sections were incubated with 1:300 of Alexa fluor rabbit anti-goat 647 in 10-fold diluted blocker. The mouse antibody-based section was incubated with 1:300 of Alexa fluor goat anti-mouse 647. After 4 h, the slides were washed, mounted with Mowiol, and examined using a laser confocal scanning microscope. Images were captured using the Laser-sharp software (Bio-Rad, Hercules, CA), and signals were measured by MetaVue analysis (Boyce Scientific, Grey Summit, MO) (43, 44).
Evaluation of myocardial oxidative stress (3-nitrotyrosine).
Peroxynitrite (ONOO−), resulting from ROS scavenging of nitric oxide, binds to protein to produce stable 3-nitrotyrosine, which serves as a surrogate marker of oxidative stress (16). To assess myocardial 3-nitrotyrosine content, 4-μm LV sections were deparaffinized and rehydrated, and epitopes were retrieved in citrate buffer (16, 44). Endogenous peroxidases were quenched with 3% H2O2, and nonspecific binding sites were blocked with avidin, biotin, and protein block (Dako, Carpinteria, CA). The sections were then incubated overnight with 1:200 primary rabbit polyclonal anti-nitrotyrosine antibody (Chemicon, Temecula, CA). Subsequently, sections were washed and incubated with secondary antibodies, linked, and labeled with Streptavidin for 30 min each. After several rinses with distilled water, diaminobenzidine was applied for 10 min. The sections were again rinsed with distilled water, stained with hematoxylin for 1 min, rehydrated, and mounted with a permanent medium. The slides were evaluated under a bright field (Nikon 50i) microscope, and ×40 images were captured with a Cool Snapcf camera. Images were analyzed and the signal intensities measured as described previously.
Perivascular fibrosis.
To evaluate perivascular fibrosis, fixed paraffin sections of LV were evaluated with Verhoeff von Gieson (VVG), which stains elastin (black), nuclei (blue-black), collagen (red), and connective tissue (yellow) (38, 44). Slides were viewed with a Nikon50i microscope and ×4, ×10, and ×40 images were captured with a Cool Snapcf camera. Morphometric analysis was performed using MetaVue software. In each image, the perimeters of the adventitia, medium, and lumen of 15–20 coronary arteries of approximately equal cross-sectional area were traced, and the percentage area was then calculated by subtracting the combined areas for medium and lumen from the total area and then dividing by the total area. To evaluate interstitial fibrosis, morphometric analysis was applied to ×40 images to discriminate collagen from elastin on the basis of the VVG staining (43). Results are expressed as percent total area.
Mitochondrial quantification.
Left ventricle sections were fixed in 3% fresh paraformaldehyde and infiltrated and embedded in paraplast. Four-micrometer sections were deparaffinized in CitriSolv and rehydrated in ethanol series and HEPES wash buffer. The epitopes were retrieved in citrate buffer at 95°C for 25 min. Nonspecific binding sites were blocked with goat blocker for 4 h. Sections were then incubated with mouse anti-complex IV subunit 1 monoclonal antibody, 3 μg/ml in 10-fold diluted blocker (Mitosciences, Eugene, OR), or Mitotracker Deep-Red, 200 nm in 10-fold diluted blocker (Invitrogen, Eugene, OR), overnight. After being washed with HEPES wash buffer, the sections were incubated with 1:300 goat-anti mouse Alexa fluor 647. Four hours later the slides were washed and incubated with 1:2,000 4,6-diamidino-2-phenylindole to counterstain nuclei. After 10 min, the slides were washed and mounted with Mowiol. Slides were checked under a laser confocal microscope (Bio-Rad) and a multiphoton confocal system (44). Images were captured with Laser-sharp software and the mitochondria quantified using MetaMorph (Boyce Scientific, St. Louis, MO).
TEM.
Heart tissue was thinly sliced and placed in primary EM fixative as described (16). Following secondary fixation, specimens were placed on a rocker overnight, embedded, and polymerized at 60°C for 24 h. Eighty-five-nanometer sections were then stained with 5% uranyl acetate and Satos triple lead stain for viewing by a JOEL transmission electron microscope.
Statistical analysis.
All values are expressed as means ± SE. Statistical analyses were performed in SPSS 15.0 (SPSS, Chicago, IL) using unpaired Student's t-tests or ANOVA with Fisher's least significant difference test as appropriate.
RESULTS
SBP.
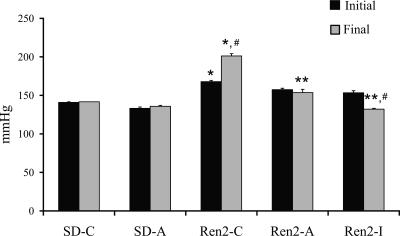
At the end of the treatment, Ren2 controls (Ren2-C) had a significantly higher systolic blood pressures compared with SD controls (SD-C; 189.6 ± 3.0 vs. 141.7 ± 0.1, P < 0.05), whereas rats treated with aliskiren (Ren2-A) did not develop further elevations in SBP over baseline levels during the 3 wk of treatment (153.6 ± 4.3 mmHg, P < 0.05 compared with Ren2-C) (Fig. 1). Treatment with irbesartan (Ren2-I) reduced BP more than what was observed with aliskiren treatment (132.1 ± 1.2 mmHg, Δ−21.2 mmHg, P < 0.05).
Fig. 1.
Systolic Blood Pressure (SBP) in the transgenic Ren2 rat. SBP was measured prior to the experimental protocol being started and on days 19 and 20 prior to the rats being killed (day 21). Values are presented as means ± SE. *P < 0.05 compared with Sprague-Dawley control (SD-C); **P < 0.05 compared with Ren2 control (Ren2-C); #P < 0.05 compared with aliskiren-treated Ren2 (Ren2-A). SD-A, aliskiren-treated Sprague-Dawley; Ren2-I, Ren2 rats treated with irbesartan.
NADPH oxidase.
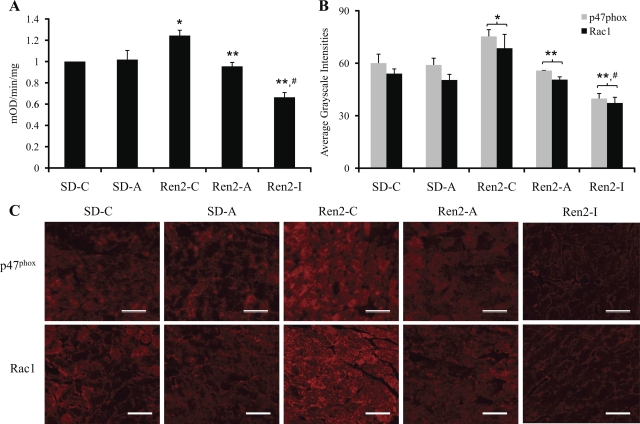
NADPH oxidase activity was elevated in Ren2 (1.24 ± 0.05 mOD·mg−1·min−1) normalized to SD controls (P < 0.05) (Fig. 2A). This activity was reduced with aliskiren treatment (0.95 ± 0.04 mOD·mg−1·min−1, P < 0.05) and to a greater extent with irbesartan (0.66 ± 0.05 mOD·mg−1·min−1, P < 0.05 compared with Ren2-C and Ren2-A). NADPH oxidase subunits p47phox and Rac1 were increased in the Ren2 control (75.3 ± 3.8 and 68.6 ± 7.9 average grayscale intensities, respectively) compared with SD control (60.0 ± 5.1 and 54.0 ± 2.7 average grayscale intensities, respectively, P < 0.05) (Fig. 2, B and C). There were reductions in both subunits in the aliskiren-treated Ren2 (55.8 ± 0.1 and 50.6 ± 1.6 average grayscale intensities, respectively, P < 0.05), and these reductions were even greater with irbesartan treatment (39.8 ± 2.9 and 37.3 ± 3.3 average grayscale intensities, P < 0.05 compared with Ren2-C and Ren2-A).
Fig. 2.
Myocardial NADPH oxidase in the transgenic Ren2 rat NADPH oxidase activity (A) and immunostaining of subunits p47phox and Rac1 and quantification of converted signal intensities in average grayscale intensities to the bottom (B and C). *P < 0.05 compared with SD-C; **P < 0.05 compared with Ren2-C; #P < 0.05 compared with aliskiren-treated Ren2 (Ren2-A). Scale bar, 50 um.
Oxidative stress measures.
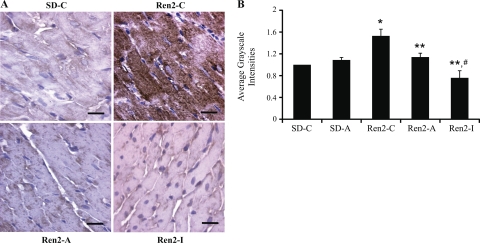
Immunostaining for 3-nitrotyrosine, a surrogate marker of oxidative and nitroso stress (13), was elevated in Ren2-C normalized to SD-C (1.53 ± 0.12 average grayscale intensities, P < 0.05; Fig. 3). 3-Nitrotyrosine levels were reduced with aliskiren treatment (1.14 ± 0.07 average grayscale intensities, P < 0.05), and this reduction was even greater with irbesartan (0.76 ± 0.13 average grayscale intensities, P < 0.05 compared with Ren2-C and Ren2-A).
Fig. 3.
Myocardial oxidative stress. A: 3-nitrotyrosine staining representative of oxidative and nitroso stress in left ventricle sections and quantification of converted signal intensities in average grayscale intensities to the right. *P < 0.05 compared with SD-C; **P < 0.05 compared with Ren2-C; #P < 0.05 compared with Ren2-A. Scale bar, 100 um.
Myocardial remodeling.
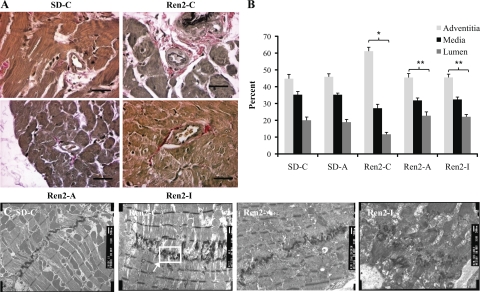
VVG staining was used to evaluate perivascular fibrosis in LV tissue. Perivascular fibrosis, represented by percent adventitial area per vessel, was significantly increased in the untreated Ren2-C compared with the SD-C (61.1 ± 2.4 vs. 44.7 ± 2.6%, P < 0.05) and abrogated by both aliskiren (45.4 ± 2.4%, P < 0.05) and irbesartan treatment (45.5 ± 2.4%, P < 0.05) (Fig 4A).
Fig. 4.
Myocardial remodeling in the transgenic Ren2 rat. Perivascular fibrosis visible by VVG staining (A) and quantification by %area to the right (B). *P < 0.05 compared with SD-C. **P < 0.05 compared with Ren2-C; scale bar, 100 um. C: remodeling of the intercalated discs (ID) in left ventricle tissue. 10K images demonstrate increased convolutions and duplication of the ID in Ren2-C that are not present in SD-C or treated animals (Ren2-A or SD-A). Scale bar, 500 nm.
Ultrastructural analysis of the myocardium utilizing TEM revealed qualitatively abnormal intercalated discs (ID) in the Ren2 left ventricle. There was a prominent appearance of ID duplication in the Ren2-C compared with the SD-C (Fig 4C). Duplication of the ID may reflect an increasing number of convolutions to compensate for the lengthening of this structure and the increase strength of adhesion with adjacent myofibrils (26). ID duplication was qualitatively diminished by treatment with both renin inhibition and AT1R blockade. Both treatments normalized the ultrastructural appearance of the myocardial tissue, resulting in fewer convolutions and less ID duplication.
Mitochondria.
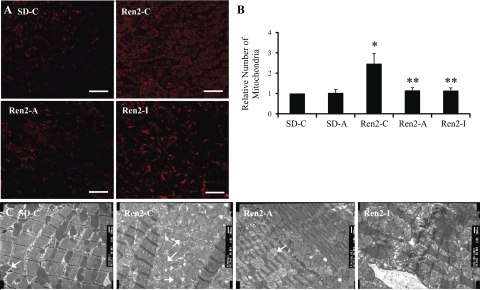
Quantification via immunostaining for mitochondria-specific protein complex IV subunit also revealed significant increases in the Ren2-C normalized to SD-C (2.47 ± 0.51 relative number, P < 0.05; Fig. 5, A and B) as well as attenuation of this phenomena following renin inhibition (1.15 ± 0.13 relative number, P < 0.05) and irbesartan treatment (1.14 ± 0.13 relative number, P < 0.05 compared with Ren2-C). Mitochondrial biogenesis was evaluated by TEM (16, 43) and quantified with immunostaining. 10K TEM images (Fig. 5C) of the Ren2-C heart revealed striking changes in the mitochondria. There were increased numbers of smaller mitochondria as well as increased convolutions (duplications) of ID in Ren2-C compared with the SD-C myocardial tissue (Fig. 5A). The structurally abnormal convoluted ID of the Ren2-C also seem to interfere with the normal orderly linear arrangement of the myofibrils. There was also an increase in complex IV (cytochrome c oxidase) in the mitochondria of Ren2 myocardial tissue reflecting overall increases in mitochondrial activity, likely a compensatory response to establish a transmembrane differential proton electrochemical potential used to generate ATP. Thus these mitochondrial changes likely reflect increased energy requirements in the untreated Ren2 myocardium (20). Following treatment with both the renin inhibitor and the AT1R blocker, the mitochondria number structural makeup and complex IV levels were similar to those observed in SD control myocardium.
Fig. 5.
Myocardial mitochondria in the transgenic Ren2 rat. A: left ventricular sections immunostained for mitochondrial complex 4 subunit 1. B: quantification of converted signal intensities in average grayscale intensities to the right. C: mitochondrial biogenesis within the left ventricle of the Ren2 on transmission electron microscopy. Ren2-C ventricle possesses increased numbers of mitochondria compared with SD-C or treated animals (Ren2-A and SD-A). Scale bar, 500 nm.
DISCUSSION
The purpose of this investigation was to evaluate the impact of two treatments to reduce tissue RAS, renin inhibition and AT1R blockade, on cardiac oxidative stress and remodeling in a transgenic rat manisfesting mouse renin transgene overexpression. Compared with its SD littermate, Ren2 rats are characterized by increased plasma and tissue total renin, prorenin, and Ang II despite lower kidney levels of renin (7). Previous reports have implicated elevated tissue Ang II levels in the activation of NADPH oxidase, which produces increased levels of oxidative stress that contribute to cardiac remodeling and dysfunction (4, 6, 16). Similarly to previous work with AT1R blockade (44), results of this investigation show that in vivo inhibition of renin, the rate-limiting step in Ang II formation, abrogates RAS-mediated enhanced activation of NADPH oxidase/increased oxidative stress and reduces left ventricular perivascular fibrosis, mitochondrial content, and remodeling. Although aliskerin and irbresatan had similar overall effects, irbesartan reduced SBP and parameters of oxidative stress to a greater extent than aliskerin in this investigation. Indeed, there is evidence that the degree of BP lowering, independently of RAS effects, may impact reductions in cardiovascular NADPH oxidase activity (42). Thus, it is likely that the greater BP-lowering effects of irbesartan, in the dose used in this study, accounted for the greater lowering of NADPH oxidase activity.
The heart has a limited endogenous antioxidant capacity, which renders it highly susceptible to ROS-induced tissue damage (12). In fact, oxidative stress has been causally associated with cardiac hypertrophy, remodeling, and ventricular dysfunction (10, 38, 44). Data from our laboratory have demonstrated that myocardial activation of NADPH oxidase and resultant increases in oxidative stress contribute to remodeling and dysfunction (16, 44). This is critical to attribute, in large part, due to the structural and functional changes observed with oxidative stress rather than physical stress induced by hypertension.
Immunostaining for 3-nitrotyrosine demonstrated that left ventricular tissues harvested from untreated heterozygous male Ren2 transgenic rats manifested significantly increased NADPH oxidase activity and ROS compared with age-matched SD controls. Three weeks of treatment with aliskiren and irbesartan effectively reduced ROS and reactive nitrogen species, as evidenced by reduced 3-nitrotyrosine immunostaining in Ren2 rat myocardium. Untreated Ren2 myocardium also displayed increases in immunostaining for the NADPH subunit p47phox and Rac1, which together with the nitrotyrosine data are indicative of increased NADPH oxidase-induced oxidative stress in Ren2 myocardium. Translocation of the small GTP-binding protein Rac1 and p47phox to the cell membrane is necessary for assembly and activation of membrane NADPH oxidase and enhanced ROS and reactive nitrogen species generation that has been directly implicated in Ang II-induced cardiac hypertrophy and fibrosis (24, 31, 37). In accordance with previous studies (16, 38, 43), histological examination using VVG staining showed prominent increases in myocardial perivascular fibrosis and ultrastructural remodeling in the untreated Ren2. Both structural and biochemical changes were normalized by 3 wk of both aliskiren and irbesartan treatment. Thus, both renin inhibition and AT1R blockade in young Ren2 rats effectively attenuated myocardial NADPH oxidase overactivity and reduced markers of oxidative stress; this was accompanied by reduced myocardial fibrosis and cellular ultrastructural TEM abnormalities.
TEM showed that heterozygous male Ren2 hearts possess increased numbers of smaller mitochondria, consistent with staining for mitochondrial protein, as published previously from our laboratory (38, 44). There were also increased convolutions (duplication) of myocardial ID, suggesting a structural response to increased mechanical requirements in Ren2 heart tissue. Increased numbers of smaller mitochondrial are suggestive of altered mitochondrial function and biogenesis/or enhanced energy requirements in hearts subjected to excessive oxidative stress and cardiac afterload (17, 26, 36). Additionally, the increased convolutions (duplication) of the ID observed may also serve to increase contact between cardiomyocytes as an adaptive response to increased cardiac energy requirements and reduced contractile efficiency accompanying increased afterload (38, 44). The reduction in complex IV with renin inhibition and AT1R blockade may reflect a lesser work load on the Ren2 heart following either RAS inhibition therapeutic strategy.
Oxidative stress can adversely affect mitochondria through numerous mechanisms, such as mitochondrial DNA (mtDNA) damage (26, 36). Damaged mtDNA, such as mutant mtDNA with large deletions, has been shown to quickly and predominantly repopulate cellular mitochondria (17). Additionally, low myocardial ATP concentrations, due to dysfunctional mitochondria, have been shown to stimulate proliferation of defective mitochondria in an attempt to meet the energy requirements of the tissue (17, 26, 36). This notion is consistent with the idea that mitochondria in highly oxidative environments generally proliferate more rapidly and thus tend to be smaller and more plentiful. This concept is reinforced by our observations that these mitochondrial morphological, as well as functional, alterations are markedly attenuated, in conjunction with contemporaneous reductions in oxidative stress, in young Ren2 rats treated following in vivo treatment with agents reducing RAS activity.
GRANTS
This research was supported by an NHLBI grant (R01-HL-73101-01A1) for J. R. Sowers, the Veterans Affairs Merit System (0018) for J. R. Sowers, an Advanced Research Career Development Award for C. S. Stump, a Veterans Integrated Service Network 15 Award for A. Whaley-Connell, the Missouri Kidney Program for A. Whaley-Connell, and Novartis Pharmaceuticals (J. R. Sowers and A. Whaley-Connell).
Acknowledgments
Male transgenic Ren2 rats and male Sprague-Dawley controls were kindly provided by Wake Forest University School of Medicine, Winston-Salem, NC, through the Transgenic Core Facility supported in part by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-51952. We also acknowledge the electron microscope core facility for help and preparation of transmission electron micrographs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J 15: 2548–2550, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21 rac1. Nature 353: 668–670, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Barlucchi L, Leri A, Dostal DE, Fiordaliso F, Tada H, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. Canine ventricular myocytes possess a renin-angiotensin system that is upregulated with heart failure. Circ Res 88: 298–304, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res 98: 730–742, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802–805, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension 25: 1014–1020, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Crabos M, Roth M, Hahn AW, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest 93: 2372–2378, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Custodis F, Eberl M, Kilter H, Bohm M, Laufs U. Association of RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy. Cardiovasc Res 71: 342–351, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Das DK, Maulik N, Engelman RM. Redox regulation of angiotensin II signaling in the heart. J Cell Mol Med 8: 144–152, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer RA, Pokharel S, Flesch M, van Kampen DA, Suurmeijer AJ, Boomsma F, van Gilst WH, van Veldhuisen DJ, Pinto YM. Extracellular signal regulated kinase and SMAD signaling both mediate the angiotensin II driven progression towards overt heart failure in homozygous TGR(mRen2)27. J Mol Med 82: 678–687, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol 141: 105–113, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman D Aliskiren ameliorates diabetic nephropathy in diabetic TG(mRen-2)27 rats (Abstract). In: Council for High Blood Pressure Research. Tucson, AZ: American Heart Association, 2007.
- 14.Giordano FJ Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res 40: 352–363, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Tramonato A, Thyfault J, Stump C, Ferrario C, Muniyappa R, Sowers JR. Rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology 148: 2181–2188, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hofhaus G, Gattermann N. Mitochondria harbouring mutant mtDNA—a cuckoo in the nest? Biol Chem 380: 871–877, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Jalil JE, Perez A, Ocaranza MP, Bargetto J, Galaz A, Lavandero S. Increased aortic NADPH oxidase activity in rats with genetically high angiotensin-converting enzyme levels. Hypertension 46: 1362–1367, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia 50: 2398–2404, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 102: 401–414, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MA, Bohm M, Kim S, Bachmann S, Bachmann J, Bader M, Ganten D. Differential gene expression of renin and angiotensinogen in the TGR(mREN-2)27 transgenic rat. Hypertension 25: 570–580, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, Silver RB, Levi R. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 116: 1063–1070, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra R, Sadoshima J, Brosius FC 3rd, Izumo S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes In vitro. Circ Res 85: 137–146, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol 35: 851–859, 2003. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien E, Barton J, Nussberger J, Mulcahy D, Jensen C, Dicker P, Stanton A. Aliskiren reduces blood pressure and suppresses plasma renin activity in combination with a thiazide diuretic, an angiotensin-converting enzyme inhibitor, or an angiotensin receptor blocker. Hypertension 49: 276–284, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28: 272–275, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Pilz B, Shagdarsuren E, Wellner M, Fiebeler A, Dechend R, Gratze P, Meiners S, Feldman DL, Webb RL, Garrelds IM, Jan Danser AH, Luft FC, Muller DN. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension 46: 569–576, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Pinto YM, Pinto-Sietsma SJ, Philipp T, Engler S, Kossamehl P, Hocher B, Marquardt H, Sethmann S, Lauster R, Merker HJ, Paul M. Reduction in left ventricular messenger RNA for transforming growth factor beta(1) attenuates left ventricular fibrosis and improves survival without lowering blood pressure in the hypertensive TGR(mRen2)27 rat. Hypertension 36: 747–754, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, Chiang Y, Satlin A. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens 20: 11–20, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42: 206–212, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Qin F, Patel R, Yan C, Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic Biol Med 40: 236–246, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Rahuel J, Rasetti V, Maibaum J, Rueger H, Goschke R, Cohen NC, Stutz S, Cumin F, Fuhrer W, Wood JM, Grutter MG. Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin. Chem Biol 7: 493–504, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Rossi GP, Sacchetto A, Rizzoni D, Bova S, Porteri E, Mazzocchi G, Belloni AS, Bahcelioglu M, Nussdorfer GG, Pessina AC. Blockade of angiotensin II type 1 receptor and not of endothelin receptor prevents hypertension and cardiovascular disease in transgenic (mREN2)27 rats via adrenocortical steroid-independent mechanisms. Arterioscler Thromb Vasc Biol 20: 949–956, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73: 413–423, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Seccia TM, Belloni AS, Kreutz R, Paul M, Nussdorfer GG, Pessina AC, Rossi GP. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. J Am Coll Cardiol 41: 666–673, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 91: 10771–10778, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sowers JR Hypertension, angiotensin II, and oxidative stress. N Engl J Med 346: 1999–2001, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi P, Qazi M, Morris EM, Cooper SA, Link CD, Stump CS, Hay M, Ferrario C, Sowers JR. Mineralcorticoid receptor blockade attenuates chronic overexpression of the renin angiotensin aldosterone system stimulation of NADPH oxidase and cardiac remodeling. Endocrinology 148: 3771–3780, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Tokuda K, Kai H, Kuwahara F, Yasukawa H, Tahara N, Kudo H, Takemiya K, Koga M, Yamamoto T, Imaizumi T. Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension 43: 499–503, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Villamil A, Chrysant SG, Calhoun D, Schober B, Hsu H, Matrisciano-Dimichino L, Zhang J. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 25: 217–226, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Villarreal FJ, MacKenna DA, Omens JH, Dillmann WH. Myocardial remodeling in hypertensive Ren-2 transgenic rats. Hypertension 25: 98–104, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 40: 504–510, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Whaley-Connell A, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario CM, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 trangenic rat. Am J Physiol Renal Physiol 291: F1308–F1314, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karupathi PR, Stump C, Ferrario C, Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic TG (mRen2)27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Wood JM, Schnell CR, Cumin F, Menard J, Webb RL. Aliskiren, a novel, orally effective renin inhibitor, lowers blood pressure in marmosets and spontaneously hypertensive rats. J Hypertens 23: 417–426, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res 92: 322–329, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Bader M, Kreutz R, Fernandez-Alfonso M, Zimmermann F, Ganten U, Metzger R, Ganten D, Mullins JJ, Peters J. Ontogenetic regulation of mouse Ren-2d renin gene in transgenic hypertensive rats, TGR(mREN2)27. Am J Physiol Endocrinol Metab 265: E699–E707, 1993. [DOI] [PubMed] [Google Scholar]